Abstract
Little is known about iron efflux transporters within bacterial systems. Recently, the participation of Bacillus subtilis PfeT, a P1B4-ATPase, in cytoplasmic Fe2+ efflux has been proposed. We report here the distinct roles of mycobacterial P1B4-ATPases in the homeostasis of Co2+ and Fe2+. Mutation of Mycobacterium smegmatis ctpJ affects the homeostasis of both ions. Alternatively, an M. tuberculosis ctpJ mutant is more sensitive to Co2+ than Fe2+, whereas mutation of the homologous M. tuberculosis ctpD leads to Fe2+ sensitivity but no alterations in Co2+ homeostasis. In vitro, the three enzymes are activated by both Fe2+ and Co2+ and bind 1 eq of either ion at their transport site. However, equilibrium binding affinities and activity kinetics show that M. tuberculosis CtpD has higher affinity for Fe2+ and twice the Fe2+-stimulated activity than the CtpJs. These parameters are paralleled by a lower activation and affinity for Co2+. Analysis of Fe2+ and Co2+ binding to CtpD by x-ray absorption spectroscopy shows that both ions are five- to six-coordinate, constrained within oxygen/nitrogen environments with similar geometries. Mutagenesis studies suggest the involvement of invariant Ser, His, and Glu residues in metal coordination. Interestingly, replacement of the conserved Cys at the metal binding pocket leads to a large reduction in Fe2+ but not Co2+ binding affinity. We propose that CtpJ ATPases participate in the control of steady state Fe2+ levels. CtpD, required for M. tuberculosis virulence, is a high affinity Fe2+ transporter involved in the rapid response to iron dyshomeostasis generated upon redox stress.
Keywords: ATPase, iron, metal homeostasis, metal ion-protein interaction, Mycobacterium tuberculosis, transport metal, P1B4-ATPase
Introduction
Iron is an essential micronutrient required for numerous biological processes as it is used as a prosthetic group by several different enzymes (1, 2). However, in excess, it can be toxic due to its participation in Fenton chemistry and potential mismetallation in non-iron-containing metalloproteins. In this context, damage of iron-sulfur centers and mononuclear iron enzymes produced by various redox stresses are particular contributors to iron dyshomeostasis and consequent toxicity (3–6). Characterization of bacterial Fe2+ homeostasis has mainly been focused in mechanisms of uptake (by divalent metal, siderophore, and heme transporters), transcriptional regulation (by Fur and IdeR systems), and Fe2+ sequestration (by bacterioferritin and Dps proteins) (2, 7–9). Nevertheless, studies have suggested that cation diffusion facilitators and iron-citrate transporters participate in Fe2+ efflux (10–12). We recently observed that Bacillus subtilis PfeT, a P1B4-ATPase, confers Fe2+ tolerance (13). PfeT is expressed under the control of PerR in response to peroxide exposure (14). Initial biochemical characterization showed that Fe2+ activates isolated PfeT ATPase, leading to a higher Vmax than generated by Co2+, which is the proposed substrate of P1B4-ATPases (13, 15–17). Interestingly, phenotypic analysis of Listeria monocytogenes lacking the P1B4-ATPase FrvA showed a role of this ATPase in resistance to heme toxicity (18). These observations suggest a significant role of this subfamily of P-type ATPases in Fe2+ homeostasis (13, 14).
P1B4-ATPases present in prokaryotes and plant chloroplasts are part of the large family of P-type ATPases (15, 19, 20). P-type ATPases are polytopic membrane proteins that transport a variety of ions using the energy provided by ATP hydrolysis (21–23). The P1B subgroup includes proteins responsible for the efflux of cytoplasmic transition metals including Cu+, Zn2+, Co2+, and Ni2+ (19, 22, 23). The specificity of their transmembrane metal binding sites (TM-MBSs)2 is determined by invariant amino acid sequences in their last three transmembrane segments (TMs) (17, 19, 24–26). However, activation by non-cognate substrates has been reported for most P1B-ATPase subgroups (22, 27). In particular, activation of P1B4-ATPases by Co2+, Ni2+, Ca2+, Cu+, Zn2+, and Cd2+ has been proposed (15–17, 28–30). We previously reported in vivo and in vitro functional studies directed at understanding the metal selectivity and consequent physiological roles of mycobacterial P1B4-ATPases (15, 16). The presence of one or two P1B4-ATPase-coding genes in mycobacterial species enabled comparative studies of Mycobacterium smegmatis CtpJ and Mycobacterium tuberculosis CtpJ and CtpD. In vitro, MsCtpJ and MtCtpJ display a higher activation by Co2+ and Ni2+ compared with Zn2+, although equilibrium binding affinities show KD values for Zn2+ < Co2+ = Ni2+ (15, 16). In vivo, ctpJ expression is induced by Co2+, whereas mutant strains show accumulation and sensitivity to the metal. On the contrary, the expression of the homologous MtctpD is not induced by Co2+ but rather by redox stress. Mutation of MtctpD does not lead to Co2+ sensitivity or higher intracellular levels of this metal. Nevertheless, MtCtpD ATPase activity is partially activated by Co2+. Surprisingly, MtCtpD but not MtCtpJ is required for M. tuberculosis virulence.
Previous studies have not explored the activation of mycobacterial P1B4-ATPases by Fe2+. Could a differential activation by Co2+/Fe2+ explain the presence of paralogous genes in M. tuberculosis? Why is MtCtpD but not MtCtpJ required for virulence? To address these questions, we examined the activation of M. smegmatis and M. tuberculosis P1B4-ATPases by Fe2+ and their participation in Fe2+ homeostasis and stress response. In addition, we explored the molecular basis of the different Fe2+ and Co2+-ATPase activities by determining the coordination of these metals during transport by MtCtpD.
Experimental Procedures
Mycobacterium Strains and Culture Conditions
M. smegmatis mc2155, M. tuberculosis H37Rv, and derived strains were grown in 7H9 liquid medium (BD Biosciences, Difco) supplemented with 0.2% glycerol, 0.05% Tween 80, and 10% ADN supplement (0.5% bovine serum albumin, 0.2% dextrose, and 0.085% NaCl) or in low iron defined medium (LIMM) containing 0.5% l-asparagine, 0.5% KH2PO4, 2% glycerol, 0.05% Tween 80, and 10% ADN, pH 6.8 (31). LIMM was treated with Chelex-100 (Sigma) and before use supplemented with 3.7 μm ZnCl2, 0.8 μm MnCl2, and 0.4 mm MgCl2. This medium contained less than 1 μm residual iron as determined by atomic absorption spectroscopy (AAS) (PerkinElmer Life Sciences PinAAcle 900z). Construction of MsΔctpJ (MSMEG_5403), MtΔctpD (Rv1469), MtΔctpJ (Rv3743), and MtΔctpD:ΔctpJ mutant and complemented strains was described previously (15, 16).
Iron and Hemin Sensitivity Tests
Liquid LIMM cultures of M. smegmatis mc2155, M. tuberculosis H37Rv, mutant, and complemented strains were inoculated at 0.05 A600 from late exponential phase cultures and supplemented with the desired concentration of FeCl3 or hemin (Sigma). A hemin stock solution was prepared at 25 mg/ml in 1.4 m NaOH. Cells were incubated for 16 h (M. smegmatis) or 5 days (M. tuberculosis), and A600 was measured. To avoid hemin interference in A600 readings, cells grown in hemin-containing medium were collected, washed twice with LIMM, and suspended in the original LIMM volume, and A600 was measured.
Streptonigrin Sensitivity Tests
M. smegmatis mc2155, M. tuberculosis H37Rv, mutant, and complemented strains grown in LIMM to midlog phase were diluted to 0.05 A600 in LIMM. The cultures were supplemented with 1 μg ml−1 streptonigrin (STN) and 10 μm FeCl3 as indicated in the figures. Cells were incubated for 16 h (M. smegmatis) or 5 days (M. tuberculosis), and A600 was measured.
Metal Accumulation Assays
Liquid LIMM cultures in midexponential phase (A600 ∼ 1.0) were supplemented with increasing concentrations of FeCl3 and incubated for 4 (M. smegmatis) or 8 h (M. tuberculosis). After this incubation, cells were harvested and washed with 5 mm EDTA and 0.9% NaCl. Aliquots were taken for protein determinations (32). Pellets were acid-digested with 0.5 ml of NO3H for 1 h at 80 °C and then overnight at 20 °C. Digestions were concluded by adding ⅛ volume of 30% (v/v) H2O2 followed by a 1:5 dilution with water. Metal contents in digested samples were measured by AAS.
Protein Expression and Purification
Preparation of E. coli LMG194 ΔcopA strains carrying M. smegmatis ctpJ (MSMEG_5403) or M. tuberculosis ctpD (Rv1469) or ctpJ (Rv3743) in pBAD-TOPO/His vectors was described previously (15, 16). Cells were grown at 37 °C in ZYP-505 autoinduction medium supplemented with 0.05% arabinose, 100 mg ml−1 ampicillin, and 50 mg ml−1 kanamycin (33). Cells were harvested at 16 h postinoculation; washed with 25 mm Tris, pH 7.0, 100 mm KCl, and 20% glycerol; and stored at −70 °C. Expressed mycobacterial proteins contained a C-terminal His6 tag sequence preceded by a tobacco etch virus protease recognition sequence. Protein purification was carried out as described previously (15, 24, 34). Briefly, cells were disrupted in a French press, and membranes were isolated by centrifugation. Membranes were treated with 0.75% dodecyl β-d-maltoside (Calbiochem), 25 mm Tris, pH 8.0, 100 mm sucrose, 500 mm NaCl, and 1 mm phenylmethylsulfonyl fluoride. The solubilized membrane protein suspension was cleared by centrifugation at 163,000 × g for 1 h, and proteins were affinity-purified using Ni2+-nitrilotriacetic acid resin. The His6 tag was removed from the C terminus by treatment with His6-tagged tobacco etch virus protease (35). Tobacco etch virus-His6 was removed by affinity purification with Ni2+-nitrilotriacetic acid resin. Protein purity was analyzed by 10% SDS-PAGE followed by Coomassie Brilliant Blue staining or Western blotting using an anti-His6 tag antibody (GenScript, Piscataway, NJ). Isolated proteins (3 mg/ml) were stored at −20 °C in 25 mm Tris, pH 8.0, 100 mm sucrose, 50 mm NaCl, 0.01% dodecyl β-d-maltoside, 0.01% asolectin, 1 mm phenylmethylsulfonyl fluoride, and 20% glycerol. Prior to ATPase activity determinations, proteins (1 mg/ml) were treated with 0.5 mm EDTA and 0.5 mm tetrathiomolybdate for 45 min at room temperature. Chelators were removed using Ultra-30 Centricon (Millipore, Darmstadt, Germany) filtration devices.
Mutagenesis of MtCtpD Metal Binding Site
MtctpD cloned into pBAD-TOPO/His vector was used as a template to introduce the mutations coding for the single substitutions Ser-316 (S316A), Cys-318 (C318A), His-642 (H642A), Glu-643 (E643A and E643D), Gly-644 (G644A), Ser-645 (S645A), and Thr-646 (T646A and T646S) and the multiple replacements S316C/C318S. All mutations were introduced using a Q5® site-directed mutagenesis kit (New England Biolabs, Ipswich, MA). The sequences of primers used in this study are available upon request to the corresponding author. DNA sequences were confirmed by automated sequencing.
Fe2+ Binding to Proteins
Metal binding to isolated enzymes was measured as described previously (15, 36). Five micromolar His-less enzyme was incubated for 1 min at 4 °C in 25 mm HEPES-NaOH, pH 7.5, 150 mm NaCl, 1 mm tris(2-carboxyethyl)phosphine (TCEP), and 20 μm either FeSO4 or CoCl2. Excess metal was removed by washing in a 30-kDa-cutoff Centricon filtration device. Protein samples were acid-digested as described above, and metal concentrations were measured using AAS.
Metal binding affinities were determined using the divalent metal-binding chromophore mag-fura-2 (Invitrogen) (15, 36). Five micromolar His-less protein and 10 μm mag-fura-2 were titrated with 1 mm Fe2+ or Co2+ in Chelex-treated buffer (25 mm HEPES-NaOH, pH 7.5, 150 mm NaCl, and 1 mm TCEP). Free mag-fura-2 was determined by monitoring A366 (ϵ366 29,900 1/m·cm). Free metal concentrations were calculated from KI = [I·Me2+]/[Ifree][Me2+free] where I is mag-fura-2, Me is the metal ion, and KI is the association constant of mag-fura-2 with each metal. KD values of 1.5 μm for Fe2+ and 2.8 μm for Co2+ were experimentally determined for the metal/mag-fura-2 interactions. The metal-protein KD values were calculated from ν = n[Me2+free]/KD(1 + ([Me2+free]/KD) where ν is the molar ratio of metal bound to protein and n is the apparent stoichiometry (37). Reported errors for KD and n are asymptotic standard errors provided by the fitting software KaleidaGraph (Synergy, Reading, PA).
ATPase Assays
ATPase assays were performed as described (15, 24, 34). The assay mixture contained 50 mm Tris, pH 7.4, 50 mm NaCl, 3 mm MgCl2, 3 mm ATP, 0.01% asolectin, 0.01% dodecyl β-d-maltoside, 2.5 mm TCEP, 20 μg/ml purified protein, and freshly prepared transition metal ions at the desired concentrations. Fe3+ was added as FeCl3, Cu2+ was added as CuSO4, and in both cases TCEP was not included in the assay medium. Cu+ was obtained by including TCEP with CuSO4 salt. Fe2+ and Zn2+ were included in the assay medium as the sulfate salts, whereas Co2+, Ni2+, and Mn2+ were included as their chloride salts. ATPase activity was stopped after a 20-min incubation at 37 °C, and released Pi was determined (38). ATPase activity measured in the absence of transition metals was subtracted from plotted values. Curves of ATPase activity metal dependence were fit to v = Vmax[Me+/2+]/([Me+/2+] + K1/2). The reported standard errors for Vmax and K1/2 are asymptotic standard errors reported by the fitting software KaleidaGraph.
X-ray Absorption Spectroscopy (XAS)
XAS samples were loaded in a Coy anaerobic chamber at a 1:1 metal:protein molar ratio (1.2 mm Fe2+ or 0.43 mm Co2+). Sample was injected into a Kapton-wrapped Lucite cell, flash frozen, and stored in liquid nitrogen. XAS data were collected at the Stanford Synchrotron Radiation Lightsource on beamline 7-3 equipped with a Si(220) double crystal monochromator with a harmonic rejection mirror. Fluorescence spectra were collected using a 30-element germanium solid-state detector (Canberra, Meriden, CT). During data collection, the continuous flow liquid helium cryostat (Oxford Instruments, Concord, MA) was stabilized at 10 K. Iron and cobalt data were collected using a 6-μm magnesium or a 3-μm iron filter, respectively, placed between the cryostat and the detector to reduce unassociated scattering. Iron and cobalt foil spectra were collected simultaneously with protein data for direct energy calibration of the data. The first inflection points for iron and cobalt were set at 7111.3 and 7709.5 eV, respectively. Iron XAS spectra were recorded using 5-eV steps in the pre-edge regions (6900–7094 eV), 0.25-eV steps in the edge regions (7095–7135 eV), and 0.05-Å−1 increments in the extended x-ray absorption fine structure (EXAFS) region (to k = 13.5 Å−1), integrating from 1 to 20 s in a k3-weighted manner. Cobalt XAS spectra were recorded using 5-eV steps in the pre-edge regions (7542–7702 eV), 0.25-eV steps in the edge regions (7702–7780 eV), and 0.05-Å−1 increments in the extended EXAFS region (to k = 13.5 Å−1), integrating from 1 to 25 s in a k3-weighted manner. A total of eight scans were taken for each sample, and these were then averaged.
XAS spectra were processed and analyzed using the EXAFSPAK program suite for Macintosh OSX (39). A Gaussian function was used in the pre-edge region, and a three-region cubic spline was used in the EXAFS region. EXAFS data were converted to k space using E0 values of 7130 and 7745 eV for iron and cobalt, respectively. Spectra were simulated using single and multiple scattering amplitude and phase functions generated using the Feff v8 software integrated within EXAFSPAK. Single scattering models were calculated for oxygen, nitrogen, and carbon to simulate possible iron- or cobalt-ligand environment. Calibrated scale factors and model E0 values were not allowed to vary during fitting; the scale factor for iron was 0.95, and that for cobalt was 0.98. Iron data were fit out to a k value of 13.5 Å−1. Calibration from Fe2+ and Fe3+ model compounds was used for determination of E0 and scale factor parameters for iron. E0 values for Fe–O and Fe–C were set at −10 eV. Cobalt data were fit out to a k value of 13.5 Å−1. Calibration from Co2+ model compounds was used for determination of E0 and scale factor parameters. E0 values for Co–O and Co–C were set at −11.3 eV. EXAFS spectra were simulated using both filtered and unfiltered data; however, simulation results are presented only for fits to unfiltered (raw) data. Simulation protocols and criteria for determining the best fit have been described elsewhere (25).
Results
Mycobacterial P1B4-ATPases Confer Fe2+ Tolerance
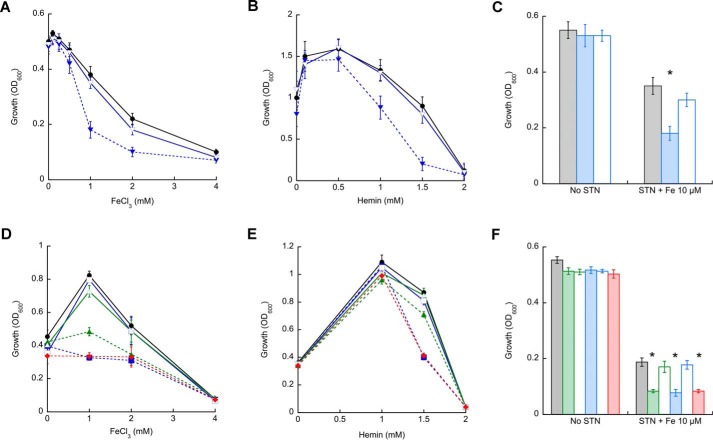
We previously reported the activation of mycobacterial P1B4-ATPases by Co2+ (15, 16). However, M. tuberculosis CtpJ and CtpD appear to have distinct roles in Co2+ tolerance and cellular response to redox stress (16). We recently observed that B. subtilis PfeT, a P1B4-ATPase in the PerR regulon, transports and confers tolerance to Fe2+ in addition to Co2+ (13). Similarly, the L. monocytogenes P1B4-ATPase, FrvA, confers resistance to heme toxicity (18). Thus, we hypothesized that selective activation by Fe2+ might explain the presence of the ctpJ and ctpD paralogs in the M. tuberculosis genome. To test this idea, the capability of mycobacterial P1B4-ATPases to confer tolerance to Fe2+ was assessed. Fig. 1 shows the growth of MsΔctpJ, MtΔctpD, MtΔctpJ, and MtΔctpD:ΔctpJ double mutant strains in LIMM supplemented with different concentrations of FeCl3 or hemin. The MsΔctpJ strain showed a growth defect at high FeCl3 or hemin as compared with M. smegmatis WT (Fig. 1, A and B). The complemented MsΔctpJ strain, carrying the plasmid pMV306 harboring the full-length MsctpJ gene under the regulation of its native promoter, showed similar growth as the M. smegmatis WT. A comparable deficiency was observed in the MtΔctpJ strain grown in the presence of 1 mm FeCl3 (Fig. 1D). However, this mutant strain was not affected by the presence of hemin in the medium. On the contrary, mutation of the MtctpD gene significantly affected the growth in both FeCl3- and hemin-supplemented medium. Interestingly, the MtΔctpD:ΔctpJ double mutant strain showed a behavior identical to that of the MtΔctpD strain. In all cases, complemented strains showed the growth phenotype of M. tuberculosis WT (Fig. 1, D and E). The data indicate that MsCtpJ and MtCtpD confer iron tolerance when cells are exposed to relatively high metal levels. Exploring their role at lower iron levels, the sensitivity to STN was tested. STN is a quinone antibiotic whose activity is correlated with intracellular iron availability (40). The MsΔctpJ, MtΔctpD, MtΔctpJ, and MtΔctpD:ΔctpJ mutant strains displayed a significantly increased STN sensitivity in LIMM supplemented with 1 μg ml−1 STN and only 10 μm FeCl3 (Fig. 1, C and F). In contrast to their distinct tolerance to high Fe3+ in the medium, there was no significant difference in the sensitivity of MtΔctpD and MtΔctpJ strains to STN-Fe2+.
FIGURE 1.
Growth response of mycobacterial P1B4-ATPase mutant strains to iron, hemin, and streptonigrin/iron. A and B, M. smegmatis WT (●; black) and ΔctpJ (▾; blue) and complemented (▿; blue) strains were grown in the presence of increasing concentrations of FeCl3 (A) or hemin (B) in the LIMM for 16 h, and A600 was measured. C, M. smegmatis WT (gray bars), ΔctpJ (blue bars), and complemented (white bars) strains were grown in LIMM supplemented with 1 μg ml−1 STN and 10 μm FeCl3, and growth was measured at 16 h. D and E, M. tuberculosis WT (●; black), ΔctpD (■; blue) and complemented (□; blue), ΔctpJ (▴; green) and complemented (▵; green), and ΔctpD:ΔctpJ (♦; red) strains were grown in the presence of increasing concentrations of FeCl3 (D) or hemin (E) in LIMM for 5 days, and A600 was measured. F, M. tuberculosis WT (gray bars), ΔctpD (green bars), ΔctpD complemented (white bars), ΔctpJ (blue bars), ΔctpJ complemented (white bars), and ΔctpD:ΔctpJ (red bars) strains were grown in LIMM supplemented with 1 μg ml−1 STN and 10 μm FeCl3, growth was measured at 5 days, and A600 was measured. Data are the mean ± S.E. (error bars) of three independent experiments. Significant differences from the WT as determined by Student's t test are indicated (*, p < 0.05).
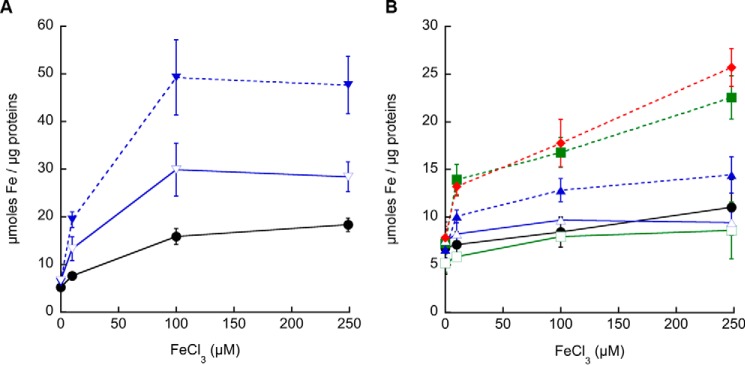
These results suggest that to different extents mycobacterial P1B4-ATPases contribute to Fe2+ homeostasis by driving this metal efflux. To further explore this hypothesis, MsΔctpJ, MtΔctpD, MtΔctpJ, and MtΔctpD:ΔctpJ mutant strains were challenged with sublethal concentrations of FeCl3, and the resulting cellular Fe2+ levels were determined. Consistent with the iron sensitivity phenotypes (Fig. 1), Fe2+ accumulation was observed in mutant strains (Fig. 2, A and B). Iron levels in the MsΔctpJ strain were approximately 5 times higher than those in M. smegmatis WT (Fig. 2A). The partial recovery observed in the MsΔctpJ mutant strain complemented with MsctpJ appears to be associated with lower levels of transcript (35% of WT; not shown). Reinforcing the predominant role of MtCtpD in Fe2+ homeostasis, a significant increase of Fe2+ content was observed in the MtΔctpD strain, whereas 50% smaller changes were observed in the MtΔctpJ strain. Similar Fe2+ accumulation was observed in the MtΔctpD and MtΔctpD:ΔctpJ double mutant strains (Fig. 2B). These results suggest that although mycobacterial CtpJs are involved in controlling Co2+ levels they also participate in Fe2+ efflux, particularly when they are the only P1B4-ATPase in the organism as in M. smegmatis. In contrast, MtCtpD appears to play a dominant role in maintaining the cytoplasmic Fe2+ level in this organism.
FIGURE 2.
Iron levels in mycobacterial P1B4-ATPase mutant strains. A, M. smegmatis WT (●; black), ΔctpJ (▾; blue), and complemented (▿; blue) strains grown in LIMM supplemented with increasing concentrations of FeCl3 for 4 h. B, M. tuberculosis WT (●; black), ΔctpD (■; green), ΔctpD complemented (□; green), ΔctpJ (▴; blue), ΔctpJ complemented (▵; blue), and ΔctpD:ΔctpJ (♦; red) strains grown in LIMM supplemented with increasing concentrations of FeCl3 for 8 h. Data are the mean ± S.E. (error bars) of three independent experiments.
Distinct Biochemical Properties of Mycobacterial P1B4-ATPase
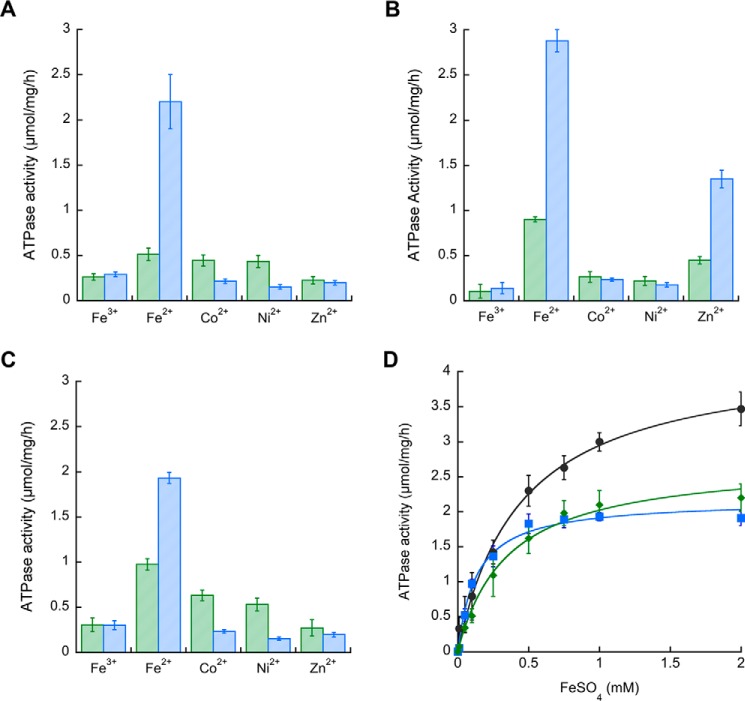
P-ATPases couple the transmembrane transport of their substrate to ATP hydrolysis following the Albers-Post E1/E2-like mechanism (23). Consequently, the metal dependence of ATPase activity provides a starting point to analyze substrate selectivity. Previous reports showed that MsCtpJ, MtCtpD, and MtCtpJ are differently activated by Co2+, Ni2+, and to a lesser extent Zn2+ (15, 16). The activation of mycobacterial P1B4-ATPases by Fe2+/3+ was tested using purified proteins stabilized in lipid/detergent micelles. All three proteins were strongly activated by Fe2+ and only minimally by Fe3+ (Fig. 3). For comparison, activation by Co2+, Ni2+, and Zn2+ at 0.1 and 1 mm concentrations is shown. MtCtpD Fe2+-dependent activity was ∼2-fold higher than those observed in MtCtpJ and MsCtpJ (Table 1 and Fig. 3D) and quite similar to that of B. subtilis PfeT (3.25 ± 0.21 μmol/mg/h) (13). MtCtpD also showed significant activation at 1 mm Zn2+ (Fig. 3B). Zn2+ binding to P1B4-ATPases as well as Zn2+ transport has been reported (15, 30). The K1/2 for Fe2+ activation of the mycobacterial enzymes confirmed a tendency observed in B. subtilis PfeT: the larger activation by Fe2+ is associated with a K1/2 much larger than that of Co2+ (Table 1). However, the observed K1/2 values do not describe the selectivity to the enzymes. These parameters result from the kon/koff of the metals binding the cytoplasmic facing transmembrane sites and the kon/koff for the release/backward binding of the metal to the periplasmic facing sites (41). As shown below, equilibrium binding determinations of KD better report the relative selectivity for the activating metals.
FIGURE 3.
Activation of mycobacterial P1B4-ATPases by Fe2+. ATPase activity of purified MsCtpJ (A), MtCtpD (B), and MtCtpJ (C) in the presence of a 0.1 (green bars) or 1.0 mm (blue bars) concentration of the indicated metal ions was determined. D, Fe2+ dependence of MsCtpJ (■; blue), MtCtpD (●; black), and MtCtpJ (♦; green) ATPase activities. Data are the mean ± S.E. (error bars) of three independent experiments performed in duplicate.
TABLE 1.
Summary of kinetic parameters, metal binding stoichiometry, and affinity of mycobacterial P1B4-ATPases
|
M. smegmatis CtpJ |
M. tuberculosis CtpD |
M. tuberculosis CtpJ |
||||
|---|---|---|---|---|---|---|
| Fe2+ | Co2+ | Fe2+ | Co2+ | Fe2+ | Co2+ | |
| ATPase activitya | ||||||
| Vmax (μmol/mg/h) | 2.73 ± 0.12 | 0.62 ± 0.03b | 4.25 ± 0.19 | 0.35 ± 0.03b | 2.16 ± 0.07 | 1.38 ± 0.12b |
| K1/2 (μm) | 350 ± 45 | 4.1 ± 0.9b | 443 ± 54 | 4.8 ± 1.4b | 143 ± 18 | 6.3 ± 2.2b |
| Metal stoichiometryc | 1.0 ± 0.1 | 0.9 ± 0.1b | 1.1 ± 0.1 | 1.1 ± 0.1b | 1.1 ± 0.1 | 1.1 ± 0.3b |
| Metal binding affinityd | ||||||
| KD (μm) | 2.24 ± 0.43 | 2.59 ± 0.32b | 0.12 ± 0.04 | 0.33 ± 0.04 | 2.41 ± 0.27 | 1.60 ± 0.16 |
| N | 1.44 ± 0.17 | 1.39 ± 0.22b | 1.32 ± 0.11 | 1.26 ± 0.04 | 1.35 ± 0.06 | 1.38 ± 0.05 |
a Values are the best fit parameters of activity versus Fe2+ curves. Errors are asymptotic errors provided by the fitting software (Fig. 3D).
c Values are the mean ± S.E. (n = 3).
d Values obtained by competitive metal binding with mag-fura-2. Values are the mean ± S.D. (n = 3).
The described Fe2+-ATPase activities require the binding of the transported substrate to the TM-MBS. The stoichiometry of this interaction was verified by measuring Fe2+ binding to MsCtpJ, MtCtpD, and MtCtpJ in non-turnover conditions lacking ATP (Table 1). The His6-less enzymes were incubated with excess Fe2+, unbound metal was removed by filtration, and bound metal was quantified by AAS. As expected, the proteins bind Fe2+ in a 1:1 molar ratio. Discarding the possibility of nonspecific interactions, metal binding was largely abolished in the presence of 1.5 mm vanadate (not shown). This binding stoichiometry is similar to that previously observed for Zn2+, Ni2+, and Co2+ binding to P1B4-ATPases (15, 17). Notably, although the TM-MBSs of these enzymes appear to accommodate divalent cations, no significant binding of Fe3+ was observed (not shown).
Mycobacterial P1B4-ATPase affinities for Fe2+ and Co2+ under equilibrium conditions were determined by titration of isolated enzymes in the presence of the fluorescence indicator mag-fura-2 (15, 42). In these experiments, mag-fura-2 forms 1:1 indicator-metal complexes of known KD. The concentration of free indicator can be spectrophotometrically monitored, and the free metal and metal-protein complex levels can be calculated. The enzyme-metal KD and the apparent stoichiometry of the interactions were obtained by fitting mag-fura-2 A366 versus free metal concentration curves (Table 1). MsCtpJ and MtCtpJ showed a similar KD for Fe2+. These were also comparable with those previously reported for Co2+ (included in Table 1 for comparison). Notably, MtCtpD has ∼3-fold higher affinity for Fe2+ compared with Co2+. Moreover, the affinity of MtCtpD is 20 times higher for Fe2+ (lower KD) and 5 times higher for Co2+ when compared with those observed in the CtpJ enzymes. The relative preference of MtCtpD for Fe2+ when compared with MtCtpJ further supports a dominant role of MtCtpD in Fe2+ tolerance (Fig. 1).
Distinct Co2+ and Fe2+ Coordination by MtCtpD
Full appreciation of the different enzymatic activities and metal selectivity observed in P1B4-ATPases requires understanding of the structural basis of these phenomena. P1B4-ATPases share a number of invariant residues in the transmembrane region proposed to participate in metal coordination (19, 24–26). A six-coordinate Co2+ species by the Sulfitobacter sp. P1B4-ATPase has been postulated with participation of a Ser in the conserved SPC in the fourth TM and invariant His, Glu, and Thr in the sixth TM (17). Surprisingly, this coordination did not include the invariant Cys located in the fourth transmembrane segment of all P1B-ATPases.
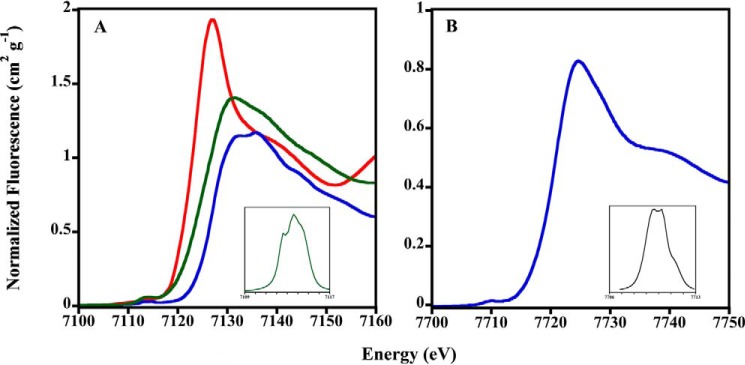
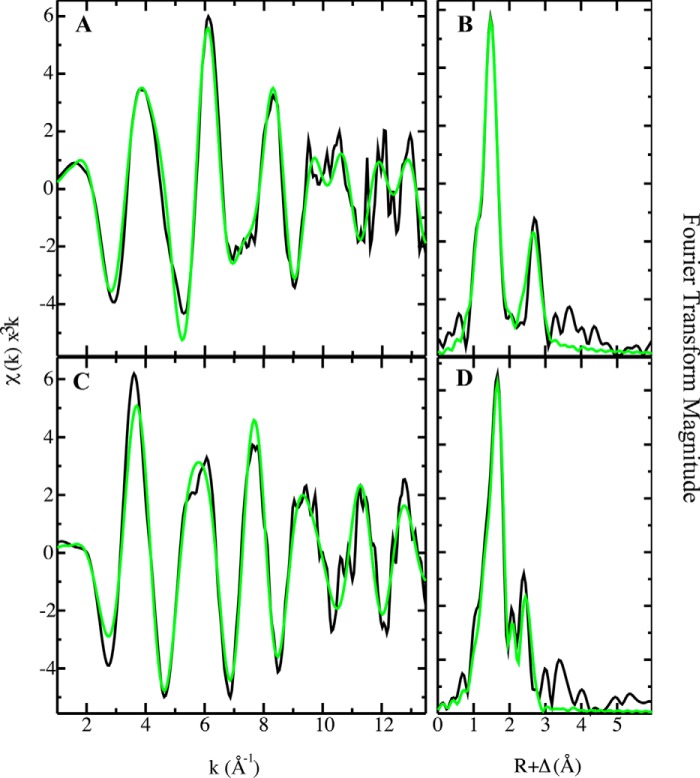
Considering the results shown above and the possible distinct Fe2+/Co2+ coordination, the binding environment of Fe2+ and Co2+ in MtCtpD was analyzed by XAS. The x-ray absorption near edge spectroscopy (XANES) portion of the XAS spectrum is element-specific and local bonding-sensitive; therefore it is useful for reporting the oxidation and coordination states of metals bound to the enzyme. The spectra of Fe2+-loaded protein were compared with Fe2+ and Fe3+ model systems (Fig. 4A). The first inflection point energy for protein-bound iron occurs at 7127.6 eV, consistent with a 50%/50% Fe2+/Fe3+ redox state mixture. Although all spectra were closely screened for photoreduction, iron oxidation during protein concentration postmetal loading may have led to this observation. Pre-edge features observed in the XANES of iron-MtCtpD are characteristic of 1s → 3d electronic transitions. These pre-edge features are consistent with pseudosymmetric six-coordinate iron-ligand systems (43). Cobalt-MtCtpD XANES pre-edge features and the general edge structure are consistent with Co2+ bound to protein systems in a six-coordinate ligand environment as observed previously (Fig. 4B) (17, 44). The EXAFS region of an XAS spectrum provides high resolution distances for ligand coordination environments of metals bound to metalloproteins. Fourier transform of the EXAFS provides a pseudoradial distribution function of ligand environments surrounding the metal. EXAFS analysis was used to compare differences in coordination of iron and cobalt to MtCtpD (Fig. 5). The resulting coordination number, ligand identity, bond lengths, and statistical fitting parameters are described in Table 2. The iron-MtCtpD spectrum was best simulated by a ligand environment containing five oxygen/nitrogen ligands at two sets of coordinating distances. Long range iron-ligand scattering was best fit with four Fe–C ligands. The general features in the iron EXAFS and bond lengths obtained from the simulations suggest that iron is most likely coordinated by six oxygen/nitrogen-based side chain ligands from amino acids and water molecules. The cobalt-MtCtpD EXAFS served as a comparison for the iron bound to MtCtpD. The best fit simulation contained two unique Co–O/N environments (Table 2). The Co–O/N bond lengths, compared with crystallographically characterized model compounds in the Cambridge Structural Database, are again consistent with five- to six-coordinate Co–(O/N)6 compounds (45). This is similar to what has been observed in the Sulfitobacter sp. P1B4-ATPase (17).
FIGURE 4.
Normalized iron and cobalt XANES spectra of MtCtpD. A, XANES plot for iron-MtCtpD (green), FeSO4 (red), and Fe2(SO4)3 (blue). Peak maximum, 7113.5 eV; total peak area, 7.91. B, XANES plot for cobalt-MtCtpD (blue). Peak maximum, 7709.6 eV; total peak area, 1.92. Insets show expansion of spline-subtracted pre-edge feature for the 1s → 3d transition for each element.
FIGURE 5.
Iron and cobalt EXAFS and Fourier transform with best fit simulations. Iron-MtCtpD raw k3-weighted EXAFS (A) and phase-shifted Fourier transform (B) are shown. Cobalt-MtCtpD raw k3-weighted EXAFS (C) and phase-shifted Fourier transform (D) are shown. Raw unfiltered data are shown in black, and best fit simulations are shown in green.
TABLE 2.
Summary of the iron and cobalt EXAFS simulations for metals bound to MtCtpD
Data utilized a k range of 1–13.5 Å−1.
| Nearest neighbor ligand environmenta |
Long range ligand environmenta |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Metal | Atomb | Rc | C.N.d | σ2e | Atomb | Rc | C.N.d | σ2e | F′f |
| Å | Å | ||||||||
| Iron | Oxygen/nitrogen | 1.93 | 2.5 | 1.93 | Carbon | 3.09 | 4.0 | 2.46 | 0.32 |
| Oxygen/nitrogen | 2.07 | 2.5 | 2.41 | ||||||
| Cobalt | Oxygen/nitrogen | 2.09 | 4.0 | 3.73 | Carbon | 2.91 | 4.0 | 4.43 | 0.33 |
| Oxygen/nitrogen | 1.97 | 1.0 | 3.91 | ||||||
a Independent metal-ligand scattering environment.
b Scattering atoms.
c Average metal-ligand bond length.
d Average metal-ligand coordination number.
e Average Debye-Waller factor (Å × 103).
f Number of degrees of freedom weighted mean square deviation between data and fit.
The spectroscopy analysis points to a distinct coordination for Co2+ and Fe2+. However, the spectroscopy is not able to reveal alternative ligands. Conserved residues of MtCtpD possibly involved in metal coordination are Ser-316 and Cys-318 in TM4 and His-642, Glu-643, Gly-644, Ser-645, and Thr-646 in TM6. Seeking a more detailed understanding of the Fe2+ and Co2+ coordination at the TM-MBS, residues likely involved in the metal coordination were exchanged by site-directed mutagenesis, and the resulting proteins were functionally characterized. The single mutants E643D and T646S (included as conservative control modifications) and G644A and S645A did not alter the Co2+- or Fe2+-ATPase activities of MtCtpD (Fig. 6). In agreement with previous reports, mutation of S316A, H642A, E643A, or T646A led to significant loss of Co2+ ATPase activity (17). The single substitutions S316A, E643A, and T646A equally affected the Co2+- and Fe2+-ATPase activities. However, the H642A mutation largely abolished Co2+ stimulation (6% of WT activity) while preserving significant Fe2+ sensitivity (33%) at saturating metal concentrations. In contrast, the C318A mutation had diminished Fe2+ activation (18%) while retaining 39% of the Co2+-ATPase activity. The differential effects of H642A and C318A mutations on the ATPase activity point toward a plausible mechanism. It appears that CtpD differentiates Co2+ and Fe2+ as substrates perhaps via alternative coordination despite binding these ions with quite similar affinities.
FIGURE 6.
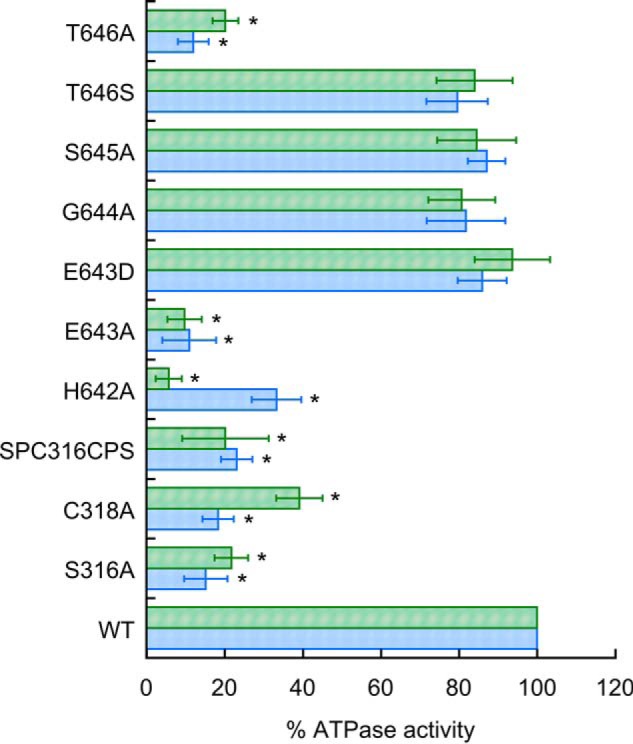
ATPase activity of MtCtpD proteins carrying substitutions of residues likely participating in the metal binding site in the presence of 0.1 mm Co2+ (green bars) or 1 mm Fe2+ (blue bars). Replacement SPC16CPS indicates the double mutant S316C/C318S. Activities were normalized to those of the wild type MtCtpD. Data are the mean ± S.E. (error bars) of three independent experiments performed in duplicate. Significant differences from the WT as determined by Student's t test are indicated (*, p < 0.01).
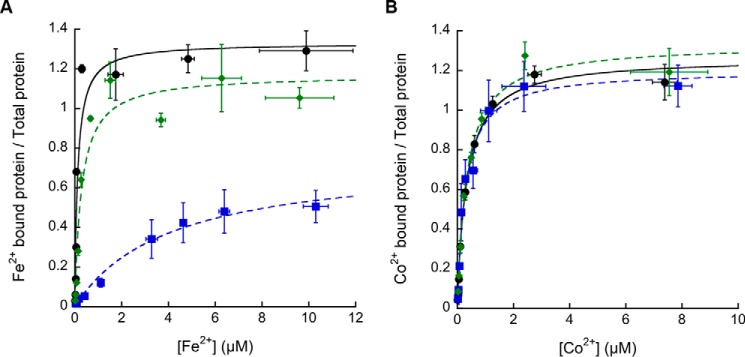
Considering that the ATPase activities might be affected by the removal of a ligating group or by the inability to undergo structural changes required for transport, the capability of the C318A and H642A mutant proteins to bind Co2+ and Fe2+ was tested. In comparison, the C318A mutant showed significantly lowered MtCtpD affinity for Fe2+, but it did not change the binding affinity for Co2+, suggesting an important role of this conserved Cys in Fe2+ binding (Fig. 7 and Table 3). The critical role of Cys-318 was further confirmed by the determination of the metal binding by AAS after incubation of the C318A mutant protein with metals at concentrations 10 times over the observed KD. The C318A mutant protein was able to bind 1.05 ± 0.14 Co2+ but only 0.32 ± 0.06 Fe2+. Finally, the H642A mutant had no detectable effect on Fe2+ or Co2+ binding affinities compared with WT (Table 3), raising the possibility that His-642 has a role other than the direct participation in the TM-MBS.
FIGURE 7.
Fe2+ and Co2+ binding affinities to MtCtpD WT, C318A, and H642A protein variants. Dissociation constants of MtCtpD WT (●; black), C318A (■; blue), and H642A (♦; green) for Fe2+ (A) and Co2+ (B) were determined. The data were fit to ν = n[Me2+free]/KD(1 + ([Me2+free]/KD) using values shown in Table 3. Data are the mean ± S.D. (error bars) of three independent experiments.
TABLE 3.
Comparison of Fe2+ and Co2+ dissociation constants of MtCtpD and C318A and H642A variants
| MtCtpD | Fe2+ |
Co2+ |
||
|---|---|---|---|---|
| KDa | na | KD | n | |
| μm | μm | |||
| WT | 0.12 ± 0.04 | 1.32 ± 0.11 | 0.33 ± 0.04 | 1.26 ± 0.04 |
| C318A | 4.01 ± 0.89 | 0.74 ± 0.07 | 0.28 ± 0.08 | 1.20 ± 0.05 |
| H642A | 0.28 ± 0.08 | 1.14 ± 0.10 | 0.34 ± 0.05 | 1.33 ± 0.14 |
a Values obtained by fitting curves resulting from competitive metal binding with mag-fura-2 (Fig. 7). Values are the mean ± S.D. (n = 3).
Discussion
The substrates and consequent functional roles of bacterial and eukaryotic P1B4-ATPases have remained elusive as their capabilities to transport different transition metals have been reported (15–17, 28–30). This is relevant as some of these transporters are required for bacterial virulence (16, 18) and critical for metal homeostasis in chloroplasts (29, 46). An extra layer of complexity is present in bacterial systems containing homologous non-redundant P1B4-ATPases (16). From a different perspective, defining P1B4-ATPases substrates is significant to understanding the coordination of transition metals by transport proteins as well as likely additional selectivity mechanisms acting in vivo. Here we describe the roles of mycobacterial P14B-ATPases in Fe2+ homeostasis, the kinetics of transport, and the structural elements that in part determine the selectivity of these enzymes. These results indicate that mycobacterial P14B-ATPases are Fe2+/Co2+-ATPases; however, the various isoforms show differential participation in the homeostasis of these ions.
Mycobacterial P1B4-ATPases Participates in Both Fe2+ and Co2+ Homeostasis
We observed that mycobacterial CtpJ proteins contribute to the homeostasis of Fe2+ and Co2+ to different extents. M. smegmatis has a single P1B4-ATPase, MsCtpJ. Expression of the coding gene is induced by Co2+ and partially by the superoxide generator paraquat but not by H2O2 (15). Deletion of MsctpJ leads to lower tolerance to Co2+, Fe2+, and hemin as well as increments in intracellular Co2+ and Fe2+ levels (Fig. 1) (15). The M. tuberculosis genome encodes two P1B4-ATPases. MtCtpJ expression, like MsCtpJ, is induced by Co2+ and to a lesser extent by redox stressors and Fe2+ (16). The MtΔctpJ strain accumulates higher levels of both Co2+ and Fe2+ (Fig. 1) (16), again in a fashion similar to that of the MsΔctpJ strain. These characteristics appear similar to those observed for PfteT, the single P1B4-ATPase present in B. subtilis (13). The comparable functions suggested by the observed phenotypes correlate with the analogous biochemistry of MsCtpJ, MtCtpJ, and B. subtilis PfteT. These three ATPases transport Fe2+ and Co2+ with surprisingly similar Vmax and K1/2 for activation. Moreover, both CtpJs bind Fe2+ and Co2+ with micromolar affinities (equilibrium binding determinations have not been performed for PfeT). These affinities explain the observed capability of these enzymes to influence the cellular response to STN when extracellular iron is maintained at just 10 μm. More importantly, 2–3 μm Fe2+ affinities appear consistent with reported free Fe2+ levels in the 1–10 μm region (4). In fact, the iron-sensing transcriptional regulators Fur and IdeR have 9 μm KD for Fe2+ (47, 48), indicating that these regulators are sensitive to the same concentration of Fe2+ as the P1B4-ATPases. Consequently, efflux CtpJ ATPases and influx transporter regulators are likely to coordinately respond to changes in metal levels not only under Fe2+ stress conditions but also under normal conditions.
Homologous P1B4-ATPases Present in Mycobacterial Genomes Have Distinct Roles
M. tuberculosis, as in other mycobacteria, has an additional P1B4-ATPase, CtpD. Notably, MtCtpD, but not MtCtpJ, is required for bacterial virulence. What unique function does CtpD provide? The MtΔctpD strain is more sensitive to iron stress and accumulates higher levels of this metal than MtΔctpJ. The phenotypic differences between the MtΔctpD and MtΔctpJ strains should have a molecular basis either in the biochemistry of these enzymes or the iron pool that they transport. Notably, the phenotype of the MtΔctpD:ΔctpJ double mutant strain is similar to that of the MtΔctpD cells, suggesting that CtpD and CtpJ use the same iron pool as a substrate, and this can be controlled by MtCtpD alone. Alternatively, the molecular activities of MtCtpD and MtCtpJ appear distinct. MtCtpD has significantly higher Fe2+-ATPase activity. Moreover, if the relative activation induced by Fe2+/Co2+ is considered, MtCtpD shows Fe2+ activity 12 times larger than that generated by Co2+. On the contrary, MtCtpJ shows higher activation by Co2+ than MtCtpD and only a 1.5 Fe2+-ATPase:Co2+-ATPase ratio. Although these relative activities approximately correlate with the observed phenotypes, the higher affinity of MtCtpD for Fe2+ (KD of 0.1 μm) appears to confer its dominant role in Fe2+ homeostasis. This KD is 1 order of magnitude smaller than that reported for Fe2+-sensing transcriptional regulators of influx systems (47, 48). Distinct from CtpJ, CtpD is not induced by divalent metals but by redox stressors, such as the nitric oxide generator nitroprusside and the respiratory poison cyanide (16). In fact, the region upstream of ctpD contains the TTGXXXXTTCXXG operator sequence for the redox-sensing MtFurA regulator (49). Considering the release of Fe2+ from iron-sulfur and mononuclear iron-containing proteins upon redox stress, it can be hypothesized that CtpD constitutes an early response to Fe2+ dyshomeostasis that is independent of efflux (CtpJ), storage (bacterioferritin), and regulators (IdeR) that respond to higher free Fe2+ levels.
The Coordination of Fe2+ by P1B4-ATPases Likely Requires the Invariant Cys in the Fourth TM
Metal selectivity is central to the physiological roles of P1B-ATPases. In early studies, invariant Cys in the sixth TM (fourth TM in P1B4-ATPases) were instrumental in defining P1B-ATPases. Detection of other conserve residues in the transmembrane region led to the identification of P1B-ATPases subgroups (19). The participation of these signature residues in the binding sites of P1B1 Cu+-ATPases and P1B2 Zn2+-ATPases was later established (25, 26). Then it was relevant to establish the metal coordination in P1B4-ATPases. Previous studies proposed that P1B4-ATPases coordinate Co2+ with a Ser in the conserved SPC in the fourth TM and invariant His, Glu, and Thr in the sixth TM of these proteins (17). Surprisingly, no participation of the archetypical Cys in the fourth TM in Co2+ coordination was observed. However, a different coordination of Fe2+ by MtCtpD might explain its distinct biochemistry, i.e. higher affinity for Fe2+ and Co2+ and higher activity in the presence of Fe2+. We studied the coordination of Fe2+ and Co2+ while bound to MtCtpD TM-MBS by XAS and functionally analyzed variants carrying mutations in putative coordinating groups. XAS data indicate that both Fe2+ and Co2+ are coordinated by five to six oxygen/nitrogen ligands in a manner similar to that described previously for the Sulfitobacter sp. P1B4-ATPase. That is, the spectroscopy does not show the participation of sulfur atoms from the invariant Cys in the fourth TM as a metal ligand.
Notably, mutagenesis studies showed an alternative portrait of MtCtpD TM-MBS. As shown in the case the Sulfitobacter sp. P1B4-ATPase, we observed that mutation of S316A, H642A, E643A, and T646A led to an almost complete inhibition of Co2+ activation, whereas replacement C318A retains significant (39%) Co2+-ATPase activity. A different pattern is observed, however, for the effects of these mutations on the Fe2+-ATPase. In this case, H642A retains some activity, whereas C318A causes a larger decrease in the activation by Fe2+. Although these differences are not dramatic, they suggest a putative differential involvement of these residues. Determination of the equilibrium binding affinities provided a more detailed view. Surprisingly, mutation H642A did not affect the metal binding to MtCtpD, suggesting that the reduced Vmax of this mutant is associated with alterations in rate-limiting conformational steps rather than ion coordination. Keep in mind that metal release is the rate-limiting step in P-type ATPases (21, 50). More remarkably, replacement of C318A leads to a large reduction in the affinity for Fe2+ without affecting Co2+ binding. This datum in itself does not show a role of the conserved Cys in coordinating metals but suggests a direct effect, perhaps steric or through the second coordination sphere, in determining the affinity for Fe2+. In this case, the conservation of this Cys in the CPS signature sequence appears to be a logical consequence of the need to maintain a high binding affinity for Fe2+.
In summary, our observations suggest that mycobacterial P1B4-ATPases play a central role in Fe2+ homeostasis. CtpD in particular, likely regulated by FurA, constitutes part of the cellular response to redox-induced damage of iron centers.
Author Contributions
S. J. P. conducted most of the experiments, analyzed the results, and wrote the initial draft of the manuscript. B. E. L. performed X-ray spectroscopy analysis. J. E. L. and S. N. conducted growth and metal tolerance experiments using M. tuberculosis strains. C. M. S. oversaw in vivo experiments with M. tuberculosis and participated in manuscript revision. T. L. S. supervised x-ray spectroscopic analysis and participated in manuscript revision. J. M. A. conceived the idea for the project, directed the project, and wrote the paper with S. J. P.
Acknowledgments
We thank Andrew Baez (Worcester Polytechnic Institute) for assistance with early experiments. X-ray absorption spectroscopic studies were performed at the Stanford Synchrotron Radiation Lightsource (SSRL). SSRL is a national user facility operated by Stanford University on behalf of the United States Department of Energy, Office of Basic Energy Sciences. The SSRL Structural Molecular Biology Program is supported by the Department of Energy, Office of Biological and Environmental Research, and by the National Institutes of Health, National Center for Research Resources, Biomedical Technology Program.
This work was supported by National Institutes of Health Grant DK068139 (to T. L. S.). The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
- TM-MBS
- transmembrane metal binding site
- AAS
- atomic absorbance spectroscopy
- EXAFS
- extended x-ray absorption fine structure
- LIMM
- low iron defined medium
- STN
- streptonigrin
- TM
- transmembrane segment
- XANES
- x-ray absorption near edge spectroscopy
- XAS
- x-ray absorption spectroscopy
- Ms
- M. smegmatis
- Mt
- M. tuberculosis
- TCEP
- tris(2-carboxyethyl)phosphine.
References
- 1. Fraústo da Silva J. J. R., and Williams R. J. P. (2001) The Biological Chemistry of the Elements: the Inorganic Chemistry of Life, 2nd Ed., Oxford University Press, Oxford [Google Scholar]
- 2. Andrews S. C., Robinson A. K., and Rodríguez-Quiñones F. (2003) Bacterial iron homeostasis. FEMS Microbiol. Rev. 27, 215–237 [DOI] [PubMed] [Google Scholar]
- 3. Anjem A., and Imlay J. A. (2012) Mononuclear iron enzymes are primary targets of hydrogen peroxide stress. J. Biol. Chem. 287, 15544–15556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Keyer K., and Imlay J. A. (1996) Superoxide accelerates DNA damage by elevating free-iron levels. Proc. Natl. Acad. Sci. U.S.A. 93, 13635–13640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jang S., and Imlay J. A. (2007) Micromolar intracellular hydrogen peroxide disrupts metabolism by damaging iron-sulfur enzymes. J. Biol. Chem. 282, 929–937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Varghese S., Wu A., Park S., Imlay K. R., and Imlay J. A. (2007) Submicromolar hydrogen peroxide disrupts the ability of Fur protein to control free-iron levels in Escherichia coli. Mol. Microbiol. 64, 822–830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rodriguez G. M. (2006) Control of iron metabolism in Mycobacterium tuberculosis. Trends Microbiol. 14, 320–327 [DOI] [PubMed] [Google Scholar]
- 8. Lucarelli D., Vasil M. L., Meyer-Klaucke W., and Pohl E. (2008) The metal-dependent regulators FurA and FurB from Mycobacterium tuberculosis. Int. J. Mol. Sci. 9, 1548–1560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pandey R., and Rodriguez G. M. (2014) IdeR is required for iron homeostasis and virulence in Mycobacterium tuberculosis. Mol. Microbiol. 91, 98–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Grass G., Otto M., Fricke B., Haney C. J., Rensing C., Nies D. H., and Munkelt D. (2005) FieF (YiiP) from Escherichia coli mediates decreased cellular accumulation of iron and relieves iron stress. Arch. Microbiol. 183, 9–18 [DOI] [PubMed] [Google Scholar]
- 11. Bennett B. D., Brutinel E. D., and Gralnick J. A. (2015) A ferrous iron exporter mediates iron resistance in Shewanella oneidensis MR-1. Appl. Environ. Microbiol. 81, 7938–7944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Frawley E. R., and Fang F. C. (2014) The ins and outs of bacterial iron metabolism. Mol. Microbiol. 93, 609–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Guan G., Pinochet-Barros A., Gaballa A., Patel S. J., Argüello J. M., and Helmann J. D. (2015) PfeT, a P1B4-type ATPase, effluxes ferrous iron and protects Bacillus subtilis against iron intoxication. Mol. Microbiol. 98, 787–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gaballa A., and Helmann J. D. (2002) A peroxide-induced zinc uptake system plays an important role in protection against oxidative stress in Bacillus subtilis. Mol. Microbiol. 45, 997–1005 [DOI] [PubMed] [Google Scholar]
- 15. Raimunda D., Long J. E., Sassetti C. M., and Argüello J. M. (2012) Role in metal homeostasis of ctpd, a Co2+ transporting P1B4-ATPase of Mycobacterium smegmatis. Mol. Microbiol. 84, 1139–1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Raimunda D., Long J. E., Padilla-Benavides T., Sassetti C. M., and Argüello J. M. (2014) Differential roles for the Co2+/Ni2+ transporting ATPases, CtpD and CtpJ, in Mycobacterium tuberculosis virulence. Mol. Microbiol. 91, 185–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zielazinski E. L., Cutsail G. E. 3rd, Hoffman B. M., Stemmler T. L., and Rosenzweig A. C. (2012) Characterization of a cobalt-specific P1B-ATPase. Biochemistry 51, 7891–7900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McLaughlin H. P., Xiao Q., Rea R. B., Pi H., Casey P. G., Darby T., Charbit A., Sleator R. D., Joyce S. A., Cowart R. E., Hill C., Klebba P. E., and Gahan C. G. (2012) A putative P-type ATPase required for virulence and resistance to haem toxicity in Listeria monocytogenes. PLoS One 7, e30928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Argüello J. M. (2003) Identification of ion selectivity determinants in heavy metal transport P1B-type ATPases. J. Membr. Biol. 195, 93–108 [DOI] [PubMed] [Google Scholar]
- 20. Smith A. T., Barupala D., Stemmler T. L., and Rosenzweig A. C. (2015) A new metal binding domain involved in cadmium, cobalt and zinc transport. Nat. Chem. Biol. 11, 678–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Argüello J. M., Eren E., and González-Guerrero M. (2007) The structure and function of heavy metal transport P1B-ATPases. Biometals 20, 233–248 [DOI] [PubMed] [Google Scholar]
- 22. Rosenzweig A. C., and Argüello J. M. (2012) Toward a molecular understanding of metal transport by P1B-type ATPases. Curr. Top. Membr. 69, 113–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Argüello J. M., González-Guerrero M., and Raimunda D. (2011) Bacterial transition metal P1B-ATPases: transport mechanism and roles in virulence. Biochemistry 50, 9940–9949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Padilla-Benavides T., Long J. E., Raimunda D., Sassetti C. M., and Argüello J. M. (2013) A novel P1B-type Mn2+-transporting ATPase is required for secreted protein metallation in mycobacteria. J. Biol. Chem. 288, 11334–11347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Raimunda D., Subramanian P., Stemmler T., and Argüello J. M. (2012) A tetrahedral coordination of zinc during transmembrane transport by P-type Zn2+-ATPases. Biochim. Biophys. Acta 1818, 1374–1377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. González-Guerrero M., Eren E., Rawat S., Stemmler T. L., and Argüello J. M. (2008) Cu+ transporting ATPases: structure of the two transmembrane Cu+ transport sites. J. Biol. Chem. 283, 29753–29759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Argüello J. M., Raimunda D., and González-Guerrero M. (2012) Metal transport across biomembranes: emerging models for a distinct chemistry. J. Biol. Chem. 287, 13510–13517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Moreno I., Norambuena L., Maturana D., Toro M., Vergara C., Orellana A., Zurita-Silva A., and Ordenes V. R. (2008) AtHMA1 is a thapsigargin-sensitive Ca2+/heavy metal pump. J. Biol. Chem. 283, 9633–9641 [DOI] [PubMed] [Google Scholar]
- 29. Seigneurin-Berny D., Gravot A., Auroy P., Mazard C., Kraut A., Finazzi G., Grunwald D., Rappaport F., Vavasseur A., Joyard J., Richaud P., and Rolland N. (2006) HMA1, a new cu-ATPase of the chloroplast envelope, is essential for growth under adverse light conditions. J. Biol. Chem. 281, 2882–2892 [DOI] [PubMed] [Google Scholar]
- 30. Scherer J., and Nies D. H. (2009) CzcP is a novel efflux system contributing to transition metal resistance in Cupriavidus metallidurans CH34. Mol. Microbiol. 73, 601–621 [DOI] [PubMed] [Google Scholar]
- 31. Rodriguez G. M., and Smith I. (2006) Identification of an ABC transporter required for iron acquisition and virulence in Mycobacterium tuberculosis. J. Bacteriol. 188, 424–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bradford M. M. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254 [DOI] [PubMed] [Google Scholar]
- 33. Studier F. W. (2005) Protein production by auto-induction in high density shaking cultures. Protein Expr. Purif. 41, 207–234 [DOI] [PubMed] [Google Scholar]
- 34. Mandal A. K., Cheung W. D., and Argüello J. M. (2002) Characterization of a thermophilic P-type Ag+/Cu+-ATPase from the extremophile Archaeglobus fulgidus. J. Biol. Chem. 277, 7201–7208 [DOI] [PubMed] [Google Scholar]
- 35. Rosadini C. V., Gawronski J. D., Raimunda D., Argüello J. M., and Akerley B. J. (2011) A novel zinc binding system, ZevAB, is critical for survival of nontypeable Haemophilus influenzae in a murine lung infection model. Infect. Immun. 79, 3366–3376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Eren E., Kennedy D. C., Maroney M. J., and Argüello J. M. (2006) A novel regulatory metal binding domain is present in the C terminus of Arabidopsis Zn2+-ATPase HMA2. J. Biol. Chem. 281, 33881–33891 [DOI] [PubMed] [Google Scholar]
- 37. Guo J., Giedroc D.P. (1997) Zinc site redesign in t4 gene 32 protein: structure and stability of Co(II) complexes formed by wild-type and metal ligand substitution mutants. Biochemistry 36, 730–742 [DOI] [PubMed] [Google Scholar]
- 38. Lanzetta P. A., Alvarez L. J., Reinach P. S., and Candia O. A. (1979) An improved assay for nanomole amounts of inorganic phosphate. Anal. Biochem. 100, 95–97 [DOI] [PubMed] [Google Scholar]
- 39. George G., and Pickering I. (1995) EXAFSPAK: a Suite of Computer Programs for Analysis of X-ray Absorption Spectra, Stanford Synchrotron Radiation Lightsource, Menlo Park, CA [Google Scholar]
- 40. Yeowell H. N., and White J. R. (1982) Iron requirement in the bactericidal mechanism of streptonigrin. Antimicrob. Agents Chemother. 22, 961–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Segel I. H. (1993) Enzyme Kinetics, pp. 534–543, John Wiley and Sons, Inc., New York [Google Scholar]
- 42. Eren E., González-Guerrero M., Kaufman B. M., and Argüello J. M. (2007) Novel Zn2+ coordination by the regulatory N-terminus metal binding domain is present of Arabidopsis thaliana Zn2+-ATPase HMA2. Biochemistry 46, 7754–7764 [DOI] [PubMed] [Google Scholar]
- 43. Westre T. E., Kennepohl P., DeWitt J. G., Hedman B., Hodgson K. O., and Solomon E. I. (1997) A multiplet analysis of Fe k-edge 1s → 3d pre-edge features of iron complexes. J. Am. Chem. Soc. 119, 6297–6314 [Google Scholar]
- 44. Rhine M. A., Rodrigues A. V., Bieber Urbauer R. J., Urbauer J. L., Stemmler T. L., and Harrop T. C. (2014) Proton-induced reactivity of NO− from a {CoNO}8 complex. J. Am. Chem. Soc. 136, 12560–12563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Allen F. H. (2002) The Cambridge Structural Database: a quarter of a million crystal structures and rising. Acta Crystallogr. B 58, 380–388 [DOI] [PubMed] [Google Scholar]
- 46. Boutigny S., Sautron E., Finazzi G., Rivasseau C., Frelet-Barrand A., Pilon M., Rolland N., and Seigneurin-Berny D. (2014) HMA1 and PAA1, two chloroplast-envelope P1B-ATPases, play distinct roles in chloroplast copper homeostasis. J. Exp. Bot. 65, 1529–1540 [DOI] [PubMed] [Google Scholar]
- 47. Mills S. A., and Marletta M. A. (2005) Metal binding characteristics and role of iron oxidation in the ferric uptake regulator from Escherichia coli. Biochemistry 44, 13553–13559 [DOI] [PubMed] [Google Scholar]
- 48. Chou C. J., Wisedchaisri G., Monfeli R. R., Oram D. M., Holmes R. K., Hol W. G., and Beeson C. (2004) Functional studies of the Mycobacterium tuberculosis iron-dependent regulator. J. Biol. Chem. 279, 53554–53561 [DOI] [PubMed] [Google Scholar]
- 49. Sala C., Forti F., Di Florio E., Canneva F., Milano A., Riccardi G., and Ghisotti D. (2003) Mycobacterium tuberculosis FurA autoregulates its own expression. J. Bacteriol. 185, 5357–5362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Heyse S., Wuddel I., Apell H. J., and Stürmer W. (1994) Partial reactions of the Na,K-ATPase: determination of rate constants. J. Gen. Physiol. 104, 197–240 [DOI] [PMC free article] [PubMed] [Google Scholar]