Abstract
This study investigated the energy expenditure (EE) and substrate utilization reflected by the respiratory-exchange ratio (RER) during and after resistance exercises performed with different muscle mass. Ten male volunteers (mean±SD; 26±4yr, 179±6cm, 77±8kg) performed multiple sets of the horizontal leg press (LP) and chest fly (CF) (5 sets of 10 repetitions with 15 repetition-maximum, 1-minute between-set intervals) in a counterbalanced design. Oxygen uptake and carbon dioxide production were measured during 40 minutes of resting; resistance exercise protocols (sets and intervals); 90 minutes of post-exercise recovery. Total fat and carbohydrate oxidation rates were calculated according to the non-protein respiratory quotient. Both exercise conditions elicited net excess post-exercise oxygen consumption (EPOC) of similar duration (approximately 40min). The EPOC magnitude at 40 minutes was greater after LP than after CF (7.36±1.10L vs. 4.73±0.99L; P<0.001). The RER was higher in LP (1.30±0.04) than CF (1.16±0.05, P=0.0003) during exercise. During recovery the RER was similar in LP and CF (P>0.05) and lower than pre-exercise (Pre-exercise=0.78±0.04 vs. CF40min=0.74±0.04; CF90min=0.68±0.02 and LP50min=0.73±0.06; LP90min=0.65±0.04, P<0.05). However, fat oxidation after LP was greater than CF between 30–90 minutes of recovery (mean total fat oxidation: LP=10.9 g vs. CF=8.4 g; P<0.01). The increases of EE and fat oxidation during post-exercise recovery were greater after multiple sets of resistance exercises performed with larger muscle mass than smaller muscle mass. This finding has practical implications for resistance training designed as part of weight management programs.
Keywords: Physical activity, strength training, metabolism, exercise physiology, rate of respiratory ratio
INTRODUCTION
Resistance exercise has been shown to intensify the resting energy expenditure (EE) (25, 26) and increase post-exercise lipid oxidation (20), which would be desirable for weight loss (15, 18). The respiratory exchange ratio (RER) is a surrogate of substrate utilization, and can be used to identify the rate of lipid oxidation (2, 11, 15, 22, 25). However, its response to resistance exercise sessions has not been extensively investigated, and data from a few available studies are varied (2, 20, 21, 29, 33).
Prior researchers investigating the influence of resistance training variables upon EE, lipid oxidation, or RER have focused on the intensity (33), volume (11, 12, 22), rest intervals (29), type of exercise (15), or session design (25). Some studies tested the influence of complete sessions of resistance exercises (2, 15, 21, 25) or compared resistance and aerobic exercise (3, 9, 20). A previous study by our group (8) demonstrated that EE estimated from oxygen uptake net (VO2net) during and after exercise was higher after exercises performed with larger than smaller muscle mass. On the other hand, differences of substrate utilization between exercises with different muscle mass have not been yet addressed. This information would be valuable, since it is acknowledged that the amount of muscle mass influences EE and substrate utilization during and after exercise sessions (7). The assessment of RER during and after resistance exercise sessions would be useful to address this issue, as demonstrated by several previous studies (1, 2, 12, 21).
Carbohydrates are used for glycogen resynthesis following exercise, which helps to explain the increased lipid oxidation during the excess post-exercise consumption (EPOC) (13). It can be speculated that EE and the need for posterior glycogenesis would be higher if a greater amount of muscle mass is exercised. Thus, it is feasible to think that the amount of muscle mass used for exercises may determine the substrate utilization during post-exercise recovery and that the RER would reflect differences in this regard. However, this premise has not been experimentally confirmed. This study compared the impact of multiple sets of acute resistance exercises performed with similar intensity but different muscle mass upon EE and substrate utilization, as reflected by RER during and after exercise. The hypothesis was that the exercised muscle mass would influence the total VO2 and rate of oxidation of nutrients estimated by means of the RER, especially lipid oxidation.
METHODS
Participants
Ten healthy men with at least one year of experience with resistance training (1.6±0.4 yrs.) volunteered for the study (26±3 yrs.; 179±6 cm; 78±7 kg). Subjects were not taking any drugs or nutritional supplements influencing metabolism or performance. The Institutional Ethical Committee approved the study and participants signed written informed consent prior to enrolling in the study.
Protocol
Data were assessed on four non-consecutive days, interspersed with intervals of 48–72 hours, using a within-group study design. On the first day, volunteers went through anthropometric measurements and anamnesis, including 24-hours food intake recall. Researchers determined the resting VO2, RER, and load corresponding to 15 repetition-maximum (15RM) for the horizontal leg press (LP) and the chest fly (CF). On the second day, resting measures and 15RM determination protocols were repeated to check for reliability. Subjects performed two exercise sessions in a counterbalanced design on the third and fourth days.
Upon arrival at the laboratory, subjects were positioned to perform the exercise, installing the VO2 mask and equipment before the standardized warm-up (12 repetitions with 30% 15RM). The exercise protocols began five minutes after the warm-up (5 sets of 10 repetitions with 15RM workloads and 1-min interval between sets). All subjects performed LP (larger muscle mass) and CF (smaller muscle mass) protocols on different days.
Prior to the 15RM tests, a warm-up of 12 repetitions with 30% of pre-determined 15RM was performed. After five minutes, three to four maximal trials with 5-minute rest intervals were allowed to determine 15RM either for LP or CF (TechnogymTM, Gambettola, FC, Italy). The order of exercises was counterbalanced and interspersed by intervals of 20 minutes. Similar procedures were adopted for determining the load corresponding to 15 RM in LP and CF, being repeated after 48–72 hours to assess the load reliability. Test-retest intraclass correlation (ICC) and coefficient of variation were considered satisfactory (LP: ICC=0.96, P<0.001 and CV=4.2%; CF: ICC=0.97, P<0.001 and CV=4.8%).
Weight was measured to the nearest 1gram and height was measured in millimeters using a wall mounted stadiometer (HarpendenTM, Cambridge, UK). Body surface (m2) was determined using classic standard protocol (6), and body composition was calculated based on chest, abdomen and thigh skinfolds (14, 32). In order to avoid bias due to the thermic effect of food, all participants gave a 24-hours dietary recall by direct interview. The proportion of macronutrients in total energetic value (TEV) recommended by the World Health Organization (24) and daily EE of approximately 2400 kcal/day were adopted as references (15, 23).
On the day before the exercise sessions, participants were instructed to have dinner at 8–9 PM to minimize possible metabolic effects of previous nutritional intake. All tests were performed in the morning, and 2 hours prior to the experiment, a standard food intake was provided (16): a glass of fruit juice (200 ml) and 6 salt crackers. Energy values of fruit juice and crackers were 88 kcal (22 g of carbohydrate) and 240 kcal (38 g of carbohydrate, 8 g of fat, and 4 g of protein), respectively. Adequate hydration was provided throughout the entire experiment.
Prior to respiratory measurements at rest, subjects abstained from physical exercise, alcohol, soft drinks, and caffeine for 48 hours, fasted for 8–10 hours, and made minimum effort when travelling to the laboratory. Upon arrival, they laid in a calm environment for 20 minutes. The VO2 and ventilation (VE) were measured using a VO2000 analyzer (Medical GraphicsTM, Saint Louis, MO, USA) with subjects at the supine position. A facemask (Hans Rudolph V MaskTM; Hans Rudolph Inc., Shawnee, KS, USA) covering the mouth and nose was attached to a bidirectional digital flow valve and fastened using a mesh hairnet and Velcro straps. Careful calibrations of flow sensors and gas analyzers were performed before each measurement. Standard conditions were respected during the assessment, including length of fasting and resting period before measurement to allow subjects to achieve a steady state.
The RER under typical metabolic conditions with stable respiratory function ranges from 0.7 to 1.0. If RER is lower than 0.7 or higher than 1.0, prolonged starvation or excessive recent energy consumption should be suspected, both events representing protocol violation of resting metabolic rate (4). The RER at rest was assessed using a low-flow pneumotachometer (2–30 L/min) and data output frequency of three respiratory cycles. The VO2 (ml/kg/min) was measured for 40 minutes, and analyses were performed using five minutes of steady state data after the 10-minute stabilization period. All resting measurements were made in a temperature and humidity controlled environment (20–22 ºC and 60–70% respectively). After 48–72h, respiratory measurements were repeated to determine RER reliability. Intraclass correlations and coefficients of variation were calculated (ICC=0.89, P=0.023 and CV=5.2%).
Before the exercise protocols, subjects remained at rest in a quiet environment for 15 minutes or until RER matched the previously assessed resting values (variation could not exceed 5%). During exercise, VO2, VCO2, and VE were assessed using a medium-flow pneumotachometer (10–120 L/min). Upon completion of the exercise sequences, the RER was recorded for another 90 minutes. The same procedures adopted for RER assessment at rest were applied to measurements during post-exercise recovery. However, it was critical to change the pneumotachometer from medium- to low-flow. The change was made within five to ten minutes of recovery and approximately three minutes were required to reprogram the software, recalibrate the system, and resume assessment. Data from the first minute of measurement after the pneumotachometer replacement were discarded. In brief, post-exercise breath-by-breath VO2 data were averaged immediately following the last set of a given exercise, and at 1, 2, 3, 4, 5, 10, 20, 30, 40, 50, 60, 70, 80, and 90 minutes of recovery.
Individual breath-by-breath data points for VO2 (ml/kg/min) were averaged for the entire set and each minute of resting intervals. The total VO2 (L) for a given exercise was calculated by adding the values obtained during the sets and rest intervals. The absolute VO2 (L/min) and RER during each set, between set intervals, and post-exercise recovery were used to calculate the total VO2 (defined as the sum of VO2 obtained during the exercises, recovery intervals, and 90-minute EPOC). The net VO2 (total VO2 – resting VO2) was adopted as a surrogate of total EE.
The occurrence of lipid oxidation was related to RER < 0.71, whereas a value > 1.00 was considered to reflect carbohydrate oxidation. Considering a RER > 0.85 as an index of fat to carbohydrate oxidation, total fat and carbohydrate (CHO) oxidation rates were calculated according to the non-protein respiratory quotient (28): CHO oxidation rate = 4.585 V̇CO2 – 3.226 V̇O2; Fat oxidation rate = 1.695 V̇O2 – 1.701 V̇CO2, with V̇O2 and V̇CO2 expressed in liters per minute (L/min) and oxidation rates in grams per minute (g/min). The non-protein respiratory quotient approach has been extensively applied. It assumes that the amount of protein oxidized is negligible, and that other metabolic processes involving the production or utilization of O2 or CO2 are also quantitatively negligible compared to glucose and fatty acid oxidation (28).
The CHO and fat consumption were averaged for each minute of resting intervals. The post-exercise CHO and fat consumption were averaged at each five minutes of recovery, starting with 11–15 until 86–90 minutes. The first 10 minutes of recovery were discarded to avoid influence of the pneumotachometer change, as well as bias due to peak VO2 attained during the exercise sessions.
Statistical Analysis
The assumption of data normality was proven by univariate analysis. Therefore total VO2, CHO and fat consumption during and after exercises were compared using student t-tests for paired samples. The RER measured during exercise was compared by 2-way repeated measures ANOVA (exercises and sets as main factors). Differences between RER, CHO, and fat consumption at baseline and during recovery were tested by repeated measures ANOVA. In all cases, Fisher post hoc tests were applied in the event of significant F ratios. A probability level of P ≤ 0.05 was adopted for statistical significance. The same statistical software was used for all calculations (STATA/SETM 8.2, College Station, TX, USA).
RESULTS
Table 1. presents subjects’ characteristics concerning anthropometric data, diet recall, physiological measurements at rest, and loads corresponding to 15RM.
Table 1.
Subjects’ characteristics (n = 10)
| Characteristics | Mean ± SD |
|---|---|
| Age (years) | 26 ± 3 |
| Body surface area (m2) | 2.0 ± 0.1 |
| TEV (kcal) | 2410.1±86.0 |
| Lipids (%) | 22.0 ± 3.4 |
| Carbohydrates (%) | 69.1 ± 3.8 |
| Protein (%) | 11.6 ± 3.7 |
| TEV breakfast usual (kcal) | 332.5 ± 33.0 |
| TEV standardized breakfast (kcal) | 328.0 |
| Resting RER (VCO2/VO2) | 0.78 ± 0.04 |
| Chest fly - 15RM (kg) | 30.2 ± 4.3 |
| Leg press - 15RM (kg) | 69.8 ± 9.0 |
TEV = total energetic value considering the summation of macro-nutrient intake per day; RER = respiratory exchange ratio; RM = repetition-maximum; SD = standard deviation.
The VO2 during exercise was higher in LP than in CF (6.54±1.26 L vs. 3.31±0.71 LP=0.006). The EPOC was also higher after LP than after CF until four minutes of recovery (P<0.001), producing greater EPOC net in LP than in CF (7.36±1.10 L vs. 4.73±0.99 L, respectively; P<0.001 L at 40 min). On the other hand, the type of exercise did not affect the EPOC duration. The VO2 remained significantly higher compared to pre-exercise until 40 minutes of recovery after CF and LP (P<0.0001). In the two exercises, approximately 45% of EPOC net was recorded until five minutes of recovery, whereas another 55% corresponded to measurements taken within 10–90 minutes. The total VO2 net in LP was significantly higher than CF (LP=17.57±3.63 L vs. CF=9.96±2.86 L at 40 min recovery; P=0.003).
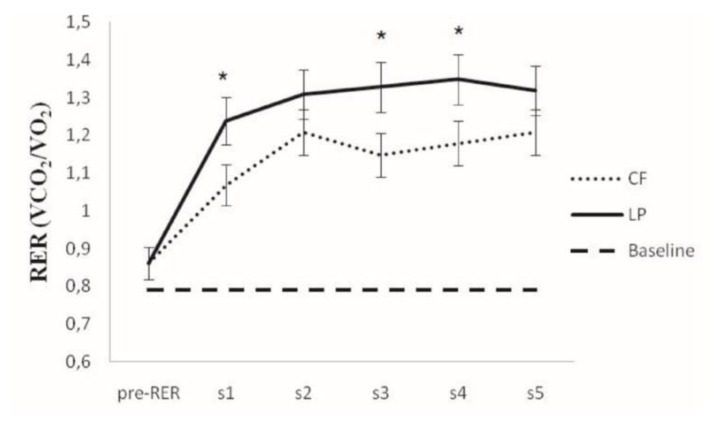
Results for RER before and during exercises are shown in Figure 1. A considerable increase occurred during both protocols vs. baseline (P<0.0001). However, RER was in general higher in LP than in CF (1st set, P=0.005; 3rd set, P=0.0003; 4th set, P=0.0003).
Figure 1.
Mean values and ANOVA results for the respiratory exchange ratio at rest and during exercise (n = 10). CF = chest fly machine; LP = horizontal leg press; Pre-RER = RER measured at beginning of exercise; s1–s5 = 1st to 5th sets. *: Significant difference between exercises (P<0.05). Bars around the means indicate confidence intervals at 95%.
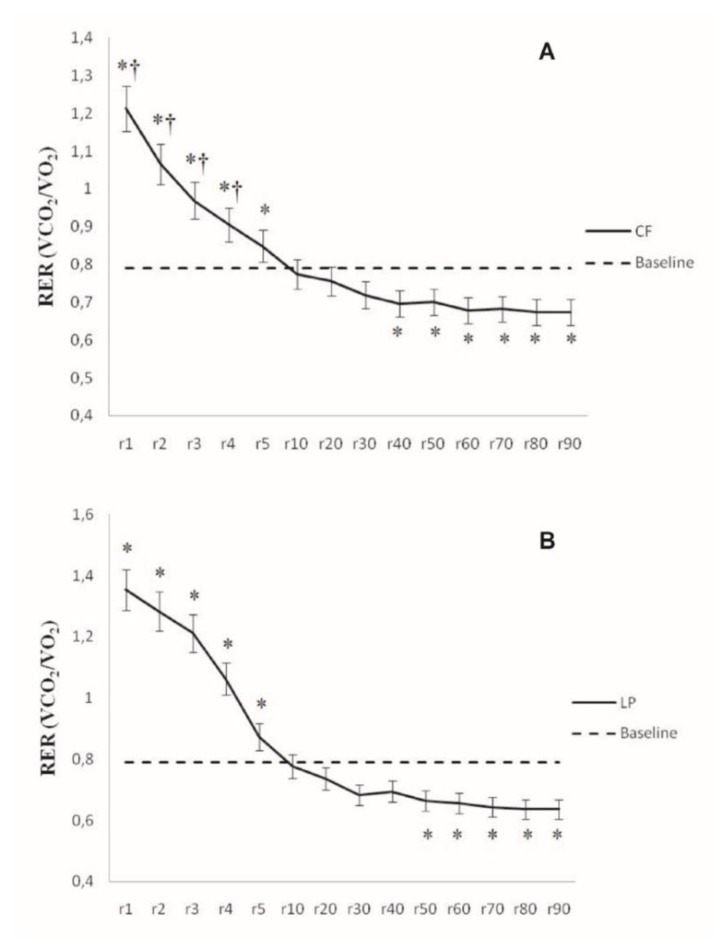
Figure 2. depicts RER during post-exercise recovery. In the first minutes of recovery, the RER was higher after LP than CF (first four minutes, P=0.019 to P=0.009), but data indicate that lipid oxidation occurred after both exercise protocols. Three marked phases were identified during EPOC: 1) In the first four minutes, the RER remained significantly higher compared to baseline (P=0.02 to P<0.0001); 2) The RER decreased steadily within 10–40 minutes and no difference was detected against pre-exercise (P>0.05); 3) After 40 (CF) or 50 (LP) minutes until the end of recovery, the RER was significantly lower than pre-exercise (RER=0.78±0.04) and values were compatible with lipid oxidation (at 90 min: RER-CF=0.68±0.02, P=0.03; RER-LP= 0.65±0.04; P=0.001).
Figure 2.
Mean values and ANOVA results for the RER during 90 minutes recovery (n = 10). A) CF = chest fly machine. B) LP = horizontal leg press. *: significant difference with baseline values (P<0.05); †: significant difference between exercises (P<0.05). Bars around the means indicate confidence intervals at 95%.
Macronutrient analyses during exercise from baseline to the last interval between sets (S1 to S4) are presented in Table 2. As expected, the CHO utilization increased from the first to fourth intervals in both exercises (P<0.01), with higher values being found for LP (P<0.01). No significant difference between exercises was found for fat utilization (P=0.69).
Table 2.
Carbohydrate and fat oxidation for the chest fly and leg press from baseline to the last interval between sets (Mean ± SD) (n = 10)
| Intervals between sets | Chest Fly | Leg Press | ||
|---|---|---|---|---|
| CHO (g/min) | TF (g/min) | CHO (g/min) | TF (g/min) | |
| Baseline | 0.074±0.027† | 0.068±0.014 | 0.074±0.027† | 0.068±0.014 |
| S1 | 0.621±0.188* | 0.090±0.059 | 1.705±0.541 | 0 |
| S2 | 0.782±0.237* | 0 | 3.041±1.026 | 0 |
| S3 | 1.229±0.258* | 0 | 3.488±1.193 | 0 |
| S4 | 1.427±0.386* | 0 | 3.643±0.957 | 0 |
CHO: Carbohydrate oxidation; FT: Fat oxidation;
Significant difference of CHO between CF and LP (P < 0.01);
Significant difference between CHO at baseline and all between-set intervals (P < 0.01).
Table 3 exhibits CHO and fat utilization during post-exercise recovery. The CHO consumption reached zero at 35 minutes and 45 minutes following LP and CF, respectively. The CHO consumption did not differ between exercises (P=0.62). The fat consumption predominated until the last interval with slightly higher values being detected in LP than in CF (mean total fat oxidation = 10.9 g vs. 8.4 g, after LP and CF, respectively; P<0.01). In both exercises, CHO utilization in the first interval (e.g., 11–15 minutes of recovery) was lower and fat oxidation was higher compared to all intervals after 20 minutes of recovery (P<0.001).
Table 3.
Carbohydrate and fat oxidation for the chest fly and leg press within 5-min intervals during 90 min of post-exercise recovery. (Mean ± SD) (n = 10)
| Post-exercise (min) | Chest Fly | Leg Press | ||
|---|---|---|---|---|
|
| ||||
| CHO (g/min) | Fat (g/min) | CHO (g/min) | Fat (g/min) | |
| 11 to 15 | 0.136±0.047† | 0.082±0.010†† | 0.184±0.160† | 0.093±0.073†† |
| 16 to 20 | 0.088±0.051 | 0.095±0.014 | 0.088±0.166 | 0.110±0.73 |
| 21 to 25 | 0.047±0.041 | 0.110±0.025 | 0.056±0.109 | 0.121±0.053 |
| 26 to 30 | 0.055±0.056 | 0.101±0.032 | 0.036±0.093 | 0.121±0.042 |
| 31 to 35 | 0.034±0.041 | 0.108±0.023 | 0.012±0.067 | 0.128±0.029 |
| 36 to 40 | 0.017±0.039 | 0.112±0.028* | 0 | 0.134±0.030 |
| 41 to 45 | 0.003±0.027 | 0.114±0.023* | 0 | 0.132±0.031 |
| 46 to 50 | 0 | 0.113±0.023* | 0 | 0.129±0.25 |
| 51 to 55 | 0 | 0.109±0.028* | 0 | 0.130±0.020 |
| 56 to 60 | 0 | 0.111±0.029* | 0 | 0.137±0.028 |
| 61 to 65 | 0 | 0.109±0.019* | 0 | 0.136±0.020 |
| 66 to 70 | 0 | 0.110±0.029* | 0 | 0.137±0.033 |
| 71 to 75 | 0 | 0.110±0.021* | 0 | 0.127±0.022 |
| 76 to 80 | 0 | 0.110±0.018* | 0 | 0.139±0.028 |
| 81 to 85 | 0 | 0.110±0.018* | 0 | 0.136±0.027 |
| 86 to 90 | 0 | 0.107±0.023* | 0 | 0.136±0.027 |
CHO: carbohydrate;
Significant difference of fat oxidation between CF and LP (P < 0.01);
Significant difference of CHO oxidation between baseline and 5-min recovery intervals (P < 0.001) with the exception of 16–20 interval (CF: P = 0.32; LP: P = 0.16);
Significant difference of fat oxidation between baseline and 5-min recovery intervals (P < 0.001) with the exception of 16–20 interval (CF: P = 0.19; LP: P = 0.24)
DISCUSSION
This study investigated the effects of resistance exercises performed with different muscle mass upon EE and substrate utilization, as reflected by RER. The main results were: a) Total VO2 during EPOC (and therefore overall EE) was significantly higher in exercise performed with larger than smaller muscle mass; b) Based on RER values, lipid oxidation occurred during EPOC after both exercise protocols. However, macronutrient analyses suggested that fat oxidation would be slightly more pronounced after LP than CF.
Many factors may influence the comparison of respiratory responses during resistance exercises, such as subjects’ physical activity level or dietary behavior (19). This study adopted a within-group design and subjects were homogeneous in regards to physical activity, body surface area, and caloric intake, minimizing the influence of these variables on the metabolic rate. The estimated daily caloric intake and proportion of macronutrients in relation to TEV were consistent with current available recommendations (23). Moreover, the standardized breakfast provided in the study prior to data assessment was quite similar to the usual breakfast related in the diet recall.
The utilization of indirect calorimetry during exercises with increased CO2 production to assess the substrate balance has been questioned (17). The difficulty in reaching an acid-base balance during resistance exercise could compromise an accurate determination of substrate utilization. Moreover, when recovering from intense activities, the RER may be influenced by the replenishment of bicarbonate reserves, which requires the incorporation of CO2 in their molecular structure (21). This could result in lower VCO2 and decreased RER, leading to the false idea that fat oxidation is increasing. However, this limitation mostly applies to recovery from exhaustive activities, which was not the case in the present protocols. Moreover, comparisons between indirect calorimetry and stricter techniques have shown that absolute substrate oxidation could be well determined, regardless of the stable bicarbonate pool (31). Actually, many previous studies assessing the RER during and after resistance exercises have used similar techniques and approaches adopted in the present experiment (15, 18, 21, 29).
The choice of LP and CF must be justified. First of all, resistance exercise sessions are mostly composed of exercises for lower and upper limbs. The CF was chosen instead of more common exercises, as the bench press for two reasons: a) the agonistic action of the triceps would increase the amount of recruited muscle mass compared to CF, which could compromise the comparison between exercises. Adopting a monoarticular exercise instead of a multiarticular exercise as the bench press seemed more appropriate to assure a substantial difference in the exercised muscle mass. Secondly, in the context of weight management, weight training is hardly performed with maximum repetitions. Instead, this kind of program is usually designed with submaximal load and repetitions, since subjects typically lack experience with resistance training (5). A greater number of successive sets increased the EE to levels that made easier the detection of differences between exercises.
Our results concur with previous studies suggesting that acute resistance exercise may increase the EPOC (7, 8, 11, 28). However, we failed to demonstrate differences in EPOC duration related to exercised muscle mass. After both LP and CF, the VO2 returned to resting levels after approximately 40 minutes of recovery. On the other hand, the overall EE seemed to be influenced by the type of exercise, since total VO2 net in LP was almost twice the value obtained for CF. It is well accepted that exercise volume is a major determinant of EE during this type of exercise (7). Collectively, available studies have shown that total VO2 would increase in protocols with greater vs. lower exercise volume (intensity x number of repetitions x number of sets) (8, 19, 33). It has been suggested that EE during resistance exercise would be proportional to the recruited muscle mass (8, 15, 30). Our findings ratify the premise that the amount of exercise muscle mass influence the EE elicited by resistance exercises, since the VO2 net in LP was significantly higher than CF.
Whether resistance exercise sessions are able to induce fat oxidation during recovery is an interesting issue, but its assessment is problematic. Although RER might be used for evaluating potential lipid oxidation, its assessment should be performed concomitantly to substrate utilization analysis (10) to avoid misinterpretation of metabolic responses. The RER in resistance exercises has not been extensively investigated and little is known about energy substrate utilization in recovery (11, 15, 18, 25). It is acknowledged that during resistance exercise there would be a substantial contribution of high-energy phosphate and glucose breakdown to supply energy (27), but during post-exercise, data are mixed and controversial. Some studies have shown that lipids would be the main energy substrate during post-exercise recovery (11, 18, 22, 25), while others suggested that carbohydrate oxidation would be predominant (21, 29) or that differences of substrate utilization would not exist (33).
It appears that the anaerobic nature of each set increases the CO2 levels, resulting in high RER values. On the other hand, high levels of CO2 may stimulate a central mediated increase in VE and subsequent reduction in RER during the rest intervals. Lengthening the rest intervals increases the proportion of time the subject is not exercising and consequently the recovery from anaerobic stress, lowering the RER throughout the sets in comparison with shorter intervals. It was not surprising that multiple sets with 1-min intervals, as applied in our protocol, provoked a substantial increase in RER that could not be offset by this relatively short recovery period. Ratamess et al. (29) compared the RER during and after five sets of bench press performed with five or ten repetitions at 85% or 75% 1RM, respectively. The RER values during the exercise bouts reported in that study (within 1.05–1.36) were compatible with our findings. The condition that approached the characteristics of our protocol (5 sets of 10 reps with 1–2 min intervals) elicited the highest RER values (between 1.21–1.36). The CHO oxidation in the Ratamess et al. study (29) was detected up to 10–15 minutes after the end of the exercise, but no lipid oxidation was observed until 30 minutes of recovery. However, the assessment was interrupted while the RER was still declining. The present study extended the period of observation and was able to detect lipid oxidation after 40 minutes of recovery, with fat utilization predominance until 90 minutes after both exercise protocols, but greater in LP than in CP.
Another issue that is not clear in the literature is the relative influence of training intensity and volume upon RER and substrate utilization. For instance, Thornton and Potteiger (33) failed to observe lipid oxidation during 120 minutes recovery after exercises performed with similar volume but different intensities (85% or 45% 1RM) in young men. On the other hand, Haddock and Wilkin (11) and Melby et al. (22) suggested that lipid oxidation would occur for several hours after resistance exercises performed with similar intensities, regardless of training volume. Our findings are in line with the idea that lipid oxidation may indeed occur after multiple sets of resistance exercise and that exercise volume – in the present case related to the exercised muscle mass – would be a determinant of such response. An increase in fat utilization occurred after approximately 30 minutes of recovery, irrespective of the type of exercise, which has implications to exercise programs designed for health promotion.
A limitation of this study relates to the potential application of our results to actual exercise prescription. The protocols involved just one exercise performed with moderate intensity, which is not the current practice in resistance training. It must be also acknowledged that overgeneralization of the present data should be avoided. The subjects participating in the study had normal weight and were young and experienced in resistance training, which limits the application of our findings to middle aged populations, which are frequently targets of adult weight management programs. Future research is needed to ratify the possible effects of resistance exercise performed with different intensities and volumes upon fat oxidation in overweight populations.
In brief, increases in post-exercise EE and fat oxidation were greater after resistance exercise performed with larger muscle mass. In a practical perspective, this means that lipid oxidation will occur during post-exercise recovery irrespective of the exercises included in the routine. However, larger muscle groups should be exercised if the purpose is to increase EE and perhaps optimize fat oxidation following resistance exercise sessions.
The EPOC duration was similar after protocols including multiple sets of LP and CF, but total VO2 and RER were influenced by the exercised muscle mass, being higher in LP (larger muscle mass) than in CF (smaller muscle mass). The RER after both protocols indicated the occurrence of lipid oxidation during post-exercise recovery. Additional macronutrient analyses showed that lipid oxidation after LP was greater over CF, suggesting that substrate utilization may be influenced by the amount of exercised muscle mass.
ACKOWLEDGEMENTS
This study was supported by the Carlos Chagas Filho Foundation for the Research Support in the Rio de Janeiro State (FAPERJ) and by the Brazilian Council for the Technological and Research Development (CNPq).
REFERENCES
- 1.Benton MJ, Swan PD. Effect of protein ingestion on energy expenditure and substrate utilization after exercise in middle-aged women. Int J Sport Nutr Exerc Metab. 2007;17(6):544–55. doi: 10.1123/ijsnem.17.6.544. [DOI] [PubMed] [Google Scholar]
- 2.Binzen CA, Swan PD, Manore MM. Postexercise oxygen consumption and substrate use after resistance exercise in women. Med Sci Sports Exerc. 2001;33(6):932–8. doi: 10.1097/00005768-200106000-00012. [DOI] [PubMed] [Google Scholar]
- 3.Burleson MA, O’Bryant HS, Stone MH, Collins MA, Triplett-McBride T. Effect of weight training exercise and treadmill exercise on post-exercise oxygen consumption. Med Sci Sports Exerc. 1998;30(4):518–22. doi: 10.1097/00005768-199804000-00008. [DOI] [PubMed] [Google Scholar]
- 4.Compher C, Frankenfield D, Keim N, Roth-Yousey L. Best practice methods to apply to measurement of resting metabolic rate in adults: a systematic review. J Am Diet Assoc. 2006;106(6):881–903. doi: 10.1016/j.jada.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 5.Donnelly JE, Blair SN, Jakicic JM, Manore MM, Rankin JW, Smith BK. American College of Sports Medicine Position Stand. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc. 2009;41(2):459–71. doi: 10.1249/MSS.0b013e3181949333. [DOI] [PubMed] [Google Scholar]
- 6.Du Bois D, Du Bois EF. A formula to estimate the approximate surface area if height and weight be known. Archives of Internal Medicine. 1916;17:863–71. [PubMed] [Google Scholar]
- 7.Farinatti P, Castinheiras Neto AG, da Silva NL. Influence of Resistance Training Variables on Excess Postexercise Oxygen Consumption: A Systematic Review. ISRN Physiology. 2013;2013:10. [Google Scholar]
- 8.Farinatti PT, Castinheiras Neto AG. The effect of between-set rest intervals on the oxygen uptake during and after resistance exercise sessions performed with large- and small-muscle mass. J Strength Cond Res. 2011;25(11):3181–90. doi: 10.1519/JSC.0b013e318212e415. [DOI] [PubMed] [Google Scholar]
- 9.Gillette CA, Bullough RC, Melby CL. Postexercise energy expenditure in response to acute aerobic or resistive exercise. Int J Sport Nutr. 1994;4(4):347–60. doi: 10.1123/ijsn.4.4.347. [DOI] [PubMed] [Google Scholar]
- 10.Gregory S, Wood R, Matthews T, Vanlangen D, Sawyer J, Headley S. Substrate Utilization is Influenced by Acute Dietary Carbohydrate Intake in Active, Healthy Females. J Sports Sci Med. 2011;10(1):59–65. [PMC free article] [PubMed] [Google Scholar]
- 11.Haddock BL, Wilkin LD. Resistance training volume and post exercise energy expenditure. Int J Sports Med. 2006;27(2):143–8. doi: 10.1055/s-2005-865601. [DOI] [PubMed] [Google Scholar]
- 12.Heden T, Lox C, Rose P, Reid S, Kirk EP. One-set resistance training elevates energy expenditure for 72 h similar to three sets. Eur J Appl Physiol. 2011;111(3):477–84. doi: 10.1007/s00421-010-1666-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hill JO, Melby C, Johnson SL, Peters JC. Physical activity and energy requirements. Am J Clin Nutr. 1995;62(5 Suppl):1059S–66S. doi: 10.1093/ajcn/62.5.1059S. [DOI] [PubMed] [Google Scholar]
- 14.Jackson AS, Pollock ML. Generalized equations for predicting body density of men. Br J Nutr. 1978;40(3):497–504. doi: 10.1079/bjn19780152. [DOI] [PubMed] [Google Scholar]
- 15.Jamurtas AZ, Koutedakis Y, Paschalis V, Tofas T, Yfanti C, Tsiokanos A, Koukoulis G, Kouretas D, Loupos D. The effects of a single bout of exercise on resting energy expenditure and respiratory exchange ratio. Eur J Appl Physiol. 2004;92(4–5):393–8. doi: 10.1007/s00421-004-1156-8. [DOI] [PubMed] [Google Scholar]
- 16.Jeacocke NA, Burke LM. Methods to standardize dietary intake before performance testing. Int J Sport Nutr Exerc Metab. 2010;20(2):87–103. doi: 10.1123/ijsnem.20.2.87. [DOI] [PubMed] [Google Scholar]
- 17.Kanaley JA, Mottram CD, Scanlon PD, Jensen MD. Fatty acid kinetic responses to running above or below lactate threshold. J Appl Physiol. 1985;1995;79(2):439–47. doi: 10.1152/jappl.1995.79.2.439. [DOI] [PubMed] [Google Scholar]
- 18.Kirk EP, Donnelly JE, Smith BK, Honas J, Lecheminant JD, Bailey BW, Jacobsen DJ, Washburn RA. Minimal resistance training improves daily energy expenditure and fat oxidation. Med Sci Sports Exerc. 2009;41(5):1122–9. doi: 10.1249/MSS.0b013e318193c64e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lyons S, Richardson M, Bishop P, Smith J, Heath H, Giesen J. Excess post-exercise oxygen consumption in untrained men following exercise of equal energy expenditure: comparisons of upper and lower body exercise. Diabetes Obes Metab. 2007;9(6):889–94. doi: 10.1111/j.1463-1326.2006.00679.x. [DOI] [PubMed] [Google Scholar]
- 20.Melanson EL, Sharp TA, Seagle HM, Donahoo WT, Grunwald GK, Peters JC, Hamilton JT, Hill JO. Resistance and aerobic exercise have similar effects on 24-h nutrient oxidation. Med Sci Sports Exerc. 2002;34(11):1793–800. doi: 10.1097/00005768-200211000-00016. [DOI] [PubMed] [Google Scholar]
- 21.Melanson EL, Sharp TA, Seagle HM, Donahoo WT, Grunwald GK, Peters JC, Hamilton JT, Hill JO. Twenty-four-hour metabolic responses to resistance exercise in women. J Strength Cond Res. 2005;19(1):61–6. doi: 10.1519/14293.1. [DOI] [PubMed] [Google Scholar]
- 22.Melby C, Scholl C, Edwards G, Bullough R. Effect of acute resistance exercise on postexercise energy expenditure and resting metabolic rate. J Appl Physiol. 1985;1993;75(4):1847–53. doi: 10.1152/jappl.1993.75.4.1847. [DOI] [PubMed] [Google Scholar]
- 23.National Academy of Sciences. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids (Macronutrients): A Report of the Panel on Macronutrients, Subcommittees on Upper Reference Levels of Nutrients and Interpretation and Uses of Dietary Reference Intakes, and the Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. Washington, DC: The National Academies Press; 2005. [Google Scholar]
- 24.Organization WH. Diet, Nutrition, and Prevention of Chronic Disease. Geneve: World Health Organization; 2003. (Technical Report Series 916). [PubMed] [Google Scholar]
- 25.Ormsbee MJ, Thyfault JP, Johnson EA, Kraus RM, Choi MD, Hickner RC. Fat metabolism and acute resistance exercise in trained men. J Appl Physiol. 1985;2007;102(5):1767–72. doi: 10.1152/japplphysiol.00704.2006. [DOI] [PubMed] [Google Scholar]
- 26.Osterberg KL, Melby CL. Effect of acute resistance exercise on postexercise oxygen consumption and resting metabolic rate in young women. Int J Sport Nutr Exerc Metab. 2000;10(1):71–81. doi: 10.1123/ijsnem.10.1.71. [DOI] [PubMed] [Google Scholar]
- 27.Pascoe DD, Costill DL, Fink WJ, Robergs RA, Zachwieja JJ. Glycogen resynthesis in skeletal muscle following resistive exercise. Med Sci Sports Exerc. 1993;25(3):349–54. [PubMed] [Google Scholar]
- 28.Peronnet F, Massicotte D. Table of nonprotein respiratory quotient: an update. Can J Sport Sci. 1991;16(1):23–9. [PubMed] [Google Scholar]
- 29.Ratamess NA, Falvo MJ, Mangine GT, Hoffman JR, Faigenbaum AD, Kang J. The effect of rest interval length on metabolic responses to the bench press exercise. Eur J Appl Physiol. 2007;100(1):1–17. doi: 10.1007/s00421-007-0394-y. [DOI] [PubMed] [Google Scholar]
- 30.Robergs RA, Gordon T, Reynolds J, Walker TB. Energy expenditure during bench press and squat exercises. J Strength Cond Res. 2007;21(1):123–30. doi: 10.1519/00124278-200702000-00023. [DOI] [PubMed] [Google Scholar]
- 31.Romijn JA, Coyle EF, Hibbert J, Wolfe RR. Comparison of indirect calorimetry and a new breath 13C/12C ratio method during strenuous exercise. Am J Physiol. 1992;263(1 Pt 1):E64–71. doi: 10.1152/ajpendo.1992.263.1.E64. [DOI] [PubMed] [Google Scholar]
- 32.Siri WE. Body composition from fluid space and density. In: Brozek J, Henschel A, editors. Techniques for measuring body composition. Washington, DC: National Academy of Science; 1961. pp. 223–44. [Google Scholar]
- 33.Thornton MK, Potteiger JA. Effects of resistance exercise bouts of different intensities but equal work on EPOC. Med Sci Sports Exerc. 2002;34(4):715–22. doi: 10.1097/00005768-200204000-00024. [DOI] [PubMed] [Google Scholar]