Abstract
The LasR regulator protein functions at the top of the Pseudomonas aeruginosa quorum-sensing hierarchy and is implicated in promoting bacterial virulence. Of note is recent evidence that this transcription factor may also respond to oxidative stress. Here, all cysteines in LasR were inspected to deduce their redox sensitivity and to probe the connection between stress response and LasR activity using purified LasR and individual LasR domains. Cys79 in the ligand binding domain of LasR appears to be important for ligand recognition and folding of this domain to potentiate DNA binding but does not seem to be sensitive to oxidative stress when bound to its native ligand. Two cysteines in the DNA binding domain of LasR do form a disulfide bond when treated with hydrogen peroxide, and formation of this Cys201-Cys203 disulfide bond appears to disrupt the DNA binding activity of the transcription factor. Mutagenesis of either of these cysteines leads to expression of a protein that no longer binds DNA. A cell-based reporter assay linking LasR function with β-galactosidase activity gave results consistent with those obtained with purified LasR. This work provides a possible mechanism for oxidative stress response by LasR and indicates that multiple cysteines within the protein may prove to be useful targets for disabling its activity.
Keywords: antibiotic resistance, bacterial pathogenesis, bacterial transcription, DNA binding protein, ligand-binding protein, Pseudomonas aeruginosa, quorum sensing, cysteine
Introduction
The Gram-negative bacterium Pseudomonas aeruginosa is an opportunistic human pathogen that establishes chronic infections in immunocompromised patients, with persistent pulmonary colonization in individuals with cystic fibrosis (1–3). Like many bacteria, P. aeruginosa employs quorum-sensing (QS)4 machinery to communicate local population density through the exchange of self-produced signaling molecules (4). QS contributes to the development of acute infections by coordinating the production of multiple virulence factors, including elastase and pyocyanin, and the formation of biofilms (5–7). QS in P. aeruginosa is largely mediated by N-acyl l-homoserine lactone autoinducer molecules, biosynthesized by LuxI-type synthases, and detected by LuxR-type regulator proteins (8). The two major systems responsive to acylhomoserine lactones (AHLs) in P. aeruginosa are LasI/LasR and RhlI/RhlR. LasI and RhlI are LuxI-type synthases, whereas LasR and RhlR are LuxR-type regulatory proteins. These AHL-dependent systems are also closely connected to signaling governed by 2-alkyl-4-quinolones in P. aeruginosa (9).
The regulator protein LasR, with its cognate synthase LasI, functions at the top of the P. aeruginosa quorum-sensing hierarchy (10). Like other LuxR-type proteins, this transcription factor is composed of two domains (11). The amino-terminal ligand binding domain (LBD) specifically recognizes its autoinducer, N-(3-oxo-dodecanoyl)-l-homoserine lactone (3O-C12-HSL), promoting protein folding and dimerization, whereas the DNA binding domain (DBD) recognizes target sites to activate downstream gene expression. There has been significant interest in understanding LasR, as its inhibition represents a promising antivirulence strategy for the treatment of P. aeruginosa. One prevalent mode of inhibition is the development of non-native small molecules shown to disrupt LasR function through the reduction of virulence factors produced (12–14). A number of these analogs appear to inhibit LasR through competition with the native 3O-C12-HSL because they are often structurally similar.
Recent work has also revealed a somewhat unexpected role for LasR in oxidative sensing (15). A proteome-wide screen of P. aeruginosa to detect H2O2-sensitive cysteines identified Cys79 of LasR. Subsequent experiments in P. aeruginosa indicated that a LasR-C79A mutant strain showed decreased motility and proteolysis, highlighting Cys79 as important for LasR QS function. The link between LasR and oxidative stress response was supported by Northern hybridization analysis that revealed that the RNA level of lasI, under LasR control, was reduced after cells were treated with H2O2. Complementation studies with a LasR deletion strain showed that LasR function was restored when complemented with wild-type plasmid p-lasR but diminished by about half when cells were treated with 5 mm H2O2. Interestingly, complementation with mutant plasmid p-lasR-C79S was only partially successful but did not change significantly in the presence of oxidant. Complementation with p-lasR-C79A resulted in negligible LasR activity even in the absence of oxidant. Taken together, these findings suggest that LasR responds to both its autoinducer and oxidative stress.
Numerous bacterial proteins have been shown to respond to oxidative stress through redox-sensitive thiols, including the OxyR, HypT, and MgrA transcription factors (16–18). Although these proteins are important for regulating pathogen response to potentially damaging oxidants, they are not known to be associated with QS. In addition to the recent finding that LasR may be governed by both oxidants and QS autoinducers, several other bacterial regulators have been linked to a similar dual function. Staphylococcus aureus AgrA has been shown to form an intramolecular disulfide bond within its DBD when oxidized, leading to a reduction in DNA binding affinity (19). Additionally, Escherichia coli SdiA, a LuxR homolog like LasR, forms an intermolecular disulfide bond within its DBD, covalently linking two monomers and altering promoter binding affinity when oxidized with the addition of 2 mm H2O2 in EMSAs (20). These studies provide additional evidence for a possible intersection between QS and oxidative sensing in bacterial regulator proteins.
In this work, we probed the mechanism for the reported LasR sensitivity to oxidative stress to further characterize this important transcriptional regulator at the biochemical level. Our exploration of Cys79 revealed that this residue is important for ligand recognition and protein folding, but it does not seem to be sensitive to oxidative stress when LasR is autoinducer-bound and folded. Instead, we have identified two cysteines in the DBD of LasR, Cys201 and Cys203, which appear to modulate DNA binding affinity through the formation of an intramolecular disulfide bond in the presence of H2O2. Additionally, mutagenesis of either of these residues leads to poor protein overexpression and loss of DNA binding, underscoring their critical role in functional LasR. Results from these in vitro assays with purified protein were further supported by parallel studies using E. coli transformed with a plasmid containing both the lasR gene and a lasB-lacZ detection system (21). Experiments with this reporter gene system also demonstrated the critical importance of Cys201 and Cys203 for LasR function in a cell-based assay and indicated the down-regulation of LasR activity in the presence of H2O2. Taken together, our results add to the growing collective understanding of LasR, supporting a possible additional role for this protein as an oxidative sensor and suggesting new ways in which this pathogenic regulator may be handicapped.
Experimental Procedures
Cloning, Expression, and Purification of LasR, LBD, and DBD
The P. aeruginosa PA01 lasR gene was amplified by PCR from a lasR-containing pDONR201 vector obtained from the Harvard Medical School PlasmID Repository. Using incorporated NotI and NcoI restriction sites, the lasR insert was ligated into the pET28b (Novagen) expression vector to yield plasR. Similarly, the lasR fragment (nucleotides 1–519) corresponding to the LasR LBD (amino acid residues 1–173) (22) was amplified and cloned into the NdeI/NotI sites of the pET24b vector to yield pLBD. The lasR fragment (nucleotides 523–720) corresponding to amino acid residues 175–240 with added N-terminal Met was amplified and cloned into the NotI/NcoI sites of pET28b to yield pDBD. Generation of Cys79, Cys188, Cys201, and Cys203 mutants was achieved using the QuikChange Lightning site-directed mutagenesis kit (Agilent Technologies). All constructs were sequenced to confirm correct gene insertion and desired mutations (DNA Analysis Facility, Yale University).
For expression of all proteins, BL21(DE3) cells were transformed with the expression plasmid of interest and grown in Luria broth medium supplemented with 30 μg/ml kanamycin. For the expression of LasR and LBD, growth medium was additionally supplemented with 20 μm 3O-C12-HSL (Sigma-Aldrich) or non-native AHLs. Cultures were grown to A600 = 0.8 at 37 °C, protein expression was induced by the addition of isopropyl 1-thio-β-d-galactopyranoside to 400 μm, and growth continued for an additional 24 h at 23 °C. Cells were harvested by centrifugation, with pellets stored at −80 °C.
Harvested cells resulting from 0.5 liter of culture were resuspended in 10 ml of lysis buffer (25 mm Tris (pH 7.5), 200 mm NaCl, and 10% (v/v) glycerol). Lysis buffer was supplemented with 1 mm tris(2-carboxyethyl)phosphine for the purification of LasR and DBD. Resuspended cells were lysed by sonication and clarified by centrifugation, and the resulting supernatant was incubated with 0.25 ml of nickel-nitrilotriacetic acid-agarose resin (Qiagen) for 2 h at 4 °C. The resin was decanted into a column and washed with 5 ml of 5 mm imidazole in lysis buffer. C-terminally His6-tagged LasR, LBD, or DBD protein was eluted with a step gradient of 30, 60, 100, 250, and 500 mm imidazole in lysis buffer. Fractions containing the desired protein as judged by SDS-PAGE were pooled and dialyzed against dialysis buffer (50 mm sodium phosphate (pH 7.0), 200 mm NaCl, and 10% (v/v) glycerol, with 1 mm DTT added for LasR and DBD purification). LasR purified without DTT in dialysis buffer was not an active DNA-binding protein.
Synthesis of Non-native AHLs
AHL derivatives were prepared by coupling acyl carboxylic acids with l-homoserine lactone in the presence of 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide as described previously (23). Product identity and purity were confirmed by 1H NMR (500 MHz Agilent DD2-500 NMR), with comparison to known characterization data for each compound (24).
SDS-PAGE Analysis of LBD
To measure the impact of oxidants on LBD dimerization, 25 μm LBD or LBD C79S was combined with 2.5 mm H2O2, 2.5 mm cumene hydroperoxide, or water as a control and incubated for 3 h at 25 °C. LBD and LBD C79S samples were diluted in non-reducing SDS sample buffer and separated using 12% polyacrylamide gels (Bio-Rad). Samples containing 100 mm DTT in sample buffer were incubated for 10 min at 25 °C prior to electrophoresis. Detection, excision, and elution of protein bands for mass spectrometric confirmation of molecular weight followed established methods (25). LBD expressed in the presence of non-native AHLs was also diluted in non-reducing SDS sample buffer and resolved on a 12% polyacrylamide gel.
DTNB Assay
LBD (25 μm) was combined with 0–200 μm H2O2 in native (100 mm sodium phosphate and 1 mm EDTA (pH 8.0)) or denaturing (6 m guanidine hydrochloride, 100 mm sodium phosphate, and 1 mm EDTA (pH 8.0)) buffer and incubated for 1 h at 25 °C. To detect free thiols, 180 μm 5,5′-dithiobis(2-nitrobenzoic acid) (DTNB) was added and incubated for 15 min at 25 °C, and the absorbance of each sample was measured at 412 nm. The concentration of 2-nitro-5-thiobenzoate, released upon reaction between free thiols and DTNB, was calculated using this absorbance and known extinction coefficients for 2-nitro-5-thiobenzoate under native and denaturing conditions (26).
Circular Dichroism Spectroscopy
Far-UV CD spectra of wild-type and mutant LBD (15 μm in 100 mm sodium phosphate (pH 7.0)) expressed in the presence of varied AHLs were measured with a Jasco J-810 spectropolarimeter using a 0.1-cm path length quartz cuvette. Data were acquired at 10 °C, and spectra represent the average of four scans for each sample. Temperature-dependent CD analysis at 222 nm from 10–90 °C at a rate of 1 °C/min permitted determination of melting temperature (Tm) using the Jasco Spectra Analysis software to fit data.
Electrophoretic Mobility Shift Assays
Binding reactions were performed in binding buffer (0.7 mm KH2PO4, 2.15 mm Na2HPO4, 1.35 mm KCl, 20 mm NaCl, 0.5 mm EDTA, 0.05% (v/v) Triton X-100, 5% (v/v) glycerol, and 10 μg/ml salmon sperm DNA (pH 7.4)). A 43-mer DNA duplex containing the lasB OP1 site (5′-ATCAAGGCTACCTGCCAGTTCTGGCAGGTTTGGCCGCGGGTTC-3′ annealed to its complementary strand) with 5′ modification of the top strand with fluorescein (6-FAM, Integrated DNA Technologies) was used for all binding assays. For quantitative binding assays, serial dilutions of LasR, LasR mutants, and DBD were incubated with 100 nm lasB OP1 for 30 min at 25 °C before binding reactions were applied to a nondenaturing 6% polyacrylamide DNA retardation gel (Invitrogen) with 0.5× Tris borate-EDTA as the electrophoresis running buffer. The amounts of complexed and free DNA were visualized and quantified using a Gel Doc EZ system (Bio-Rad). Equilibrium dissociation constants were determined as described previously (27). Assays to test the impact of H2O2 on DNA binding were performed under the same conditions, with incubation with oxidant for 30 min at 25 °C either before or after incubation with target DNA as noted. The low millimolar concentration of H2O2 used in these EMSA assays is common practice for the in vitro evaluation of the impact of oxidant on regulator protein-DNA binding affinity (18–20, 28–30) and is required in part to overcome the 1 mm DTT necessary for the purification and storage of active LasR. To evaluate recovery of binding with DTT, samples were incubated for an additional 30 min at 25 °C in the presence of excess reducing agent (25 mm DTT). DBD (64 μm) was reacted with 45 mm iodoacetamide for 1 h at 25 °C to give alkylated DBD for gel shift analysis.
Mass Spectrometric Characterization of LasR
All mass spectral analyses were performed by tandem HPLC-electrospray ionization mass spectrometry (LC-MS) using a Thermo Finnigan Surveyor HPLC system and a Thermo Scientific LTQ XL mass spectrometer. In preparation for LC-MS analysis, 2.5 μm LasR C79S/C188S and LasR C79S each were incubated with 3 mm H2O2 for 2 h at 25 °C, followed by reaction with 45 mm iodoacetamide for 1 h at 25 °C to cap any remaining free thiols. Digestion of LasR C79S/C188S proceeded with the addition of Glu-C (Promega) to 3.5 ng/μl in 50 mm NH4HCO3 (pH 8.0) with incubation at 37 °C for 18 h. Digested proteins were subjected to LC-MS, with ions of interest selected and subjected to collision-induced dissociation (MS/MS) to confirm peptide identity.
β-Galactosidase Reporter Assays
XL10-Gold E. coli cells (Agilent Technologies) were transformed with plasmid pKDT17 to yield a reporter strain coupling the lasR gene with a lasB::lacZ translational fusion (21). pKDT17 was a gift from Peter Greenberg (Addgene plasmid 27503). Site-directed mutagenesis of pKDT17 to convert LasR Cys79, Cys188, Cys201, and Cys203 to serine was achieved using the QuikChange Lightning site-directed mutagenesis kit (Agilent Technologies) and confirmed by DNA sequencing (DNA Analysis Facility, Yale University).
Using this reporter strain, LasR activity was quantified as a function of β-galactosidase activity as measured in Miller assays (31) using the modified procedure reported previously (32). Starting with a 1/1000-fold dilution of an overnight culture, the reporter strain was grown at 37 °C with shaking in LB medium supplemented with ampicillin (200 μg/ml) to A600 = 0.8–1.2. Cultures also included 20 μm 3O-C12-HSL and 10–100 μm H2O2 as noted. Subsequent dilution, permeabilization, and β-galactosidase activity assays were performed as described previously (32), with measured enzyme activity similarly converted to Miller units to account for any variance in culture density or reaction times.
Results
Impact of Oxidative Stress on the LasR Ligand Binding Domain
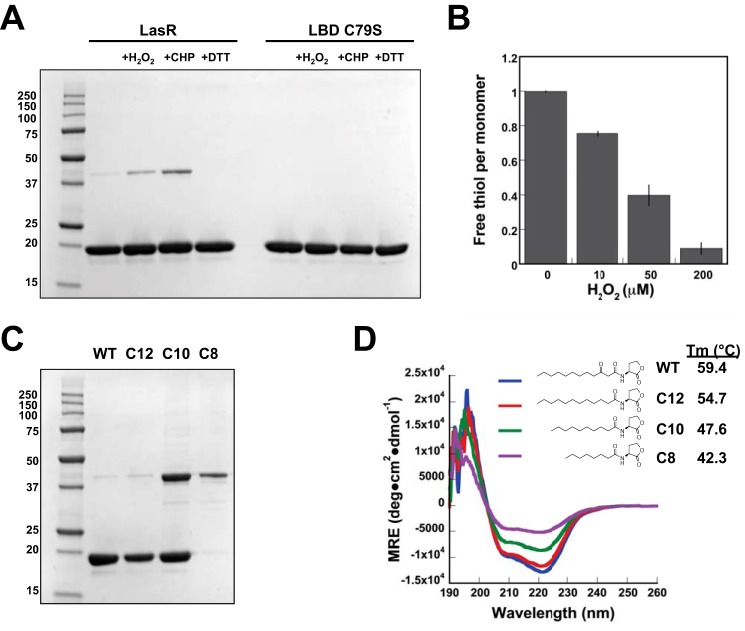
Previous work identified Cys79 of LasR, the sole cysteine within the LasR ligand binding domain (Fig. 1), as sensitive to oxidative stress in a proteome-wide screen of P. aeruginosa (15). This report provided evidence that LasR activity is impacted by oxidative stress, particularly in cell-based assays, but did not delineate the underlying protein chemistry of this redox response. We were interested in probing this biochemical mechanism further and learning the specifics of how LasR function may span both quorum-sensing and redox-sensing pathways. To take a close look at Cys79, we expressed and purified the LasR LBD (amino acids 1–173) in the presence of its native autoinducer, 3O-C12-HSL, yielding soluble protein. Although there are numerous possible outcomes for the reaction of a thiol such as Cys79 with an oxidizing agent, one conceivable oxidative stress response mechanism is the formation of a disulfide bond between two reactive thiols, here the linking of two Cys79 residues. Interestingly, we saw evidence of some dimeric LBD when the protein was purified under non-reducing conditions and evaluated on a denaturing, non-reducing SDS-PAGE gel (Fig. 2A). This result suggests a disulfide bond between monomers. Dimer formation was promoted in the presence of oxidants such as H2O2 and cumene hydroperoxide but reversed in the presence of the reducing agent DTT. Additionally, mutagenesis of Cys79 to serine (LBD C79S) confirmed that Cys79 was required for formation of the observed covalent dimer. These results led us to probe this dimer further to determine its importance for oxidative sensing by LasR. Because the two Cys79 in the known dimeric, autoinducer-bound LBD crystal structures (22, 33) are ∼21 Å apart (Fig. 1B), a significant conformational change or unfolding of the protein would be necessary to permit the formation of a disulfide bond. Such a structural change, though, could impact the ability of LasR to activate transcription.
FIGURE 1.
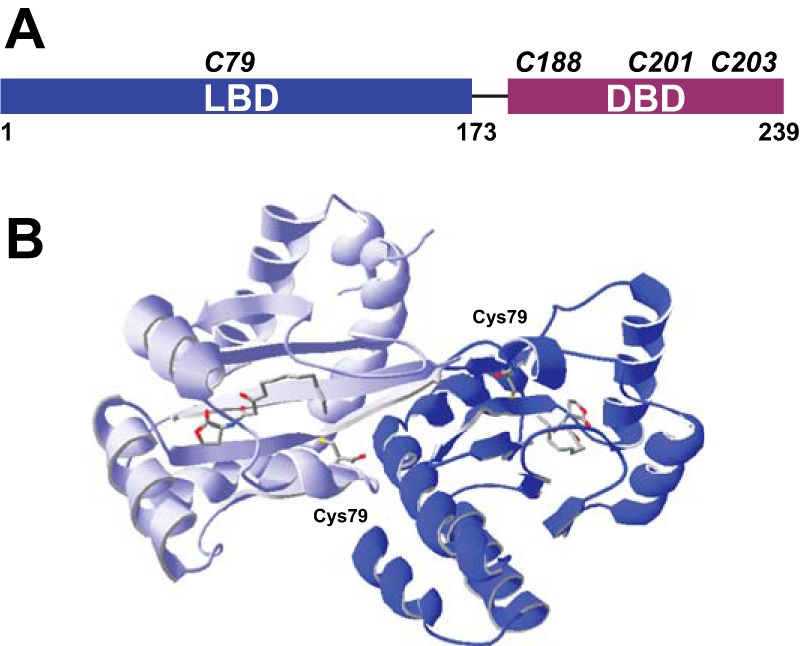
Cysteine composition of LasR. A, domain structure of LasR with the relative positions of all LasR cysteines shown. B, dimeric crystal structure (22) of the LBD with 3O-C12-HSL bound by each monomer and both Cys79 residues shown. Within each monomer, the terminal carbon of 3O-C12-HSL is proximal to Cys79 (4.3 Å), whereas the two cysteines in the dimeric complex are 21.6 Å apart.
FIGURE 2.
Impact of oxidative stress and unnatural ligands on LasR LBD. A, SDS-PAGE of 25 μm LBD under non-reducing conditions showing both monomer and dimer bands. Protein bands were excised from the third lane of a duplicate gel and subjected to LC-MS to confirm the predicted molecular weight of monomer (20,453.0 Da observed and 20,451.1 Da calculated) and dimer (40,906.5 Da observed and 40,900.2 Da calculated) LBD. Dimer formation is enhanced in the presence of 2.5 mm H2O2 and cumene hydroperoxide (CHP), whereas only monomer LBD is observed in the presence of 100 mm DTT or with the LBD C79S mutant. B, DTNB quantification of free thiols under denaturing conditions illustrates the impact of 10–200 μm H2O2 on 25 μm LBD. Error bars denote standard deviation for triplicate data. C, non-reducing SDS-PAGE of LBD expressed in the presence of 3O-C12-HSL (WT) and the non-native ligands C12-HSL, C10-HSL, and C8-HSL. D, wavelength-dependent CD spectra comparing relative folding of 15 μm LBD expressed in the presence of varied AHLs. The thermal stability (Tm) of each was also determined.
To further characterize the reactivity of the Cys79 thiol, we completed a DTNB assay for free thiols under native and denaturing conditions with 25 μm LBD. No labeling was observed for native LBD, but denaturation of the protein led to stoichiometric labeling of one thiol per monomer as anticipated. Addition of H2O2 from 10–200 μm readily protected the denatured LBD from labeling by DTNB (Fig. 2B), promoting the formation of dimeric LBD as detected by LC-MS (40,899.8 Da observed and 40,900.2 Da calculated). Similar experiments with H2O2 were performed with native LBD, revealing neither the formation of dimeric LBD nor any other change in mass, suggesting that the thiol is largely protected from oxidation when the LBD is folded and ligand-bound. Additionally, no alkylation was observed by LC-MS when the native LBD was treated with excess iodoacetamide. The proximity of Cys79 to the terminal carbon of 3O-C12-HSL (22) (Fig. 1B) suggests that this thiol might be important for ligand recognition, and others have reported covalent modification of Cys79 when LBD is expressed in the presence of electrophilic probes derived from the native AHL structure, placing reactive functional groups proximal to the thiol (34, 35). Cys79 appears to be most sensitive to oxidative stress when not bound to its native ligand, and perhaps the dimer observed by SDS-PAGE (Fig. 2A) reflects the reaction of two LBD monomers denatured by SDS gel sample buffer.
Although we and others (22) have found that it is not possible to purify soluble LBD expressed in the absence of ligand, we moved toward an approximation of ligand-free LBD by incrementally shortening the acyl chain length of ligands added to LBD expression cultures. We successfully expressed and purified the LBD in the presence of a series of AHLs differing in acyl chain length and presence of the 3-oxo group. Gel analysis (Fig. 2C) of these samples under non-reducing conditions revealed a trend toward dimer formation as AHL acyl chains shortened, suggesting a role for the native 3O-C12-HSL in mediating native LBD folding and perhaps blocking disulfide bond formation. The yield of soluble protein also decreased with shorter AHLs, and no soluble protein was produced with C6-HSL or C4-HSL. CD results also indicate that the secondary structure and thermal stability of LasR LBD are impacted by changes to acyl chain length (Fig. 2D). Our results join previous findings suggesting that autoinducer acyl side chain length is particularly important in dictating the stimulation of LasR activity (36) and recent work cataloging hydrogen-bonding interactions in the LasR LBD critical for LasR activation and inhibition (37, 38).
We also investigated the impact of Cys79 mutagenesis on LBD structure by measuring the thermal stability of LBD mutants C79S (Tm = 55.1 °C) and C79A (Tm = 55.7 °C), finding that replacement of Cys79 does lower thermal stability for these mutants expressed in the presence of native 3O-C12-HSL compared with wild-type LBD (Tm = 59.4 °C). Expression and purification of C79A LBD in the presence of non-native C10-HSL gave still lower thermal stability (Tm = 43.8 °C), a finding that parallels the diminished stability observed for wild-type LBD in the presence of shortened ligands (Fig. 2D). Taken together, our experiments with the LasR LBD suggest that Cys79 is important for protein folding and ligand recognition, and may form a disulfide-linked dimer in the presence of oxidative stress. Additional experiments with full-length LasR were required to probe how Cys79 oxidation might be linked to the function of LasR as a transcriptional activator.
Oxidative Stress Impacts LasR-DNA Binding Affinity
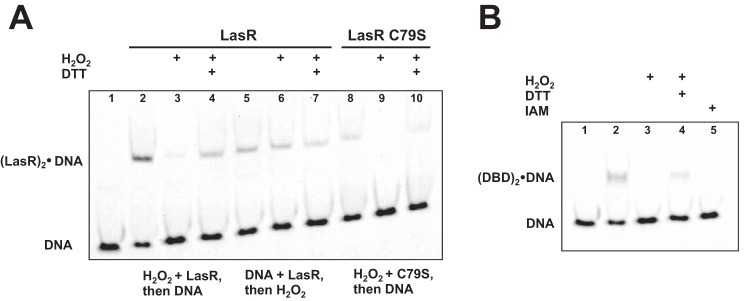
The full-length LasR protein with a C-terminal His6 tag was expressed and purified in the presence of 3O-C12-HSL, yielding soluble protein. EMSA was used to measure the interaction between LasR and one of its known target sequences, the lasB operator 1 (OP1) site (39). Addition of H2O2 to binding reactions reduced LasR-DNA binding, particularly when oxidant was added to LasR prior to the addition of DNA (Fig. 3A). Binding was recovered when excess DTT was added to the reaction, indicating that the impact of H2O2 was thiol-specific and reversible. These results suggest that one possible mechanism for the impact of oxidative stress on LasR as a transcriptional activator is through reduction in DNA binding affinity. Our next aim was to catalogue the biochemical transformations explaining this observed change in binding affinity.
FIGURE 3.
Oxidative stress impacts LasR-DNA binding. A, representative EMSA with 0.5 μm LasR and 5.0 μm LasR C79S. Target lasB OP1 is shown alone (lane 1). Both proteins shifted DNA in the absence of oxidant (lanes 2, 5, and 8), and the addition of 7.5 mm H2O2 significantly reduced DNA binding observed (lanes 3 and 9). The concentration of H2O2 used in these assays is similar to that reported in prior EMSA studies to evaluate the impact of oxidant on transcription factor-DNA binding (18–20, 28–30). The incubation of LasR with DNA for 30 min prior to the addition of oxidant appears to protect LasR from oxidation, preserving DNA binding (compare lanes 3 and 6). DNA binding for both proteins is restored with the addition of 25 mm DTT following incubation with H2O2 (compare lanes 3 and 4 for LasR and lanes 9 and 10 for LasR C79S). B, representative EMSA with 14 μm LasR DBD indicates binding of this domain to lasB OP1 (lane 2) that is disrupted in the presence of 7.5 mm H2O2 (lane 3) but restored with the addition of 25 mm DTT (lane 4). DBD reacted with iodoacetamide (IAM) prior to the binding assay did not show DNA binding (lane 5).
The relevance of Cys79 in connecting oxidative stress to LasR-DNA binding affinity was measured using mutant LasR C79S in similar EMSA experiments. The addition of H2O2 also reduced DNA binding affinity for this mutant (Fig. 3A), suggesting that Cys79 was not completely responsible for the observed reduction in binding affinity. This somewhat unexpected finding led us to investigate the three additional cysteines found outside the ligand binding domain of LasR, as detailed below.
Interestingly, mutagenesis of Cys79 does have a significant impact on DNA binding affinity based on results from quantitative EMSA. Replacement of Cys79 with serine increased the apparent equilibrium dissociation constant (Kd) from 0.400 ± 0.086 μm to 48.4 ± 8.0 μm (Fig. 4). The Kd for C79A LasR also increased to 4.89 ± 0.84 μm. Although Cys79 may not represent the thiol that is mediating oxidative stress response in native LasR, it does appear to be important for DNA binding, likely by promoting correct protein folding and dimerization in response to native autoinducer binding.
FIGURE 4.
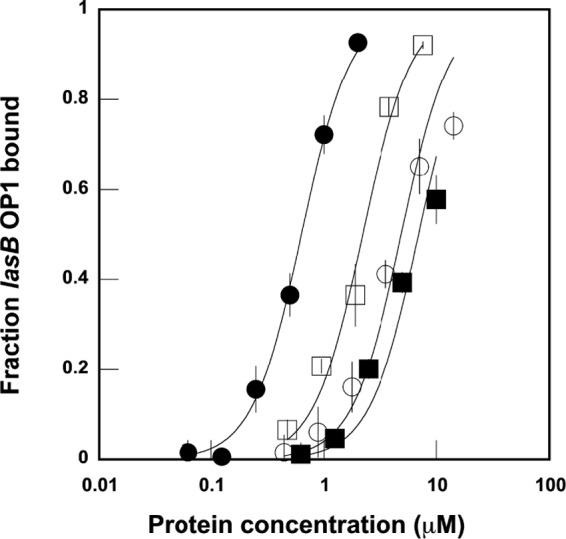
Quantitative EMSA comparing equilibrium binding affinity for wild-type LasR (●), LasR C79S (■), LasR C79A (□), and LasR DBD (○). The apparent Kd for each was determined by fitting data to the Langmuir equation as described in Ref. 27. Error bars denote standard deviation for triplicate data.
DNA Binding Domain Cys201 and Cys203 Respond to Oxidative Stress
Based on the EMSA experiments reported above, it seemed likely that one or more of the three cysteines within the LasR DNA binding domain (Fig. 1A) might be linked to the observed change in DNA-binding affinity in the presence of oxidant. We generated a construct representing only the LasR DBD (residues 175–240) to explore this portion of the protein more closely. Expression and purification of this domain with C-terminal His6 tag yielded a soluble DNA-binding protein. Although this domain alone was sufficient to bind DNA, the Kd (23.8 ± 3.6 μm) was again increased over that observed for full-length LasR (Fig. 4).
Addition of H2O2 to DNA binding reactions containing LasR DBD yielded similar results to that seen with full-length LasR (Fig. 3B), confirming that one or more of the cysteines within this domain mediates LasR oxidative response. Reacting the DBD with iodoacetamide prior to EMSA experiments resulted in a triply alkylated protein (8874.4 Da observed, 8873.14 Da calculated) that no longer bound DNA (Fig. 3B). These results indicate that cysteines in the DBD are important for DNA binding affinity and that chemical modification of these thiols, either through oxidation or alkylation, has a decided effect on DNA binding.
To inspect each of the three DBD cysteines in turn, each was mutated to alanine in full-length LasR. C188A yielded a protein that weakly bound DNA at 6 μm, forming complexes that appeared to dissociate during electrophoresis. C201A and C203A expression resulted in proteins that gave no discernible DNA binding at 3 μm compared with completely saturated wild-type LasR binding observed at ∼1 μm (Fig. 4). Generation of C201S and C203S mutants also gave proteins with no detectable DNA binding even at 7.6 μm. The mutation of Cys201 or Cys203 consistently resulted in poor protein overexpression yields as well, at most one-third of the yield observed for wild-type LasR and Cys79 or Cys188 mutants (typically 3 mg/liter). Based on these experiments that highlight the importance of proximal Cys201 and Cys203, we hypothesized that a disulfide bond between these two residues might represent a redox switch impacting the DNA binding affinity of wild-type LasR.
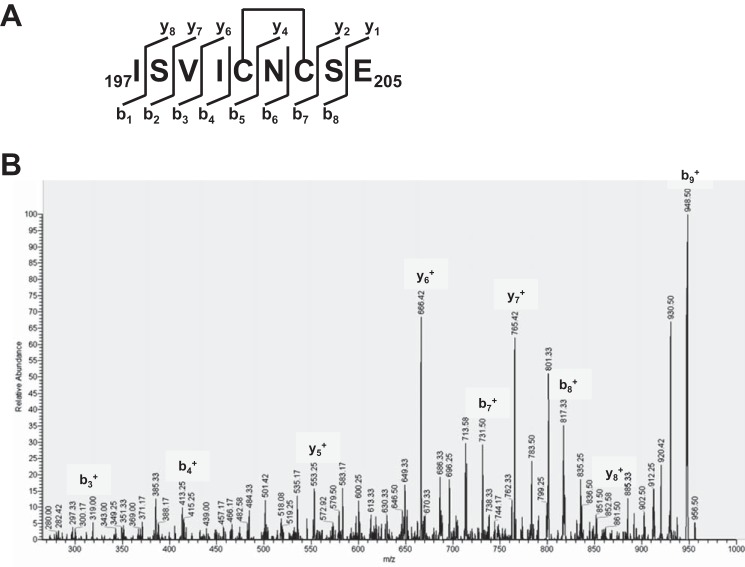
Cys201 and Cys203, seemingly important for LasR oxidative sensing, were probed further through a series of mass spectrometry experiments designed to assess the possibility of disulfide bond formation. LasR C79S/C188S, retaining only Cys201 and Cys203, was treated with H2O2, followed by excess iodoacetamide to cap any free thiols. LC-MS analysis revealed only the mass anticipated for LasR C79S/C188S with one disulfide bond (27,788.8 Da observed and 27,788.6 Da calculated). As a control experiment, treatment of LasR C79S/C188S with water followed by iodoacetamide yielded the mass expected for alkylation of the two free thiols (27,906.2 Da observed and 27,904.6 Da calculated). Similar experiments with LasR C79S yielded results that also supported the formation of a Cys201-Cys203 disulfide bond. Here, treatment of the protein with H2O2 followed by iodoacetamide gave a mass equal to that predicted for one alkylated cysteine and one disulfide bond (27,860.8 Da observed and 27,861.7 Da calculated), whereas a water control experiment in this case gave a mass indicative of alkylation of all three thiols (27,980.4 Da observed and 27,977.7 Da calculated). Taken together, these results suggest that Cys201 and Cys203 form a disulfide bond under these oxidative conditions, whereas Cys188 is not similarly oxidized, remaining reactive toward iodoacetamide. To further characterize the possible Cys201-Cys203 disulfide bond, LasR C79S/C188S that had been treated with H2O2 and iodoacetamide was digested with Glu-C protease. The expected mass for the disulfide-linked fragment containing Cys201 and Cys203 was discerned ((M+H)+ observed 965.40, calculated 965.42). LC-MS/MS fragmentation analysis confirmed the identity of this peptide fragment (Fig. 5).
FIGURE 5.
Mass spectrometric mapping of the Cys201-Cys203 disulfide bond in LasR. A, graphical fragment map correlating the relevant peptide sequence with the observed fragmentation ions. B, representative MS/MS fragmentation of the ion at m/z 965.40 corresponding to the disulfide-linked Glu-C peptide fragment 197–205.
LasR Reporter Assay Results Parallel in Vitro Experiments
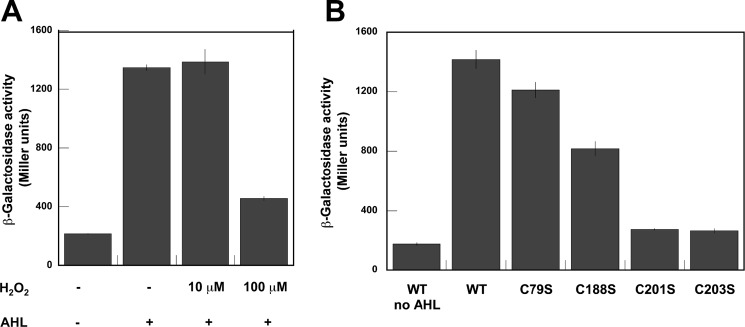
We expanded our in vitro characterization of LasR by performing parallel experiments using an E. coli-based reporter assay. The known plasmid pKDT17 (21) includes the P. aeruginosa lasR gene and a lasB::lacZ fusion, allowing monitoring of LasR activity as reflected by its binding to the lasB promoter, activating production of an elastase-β-galactosidase fusion protein with resultant enzyme activity detected in a Miller assay. Here, LasR and its target lasB promoter are the same as components of our EMSA experiments, but this cell-based assay allowed us to interrogate our findings in a different type of system. E. coli (pKDT17) was cultured under a variety of conditions, testing the impact of 3O-C12-HSL and H2O2 on lasB induction mediated by LasR. Inclusion of AHL ligand was important for detection of robust β-galactosidase activity as anticipated, and the addition of 100 μm H2O2 decreased observed β-galactosidase activity (Fig. 6A). These results are consistent with our findings that H2O2 impacts the DNA binding affinity of LasR. Mutagenesis of pKDT17 to convert each LasR cysteine to serine allowed inspection of the importance of each for LasR activity in this assay (Fig. 6B). The activity of all of the mutants was reduced compared with wild-type LasR, but C201S and C203S both showed especially low activity, similar to the wild-type construct assayed in the absence of AHL ligand. Again, these results are comparable with our findings with purified LasR mutants, particularly highlighting the essential nature of Cys201 and Cys203 for functional LasR. Because of the fact that Cys201 and Cys203 mutagenesis resulted in LasR with negligible activity under normal growth conditions, further studies to test the impact of oxidative stress on these mutants were not likely to be illustrative because their level of activity is already so low.
FIGURE 6.
Miller assay to detect the activity of β-galactosidase produced by the E. coli lasR lasB::lacZ reporter gene system. A, impact of adding H2O2 and 20 μm AHL (3O-C12-HSL) to cell cultures. Error bars denote standard deviation for triplicate data. B, impact of the indicated lasR gene mutations reflected in the reporter assay. Cultures were grown in the presence of 20 μm 3O-C12-HSL except where noted. Error bars denote standard deviation for triplicate data.
Modeling LasR DBD Structure
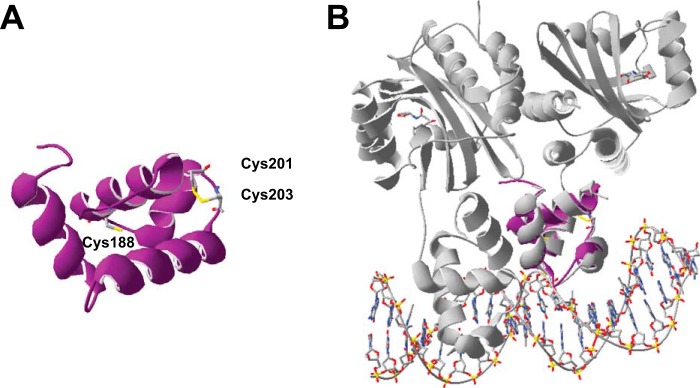
Although the structure of the LasR DBD is not known because of reported difficulties with full-length LasR protein solubility (22), we generated and evaluated a homology model of this domain using the Phyre2 protein fold recognition server (40) to consider the placement of this disulfide bond. The model, shown in Fig. 7A, illustrates the bond between Cys201 and Cys203 and predicts its position between the two helices of the helix-turn-helix motif. These helices correlate to “scaffold” helix α8 and “recognition” helix α9 in sequence alignment with TraR, a LuxR-type regulator from Agrobacterium tumefaciens whose dimeric crystal structure bound to both autoinducer and target DNA has been solved (41). To roughly approximate the position of Cys201 and Cys203 when the DBD associates with DNA, we superimposed the LasR DBD with one of the helix-turn-helix motifs in the TraR crystal structure (Fig. 7B). It is conceivable that the relative orientation of these helices could be impacted by the formation of a Cys201-Cys203 disulfide bond, altering DNA binding affinity. It is also likely that one or both of these cysteines is/are important for DNA recognition or proper protein folding because conversion of either to alanine or serine yields a protein that is poorly expressed and no longer binds DNA, and alkylation of these thiols also disrupts DNA binding. Combining LasR and target DNA prior to the addition of H2O2 reduces the impact of the oxidant, also supporting the possibility that one or both of these cysteines interact(s) closely with DNA. We anticipate that future structural studies will permit further understanding of the roles of Cys201 and Cys203 in target site recognition.
FIGURE 7.
Modeling the LasR DBD. A, homology model of DBD generated using the Phyre2 web server (40). The three DBD cysteines are shown, including the Cys201-Cys203 disulfide bond. B, overlay of the DBD homology model (purple) with the TraR DBD (right monomer, shown in gray) within the crystal structure (41) of TraR bound to its autoinducer and target DNA.
Discussion
The QS regulator LasR is of interest because of its role in controlling P. aeruginosa response to increased population density, linking autoinducer concentration to up-regulation of processes that lead to heightened bacterial virulence. Inhibition of LasR, therefore, represents an attractive antivirulence strategy, and additional understanding of LasR function and activity should enable more effective inhibition. The possibility that LasR responds to oxidative stress in addition to its known function as a QS regulator is intriguing, and we have worked to characterize this possible additional LasR function at the molecular level. Our work expands on earlier studies that demonstrated a link between oxidative stress and LasR QS efficacy in P. aeruginosa (15). Here we developed in vitro assays with purified LasR and individual LasR domains to enable us to probe the biochemical mechanism that may permit LasR to act as a redox sensor.
Systematic inspection of the four cysteines within LasR finds that Cys79 is important for protein folding and DNA binding but does not suggest a likely mechanism for this residue to act as an oxidative sensor in native, ligand-bound LasR. Although native LBD with 3O-C12-HSL bound does not react with DTNB or H2O2, denatured LBD does form a disulfide-linked dimer when exposed to oxidative stress, indicating that Cys79 is a reactive thiol that appears to be inaccessible when native autoinducer is bound. Experiments with non-native AHLs with shorter acyl chains further demonstrate that the absence of native autoinducer may permit LasR to adopt a conformation that more readily forms a disulfide dimer. Cys79 is conserved in some LasR homologs that similarly recognize 3O-C12-HSL (Fig. 8), underscoring our finding that this residue aids in specific ligand recognition. We note that mutating Cys79 significantly disrupts the enhancement in binding affinity offered by the properly folded, dimeric LasR ligand binding domain because the observed DNA binding affinity for the DBD alone only differs by a factor of two from that measured for LasR C79S, 2 orders of magnitude greater than that for wild-type LasR. It is interesting that the measured DNA binding affinity is so similar for LasR completely lacking the ligand binding domain (DBD alone) and LasR with a single ligand binding domain mutation (C79S), and these findings highlight the importance of Cys79. Mutagenesis of Cys79 to serine or alanine does not abolish sensitivity of LasR to oxidative stress, though, because DNA binding affinity is still reduced for these mutant proteins under oxidizing conditions.
FIGURE 8.
Multiple sequence alignment of LasR and its homologs. Protein sequences shown include P. aeruginosa LasR (UniProt P25084), Vibrio fischeri LuxR (UniProt P12746), Burkholderia kururiensis BraR (UniProt B1VD86), Pseudomonas putida PpuR (UniProt Q348H6), Pseudomonas fluorescens MupR (UniProt Q9AH79), A. tumefaciens TraR (UniProt A5WYC9), E. coli SdiA (UniProt P07026), P. aeruginosa RhlR (UniProt B6E4Z5), and Chromobacterium violaceum CviR (UniProt D3W065). The positions of the four LasR cysteines are indicated (stars). Cys79 is shared by BraR (47), PpuR (48), and MupR (49), all believed to recognize 3O-C12-HSL. This is not the autoinducer for the other proteins shown. With LuxR, these proteins also share Cys203. Interestingly, there is no sequence homology to Cys201 even in proteins that share Cys203, suggesting that the potential for a disulfide bond at that position is unique to LasR among known regulator sequences. It is possible that Cys201 in its reduced state may be an important hydrophobic amino acid, based on the conserved leucine at this position in most homologous sequences and the known hydrophobic nature of free cysteines (50).
Although our results do not suggest that LasR Cys79 is accessible or redox-sensitive in folded LasR, others have noted the possibility that conserved LBD cysteines might mediate the oxidative stress response in other LuxR-type regulator proteins. Recent efforts to catalogue solo luxR genes indicate that many of the LuxR proteins identified feature multiple conserved cysteines, particular in their LBDs, and it has been postulated that these cysteines, including Cys79 in solo LuxR proteins, are positioned well for disulfide bond formation and possible redox regulation (42). Based on prior reports (15) and our studies, it is certainly conceivable that LasR Cys79 has an important role in vivo as a reactive thiol, particularly in poorly folded LasR, and continued work will enable a full understanding of this residue.
Our experiments with the LasR DBD indicate that oxidative stress does impact the DNA binding affinity of this domain under in vitro reaction conditions similar to those used by others in characterizing oxidation-sensitive DNA binding proteins (18–20, 28–30). To further explore this result, we employed a cell-based reporter assay that also indicated a reduction in LasR activity in the presence of H2O2. These findings intersect with prior work describing the influence of oxidative stress on LasR activity in P. aeruginosa assays (15). Although this previous report focused on Cys79, their results indicating a reduction in wild-type LasR activity in the presence of H2O2 did not delineate which of the four LasR cysteines is critical for this response and did not consider any of the DBD cysteines. Based on our studies, we now propose that the formation of a disulfide bond between Cys201 and Cys203 might regulate that stress response. Because mutagenesis of Cys201 or Cys203 yields inactive LasR in both EMSA and cell-based reporter assays, it is difficult to completely characterize their response to oxidative stress or investigate a direct link between disulfide bond formation and reduced quorum sensing in P. aeruginosa because complementation studies with these mutants in a LasR deletion strain are likely to show deficient LasR-dependent quorum sensing with or without oxidative stress.
There is additional evidence that Cys203 is important for LasR function. Although we find that both Cys201 and Cys203 are essential for LasR activity, Cys203 appears to be more highly conserved based on comparison with homologous sequences (Fig. 8) and is even shared by LuxR. Furthermore, one LasR mutation observed in two different studies of clinical P. aeruginosa isolates is C203R, correlated with loss of LasR function as measured by elastase activity (3, 6). These clinical findings further support an important role for Cys203. It is not surprising that the formation of a disulfide dimer directly involving an essential DNA contact cysteine could have a significant impact on DNA binding affinity. Similar findings with transcription factor p53 have recently been reported, as Cys275, critical for p53-DNA binding affinity, is believed to form a disulfide bond with Cys277 upon oxidation, leading to a decrease in binding affinity (43).
As a possible oxidative sensor, LasR joins QS regulators AgrA (19) and SdiA (20), shown to have dual roles in both QS and oxidative sensing. The linking of quorum sensing to oxidative stress response through LasR may represent one of the traits that enables P. aeruginosa to coordinate growth and flourish under a variety of conditions (44). It is not yet clear whether redox sensing by QS regulators is important in responding to the burst of reactive oxidative species generated by the host immune system or whether this type of redox switch allows regulators to balance up-regulation of QS pathways with the potentially damaging reactive oxidative species produced through heightened metabolic activity resulting from QS activation. QS in P. aeruginosa has been linked to the expression of catalase and superoxide dismutase genes for protection against H2O2 (45), and other recent reports provide increased evidence for the close connection between QS and central metabolism in P. aeruginosa (46). Although it will be interesting to know precisely how oxidative sensing by LasR might serve P. aeruginosa, it is perhaps more valuable to have learned more about this protein at the chemical level, noting multiple critical cysteines that may prove to be useful targets in working toward effective treatment of this pathogen.
Author Contributions
K. A. T. cloned and expressed LasR and LBD. P. K., A. N. A., J. M. R., and T. L. S. prepared and characterized the LasR, DBD, and LBD variants. R. L. T. synthesized and characterized AHLs. E. G. S. and T. L. S. performed the LasR reporter assays. T. L. S. supervised the project and wrote the paper with input and approval from all authors.
This work was supported by a Cottrell College Science Award from the Research Corporation for Science Advancement. The authors declare that they have no conflicts of interest with the contents of this article.
- QS
- quorum-sensing
- AHL
- acylhomoserine lactone
- LBD
- ligand binding domain
- 3O-C12-HSL
- N-(3-oxo-dodecanoyl)-l-homoserine lactone
- DBD
- DNA binding domain
- DTNB
- 5,5′-dithiobis(2-nitrobenzoic acid)
- Tm
- melting temperature
- OP1
- Operator 1.
References
- 1. Lyczak J. B., Cannon C. L., and Pier G. B. (2000) Establishment of Pseudomonas aeruginosa infection: lessons from a versatile opportunist. Microbes Infect. 2, 1051–1060 [DOI] [PubMed] [Google Scholar]
- 2. Sadikot R. T., Blackwell T. S., Christman J. W., and Prince A. S. (2005) Pathogen-host interactions in Pseudomonas aeruginosa pneumonia. Am. J. Respir. Crit. Care Med. 171, 1209–1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bjarnsholt T., Jensen P. Ø., Jakobsen T. H., Phipps R., Nielsen A. K., Rybtke M. T., Tolker-Nielsen T., Givskov M., Høiby N., Ciofu O., and Scandinavian Cystic Fibrosis Study Consortium (2010) Quorum sensing and virulence of Pseudomonas aeruginosa during lung infection of cystic fibrosis patients. PLoS ONE 5, e10115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Waters C. M., and Bassler B. L. (2005) Quorum sensing: cell-to-cell communication in bacteria. Annu. Rev. Cell Dev. Biol. 21, 319–346 [DOI] [PubMed] [Google Scholar]
- 5. Schuster M., Sexton D. J., Diggle S. P., and Greenberg E. P. (2013) Acyl-homoserine lactone quorum sensing: from evolution to application. Annu. Rev. Microbiol. 67, 43–63 [DOI] [PubMed] [Google Scholar]
- 6. Köhler T., Buckling A., and van Delden C. (2009) Cooperation and virulence of clinical Pseudomonas aeruginosa populations. Proc. Natl. Acad. Sci. U.S.A. 106, 6339–6344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Smith R. S., and Iglewski B. H. (2003) P. aeruginosa quorum-sensing systems and virulence. Curr. Opin. Microbiol. 6, 56–60 [DOI] [PubMed] [Google Scholar]
- 8. Fuqua C., and Greenberg E. P. (2002) Listening in on bacteria: acyl-homoserine lactone signalling. Nat. Rev. Mol. Cell Biol. 3, 685–695 [DOI] [PubMed] [Google Scholar]
- 9. Dubern J. F., and Diggle S. P. (2008) Quorum sensing by 2-alkyl-4-quinolones in Pseudomonas aeruginosa and other bacterial species. Mol. Biosyst. 4, 882–888 [DOI] [PubMed] [Google Scholar]
- 10. Pesci E. C., Pearson J. P., Seed P. C., and Iglewski B. H. (1997) Regulation of las and rhl quorum sensing in Pseudomonas aeruginosa. J. Bacteriol. 179, 3127–3132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Churchill M. E., and Chen L. (2011) Structural basis of acyl-homoserine lactone-dependent signaling. Chem. Rev. 111, 68–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Galloway W. R. J. D., Hodgkinson J. T., Bowden S. D., Welch M., and Spring D. R. (2011) Quorum sensing in Gram-negative bacteria: small-molecule modulation of AHL and AI-2 quorum sensing pathways. Chem. Rev. 111, 28–67 [DOI] [PubMed] [Google Scholar]
- 13. Mattmann M. E., and Blackwell H. E. (2010) Small molecules that modulate quorum sensing and control virulence in Pseudomonas aeruginosa. J. Org. Chem. 75, 6737–6746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Moore J. D., Rossi F. M., Welsh M. A., Nyffeler K. E., and Blackwell H. E. (2015) A comparative analysis of synthetic quorum sensing modulators in Pseudomonas aeruginosa: new insights into mechanism, active efflux susceptibility, phenotypic response, and next-generation ligand design. J. Am. Chem. Soc. 137, 14626–14639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Deng X., Weerapana E., Ulanovskaya O., Sun F., Liang H., Ji Q., Ye Y., Fu Y., Zhou L., Li J., Zhang H., Wang C., Alvarez S., Hicks L. M., Lan L., Wu M., Cravatt B. F., and He C. (2013) Proteome-wide quantification and characterization of oxidation-sensitive cysteines in pathogenic bacteria. Cell Host Microbe 13, 358–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chen P. R., Brugarolas P., and He C. (2011) Redox signaling in human pathogens. Antioxid. Redox Signal. 14, 1107–1118 [DOI] [PubMed] [Google Scholar]
- 17. Zheng M., Aslund F., and Storz G. (1998) Activation of the OxyR transcription factor by reversible disulfide bond formation. Science 279, 1718–1721 [DOI] [PubMed] [Google Scholar]
- 18. Drazic A., Tsoutsoulopoulos A., Peschek J., Gundlach J., Krause M., Bach N. C., Gebendorfer K. M., and Winter J. (2013) Role of cysteines in the stability and DNA-binding activity of the hypochlorite-specific transcription factor HypT. PLoS ONE 8, e75683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sun F., Liang H., Kong X., Xie S., Cho H., Deng X., Ji Q., Zhang H., Alvarez S., Hicks L. M., Bae T., Luo C., Jiang H., and He C. (2012) Quorum-sensing agr mediates bacterial oxidation response via an intramolecular disulfide redox switch in the response regulator AgrA. Proc. Natl. Acad. Sci. U.S.A. 109, 9095–9100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kim T., Duong T., Wu C. A., Choi J., Lan N., Kang S. W., Lokanath N. K., Shin D., Hwang H. Y., and Kim K. K. (2014) Structural insights into the molecular mechanism of Escherichia coli SdiA, a quorum-sensing receptor. Acta Crystallogr. Sect. D Biol. Crystallogr. 70, 694–707 [DOI] [PubMed] [Google Scholar]
- 21. Pearson J. P., Gray K. M., Passador L., Tucker K. D., Eberhard A., Iglewski B. H., and Greenberg E. P. (1994) Structure of the autoinducer required for expression of Pseudomonas aeruginosa virulence genes. Proc. Natl. Acad. Sci. U.S.A. 91, 197–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bottomley M. J., Muraglia E., Bazzo R., and Carfì A. (2007) Molecular insights into quorum sensing in the human pathogen Pseudomonas aeruginosa from the structure of the virulence regulator LasR bound to its autoinducer. J. Biol. Chem. 282, 13592–13600 [DOI] [PubMed] [Google Scholar]
- 23. Chhabra S. R., Stead P., Bainton N. J., Salmond G. P., Stewart G. S., Williams P., and Bycroft B. W. (1993) Autoregulation of carbapenem biosynthesis in Erwinia carotovora by analogues of N-(3-oxohexanoyl)-L-homoserine lactone. J. Antibiot. 46, 441–454 [DOI] [PubMed] [Google Scholar]
- 24. Geske G. D., Wezeman R. J., Siegel A. P., and Blackwell H. E. (2005) Small molecule inhibitors of bacterial quorum sensing and biofilm formation. J. Am. Chem. Soc. 127, 12762–12763 [DOI] [PubMed] [Google Scholar]
- 25. Castellanos-Serra L., and Hardy E. (2006) Negative detection of biomolecules separated in polyacrylamide electrophoresis gels. Nat. Protoc. 1, 1544–1551 [DOI] [PubMed] [Google Scholar]
- 26. Riddles P. W., Blakeley R. L., and Zerner B. (1979) Ellman's reagent: 5,5′-dithiobis(2-nitrobenzoic acid): a reexamination. Anal. Biochem. 94, 75–81 [DOI] [PubMed] [Google Scholar]
- 27. Baranger A. M., Palmer C. R., Hamm M. K., Giebler H. A., Brauweiler A., Nyborg J. K., and Schepartz A. (1995) Mechanism of DNA-binding enhancement by the human T-cell leukaemia virus transactivator Tax. Nature 376, 606–608 [DOI] [PubMed] [Google Scholar]
- 28. Chen P. R., Bae T., Williams W. A., Duguid E. M., Rice P. A., Schneewind O., and He C. (2006) An oxidation-sensing mechanism is used by the global regulator MgrA in Staphylococcus aureus. Nat. Chem. Biol. 2, 591–595 [DOI] [PubMed] [Google Scholar]
- 29. Ji Q., Zhang L., Sun F., Deng X., Liang H., Bae T., and He C. (2012) Staphylococcus aureus CymR is a new thiol-based oxidation-sensing regulator of stress resistance and oxidative response. J. Biol. Chem. 287, 21102–21109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schook P. O., Stohl E. A., Criss A. K., and Seifert H. S. (2011) The DNA-binding activity of the Neisseria gonorrhoeae LexA orthologue NG1427 is modulated by oxidation. Mol. Microbiol. 79, 846–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Miller J. H. (1972) Experiments in Molecular Genetics, Cold Spring Harbor Laboratory, Cold Spring Harbor, NY [Google Scholar]
- 32. Zhang X., and Bremer H. (1995) Control of the Escherichia coli rrnB P1 promoter strength by ppGpp. J. Biol. Chem. 270, 11181–11189 [DOI] [PubMed] [Google Scholar]
- 33. Zou Y., and Nair S. K. (2009) Molecular basis for the recognition of structurally distinct autoinducer mimics by the Pseudomonas aeruginosa LasR quorum-sensing signaling receptor. Chem. Biol. 16, 961–970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Amara N., Mashiach R., Amar D., Krief P., Spieser S. A., Bottomley M. J., Aharoni A., and Meijler M. M. (2009) Covalent inhibition of bacterial quorum sensing. J. Am. Chem. Soc. 131, 10610–10619 [DOI] [PubMed] [Google Scholar]
- 35. O'Brien K. T., Noto J. G., Nichols-O'Neill L., and Perez L. J. (2015) Potent irreversible inhibitors of LasR quorum sensing in Pseudomonas aeruginosa. ACS Med. Chem. Lett. 6, 162–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Passador L., Tucker K. D., Guertin K. R., Journet M. P., Kende A. S., and Iglewski B. H. (1996) Functional analysis of the Pseudomonas aeruginosa autoinducer PAI. J. Bacteriol. 178, 5995–6000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gerdt J. P., McInnis C. E., Schell T. L., and Blackwell H. E. (2015) Unraveling the contributions of hydrogen-bonding interactions to the activity of native and non-native ligands in the quorum-sensing receptor LasR. Org. Biomol. Chem. 13, 1453–1462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gerdt J. P., McInnis C. E., Schell T. L., Rossi F. M., and Blackwell H. E. (2014) Mutational analysis of the quorum-sensing receptor LasR reveals interactions that govern activation and inhibition by nonlactone ligands. Chem. Biol. 21, 1361–1369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Schuster M., Urbanowski M. L., and Greenberg E. P. (2004) Promoter specificity in Pseudomonas aeruginosa quorum sensing revealed by DNA binding of purified LasR. Proc. Natl. Acad. Sci. U.S.A. 101, 15833–15839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kelley L. A., Mezulis S., Yates C. M., Wass M. N., and Sternberg M. J. (2015) The Phyre2 web portal for protein modeling, prediction and analysis. Nat. Protoc. 10, 845–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Vannini A., Volpari C., Gargioli C., Muraglia E., Cortese R., De Francesco R., Neddermann P., and Marco S. D. (2002) The crystal structure of the quorum sensing protein TraR bound to its autoinducer and target DNA. EMBO J. 21, 4393–4401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hudaiberdiev S., Choudhary K. S., Vera Alvarez R., Gelencsér Z., Ligeti B., Lamba D., and Pongor S. (2015) Census of solo LuxR genes in prokaryotic genomes. Front. Cell. Infect. Microbiol. 5, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Schaefer K. N., Geil W. M., Sweredoski M. J., Moradian A., Hess S., and Barton J. K. (2015) Oxidation of p53 through DNA charge transport involves a network of disulfides within the DNA-binding domain. Biochemistry 54, 932–941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Schuster M., and Greenberg E. P. (2006) A network of networks: quorum-sensing gene regulation in Pseudomonas aeruginosa. Int. J. Med. Microbiol. 296, 73–81 [DOI] [PubMed] [Google Scholar]
- 45. Hassett D. J., Ma J.-F., Elkins J. G., McDermott T. R., Ochsner U. A., West S. E., Huang C.-T., Fredericks J., Burnett S., Stewart P. S., McFeters G., Passador L., and Iglewski B. H. (1999) Quorum sensing in Pseudomonas aeruginosa controls expression of catalase and superoxide dismutase genes and mediates biofilm susceptibility to hydrogen peroxide. Mol. Microbiol. 34, 1082–1093 [DOI] [PubMed] [Google Scholar]
- 46. Davenport P. W., Griffin J. L., and Welch M. (2015) Quorum sensing is accompanied by global metabolic changes in the opportunistic human pathogen Pseudomonas aeruginosa. J. Bacteriol. 197, 2072–2082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Suárez-Moreno Z. R., Caballero-Mellado J., and Venturi V. (2008) The new group of non-pathogenic plant-associated nitrogen-fixing Burkholderia spp. shares a conserved quorum-sensing system, which is tightly regulated by the RsaL repressor. Microbiology 154, 2048–2059 [DOI] [PubMed] [Google Scholar]
- 48. Dubern J. F., Lugtenberg B. J., and Bloemberg G. V. (2006) The ppuI-rsaL-ppuR quorum-sensing system regulates biofilm formation of Pseudomonas putida PCL1445 by controlling biosynthesis of the cyclic lipopeptides putisolvins I and II. J. Bacteriol. 188, 2898–2906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. El-Sayed A. K., Hothersall J., and Thomas C. M. (2001) Quorum-sensing-dependent regulation of biosynthesis of the polyketide antibiotic mupirocin in Pseudomonas fluorescens NCIMB 10586. Microbiology 147, 2127–2139 [DOI] [PubMed] [Google Scholar]
- 50. Nagano N., Ota M., and Nishikawa K. (1999) Strong hydrophobic nature of cysteine residues in proteins. FEBS Lett. 458, 69–71 [DOI] [PubMed] [Google Scholar]