Abstract
The BH3-only protein Bid is known as a critical mediator of the mitochondrial pathway of apoptosis following death receptor activation. However, since full-length Bid possesses potent apoptotic activity, the role of a caspase-mediated Bid cleavage is not established in vivo. In addition, due to the fact that multiple caspases cleave Bid at the same site in vitro, the identity of the Bid-cleaving caspase during death receptor signaling remains uncertain. Moreover, as Bid maintains its overall structure following its cleavage by caspase 8, it remains unclear how Bid is activated upon cleavage. Here, Bid-deficient (Bid KO) colon cancer cells were generated by gene editing, and were reconstituted with wild-type or mutants of Bid. While the loss of Bid blocked apoptosis following treatment by TNF-related apoptosis inducing ligand (TRAIL), this blockade was relieved by re-introduction of the wild-type Bid. In contrast, the caspase-resistant mutant BidD60E and a BH3 defective mutant BidG94E failed to restore TRAIL-induced apoptosis. By generating Bid/Bax/Bak-deficient (TKO) cells, we demonstrated that Bid is primarily cleaved by caspase 8, not by effector caspases, to give rise to truncated Bid (tBid) upon TRAIL treatment. Importantly, despite the presence of an intact BH3 domain, a tBid mutant lacking the mitochondrial targeting helices (α6 and α7) showed diminished apoptotic activity. Together, these results for the first time establish that cleavage by caspase 8 and the subsequent association with the outer mitochondrial membrane are two critical events that activate Bid during death receptor-mediated apoptosis.
Keywords: apoptosis, B-cell lymphoma 2 (Bcl-2) family, CRISPR/Cas, mitochondria, transcription activator-like effector nuclease (TALEN)
Introduction
Apoptosis, an efficient cell death program, is primarily mediated through the intrinsic or the extrinsic pathway in response to different stimuli in various cell types. Both pathways lead to the activation of a common set of effector caspases, caspase 3, 6, or 7 (1). In the intrinsic pathway, stress signals, e.g. UV irradiation, serum starvation, DNA damage, etc., elicit discrete intracellular pathways that converge on the mitochondria, causing the release of cytochrome c and other apoptogenic proteins. Once in the cytoplasm, cytochrome c triggers formation of the apoptosome, consisting of cytochrome c, Apaf-1, and pro-caspase 9, and leads to the auto-activation of caspase 9 (2). In the extrinsic pathway, the death ligands, Fas/Apo1/CD95 ligand (FasL)3 or Apo2L/TRAIL, bind to their cell surface death receptors Fas/Apo-1/CD95 receptor or death receptors 4/5 (DR4/5), respectively, and trigger their trimerization (3). The trimerized receptors then recruit pro-caspase 8 through the adaptor molecule FADD, and form a death-induced signaling complex (DISC), in which caspase 8 becomes auto-activated (4). Once activated, either caspase 8 or 9, in turn, cleaves and activates the more abundant downstream effector caspases 3, 6, and 7, initiating the execution stage of apoptosis (5).
The mitochondrial event responsible for the release of apoptogenic factors in the intrinsic pathway, which is termed mitochondrial outer membrane permeabilization (MOMP) is primarily controlled by the Bcl-2 family proteins (6). Consisting of both anti- and pro-apoptotic members, the Bcl-2 family is characterized by the presence of at least one of the four Bcl-2 homology domains. The anti-apoptotic Bcl-2 family proteins, including Bcl-2, Bcl-xL, Bcl-w, Mcl-1, and A1 contain all four BH domains, BH1–4. The pro-apoptotic family members are further divided into the multi-BH domain proteins, i.e. Bax and Bak, and the BH3-only proteins, i.e. Bad, Bim, Bid, Bik, Puma, Hrk, Bmf, and Noxa (7). Bax and Bak are recognized as the requisite effectors of MOMP, as their simultaneous loss renders cells highly resistant to most apoptotic stimuli (8). While the anti-apoptotic Bcl-2 proteins negatively regulate the activation and activities of Bax/Bak, the BH3-only proteins are known to either directly or indirectly activate Bax/Bak. In response to different apoptotic stimuli, different subsets of the BH3-only proteins are transcriptionally or post-translationally activated, and in turn trigger Bax/Bak activation (9, 10). The activated Bax/Bak are responsible for the formation of putative mitochondrial pores, which allow the release of cytochrome c and other apoptogenic factors (11).
In select cell types, e.g. hepatocytes and numerous cancer cell lines, the extrinsic pathway needs to crosstalk with the intrinsic pathway to achieve apoptosis (12). In response to extracellular death ligands, the engaged death receptors instruct formation of the DISC and the activation of caspase 8 in most cells with functional death receptors (13). These death ligand-responsive cells, however, are classified into Type I and Type II, depending on the requirement of the mitochondria for apoptosis (14). In type I cells, the classical extrinsic pathway is in operation, with active caspase 8 directly cleaving and activating the effector caspases (14). In type II cells, caspase 8 is unable to efficiently cleave and activate caspase 3, 6, or 7, primarily due to the presence of XIAP, a potent inhibitor of active effector caspases, rendering a halt to the classical extrinsic pathway (15). Yet, this blockade can be efficiently bypassed in these cells by invoking the help from the intrinsic pathway. Instead of cleaving and activating caspase 3 in these cells, active caspase 8 is able to cleave the cytosolic BH3-only protein Bid (16–18), allowing the C-terminal part of Bid, termed truncated Bid (tBid), to move to the mitochondria and directly or indirectly actives Bax/Bak. Active Bax/Bak then mediates the release of cytochrome c and Second Mitochondria-derived Activator of Caspases (SMAC), a highly potent inhibitor of the XIAP protein, and trigger the formation of the apoptosome (19, 20). The engagement of the mitochondrial pathway through Bid then allows these type II cells to be efficiently killed following the addition of the death ligands (21). Importantly, most cancer cells are type II cells in which this hybrid pathway, involving the death receptors, caspase 8, Bid, Bax/Bak, and caspase 3, is operational in response to the death ligands (22).
Bid is a cytosolic, globular shaped protein primarily composed of eight α-helices, with two central hydrophobic helices cloaked by the remaining six amphipathic helices (23, 24). An unstructured loop (amino acid residues 42–79) located between helices 2 and 3 was found to contain recognition sites for several proteases, including caspase 8, caspase 2, caspase 3, calpain, cathepsin B, granzyme B, etc (16–18, 25–34). It is generally accepted that during death receptor-mediated apoptosis, caspase 8 cleaves Bid at residue Asp-60, the C-terminal fragment of Bid then rapidly associates with the mitochondrial outer membrane, where it triggers Bax/Bak activation (17, 18, 35). However, the cleavage of Bid at Asp-60 surprisingly did not alter the overall structure of Bid, suggesting that other event(s), in addition to proteolytic cleavage, is needed to activate Bid.
Despite the well-known requirement of both caspase 8 and Bid in cell surface death receptor-mediated apoptosis in mouse liver or in most human cancer cells, the requirement for cleavage of Bid by caspase-8 in death receptor-mediated mitochondrial pathway of apoptosis has remained unclear. This is because an uncleavable full-length Bid mutant was found to possess strong apoptotic and cytochrome c releasing activity in vivo and in vitro (36). In addition, Bid can also be cleaved by the effector caspases, caspase 3/6/7, at Asp-60 during apoptosis (32). Thus, whether cleavage of Bid at Asp-60 by caspase 8 is responsible for the engagement of the mitochondrial pathway following death receptor activation in type II cells has remained unclear. Moreover, as caspase cleavage failed to elicit a conformational change of Bid (23), how Bid becomes activated remains unknown. In the current study, we generated human colon cancer cells with loss of Bid or combined loss of Bid, Bax, and Bak, and addressed these critical issues by putback experiments.
Experimental Procedures
Cell Culture
HCT116 cells (ATCC) were cultured in McCoy's 5A medium supplemented with 1% penicillin/streptomycin and 10% fetal bovine serum (FBS). 293GP cells were cultured in Dulbecco's Modified Eagle Medium (DMEM) supplemented with 1% penicillin/streptomycin and 10% FBS. Cells were maintained at 37 °C with 5% CO2.
Reagents
Human recombinant TRAIL was generated as previously described (46). Antibodies used in this study are the following: anti-Bid (18), anti-PARP (Cell Signaling Technologies, 9524), anti-β-actin (Sigma-Aldrich, A5441), anti-caspase3 (Cell Signaling Technologies, 9665), and anti-caspase 8 (Cell Signaling Technologies, 9746).
Immunoblotting
Harvested cells were lysed in EBC buffer (50 mm Tris, 120 mm NaCl, 1 mm EDTA, 0.5% Nonidet P-40, pH 7.5) supplemented with 0.1 mm PMSF and protease inhibitors (5 mg/ml pepstatin A, 10 mg/ml leupeptin) with a constant rotation for 1–2 h at 4 °C. Following centrifugation at 22,000 × g for 10 min at 4 °C, supernatant was collected. Approximately 50 μg of total protein from whole cell lysate was resolved by SDS-PAGE gel and transferred onto a nitrocellulose membrane for Western blot.
Plasmids
TALEN expression vector for Bid was designed and constructed by Seoul National University, TALEN Library Resource, order H156033. Human Bid cDNA was PCR amplified and ligated into Xho1- and EcoR1-digested pEGFP-C3 (Clontech, 6082-1) vector to generate GFC3-Bid. Standard site-directed mutagenesis was performed on GFC3-Bid plasmid to generate BidD60E and BidG94E mutants. The wild-type and mutants of Bid were then cut out of the EGFP-C3 vector by Xho1 and EcoR1, and ligated into the Xho1/EcoR1 cut retroviral expression vector plasmid pMSCV-PIG (Addgene, plasmid 21654). To construct the CRISPR expression plasmids against Bax and Bak, two pairs of DNA oligos designed for sgRNA targeting the specific site of Bax and Bak were annealed and then ligated into Bbs1 digested px330 vector (Addgene #42230). The wild-type and BidD60E mutant of Bid were cut out of the EGFP-C3 vector by XhoI and EcoRI, and ligated into the XhoI/EcoRI cut EGFP-N2 vector to generate GFN2-Bid and GFN2-BidD60E. Bid-GFP and BidD60E-GFP were then PCR amplified and ligated into XhoI and HindIII digested retroviral MSCV-IRES-GFP vector. The tBid-ΔH6&7 was generated using the PCR “gene SOEing” method (47). Wild-type and mutant tBid-GFP was PCR amplified from the GFN2 constructs and ligated into NotI and BamHI cut inducible retroviral expression vector plasmid pRetroX-Tight-Pur. The GFP, wt Bid and the BidD60E and BidG94E mutants were PCR amplified and ligated into Not1 and Ecor1 digested inducible retroviral expression vector plasmid pRetroX-Tight-Pur.
Plasmid Transfection
FugeneHD reagent (Promega, E2311) was used for transfection in HCT116 cells according to manufacturer's instructions. For human Bid TALEN transfection, 1.5 μg of TALEN construct was used along with 400 ng of mRFP-TS-2A-HYG-EGFP reporter. PcDNA3.1 was added to reach a total DNA concentration of 2 μg per transfection. The transfected cells were split equally into two plates 24 h later, and selected by Hygromycin (1 mg/ml) for 2–3 days. For Bax and Bak CRISPR transfection, 500 ng of Bax CRISPR construct or 500 ng of Bak CRISPR construct was used along with 400 ng mRFP-TS-2A-HYG-EGFP reporter. pcDNA3.1 was added to reach a total DNA of 1.8 μg per transfection. The transfected cells were split equally into two plates 24 h later, and selected by Hygromycin B (1 mg/ml) for 2–3 days.
Retrovirus Infection and the Generation of Stable Pools
Retroviruses from the pMSCV-PIG vector were produced in 293GP packaging cells as previously described (37). Virus infection was performed in the Bid KO HCT116 cells in the presence of 10 μg/ml polybrene (American Bioanalytical, AB01643). Puromycin (1 μm) was added to the cells for 3 days before being removed from the medium. The Puromycin-resistant pools were cultured and examined for expression of Bid and its mutants. Inducible cell line in Bid KO HCT116 cells (Bid KO advanced) was made by infecting of pRetro-X-Tet-On Advanced virus as described earlier (38). The cells were split equally into 2 plates 48 h later and selected by 1 mg/ml G418 for 6 days. The Bid KO advanced cells were then infected with pRetroX-Tight-Pur viruses containing GFP, tBid-GFP, tBidG94E-GFP and tBidΔH6&7-GFP, Bid, BidD60E and BidG94E cDNAs. The infected cells were then selected by 0.5 μg/ml puromycin for 2 days.
Genotyping
Genomic DNA was isolated from HCT cells using DNeasy Blood and Tissue kit (Qiagen, 69504), and subjected to polymerase chain reaction to amplify genomic target regions of interest using Phusion or Taq polymerase (New England Biolabs, E0553S and M0273L). PCR was performed according to manufacturer's instructions for each polymerase, and thermocycler settings were adjusted according to product size and optimal primer annealing temperatures. PCR products were cloned into the pGEM T-easy vector (Promega, A1360) using T4 ligase. Following transformation, blue/white selection (Fisher Scientific, BP4200-10) was used to enrich for colonies containing PCR inserts. Miniprep DNAs (Wizard Plus SV Minipreps DNA Purification System, Promega, A1460) from the selected colonies were digested by EcoR1 to determine the sizes of inserts. Plasmids with inserts were then sent for Sanger sequencing using T7 or SP6 primers (UNMC High-Throughput DNA Sequencing and Genotyping Core Facility).
Apoptosis Treatment and Assay
4–5 × 105 cells were seeded in 35-mm plates 16–20 h prior to TRAIL treatment. Human recombinant TRAIL (50 ng/ml) was added to the cells for 4 h before being harvested. Whole cell lysates were prepared as described above and subjected to Western blot with PARP antibody. Quantification of apoptotic cells according to nuclear morphology was performed as previously described (39). Briefly, following apoptotic treatment, cells were stained with 1 μg/ml Hoechst 33342 (Molecular Probes, H-3570) for 10 min at 37 °C. Pictures of three random viewing areas containing 300–600 cells were taken for each plate. The percentage of cells undergoing nuclear condensation was determined for each viewing area.
Mitochondria Staining
Immunostaining was essentially performed as described (40). Tom 20 antibody (Santa Cruz Biotechnology) was added to the paraformaldehyde-fixed cells at 1:200 dilution. The cells were then washed with PBS and incubated with Alexa Fluor 594-conjugated goat anti-rabbit secondary antibody (Molecular Probes) at 1:500. Pictures of GFP-positive and immunostained cells were taken under a Nikon Eclipse 50i Fluorescence microscope.
For mitotracker staining, 2–3 × 105 cells were plated in 35-mm plates with a coverslip at the bottom 24 h prior to staining. Mitotracker (Life Technologies, Inc., M7512) was added at 20 nm. After an incubation of 15 min, the medium was replaced with PBS. The coverslips were then placed on slides and sealed. Pictures of GFP-positive and mitotracker-stained cells were taken under a Nikon Eclipse 50i Fluorescence microscope.
Results
Loss of Bid Blocks TRAIL-induced Apoptosis in HCT116 Cells
To investigate the role of Bid and its processing by caspase-8 in death receptor-mediated apoptosis in type II cells, we employed genome editing to eliminate the Bid protein from HCT116 cells. A TALEN expressing plasmid targeting the central region of Bid (exon 4) was transfected into HCT116 cells together with a reporter plasmid expressing a hygromycin resistance gene. After hygromycin selection, single clones were isolated and screened for the loss of Bid protein by Western blot analysis. Two clones, Bid KO clones 1 and 2, with a loss of the Bid protein (Fig. 1A), were chosen for further analysis by genomic PCR and sequencing. As shown in Fig. 1B, both clones have heterozygous frameshift mutations within the TALEN target region in exon 4 of the Bid gene.
FIGURE 1.
Generation of Bid KO HCT116 cells. A, whole cell lysates from wild-type and Bid KO clones 1 and 2 of HCT116 cells were subjected to SDS-PAGE and probed with the indicated antibodies. B, sequence comparison between wild-type, Bid KO clones 1&2 around the TALEN target site in exon 4 of human Bid gene. The TALEN target site is underlined in the wild-type Bid sequence.
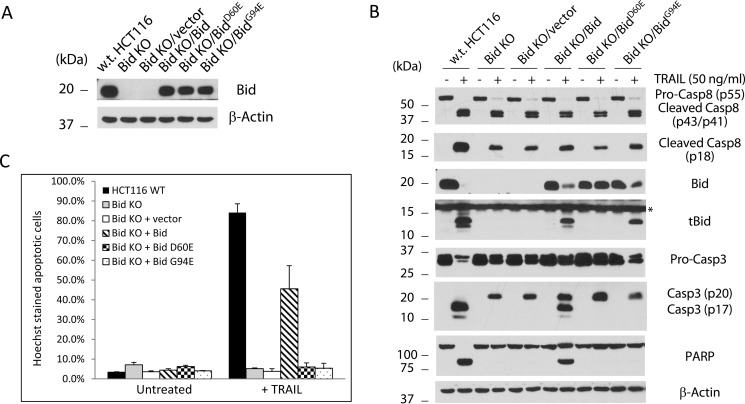
We first examined the HCT116 wild-type and the two Bid KO cells for their response to TRAIL treatment. Not surprisingly, while full scale apoptosis was observed in wild-type HCT116 cells, as judged by PARP cleavage in cell lysates, both Bid KO clones showed little apoptosis following TRAIL treatment, indicating that HCT116 cells are indeed type II cells that require Bid and the mitochondrial pathway for apoptosis in response to death ligands (Fig. 2). These results also functionally confirmed that Bid is indeed lost from the Bid KO cells. To eliminate the possibility that the TALEN plasmid used against Bid in this study might have caused unintended mutations in other genes, which may be responsible for the observed blockade of apoptosis in the Bid KO cells, we sought to re-introduce wild-type Bid into these cells and test if it can restore apoptosis. Retrovirus from empty vector or those expressing wild-type or mutants of Bid were used to infect the HCT116 Bid KO clone 1 cells. As the retroviral vector used also carries a puromycin resistance gene, we selected the infected cells with puromycin. The selected pool of Bid KO/vector and Bid KO/Bid were examined for Bid expression and response to TRAIL. Bid expression was to a large extent restored in HCT116 Bid KO/Bid cells (Fig. 3A). Following treatment by TRAIL, pro-caspase 8 was cleaved and presumably activated in w.t., Bid KO/vector, and Bid KO/Bid HCT116 cells. As expected, TRAIL treatment generated tBid in both wild-type and Bid KO/Bid cells (Fig. 3B). Interestingly, while pro-caspase 3 was cleaved, presumably by caspase 8, to generate p20 in all five Bid KO cell lines, the p17 (large subunit of caspase 3) cleavage product was only found in w.t. and Bid KO/Bid cells. Not surprisingly, apoptosis, as indicated by PARP cleavage, was restored in Bid KO/Bid cells following TRAIL treatment. These results indicated that Bid is indeed required for TRAIL-induced apoptosis in HCT116 cells.
FIGURE 2.
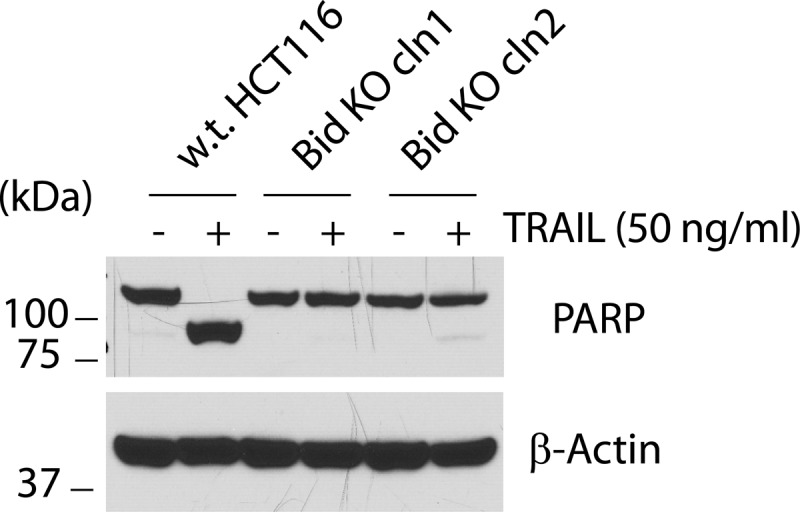
Loss of Bid blocks TRAIL-induced apoptosis. The wild-type and Bid KO clones 1 and 2 HCT116 cells were treated with TRAIL (50 ng/ml) for 4 h before being harvested. Whole cell lysates were generated and subjected to SDS-PAGE and Western blot with the indicated antibodies.
FIGURE 3.
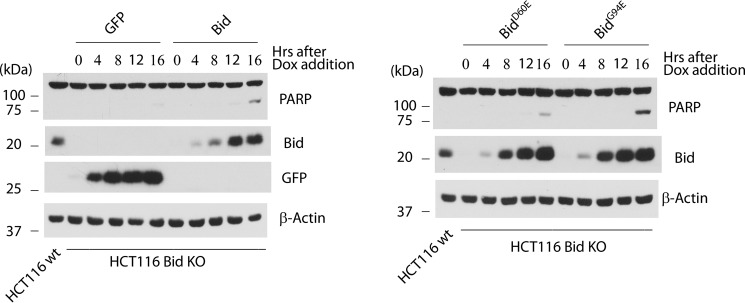
Apoptotic activities of Bid and its mutants when expressed in Bid KO cells. Bid KO HCT116 clone 1 cells carrying Dox-inducible expression viruses for GFP, Bid, Bid D60E, or Bid G94E were treated with Dox (1 μg/ml) for the indicated time. Cells were harvested at the end of the Dox treatment and assayed for protein expression and PARP cleavage by Western blot.
Cleavage of Bid at Residue Asp-60 and an Intact Bid BH3 Domain Are Required for TRAIL-induced Apoptosis
Whereas it is known that Bid can be cleaved at Asp-60 by caspase 8 (16–18), it has not been established that this cleavage happens at the endogenous level during TRAIL-induced apoptosis. Further, as full-length Bid was found to possess strong apoptotic activity (36), it is not clear whether the cleavage has any functional significance in transducing the signal to the mitochondria through tBid. We sought to address these issues by expressing a Bid mutant that is resistant to cleavage by caspase 8 (BidD60E) in the Bid KO cells. First, we established a Dox-inducible expression system in which cell pools express GFP, Bid, BidD60E (mutation at the cleavage site) or BidG94E (mutation in the BH3 domain) in response to Dox. Similar to GFP, the wild-type and mutants of Bid failed to induce apoptosis following 8 or 12 h of Dox induction, when their respective levels reached or surpassed the endogenous level (Fig. 3). However, Bid and its two mutants showed mild level of apoptosis after 16 h of Dox induction, whereas no apoptosis was observed following expression of GFP. These results indicate that full-length or non-cleavable Bid induces apoptosis only when it is overexpressed, and such an activity is not dependent on an intact BH3 domain. Second, we examined the ability of Bid and its mutants to mediate apoptosis following death receptor activation. Following infection by a retrovirus constitutively expressing Bid, BidD60E, or BidG94E, the Bid KO cells were selected by puromycin (Fig. 4A). The resulting cell pools were then examined for their response to TRAIL treatment. While caspase 8 was cleaved and presumably activated following the addition of TRAIL, the BidD60E- reconstituted Bid KO cells failed to produce tBid (Fig. 4B). Importantly, no apoptosis was observed in these cells, as demonstrated by a lack of PARP cleavage (Fig. 4B) and nuclear condensation (Fig. 4C), indicating that cleavage of Bid at residue Asp-60 is absolutely required for TRAIL-induced apoptosis in HCT116 cells. These results also indicate that uncleaved Bid is unable to mediate death receptor-mediated apoptotic pathway in type II cells.
FIGURE 4.
Requirement of cleavage at Asp-60 and an intact BH3 domain of Bid in TRAIL-induced apoptosis. A, whole cell lysates from w.t. HCT116 and stable pools of Bid KO clone 1 infected with retrovirus produced from empty vector or those expressing wild-type and the indicated mutants of human Bid were subjected to SDS-PAGE and Western blot. B, indicated cells were treated with TRAIL (50 ng/ml) for 4 h. Whole cell lysates were harvested and subjected to SDS-PAGE and Western blot with the indicated antibodies. C, HCT116 w.t. and stable pools of Bid KO HCT116cells infected with the indicated retroviruses were treated with TRAIL (50 ng/ml) for 4 h before stained with Hoechst dye. The percentage of cells that underwent nuclear condensation or fragmentation was quantified. The results are averages of two independent experiments.
Following cleavage of Bid by caspase 8, tBid is known to move to the mitochondria and directly or indirectly activate Bax/Bak. To test if a functional BH3 domain of Bid is required for TRAIL-induced apoptosis, the mutant BidG94E, carrying a mutation in the BH3 domain, was introduced into the Bid KO cells by retrovirus, followed by puromycin selection. Not surprisingly, while BidG94E was cleaved by caspase 8 following TRAIL treatment, as indicated by the generation of tBid (tBidG94E), no apoptosis was observed. Consistently, no active caspase 3 or cleaved PARP was observed, indicating that a functional BH3 domain of Bid is required for TRAIL-induced apoptosis (Fig. 4B).
An Initiator Caspase, Not Effector Caspases, Cleaves Bid at Asp-60 to Generate tBid
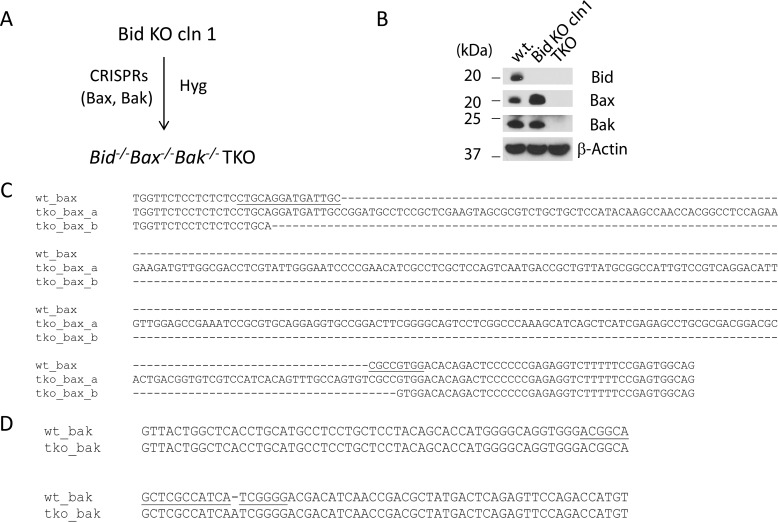
As Bid can also be cleaved by caspase 3 at Asp-60 (32), it remains possible that the 15-kDa “tBid” normally observed following death receptor activation is the result of cleavage by the downstream effector caspases (Caspases 3/6/7), which are activated by the Bax/Bak-dependent mitochondrial pathway of apoptosis (1). To block the activation of the downstream effector caspases and yet allow the initiator caspases to be activated following TRAIL treatment, we sought to generate cells that are deficient for Bax and Bak under the background of Bid KO. Bid KO clone 1 cells were therefore transfected with two CRISPR plasmids, one against Bax (human) and the other against Bak (human), together with a reporter plasmid expressing a hygromycin resistance gene. Following selection by hygromycin, a single clone with a combined loss of Bid, Bax, and Bak, was isolated based on the disappearance of all three proteins in Western blot analysis (Fig. 5), and was named the TKO.
FIGURE 5.
Generation of Bid−/−Bax−/−Bak−/− TKO cells. A, diagram of the steps involved in generating TKO cells. B, Western blots of whole cell lysates from the indicated cell lines. C and D, sequences of Bax (C) and Bak (D) of the TKO clone around the CRISPR target sites of human Bax and Bak genes. The CRISPR target sites are underlined in the wild-type Bax and Bak sequences.
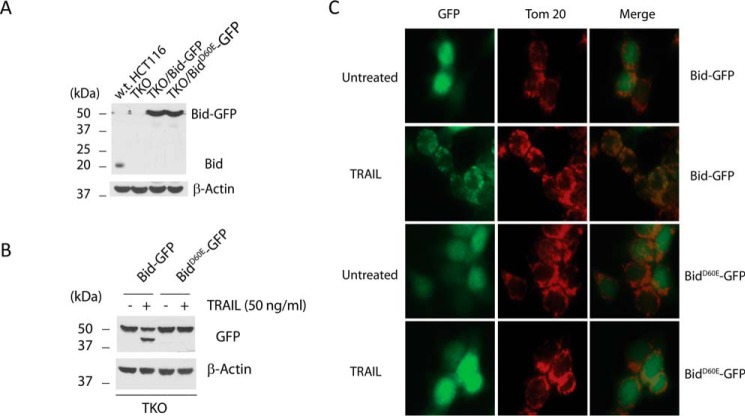
The availability of the TKO cells for the first time allows us to test if an initiator caspase, namely, caspase 8 is sufficient to cleave Bid without the participation of the effector caspases. We therefore stably expressed Bid/GFP fusion proteins with a GFP fused to the C terminus of Bid (Bid-GFP) or its cleavage defective mutant (BidD60E-GFP) in the TKO cells through retroviral infection (Fig. 6A). Five hours after TRAIL treatment, cells were harvested for Western blot analysis against GFP. As shown in Fig. 6B, Bid-GFP was cleaved into tBid-GFP, and this cleavage is completely abolished in cells expressing BidD60E-GFP, indicating that an initiator caspase, namely, caspase 8, but not effector caspases, is responsible for the cleavage of Bid following activation of the death receptor-mediated apoptotic pathway.
FIGURE 6.
TRAIL-induced cleavage of Bid at Asp-60 in the absence of Bax and Bak. A, Western blot of whole cell lysates from the indicated cell lines. B, TKO cells stably expressing the indicated proteins were treated with TRAIL (50 ng/ml) for 5 h. Cells were harvested to generate whole cell lysates, which were subjected to Western blot analysis with the indicated antibodies. C, same cells as in B were treated with TRAIL for 5 h. After treatment, the cells were fixed and immunostained with antibody against Tom20, and visualized under a fluorescence microscope.
To further investigate if the tBid-GFP is capable of localizing to the mitochondria, we observed these cells under fluorescence microscopy following TRAIL treatment. Before treatment, cells expressing Bid-GFP and cells expressing BidD60E-GFP showed cytosolic staining. However, five hours after the addition of TRAIL, the majority of cells expressing Bid-GFP displayed punctate staining that co-localizes with the mitochondria, as shown by immunostaining with an antibody against TOM20, a mitochondrial outer membrane protein. In contrast, cells expressing BidD60E-GFP display cytosolic staining following the treatment (Fig. 6C). This is the first time that the mitochondrial translocation of tBid was observed in live cells following cell surface death receptor activation. These results establish that the initiator caspases, namely, caspase 8 or 10 are sufficient for cleaving Bid into tBid, which then moves to the mitochondria.
Mitochondrial Membrane Association Is Required for the Apoptotic Activity of tBid
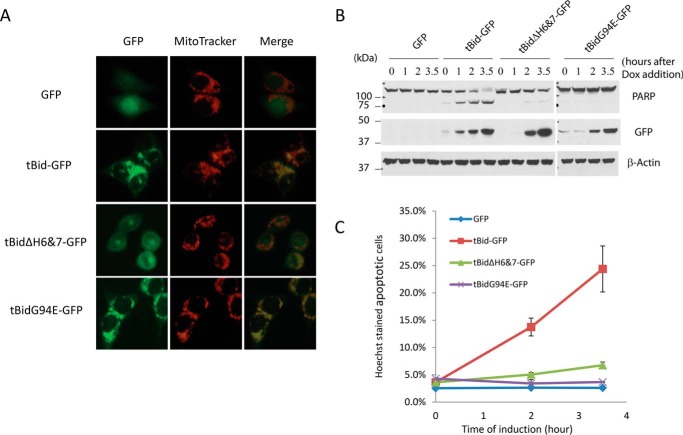
One of the surprising findings from the NMR study of full-length Bid in solution was that the cleavage by caspase-8 did not alter the structure of Bid (23). This counter-intuitive finding suggests that the proteolytic cleavage of Bid at the loop region is not sufficient to activate Bid. Then, what activated Bid? After the cleavage event, tBid becomes associated with the outer mitochondrial membrane. We therefore tested the role of membrane association in the apoptotic activity of tBid. As helices 6 and 7 have been found to be part of the mitochondrial targeting sequences of tBid (41, 42), we generated a tBid mutant that lacks these two helices (tBidΔH6&7-GFP). As expected, while wild-type tBid showed a typical mitochondrial staining, the deletion of both helix 6 and helix 7 resulted in a complete loss of mitochondrial localization (Fig. 7A). Next, we generated Dox-inducible expression systems for tBidΔH6&7 and tBid to compare their apoptotic activities. Strikingly, despite an intact BH3 domain, tBidΔH6&7 displayed greatly diminished apoptotic activity as compared with that of the wild-type tBid (Fig. 7, B and C). These results strongly suggest that mitochondrial membrane association mediated by helices 6&7 plays a critical role in activating Bid following its cleavage by caspase 8.
FIGURE 7.
Mitochondrial localization-dependent activity of tBid. The Bid KO HCT116 clone 1 cells were infected with retrovirus expressing the indicated proteins under the control of doxycyclin. A, doxycycline (1 μg/ml) and z-VAD (50 μm) were added to the cells for 5 h before the addition of MitoTracker. The cells were then visualized under a fluorescence microscope. B, same cells as in A were treated with doxycycline. At the indicated time points after the treatment, cells were harvested. The whole cell lysates were subjected to Western blot analysis with the indicated antibodies. C, Bid KO HCT116 cells with Dox inducible expression of the indicated proteins were treated with Dox (1 μg/ml). The cells were stained with Hoechst dye 0, 2, and 3.5 h after doxycyclin treatment. The percentage of cells that underwent nuclear condensation or fragmentation was quantified. The results are averages of two independent experiments.
Discussion
The BH3-only protein Bid has long been considered the molecular link between the extrinsic and the intrinsic apoptotic pathways in type II cells (17, 18, 21). However, the molecular switch that enables the engagement of the mitochondrial pathway in response to the death ligands has not been formally defined. This deficiency is partly due to the unavailability of the type II cancer cells that are deficient for Bid. In this study, through the generation of Bid KO and Bid/Bax/Bak TKO HCT116 cells, we established a putback experimental system that allows us to examine the critical elements of the molecular switch in this hybrid pathway. This system not only allows us to assay for the processing of Bid or its mutants by endogenous caspase 8, but more importantly also enabled us to observe the functional consequences (apoptosis) of this processing in response to TRAIL. Our study addressed the following three questions.
First, How Is tBid Generated?
The role of caspase 3 in the generation of tBid has remained unclear. While activated caspase 3 can directly cleave Bid at Asp-60 (32), it is also possible that activated caspase 3 can cleave and activate caspase 8, which can in turn cleave Bid at Asp-60. The latter mechanism then allows caspase 3 to indirectly cleave Bid through caspase 8. The commonly observed tBid during TRAIL-induced apoptosis is therefore likely a mixture of cleaved products from caspase 3 and caspase 8. Considering the fast kinetics of caspase 3 activation following the activation of caspase 8, there was no definitive proof that Bid can indeed be cleaved by caspase 8 before the activation of caspase 3. To further complicate the matter, Bid can also be cleaved by other proteases, including caspase 2, granzyme B, cathepsin B, and calpain, etc., and these cleavages are all mapped to the loop region, where Asp-60 is located (26–28, 30, 33, 34).
It is therefore essential to determine if activated caspase 8 following DISC formation is responsible for the generation of tBid in the absence of effector caspases or other proteases. The generation of Bid/Bax/Bak TKO cells enabled us to examine Bid cleavage in the absence of the effector caspases (Fig. 5). The translocation of tBid-GFP in the TKO cells following TRAIL treatment strongly suggests that the effector caspases are not required for the generation of tBid (Fig. 6). These results for the first time demonstrated that caspase 8 cleaves Bid in the absence of caspase 3 activation in vivo, and thus establishing the temporal and functional relationship between caspase 8 and caspase 3 during death receptor-mediated apoptosis.
Second, Is Bid Cleavage Required for Death Receptor-mediated Pathway of Apoptosis?
In other words, what is the consequence of this cleavage in vivo? Full-length Bid was found to be functional in inducing cytochrome c release and apoptosis (36). In addition, during anoikis, a form of apoptosis induced after detachment from the extracellular matrix, full-length Bid was shown to localize to the mitochondria (43). As caspase 8 was found on the mitochondria, it is possible that caspase 8 cleaves certain mitochondrial proteins, which then allow the full-length Bid to engage the mitochondria (44). In this scenario, the cleavage of Bid by caspase 8 will not be necessary for this pathway. However, such scenario is eliminated by the finding that only wild-type Bid, but not the D60E mutant, was able to restore apoptosis to the Bid KO cells in response to TRAIL (Fig. 4B). Also, while BidG94E was cleaved, it fails to mediate apoptosis (Fig. 4B). These results establish that the cleavage of Bid at Asp-60 and a functional BH3 domain in tBid are absolutely necessary for TRAIL-induced apoptosis in HCT116 cells.
Third, How Is Bid Activated After Its Cleavage by Caspase 8?
Earlier results from in vitro studies found that after cleavage, the C-terminal part of Bid (tBid) spontaneously associate with the mitochondria, whereas the N-terminal part remained soluble (18). It is reasonable to speculate that cleaved Bid, with N- and C-terminal parts still forming a complex, constantly collides with the OMM during its free diffusion, just as the uncleaved Bid does. However, due to the lack of covalent link between N- and C-terminal parts, the C-terminal part (tBid) associates with the membrane permanently, and the N-terminal part then dissociates from the C-terminal part. In this study, we tested the role of membrane association in the activity of tBid. We found that the removal of the membrane-targeting helices, H6 and H7, greatly diminished the apoptotic activity of tBid, despite the presence of an intact BH3 domain, suggesting that membrane association plays a critical role in activating tBid (Fig. 7). How does membrane activate tBid? It is possible that the N-terminal part of Bid functions as an inhibitor of tBid, and therefore the binding to membrane after cleavage changes the conformation of tBid, and help remove the N-terminal inhibitory domain. Further, the membrane association may greatly increase the local concentration of tBid, allowing it to interact with Bcl-xL, Mcl-1, or Bcl-2. Consistent with this, a previous study using liposomes demonstrated that membrane association enhanced the interaction between tBid and Bcl-xL (45). Together, these studies strongly suggest that the additional event necessary for activating Bid is the spontaneous membrane association of tBid after caspase-8-mediated Bid cleavage.
To summarize, this study for the first time established that cleavage of Bid by caspase 8 at Asp-60 and the subsequent outer mitochondrial membrane association of tBid are two critical events that activate Bid, allowing the engagement of the mitochondrial pathway during death receptor-mediated apoptosis.
Author Contributions
K. H., J. Z., and X. L. conceived the study and wrote the manuscript. K. H. carried out most of the experiments in Figs. 2–7. J. Z. generated the Bid KO clones and constructed some of the Bid expression plasmids. K. L. O. carried out part of the genomic sequencing. Y. T. participated in the design of this study. C. B. G. and R. M. Q. participated in the design and construction of the gene targeting plasmids.
This work was supported in part by the Nebraska Center for Cellular Signaling, which is funded by an Institutional Development Award (IDeA) from NIGMS, National Institutes of Health under Grant P30 GM106397, and by National Institutes of Health Grant 1R21CA193271 (to Y. T.). The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
- FasL
- Fas/Apo1/CD95 ligand
- TRAIL
- TNF-related apoptosis inducing ligand
- tBid
- truncated Bid
- TALEN
- transcription activator-like effector nuclease
- DISC
- death-induced signaling complex
- MOMP
- mitochondrial outer membrane permeabilization
- Dox
- doxycycline.
References
- 1. Danial N. N., and Korsmeyer S. J. (2004) Cell death: critical control points. Cell 116, 205–219 [DOI] [PubMed] [Google Scholar]
- 2. Jiang X., and Wang X. (2004) Cytochrome C-mediated apoptosis. Annu. Rev. Biochem. 73, 87–106 [DOI] [PubMed] [Google Scholar]
- 3. Ashkenazi A., and Dixit V. M. (1998) Death receptors: signaling and modulation. Science 281, 1305–1308 [DOI] [PubMed] [Google Scholar]
- 4. Ashkenazi A., and Dixit V. M. (1999) Apoptosis control by death and decoy receptors. Curr. Opin. Cell Biol. 11, 255–260 [DOI] [PubMed] [Google Scholar]
- 5. Strasser A., Cory S., and Adams J. M. (2011) Deciphering the rules of programmed cell death to improve therapy of cancer and other diseases. EMBO J. 30, 3667–3683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chipuk J. E., and Green D. R. (2008) How do BCL-2 proteins induce mitochondrial outer membrane permeabilization? Trends Cell Biol. 18, 157–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Youle R. J., and Strasser A. (2008) The BCL-2 protein family: opposing activities that mediate cell death. Nature reviews. Mol. Cell Biol. 9, 47–59 [DOI] [PubMed] [Google Scholar]
- 8. Wei M. C., Zong W. X., Cheng E. H., Lindsten T., Panoutsakopoulou V., Ross A. J., Roth K. A., MacGregor G. R., Thompson C. B., and Korsmeyer S. J. (2001) Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science 292, 727–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Huang D. C., and Strasser A. (2000) BH3-Only proteins-essential initiators of apoptotic cell death. Cell 103, 839–842 [DOI] [PubMed] [Google Scholar]
- 10. Lomonosova E., and Chinnadurai G. (2008) BH3-only proteins in apoptosis and beyond: an overview. Oncogene 27, S2–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chipuk J. E., Moldoveanu T., Llambi F., Parsons M. J., and Green D. R. (2010) The BCL-2 family reunion. Mol. Cell 37, 299–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ashkenazi A. (2008) Directing cancer cells to self-destruct with pro-apoptotic receptor agonists. Nature reviews. Drug Discovery 7, 1001–1012 [DOI] [PubMed] [Google Scholar]
- 13. Gonzalvez F., and Ashkenazi A. (2010) New insights into apoptosis signaling by Apo2L/TRAIL. Oncogene 29, 4752–4765 [DOI] [PubMed] [Google Scholar]
- 14. Scaffidi C., Fulda S., Srinivasan A., Friesen C., Li F., Tomaselli K. J., Debatin K. M., Krammer P. H., and Peter M. E. (1998) Two CD95 (APO-1/Fas) signaling pathways. EMBO J. 17, 1675–1687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jost P. J., Grabow S., Gray D., McKenzie M. D., Nachbur U., Huang D. C., Bouillet P., Thomas H. E., Borner C., Silke J., Strasser A., and Kaufmann T. (2009) XIAP discriminates between type I and type II FAS-induced apoptosis. Nature 460, 1035–1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gross A., Yin X. M., Wang K., Wei M. C., Jockel J., Milliman C., Erdjument-Bromage H., Tempst P., and Korsmeyer S. J. (1999) Caspase cleaved BID targets mitochondria and is required for cytochrome c release, while BCL-XL prevents this release but not tumor necrosis factor-R1/Fas death. J. Biol. Chem. 274, 1156–1163 [DOI] [PubMed] [Google Scholar]
- 17. Li H., Zhu H., Xu C. J., and Yuan J. (1998) Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell 94, 491–501 [DOI] [PubMed] [Google Scholar]
- 18. Luo X., Budihardjo I., Zou H., Slaughter C., and Wang X. (1998) Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell 94, 481–490 [DOI] [PubMed] [Google Scholar]
- 19. Du C., Fang M., Li Y., Li L., and Wang X. (2000) Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell 102, 33–42 [DOI] [PubMed] [Google Scholar]
- 20. Verhagen A. M., Ekert P. G., Pakusch M., Silke J., Connolly L. M., Reid G. E., Moritz R. L., Simpson R. J., and Vaux D. L. (2000) Identification of DIABLO, a mammalian protein that promotes apoptosis by binding to and antagonizing IAP proteins. Cell 102, 43–53 [DOI] [PubMed] [Google Scholar]
- 21. Yin X. M., Wang K., Gross A., Zhao Y., Zinkel S., Klocke B., Roth K. A., and Korsmeyer S. J. (1999) Bid-deficient mice are resistant to Fas-induced hepatocellular apoptosis. Nature 400, 886–891 [DOI] [PubMed] [Google Scholar]
- 22. Kaufmann T., Strasser A., and Jost P. J. (2012) Fas death receptor signalling: roles of Bid and XIAP. Cell Death Differ. 19, 42–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chou J. J., Li H., Salvesen G. S., Yuan J., and Wagner G. (1999) Solution structure of BID, an intracellular amplifier of apoptotic signaling. Cell 96, 615–624 [DOI] [PubMed] [Google Scholar]
- 24. McDonnell J. M., Fushman D., Milliman C. L., Korsmeyer S. J., and Cowburn D. (1999) Solution structure of the proapoptotic molecule BID: a structural basis for apoptotic agonists and antagonists. Cell 96, 625–634 [DOI] [PubMed] [Google Scholar]
- 25. Blomgran R., Zheng L., and Stendahl O. (2007) Cathepsin-cleaved Bid promotes apoptosis in human neutrophils via oxidative stress-induced lysosomal membrane permeabilization. J. Leukocyte Biol. 81, 1213–1223 [DOI] [PubMed] [Google Scholar]
- 26. Chen M., He H., Zhan S., Krajewski S., Reed J. C., and Gottlieb R. A. (2001) Bid is cleaved by calpain to an active fragment in vitro and during myocardial ischemia/reperfusion. J. Biol. Chem. 276, 30724–30728 [DOI] [PubMed] [Google Scholar]
- 27. Cirman T., Oresić K., Mazovec G. D., Turk V., Reed J. C., Myers R. M., Salvesen G. S., and Turk B. (2004) Selective disruption of lysosomes in HeLa cells triggers apoptosis mediated by cleavage of Bid by multiple papain-like lysosomal cathepsins. J. Biol. Chem. 279, 3578–3587 [DOI] [PubMed] [Google Scholar]
- 28. Gao Z., Shao Y., and Jiang X. (2005) Essential roles of the Bcl-2 family of proteins in caspase-2-induced apoptosis. J. Biol. Chem. 280, 38271–38275 [DOI] [PubMed] [Google Scholar]
- 29. Heibein J. A., Goping I. S., Barry M., Pinkoski M. J., Shore G. C., Green D. R., and Bleackley R. C. (2000) Granzyme B-mediated cytochrome c release is regulated by the Bcl-2 family members bid and Bax. The J. Exp. Med. 192, 1391–1402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mandic A., Viktorsson K., Strandberg L., Heiden T., Hansson J., Linder S., and Shoshan M. C. (2002) Calpain-mediated Bid cleavage and calpain-independent Bak modulation: two separate pathways in cisplatin-induced apoptosis. Mol. Cell. Biol. 22, 3003–3013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Reiners J. J. Jr., Caruso J. A., Mathieu P., Chelladurai B., Yin X. M., and Kessel D. (2002) Release of cytochrome c and activation of pro-caspase-9 following lysosomal photodamage involves Bid cleavage. Cell Death Differ. 9, 934–944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Slee E. A., Keogh S. A., and Martin S. J. (2000) Cleavage of BID during cytotoxic drug and UV radiation-induced apoptosis occurs downstream of the point of Bcl-2 action and is catalysed by caspase-3: a potential feedback loop for amplification of apoptosis-associated mitochondrial cytochrome c release. Cell Death Differ. 7, 556–565 [DOI] [PubMed] [Google Scholar]
- 33. Stoka V., Turk B., Schendel S. L., Kim T. H., Cirman T., Snipas S. J., Ellerby L. M., Bredesen D., Freeze H., Abrahamson M., Bromme D., Krajewski S., Reed J. C., Yin X. M., Turk V., and Salvesen G. S. (2001) Lysosomal protease pathways to apoptosis. Cleavage of bid, not pro-caspases, is the most likely route. J. Biol. Chem. 276, 3149–3157 [DOI] [PubMed] [Google Scholar]
- 34. Sutton V. R., Davis J. E., Cancilla M., Johnstone R. W., Ruefli A. A., Sedelies K., Browne K. A., and Trapani J. A. (2000) Initiation of apoptosis by granzyme B requires direct cleavage of bid, but not direct granzyme B-mediated caspase activation. J. Exp. Med. 192, 1403–1414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Billen L. P., Shamas-Din A., and Andrews D. W. (2008) Bid: a Bax-like BH3 protein. Oncogene 27, S93–104 [DOI] [PubMed] [Google Scholar]
- 36. Sarig R., Zaltsman Y., Marcellus R. C., Flavell R., Mak T. W., and Gross A. (2003) BID-D59A is a potent inducer of apoptosis in primary embryonic fibroblasts. J. Biol. Chem. 278, 10707–10715 [DOI] [PubMed] [Google Scholar]
- 37. Lopez H., Zhang L., George N. M., Liu X., Pang X., Evans J. J., Targy N. M., and Luo X. (2010) Perturbation of the Bcl-2 network and an induced Noxa/Bcl-xL interaction trigger mitochondrial dysfunction after DNA damage. J. Biol. Chem. 285, 15016–15026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhang L., Lopez H., George N. M., Liu X., Pang X., and Luo X. (2011) Selective involvement of BH3-only proteins and differential targets of Noxa in diverse apoptotic pathways. Cell Death Diff. 18, 864–873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. George N. M., Evans J. J., and Luo X. (2007) A three-helix homo-oligomerization domain containing BH3 and BH1 is responsible for the apoptotic activity of Bax. Genes Dev. 21, 1937–1948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Luo X., and Sawadogo M. (1996) Functional domains of the transcription factor USF2: atypical nuclear localization signals and context-dependent transcriptional activation domains. Mol. Cell. Biol. 16, 1367–1375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lutter M., Fang M., Luo X., Nishijima M., Xie X., and Wang X. (2000) Cardiolipin provides specificity for targeting of tBid to mitochondria. Nat. Cell Biol. 2, 754–761 [DOI] [PubMed] [Google Scholar]
- 42. Wei M. C., Lindsten T., Mootha V. K., Weiler S., Gross A., Ashiya M., Thompson C. B., and Korsmeyer S. J. (2000) tBID, a membrane-targeted death ligand, oligomerizes BAK to release cytochrome c. Genes Dev. 14, 2060–2071 [PMC free article] [PubMed] [Google Scholar]
- 43. Valentijn A. J., and Gilmore A. P. (2004) Translocation of full-length Bid to mitochondria during anoikis. J. Biol. Chem. 279, 32848–32857 [DOI] [PubMed] [Google Scholar]
- 44. Stegh A. H., Barnhart B. C., Volkland J., Algeciras-Schimnich A., Ke N., Reed J. C., and Peter M. E. (2002) Inactivation of caspase-8 on mitochondria of Bcl-xL-expressing MCF7-Fas cells: role for the bifunctional apoptosis regulator protein. J. Biol. Chem. 277, 4351–4360 [DOI] [PubMed] [Google Scholar]
- 45. Garcia-Sáez A. J., Ries J., Orzáez M., Pérez-Payà E., and Schwille P. (2009) Membrane promotes tBID interaction with BCL(XL). Nat. Struct. Mol. Biol. 16, 1178–1185 [DOI] [PubMed] [Google Scholar]
- 46. Pitti R. M., Marsters S. A., Ruppert S., Donahue C. J., Moore A., and Ashkenazi A. (1996) Induction of apoptosis by Apo-2 ligand, a new member of the tumor necrosis factor cytokine family. J. Biol. Chem. 271, 12687–12690 [DOI] [PubMed] [Google Scholar]
- 47. Horton R. M., Cai Z. L., Ho S. N., and Pease L. R. (1990) Gene splicing by overlap extension: tailor-made genes using the polymerase chain reaction. BioTechniques 8, 528–535 [PubMed] [Google Scholar]