Abstract
Objective
To describe the development and implementation of a safer conception service in a resource-limited setting.
Methods
Qualitative work to inform the design of a safer conception service was conducted with clients and providers at Witkoppen Health and Welfare Centre, a primary health center in Johannesburg, South Africa. Services began in July 2013, for HIV-affected participants planning conception within six months and included counseling about timed unprotected intercourse and home-based self-insemination, early initiation of combined antiretroviral therapy (cART) for HIV-infected individuals, pre-exposure prophylaxis (PrEP) for HIV-uninfected partners and circumcision for men. Participants were enrolled into an implementation science study evaluating method uptake, acceptability, and pregnancy and HIV transmission outcomes.
Results
Findings to-date from 51 qualitative participants and 128 clinical cohort participants (82 women and 46 men, representing 82 partnerships) are presented. All men were accompanied by female partners, whereas 56% of women attended with their male partner. Fifteen of 46 couples (33%) were in confirmed serodiscordant relationships, however of the 36 additional women attending alone, 56% were unaware of their partners’ HIV status or believed them to be HIV-uninfected. The majority of HIV-infected women (86%) and men (71%) were on cART at enrollment, however only 47% on cART were virally suppressed. Timed unprotected intercourse, self-insemination and cART were common choices for participants; few elected PrEP.
Conclusions
Lessons learned from early implementation demonstrate feasibility of safer conception services, however reaching discordant couples, cART-naïve infected partners, and men remain challenges. Creating demand for safer conception services among those at highest risk for HIV transmission is necessary.
Keywords: HIV-1, safer conception, discordant couples, treatment as prevention, fertility, prevention of mother to child transmission
INTRODUCTION
While women and men living with HIV frequently desire to have children [1–6], people living with HIV in high burden settings have limited knowledge and communication with health care providers regarding safer conception methods [3, 7, 8]. Guidelines for safer conception have been published from North American and European countries [9–11] and more recently by South Africa [12], but these guidelines have not yet been adopted by governments in resource-limited settings or incorporated as standard of care. Consequently, couples often try to become pregnant without being informed of how to prevent horizontal transmission to partners while trying to conceive and vertical transmission to infants at the early stages of conception [3].
Antiretrovirals as prevention could be an important component of safer conception strategies [13]. Both combination antiretroviral treatment (cART) and pre-exposure prophylaxis (PrEP) can reduce transmission within discordant couples [14, 15], but neither strategy has been evaluated in a resource limited setting in the context of safer conception. Those trying to conceive are more likely to have increased unprotected sex in order to become pregnant and thus evaluation of risk in the context of conception is important. Even among individuals with undetectable plasma viral load, virus has been detected in female genital tract and male semen samples [16, 17].
Data from 62 discordant couples in Spain and 46 discordant couples in Switzerland have demonstrated that cART with or without PrEP can be successfully used for conception in [18, 19], but outcomes of treatment as prevention strategies among couples who did not meet the viral suppression criteria were not reported. Other methods such as sperm-washing and in-vitro fertilization (IVF) or other assisted reproductive techniques have also been used successfully to prevent horizontal transmission in serodiscordant couples in Europe and other high resource settings [20–22], however these methods are not scalable at a population level in a resource-limited setting.
The extent to which safer conception services are being offered in high-burden, resource-limited settings is unknown, and the outcomes of such services remain unreported [23]. Building an evidence base for safer conception strategies will be necessary before services can be rolled out across Sub-Saharan African (SSA) countries. In this manuscript, we document the development of a safer conception service using an implementation science framework and describe the preliminary experiences of this service integrated within a primary care clinic in Johannesburg, South Africa.
METHODS
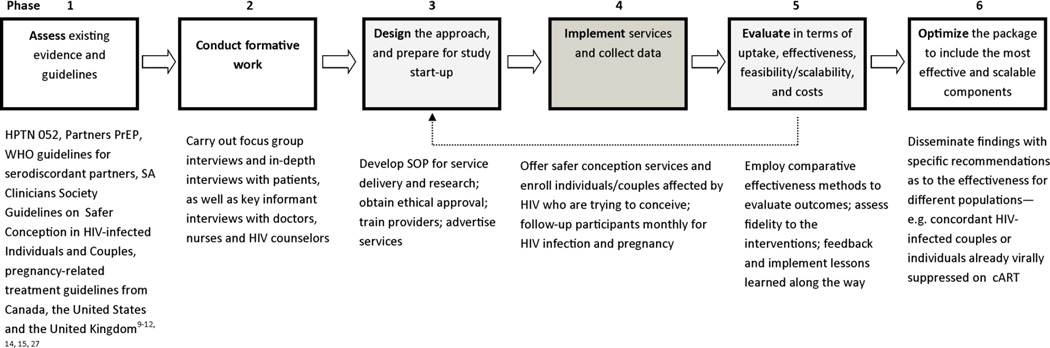
After reviewing other implementation science approaches [24–26] and considering elements relevant to this research, we developed an implementation science framework with 6 distinct phases to guide our safer conception research conducted at the non-governmental, primary care clinic, Witkoppen Health and Welfare Centre (WHWC) in Johannesburg, South Africa (Figure 1). In phase 1, we reviewed relevant guidelines and literature to collect information on possible safer conception strategies [9–12, 14, 15, 27]. In the second phase, we performed formative work to assess knowledge about safer conception strategies among clinic clients and health care workers, acceptability and preferences for safer conception approaches, logistical challenges, and safer conception concerns of patients and health care workers. The formative research included four focus group discussions (FGDs) each comprised of 6–8 participants (HIV-infected men only, HIV-uninfected men in discordant relationships, HIV-infected women only, and HIV uninfected women in discordant relationships), sixteen in-depth interviews with clinic clients, again ensuring equal representation across gender and HIV-status, and nine key informant interviews with health care providers, which included HIV doctors, nurses and lay counselors. In phase 3, we designed the safer conception service to be implemented at the clinic based on evidence collected in phases 1 and 2. Service delivery at the Sakh’umndeni (“building the family”) Safer Conception Clinic and data collection (phase 4) began in July 2013. As part of the first iterative evaluation process (phase 5), we performed a descriptive analysis of the characteristics of the first 128 individuals receiving safer conception services. We also assessed lessons learned from operating the safer conception clinic for nine months. In the future (phase 6), we will evaluate uptake, acceptability, comparative effectiveness, feasibility and scalability of the services offered to inform final recommendations for an optimized package of safer conception care integrated within a primary care clinic in a high burden, low resource setting.
Figure 1.
Implementation science approach to optimizing safer conception services
Ethics approval for this research was obtained from the University of North Carolina IRB and the Human Research Ethics Committee at the University of the Witwatersrand.
RESULTS
Formative research to inform safer conception strategies
Key findings from sixteen in-depth interviews, nine key informant interviews and four FGDs are presented in Table 1. In general, availability of safer conception options for couples affected by HIV was limited, as highlighted by the story of one male FGD participant.
“My wife she is HIV positive. We asked here if we could have a baby, they said yes. We asked how, they said they didn’t know, that we must go to a private clinic. It was very expensive. I talked to the doctor – she explained that there was a tablet that I could take, but there would be side effects and she couldn’t give it to me, I’d have to go somewhere else and also pay for the consultation fees and the tablets. She said I could also do sperm insemination in the clinic, but I couldn’t afford it. But she said the other option was to use a syringe – by not using a normal process. So I went to the pharmacy to get one and they thought I was a drug user so they just gave it to me and told me to leave without paying. So we used the syringe to inseminate her after we had sex – and then we had to boil it and sterilize it because we only had one. So now she is pregnant.” [Quote is from an HIV-uninfected man, in a relationship with an HIV-infected woman.]
Table 1.
Summary of key findings from formative work to design safer conception services
| Topics | General Themes Expressed by Patients (n=42) | |
|---|---|---|
| Women (n=21) | Men (n=21) | |
|
Knowledge around safer conception methods |
|
|
|
Acceptability of safer conception approaches |
|
|
|
Preferences for safer conception providers |
|
|
|
Logistics of offering safer conception services |
|
|
| General Themes Expressed by Health Care Workers (n=9) | ||
| HIV Lay Counselors (n=3) | HIV Clinicians (n=6) | |
|
Practice of Safer Conception Counseling |
|
|
|
Concerns & Challenges |
|
|
F+: HIV-infected female; F-: HIV-uninfected female; M+: HIV-infected male; M-: HIV-uninfected male; U: Unknown HIV status
When asked by others in the group whether he found the method to be acceptable, the participant expressed desperation and ambivalence.
“I did this because I was desperate. I previously broke up with a woman who was HIV positive. I wanted to have a kid and we tested and she was positive and I just couldn’t do it. Now again, I want to get pregnant and this is the third partner I’ve had who is positive, so I just said, maybe I am meant to be with a positive woman, just let me stay positive about it and live positively. I know many people who are HIV negative who died or are unhappy and many who are positive who are very happy and healthy, so let me just accept this…If a pill were available, I would have preferred that. Sterilizing after every use was too difficult.”
Knowledge on safer conception for individuals affected by HIV was low and mainly focused on prevention of mother-to-child transmission (PMTCT) of HIV. Some participants had heard of sperm-washing or in-vitro fertilization, but fewer were aware of home-based self-insemination methods. There was substantial interest in self-insemination among HIV-infected women and HIV-uninfected men to eliminate risk for the male uninfected partner. Some participants were aware that cART for the infected partner could be used to prevent horizontal transmission, yet HIV-infected women believed that men would not take cART and endure the side effects if they were not ill, and men had little trust in treatment as prevention. None of the women or men identified PrEP for the uninfected partner as a prevention method. Perceptions about PrEP were mixed, with participants generally feeling that the potential for side effects was a real concern. Condoms for prevention were the primary messages received or at least internalized by patients. Particularly among those already on cART, 100% condom use was often cited as the only way to ensure HIV prevention, thus pitting HIV prevention against reproductive goals.
Among health care workers, HIV clinicians demonstrated sufficient knowledge and reported prior experience counseling couples affected by HIV, but indicated that most counseling around fertility was client initiated. In contrast, counselors felt they lacked the required knowledge to effectively counsel clients and expressed concerns around the window period and re-infection. Clinicians also raised concerns regarding the difficulty of achieving sustained adherence and viral suppression, the challenge of involvement of the male partners in conception issues, and underlying fertility issues that may reduce the likelihood of conception in some HIV infected individuals.
The qualitative work also elucidated information concerning service delivery. Both men and women expressed a preference for non-integrated services, in part because it was perceived that the wait times would be shorter. Clarity and honesty related to risks and benefits of services was another key concern raised by participants.
Safer conception service model for a high-burden, resource limited setting
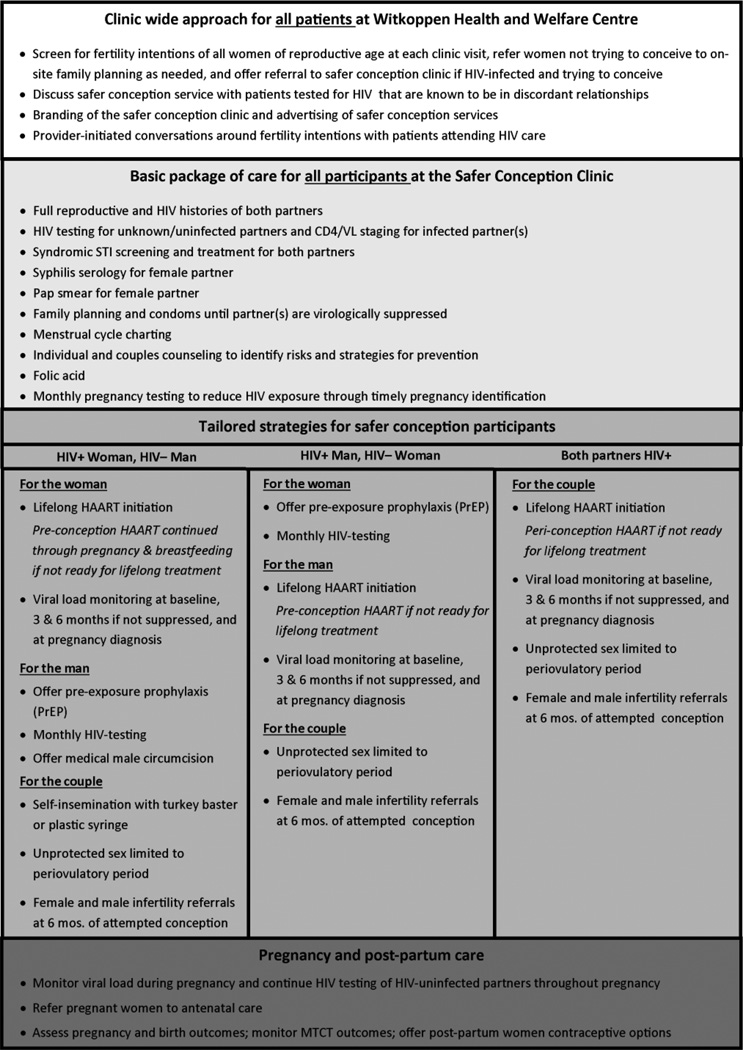
The WHWC Sakh’umndeni service delivery model (Figure 2) combines behavioral, biomedical and structural prevention tools. Key components are behavioral strategies to reduce the frequency of HIV exposure while trying to conceive and to increase male involvement in pre-conception care, biomedical interventions to reduce the probability of HIV transmission when exposure occurs, and structural components such as advertising to promote an environment in which clients are comfortable seeking services and training for health care workers to guarantee quality of services.
Figure 2.
Current safer conception service delivery model
To identify those in need of safer conception services, all WHWC clients are offered HIV counseling and testing (HCT), independent of HIV risk or reason for visit, and all women of reproductive age are asked about fertility intentions and contraceptive use and screened for pregnancy at every visit. Clients not desiring pregnancy and using a contraceptive method are referred to family planning services on-site. Providers also initiate discussions around safer conception with HIV-uninfected individuals known to be in a discordant relationship and HIV-infected individuals with pregnancy intentions. Individuals in need of safer conception services are thus identified at the vitals station, HCT program, general outpatient department, or the ARV unit and then referred to the safer conception clinic. Based on our formative work, we opted for an on-site separate service (Sakh’umndeni) with dedicated staff and patient rooms. An appointment system is used to decrease client wait times, and all clients receive comprehensive HIV and general care at Sakh’umdeni in order to avoid additional wait times and duplication of services. Services are provided by experienced professional nurses trained in cART management and HIV counselors with training in safer conception counseling.
A basic package of care is delivered to all safer conception clients. This includes full repoductive and HIV history of both partners, HIV testing if the status of a partner is unknown or negative, CD4 and viral load assessment, screening for sexually transmitted diseases, PAP smear for women, and prescription for folic acid. To reduce the frequency of unprotected intercourse, all women receive training on menstrual cycle charting and all couples are counselled on the use of condoms and offered family planning until viral suppression of the HIV infected partner is achieved. Pregnancy testing is conducted monthly to prevent unnecessary HIV exposure following succesful conception. After six months of attempted conception, couples are referred off-site for infertility assessment in order to prevent prolonged periods of unprotected intercourse if conception is unlikely.
Depending on the HIV status of the male and female partner, tailored strategies for prevention are discussed. Participants are provided with an array of options, but choose for themselves which strategies they would like to pursue. As shown in Figure 2, options include self-insemination using plastic syringes, lifelong or short term cART independent of CD4 count for the infected partner with viral load monitoring, PrEP for the uninfected partner with monthly HIV testing, and referrals to on-site male medical circumcision for HIV-uninfected men.
Following conception HIV-uninfected partners are monitored for seroconversion throughout pregnancy and viral loads of pregnant women are monitored. Pregnancy and infant outcomes are assessed and women are offered contraception in the post-partum period.
Implementation data and operational experiences
Among the first 128 safer conception participants, 64% were women and 36% were men (Table 2). All men were accompanied at some point by their female partners, but only 56% of women were accompanied to the safer conception clinic by their male partner, largely due to their partners’ work schedules. The 128 participants belonged to 82 different partnerships. Fifteen of the 46 couples in which both partners were enrolled (33%) were in confirmed serodiscordant relationships. The number of discordant couples may be higher, as of the other 36 women attending without their partners, 56% were unsure of their partners’ HIV status (n=9) or believed them to be HIV-uninfected (n=11). The majority of HIV-infected women (86%) and men (71%) attending the safer concpetion clinic were already on cART at time of enrollment - the median time on cART was two years. Overall half of the women (50%) and 36% of men were both on cART and virally suppressed at time of the first safer conception clinic visit. Median CD4 count at first visit was relatively high, 483 cells/mm3 [IQR 324–700] among HIV-infected women and 431 cells/mm3 [IQR 298–525] in HIV-infected men.
Table 2.
Characteristics of participants seeking care at the Witkoppen Health and Welfare Centre Safer Conception Clinic (n=128)
| Baseline characteristics | Women (n=82) |
Men (n=46) |
|---|---|---|
| Age, median years [IQR] | 33 [28–38] | 38 [34–43] |
| Currently employed, % | 73% | 87% |
| Number of living children, median [IQR] | 1 [0–1] | 1 [1–2] |
| Number of children with current partner, median [IQR] | 0 [0–1] | 0 [0–1] |
| STI symptoms present at baseline screening, % | 1% | 0% |
| Known to be HIV infected at time of enrollment, % | 93% | 78% |
| On HAART, % HIV+ | 86% | 71% |
| Time on HAART, median years [IQR] | 2 [1–3] | 2 [0–3] |
| CD4 cell count, % | ||
| <200 cells/mm3 | 5% | 3% |
| 200–350 cells/mm3 | 21% | 19% |
| 351–500 cells/mm3 | 23% | 22% |
| >500 cells/mm3 | 51% | 56% |
| Viral load, % | ||
| Below the detectable limit (<50 copies/ml) | 63% | 34% |
| 50 – 400 copies/ml | 22% | 29% |
| >400 – 10,000 | 13% | 21% |
| >10,000 – 50,0000 copies/ml | 0% | 8% |
| >50,000 copies/ml | 2% | 8% |
| Circumcised / male partner circumcised, % | 48% | 36% |
| Accompanied by partner to safer conception clinic, % | 56% | 100% |
| Partner’s HIV Status | ||
| Partner known to be HIV-infected at enrollment, % | 57% | 80% |
| Partner on HAART at enrollment, % of known HIV+ | 68% | 84% |
Approaches offered at the clinic are not mutually exclusive and participants choose which strategy or strategies they want to pursue after receiving safer conception counseling. All women were started on folic acid. Half (50%) of participants elected to use timed unprotected intercourse as their conception method, while 37% chose self-insemination (including 13/52 couples in which the male partner was HIV-infected), and others remained undecided at time of the first visit. Seventeen patients were initiated on cART and two started PrEP. Twelve men requested referrals for circumcision, including HIV-infected patients. Retention of those enrolled is >90%. Readiness to conceive is often several months post-enrollment in order to ensure viral suppression in both partners, with a median recommendated wait time for attempted conception of 4 months [IQR: 3–4]. To-date 12 pregnancies and no seroconversions have been observed.
Important challenges have arisen during the early months of implementation. In the first nine months, our clinic did not reach as many couples, particularly discordant couples or couples with a cART-naïve infected partner as we had anticipated. Additionally, 44% of women attending the clinic were not accompanied by their male partners, 25% of which reported that they do not know their partners’ HIV status. Adding weekend or evening hours may overcome barriers for some men. Efforts to confirm HIV status and laboratory results of consenting male partners unable to attend during clinic hours would improve tailored counseling messages. Overall, strategies to create demand among individuals likely to benefit the most from safer conception services are needed.
DISCUSSION
Despite a clear need for safer conception services, limited services are available in high burden settings and virtually no implementation data is available documenting uptake and outcomes of such services in the SSA region. We described an implemenation science approach to offering safer conception care in a primary health setting in South Africa. This work highlights the need for knowledge and access to counseling around safer conception, and demonstrates that services can be feasibly offered. Early challenges, including slow enrollment, reinforce the need for optimization, including health education at the community level, enhanced marketing of safer conception services, and systematic screening of fertility intentions and referrals for eligible candidates by all health care providers if stand alone services are going to be effective in their reach and scaleable.
Our formative work indicated that safer conception knowledge among individuals affected by HIV mainly focused on PMTCT of HIV, and 100% condom use was often cited as the only way to ensure HIV prevention, thus pitting HIV prevention against reproductive goals. Some participants had heard of assisted reproductive techniques, but few were aware of low-cost methods such as self-insemination. After education around self-insemination for discordant couples with uninfected male partners, this method was a viewed favorably by HIV-infected women with uninfected partners and HIV-uninfected men – those most likely to benefit from the methods. Additionally, few individuals in partnerships affected by HIV were aware of the possibility of HIV treatment or PrEP as strategies to prevent horizontal and vertical transmission while trying to conceive. HIV-infected women believed that men would not view early initiation of cART favorably if they were not ill, and men felt reluctant to rely on treatment as prevention as their sole safer conception approach.
In response to the request for short clinic wait times, we developed a separate on-site service with dedicated staff, used an appointment system, and provided routine HIV care to make the safer conception clinic a one-stop service. Despite these efforts, our early experience suggests that involving men remains challenging, likely reducing the effectiveness of services provided to the unaccompanied women. In addition, our experience to date indicates that concordant HIV-infected couples will comprise a large proportion of safer conception clients, including many clients already receiving cART. While this is a population in whom the benefit of safer conception services may be lower, its value may still be substantial as 50% of HIV-infected women and 64% of HIV-infected men were not virally suppressed at their first safer conception clinic visit. Evaluating the value of safer conception services when both partners are infected to prevent super-infection and vertical transmission is thus warranted.
Our initial experience concerning acceptability reiterates findings from our formative work. Despite being offered PrEP for prevention, only 2 of 16 uninfected participants to-date have elected to use PrEP. Most participants have selected more than one prevention approach and none have refused early cART initiation as a prevention strategy, although initiation is typically delayed until the first follow-up visit in order to wait for baseline safety bloodwork and administration of adherence counseling. Among those on cART, half of participants elected timed unprotected intercourse and many others elected to use self-insemination, again reinforcing that patients do not entirely trust that cART alone is sufficient for prevention purposes, even in situations in which both partners are HIV-infected. Additionally, participants frequently chose prevention options that are not recommended, such as HIV-infected men desiring medical male circumcision (MMC) and using self-insemination. Although uptake of these methods may not result in increased harm, self-insemination is less efficient as a conception method than natural intercourse and MMC has costs associated with it. Furthermore, in serodiscordant relationships in which the male partner is HIV infected, MMC could result in risk compensation in situations in which there is no corresponding risk reduction benefit, and counselling must clearly emphasize this message.
Evaluation of safer conception approaches will not be without challenges. A randomized controlled trial comparing cART for the infected partner vs. PrEP for the uninfected partner would provide efficacy data but lacks equipoise and would fail to assess the relevance of safer conception interventions for concordant HIV-infected couples. We chose an implementation science approach to evaluate a cohort of a broad range of individuals who are offered a variety of safer conception choices in a real-world operational setting. The bias introduced by the observational approach and the need to disentangle the effect of individual strategies within the combination prevention packages will neccesitate advanced epidemiologic methods and modeling. These limitations notwithstanding, this research offers the opportunitiy to assess uptake, feasibility and effectiveness of the methods.
In the era of rapidly expanding Option B and B+ PMTCT programs which treat women with cART once they have conceived, preventing horizontal transmission to partners and ensuring viral suppression prior to conception has the potential to further optimize outcomes for the mother, father and the future infant. Improved health outcomes and survival of parents will also help to improve child health outcomes [28]. Given the importance of reproduction, safer conception services should be seen as a necessary component of HIV prevention programs, rather than a luxury service. Creating demand for and knowledge of safer conception services among the population at highest risk of vertical and horizontal transmission will however require careful planning. These data show early promise that these services will be feasible, however analyses of the eventual outcome data from this cohort and others, including cost-effectiveness analyses, will be necessary to determine how to optimize use of resources and at what level scalability is warranted.
Acknowledgments
We are grateful to the clinicians and staff at Witkoppen Health and Welfare Centre for their input in the design of the safer conception clinic and to study participants for their time and willingness to participate in research. We are also grateful to the UJMT FGHF Coordinating Center and the Fogarty International Center for their support for this research.
Sources of Support:
Research reported in this publication was funded by the UJMT Fogarty Grant, supported by the Fogarty International Center of the National Institutes of Health under Award Number R25TW009340. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Nattabi B, Li J, Thompson SC, Orach CG, Earnest J. A systematic review of factors influencing fertility desires and intentions among people living with HIV/AIDS: implications for policy and service delivery. AIDS Behav. 2009;13:949–968. doi: 10.1007/s10461-009-9537-y. [DOI] [PubMed] [Google Scholar]
- 2.Cooper D, Moodley J, Zweigenthal V, Bekker LG, Shah I, Myer L. Fertility intentions and reproductive health care needs of people living with HIV in Cape Town, South Africa: implications for integrating reproductive health and HIV care services. AIDS Behav. 2009;(13 Suppl 1):38–46. doi: 10.1007/s10461-009-9550-1. [DOI] [PubMed] [Google Scholar]
- 3.Schwartz SR, Mehta SH, Taha TE, Rees HV, Venter F, Black V. High pregnancy intentions and missed opportunities for patient-provider communication about fertility in a South African cohort of HIV-positive women on antiretroviral therapy. AIDS Behav. 2012;16:69–78. doi: 10.1007/s10461-011-9981-3. [DOI] [PubMed] [Google Scholar]
- 4.Mmbaga EJ, Leyna GH, Ezekiel MJ, Kakoko DC. Fertility desire and intention of people living with HIV/AIDS in Tanzania: a call for restructuring care and treatment services. BMC Public Health. 2013;13:86. doi: 10.1186/1471-2458-13-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iliyasu Z, Abubakar IS, Kabir M, Babashani M, Shuaib F, Aliyu MH. Correlates of fertility intentions among HIV/AIDS patients in northern Nigeria. Afr J Reprod Health. 2009;13:71–83. [PubMed] [Google Scholar]
- 6.Loutfy MR, Hart TA, Mohammed SS, Su D, Ralph ED, Walmsley SL, et al. Fertility desires and intentions of HIV-positive women of reproductive age in Ontario, Canada: a cross-sectional study. PLoS One. 2009;4:e7925. doi: 10.1371/journal.pone.0007925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matthews LT, Crankshaw T, Giddy J, Kaida A, Smit JA, Ware NC, et al. Reproductive decision-making and periconception practices among HIV-positive men and women attending HIV services in Durban, South Africa. AIDS Behav. 2013;17:461–470. doi: 10.1007/s10461-011-0068-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wagner G, Linnemayr S, Kityo C, Mugyenyi P. Factors associated with intention to conceive and its communication to providers among HIV clients in Uganda. Matern Child Health J. 2012;16:510–518. doi: 10.1007/s10995-011-0761-5. [DOI] [PubMed] [Google Scholar]
- 9.Loutfy MR, Margolese S, Money DM, Gysler M, Hamilton S, Yudin MH. Canadian HIV Pregnancy Planning Guidelines: No 278, June 2012. Int J Gynaecol Obstet. 2012;119:89–99. doi: 10.1016/j.ijgo.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 10.Panel on Treatment of HIV-Infected Pregnant Women and Prevention of Perinatal Transmission. [Accessed (02 April 2014)];Recommendations for Use of Antiretroviral Drugs in Pregnant HIV-1-Infected Women for Maternal Health and Interventions to Reduce Perinatal HIV Transmission in the United States. Available at http://aidsinfo.nih.gov/contentfiles/lvguidelines/PerinatalGL.pdf.
- 11.Taylor GP, Clayden P, Dhar J, Gandhi K, Gilleece Y, Harding K, et al. British HIV Association guidelines for the management of HIV infection in pregnant women 2012. HIV Med. 2012;(13 Suppl 2):87–157. doi: 10.1111/j.1468-1293.2012.01030_2.x. [DOI] [PubMed] [Google Scholar]
- 12.Bekker LGBV, Myer L, Rees H, Cooper D, Mall S, Mnyami C, Conradie F, Mahabeer I, Gilbert L, Schwartz S. Guideline on safer conception in fertile HIV-infected individuals and couples. South Afr J HIV Med. 2011:31–44. [Google Scholar]
- 13.Matthews LT, Smit JA, Cu-Uvin S, Cohan D. Antiretrovirals and safer conception for HIV-serodiscordant couples. Curr Opin HIV AIDS. 2012;7:569–578. doi: 10.1097/COH.0b013e328358bac9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baeten JM, Donnell D, Ndase P, Mugo NR, Campbell JD, Wangisi J, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367:399–410. doi: 10.1056/NEJMoa1108524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cu-Uvin S, DeLong AK, Venkatesh KK, Hogan JW, Ingersoll J, Kurpewski J, et al. Genital tract HIV-1 RNA shedding among women with below detectable plasma viral load. AIDS. 2010;24:2489–2497. doi: 10.1097/QAD.0b013e32833e5043. [DOI] [PubMed] [Google Scholar]
- 17.Nicopoullos JD, Almeida P, Vourliotis M, Gilling-Smith C. A decade of the sperm-washing programme: correlation between markers of HIV and seminal parameters. HIV Med. 2011;12:195–201. doi: 10.1111/j.1468-1293.2010.00868.x. [DOI] [PubMed] [Google Scholar]
- 18.Barreiro P, del Romero J, Leal M, Hernando V, Asencio R, de Mendoza C, et al. Natural pregnancies in HIV-serodiscordant couples receiving successful antiretroviral therapy. J Acquir Immune Defic Syndr. 2006;43:324–326. doi: 10.1097/01.qai.0000243091.40490.fd. [DOI] [PubMed] [Google Scholar]
- 19.Vernazza PL, Graf I, Sonnenberg-Schwan U, Geit M, Meurer A. Preexposure prophylaxis and timed intercourse for HIV-discordant couples willing to conceive a child. AIDS. 2011;25:2005–2008. doi: 10.1097/QAD.0b013e32834a36d0. [DOI] [PubMed] [Google Scholar]
- 20.Bujan L, Hollander L, Coudert M, Gilling-Smith C, Vucetich A, Guibert J, et al. Safety and efficacy of sperm washing in HIV-1-serodiscordant couples where the male is infected: results from the European CREAThE network. AIDS. 2007;21:1909–1914. doi: 10.1097/QAD.0b013e3282703879. [DOI] [PubMed] [Google Scholar]
- 21.Savasi V, Ferrazzi E, Lanzani C, Oneta M, Parrilla B, Persico T. Safety of sperm washing and ART outcome in 741 HIV-1-serodiscordant couples. Hum Reprod. 2007;22:772–777. doi: 10.1093/humrep/del422. [DOI] [PubMed] [Google Scholar]
- 22.Gilling-Smith C. HIV prevention. Assisted reproduction in HIV-discordant couples. AIDS Read. 2000;10:581–587. [PubMed] [Google Scholar]
- 23.Steiner RJ, Dariotis JK, Anderson JR, Finocchario-Kessler S. Preconception care for people living with HIV: recommendations for advancing implementation. AIDS. 2013;(27 Suppl 1):S113–S119. doi: 10.1097/QAD.0000000000000059. [DOI] [PubMed] [Google Scholar]
- 24.Peters DTN, Adam T. Implementation research in health: a practical guide. 2013 [Google Scholar]
- 25.Lobb R, Colditz GA. Implementation science and its application to population health. Annu Rev Public Health. 2013;34:235–251. doi: 10.1146/annurev-publhealth-031912-114444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khoury MJ, Gwinn M, Ioannidis JP. The emergence of translational epidemiology: from scientific discovery to population health impact. Am J Epidemiol. 2010;172:517–524. doi: 10.1093/aje/kwq211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.World Health Organization. Guidance on couples HIV testing and counselling including antiretroviral therapy for treatment and prevention in serodiscordant couples: recommendations for a public health approach. 2012 [PubMed] [Google Scholar]
- 28.Ainsworth MSI, Bishai D, Dayton J, Filmer D, Heywood P, Jimenez E, Marsh K. The Impact of Adult Deaths on Children’s Health in Northwestern Tanzania. Development Research Group, World Bank, Policy research working paper no 2266. 2000 [Google Scholar]