Abstract
Most terrestrial ecosystems are nitrogen (N)-limited. The elucidation of the multivariate relationships among environmental drivers, leaf morphological traits, and foliar N of dominant species which are critical to the functioning of forests remains a critical challenge for ecologists. We sampled leaves of Quercus wutaishanica across a broad natural gradient in the Loess Plateau, China, and employed structural equation modelling to evaluate the causal pathways and the relative importance of drivers of the foliar N per unit area (Narea) and per unit mass (Nmass). We found that (1) Nmass and Narea were primarily affected by leaf morphological traits instead of environmental variables and that leaf morphological traits accounted for most of their variations; (2) the total soil potassium and phosphorus and mean annual precipitation had different effects on Nmass and Narea via different pathways and path coefficients, whereas the mean annual temperature and total soil N had non-significant effects on Nmass and Narea. Our results demonstrated that variations in Nmass and Narea within Quercus wutaishanica were strongly linked to their leaf morphological traits and that the leaf N was also influenced by mean annual precipitation and soil phosphorus and potassium instead of soil N in the Loess Plateau, China.
N comprises one of the most important limiting nutrients in plant growth and the net primary productivity of terrestrial ecosystems1,2. In forests, more than 40% of N of trees is stored within the leaves3 and foliar N exerts positive effects on their photosynthetic efficiencies and relative growth rates4,5,6,7. The N content within leaves in terrestrial ecosystems is intimately associated with environmental conditions and has been widely studied across species and at various scales5,8,9,10,11,12,13,14. Clarifying how the environment affects leaf N, which is an important predictor of light-use efficiency, is critical for predicting N status in terrestrial vegetation, especially in the context of temporal increases of N deposition over China15,16.
Plant trait variability due to both phenotypic plasticity and genetic diversity, which enables plant species to survive and reproduce under diverse environmental conditions, influences the response of species to environmental changes17,18. Fajardo and Piper19 firstly placed intraspecific variation of leaf mass per area (reciprocal of specific leaf area, SLA) and wood density of Nothofagus pumilio into the context of community ecology and assembly processes at a large scale, and found that intraspecific trait variation accounted for a large proportion of the total variation in traits. Subsequently, there is an explosion of studies on accounting for intraspecific trait variation, which may be critical for answering key questions and making predictions about plant community assembly and ecosystem functioning20,21. For example, it reported that strong but opposing responses among vs. within species for SLA and leaf N and phosphorus (P) concentrations, which are not typically accounted for in species-based measures of plant community20. With the accumulation of intraspecific trait variation researches, intraspecific trait variation accounted for 25% of the total trait variation within communities and 32% of the total trait variation among communities on average, which highlight global patterns in the relative importance of intraspecific trait variation in plant communities21.
Two indicators of leaf N content have been commonly used: mass-based (Nmass) and area-based (Narea) representations9,18,22,23,24. However, mass-based vs. area-based representations remain under discussion1,7,25. Wright et al.1 employed mass-based leaf traits to describe the universal leaf economic spectrum due to stronger correlations among the mass-based than the area-based leaf traits. Lloyd et al.7 noted that an area-based metric appears to be more logical because the primary function of leaves is to intercept light in the plant canopy; however, Westoby et al.25 noted that both representations warrant study according to different research purposes and needs.
Several environmental and leaf morphological variables, including specific leaf area (SLA), mean annual precipitation (MAP), mean annual temperature (MAT) and total soil nutrients, such as N, P and potassium (K), have been observed to affect Nmass and Narea, which were primarily derived from correlative analyses4,5,10,12,13,18,26,27. Over expansive spatial scales, however, environmental drivers simultaneously influence Nmass and Narea as well as the species composition of the studied communities, making it difficult to separate the influences of environment from inherent differences between plant species. Therefore, environmental effects on the N content of leaves may be better understood within species7,11,27. Nevertheless, intraspecific variability, which plays in a critical role in community assembly processes and ecosystem functioning20,21, could result from both genetic variability and phenotypic plasticity17. Our understanding of the controls for the intraspecific variations of Nmass and Narea remains limited.
The morphological traits of leaves, such as specific leaf area (SLA), leaf dry weight (LDW) and leaf size (LS), may be associated with Nmass and Narea. As a key morphological attribute, SLA is taken as the leaf-level cost of light interception28, which has been widely utilized as a key feature in studies of foliar N1 and plant growth strategies29. Additionally, LDW and LS may have independent effects on Nmass and Narea. SLA is calculated by dividing LS by LDW, which may omit potentially independent effects of LS and LDW on foliar N. For example, the variation in leaf size is associated with major changes in within-leaf support investments and in large modifications in integrated leaf chemical (especially N concentration) and structural characteristics30, and there is also the decline of SLA along with the increase of LDW31. This suggests that Nmass and Narea may also be associated with LS and/or LDW in addition to SLA.
To better understand multivariate determinants of the leaf content of N within a species, we studied the N content of the leaves of the Liaotung oak (Quercus wutaishanica), a widely distributed dominant species of the deciduous broad-leaved forests, along natural gradients of climate and soil nutrient variability in the Loess Plateau, Northern China32. We examined the influences of MAT, MAP, total soil N (TSN), total soil P (TSP), total soil K (TSK), SLA, LDW, and LS on Nmass and Narea using two structural equation models (Fig. 1). Specifically, we hypothesized that soil nutrient contents have positive effects on Nmass and Narea and that increasing precipitation may reduce leaf N and soil nutrients due to increasing soil and leaf nutrient leaching5,26,33,34. We hypothesize that leaf SLA, LDW, and LS have profound influences on Nmass and Narea but that their directions of influences, i.e., positive or negative, are dependent on individual traits4,5,10,12,13,18,26,27. To test these hypotheses, we collected Quercus wutaishanica foliar samples across a wide range of environmental conditions in the Loess Plateau of northern China (Fig. 2, Table 1). Understanding the relative importance of these diverse pathways should help predict how Nmass and Narea respond to variations in climate, soil nutrients and the morphological traits of leaves.
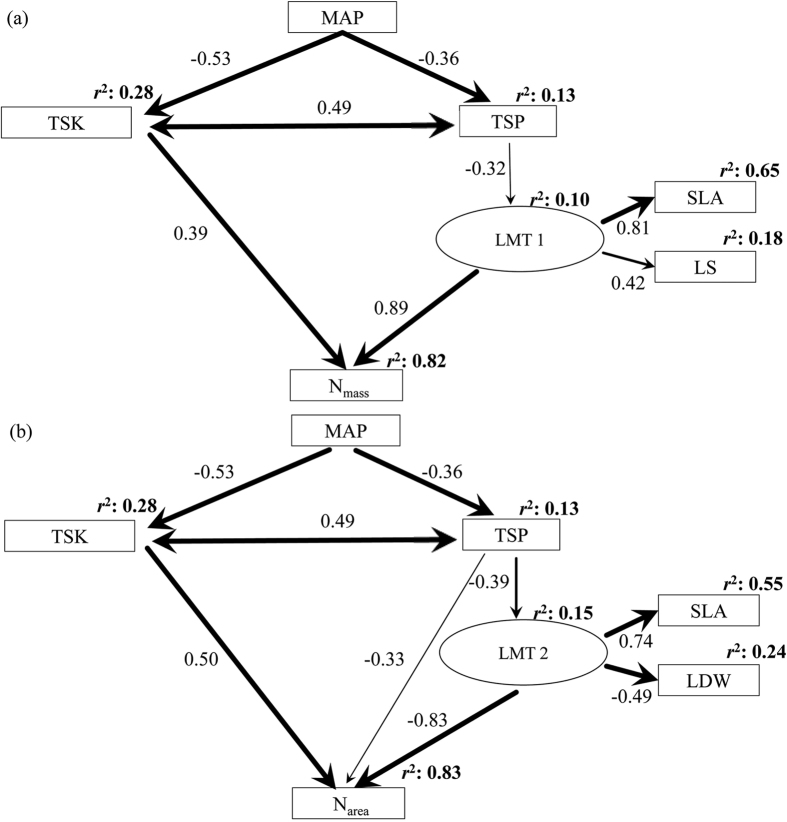
Figure 1. Results of the drivers for leaf nitrogen content.
(a) Multiple drivers for leaf nitrogen concentration per mass (Nmass). (b) Multiple drivers for leaf nitrogen concentration per area (Narea). Single headed arrows indicate a hypothesized causal effect of one variable upon another. Double headed arrows indicate correlations. Insignificant (p > 0.05) paths were eliminated. Narrow arrows indicate p < 0.05; wider arrows indicate p < 0.01; and the widest arrows indicate p < 0.001. Signs on arrows indicate standardized regression weights or correlation indices. Signs at the top-right corner of each variable are the proportion of variance explained. LMT 1, leaf morphological traits incorporated from specific leaf area and leaf size; LMT 2, leaf morphological traits incorporated from specific leaf area and leaf dry weight; other abbreviations with units are explained in Table 1.
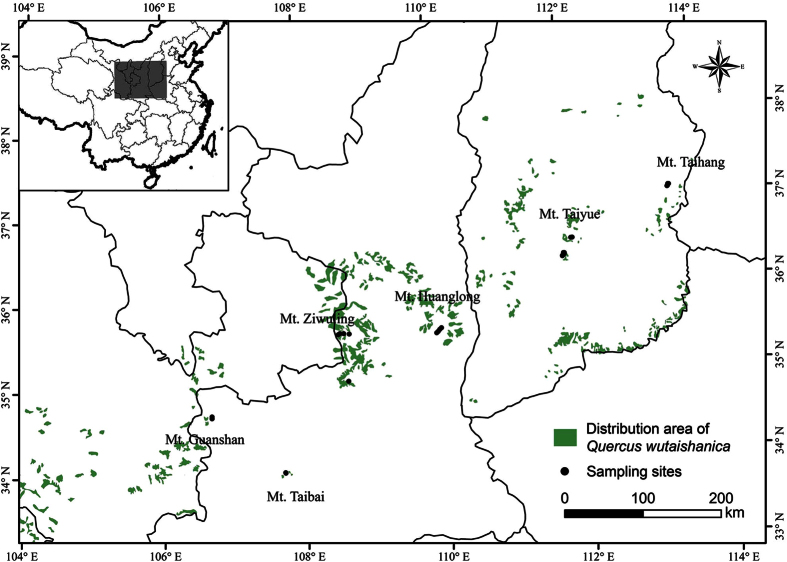
Figure 2. Locations of sampling sites.
Black dots are location of sampling sites. Green-shaded portions are Quercus wutaishanica forest in the Loess Plateau. The map is made by ArcGIS 10.2 software, http://www.arcgis.com/features/.
Table 1. Main attributes of leaf traits (90 individuals) and environmental variables.
| Variables | Mean | SE | Minimum | Maximum | CV (%) |
|---|---|---|---|---|---|
| Elevation (m) | 1700 | 36.63 | 1252 | 2303 | – |
| Longitude (°) | – | – | 106.68233 | 113.50182 | – |
| Latitude (°) | – | – | 34.04959 | 37.13302 | – |
| MAT (°C) | 6.67 | 0.22 | 4.10 | 10.30 | 27 |
| MAP (mm) | 636.80 | 11.29 | 554.00 | 889.00 | 15 |
| TSN (mg·g−1) | 2.48 | 0.22 | 0.90 | 9.60 | 71 |
| TSK (mg·g−1) | 19.57 | 0.31 | 14.10 | 25.90 | 13 |
| TSP (mg·g−1) | 0.52 | 0.02 | 0.20 | 1.30 | 39 |
| SLA (cm2·g−1) | 13.81 | 0.43 | 7.50 | 28.40 | 27 |
| LS (cm2·leaf−1) | 35.82 | 1.35 | 19.50 | 72.40 | 28 |
| LDW (g·leaf−1) | 2.70 | 0.20 | 2.70 | 10.20 | 30 |
| Nmass (mg·g−1) | 23.59 | 0.41 | 17.60 | 33.70 | 15 |
| Narea (g·m−2) | 1.79 | 0.05 | 1.00 | 3.10 | 22 |
| Leaf N:P ratio | 21.78 | 0.54 | 13.15 | 41.76 | 21 |
Abbreviations: mean annual temperature, MAT; mean annual precipitation, MAP; total soil nitrogen, TSN; total soil potassium, TSK; total soil phosphorus, TSP; specific leaf area, SLA; leaf size, LS; leaf dry weight, LDW; leaf nitrogen per unit mass, Nmass; leaf nitrogen per unit area, Narea; standard error, SE; the coefficient of variation, CV.
Results
Correlation analysis
For the wide range of environmental variations we sampled, the mean Nmass and Narea were 23.59 mg·g−1 and 1.79 g·m−2, respectively (Table 1). The coefficient of variation (CV) for Nmass (15%) was lower than that for Narea (22%) (Table 1). Correlations of Nmass and other variables differed from those of Narea (Table 2). Nmass was positively correlated with SLA (P < 0.001) and LS (P < 0.001) (Table 2). Narea was negatively correlated with SLA (P < 0.001) and positively with LDW (P < 0.001) (Table 2). Narea increased with TSK (P < 0.001) and TSP (P = 0.023) (Table 2). There were significant correlations among SLA, LS, and LDW (Table 2). TSK and TSP were positively correlated (P < 0.001); however, neither had a significant correlation with TSN. MAT and MAP had a significantly negative correlation (P = 0.004) (Table 2).
Table 2. Pearson correlation coefficients between variables.
| Nmass | Narea | SLA | LS | LDW | TSN | TSK | TSP | MAT | |
|---|---|---|---|---|---|---|---|---|---|
| Narea | 0.064 | ||||||||
| SLA | 0.63*** | −0.63*** | |||||||
| LS | 0.39*** | −0.17 | 0.35** | ||||||
| LDW | −0.03 | 0.45*** | −0.37** | 0.69*** | |||||
| TSN | −0.23 | 0.05 | −0.19 | −0.07 | 0.09 | ||||
| TSK | 0.18 | 0.55*** | −0.24* | 0.03 | 0.28* | −0.08 | |||
| TSP | −0.07 | 0.27* | −0.27* | −0.01 | 0.24 | 0.03 | 0.58*** | ||
| MAT | −0.17 | −0.04 | −0.14 | −0.11 | −0.06 | −0.07 | 0.09 | 0.13 | |
| MAP | −0.21 | −0.17 | −0.01 | −0.03 | −0.02 | 0.59*** | −0.53*** | −0.36** | −0.34** |
Significant effects are at P < 0.05 (*), <0.01 (**) and <0.001 (***).
Abbreviations are explained in Table 1.
Model for Nmass
The model for Nmass (Fig. 1a) was a good fit with the data (Table 2), and environmental variables and leaf morphological traits (LMT 1, incorporating SLA and LS) accounted for 82% of the variation in Nmass (Tables 3 and 4, Fig. 1a). MAP had negative effects on both TSK and TSP, and TSK and TSP were positively correlated (Table 4, Fig. 1a). Nmass increased with TSK, whereas TSP had no direct effect on Nmass but had an indirect negative effect on Nmass through LMT 1 (Table 4, Fig. 1a). LMT 1 had a direct positive effect on Nmass (Table 4, Fig. 1a). MAT, TSN, and LDW were not included in the model because they had neither a significant direct or indirect effect on Nmass (Fig. 1a).
Table 3. Structural equation model fit indices and evaluation criteria.
| Index | evaluation standard or critical value for fit | Model for Nmass | Model for Narea |
|---|---|---|---|
| χ2 | 5.788 | χ2 = 6.186 | |
| p | >0.05 | 0.565 | 0.403 |
| AGFI | >0.90 | 0.923 | 0.903 |
| RMSEA | <0.08 | <0.001 | 0.021 |
| CFI | >0.90 | 1.000 | 0.998 |
Abbreviations are: χ2, the chi-square test; RMSEA, the root square mean error of approximation; AGFI, adjusted goodness of fit index; CFI, the comparative fit index; Nmass, foliar N per unit mass; Narea, foliar N per unit area.
Table 4. Direct, indirect and total standardized effects on Nmass and Narea based on structural equation models (SEMs).
| SEM model | Predictor | Pathway to foliar N | Effect |
|---|---|---|---|
| Model for Nmass, Fig. 1a | Mean annual precipitation(MAP) | Total | −0.103 |
| Direct | – | ||
| Indirect | −0.103* | ||
| Total soil potassium (TSK) | Total | 0.392 | |
| Direct | 0.392*** | ||
| Indirect | – | ||
| Total soil phosphorus (TSP) | Total | −0.289 | |
| Direct | – | ||
| Indirect through LMT 1 | −0.289* | ||
| Leaf morphological traits incorporated from specific leaf area and leaf size (LMT 1) | Total | 0.891*** | |
| Direct | 0.891*** | ||
| Indirect | – | ||
| Model for Narea, Fig. 1b | Mean annual precipitation(MAP) | Total | −0.259 |
| Direct | – | ||
| Indirect | −0.259* | ||
| Total soil potassium (TSK) | Total | 0.495 | |
| Direct | 0.495*** | ||
| Indirect | – | ||
| Total soil phosphorus (TSP) | Total | −0.005 | |
| Direct | −0.326* | ||
| Indirect through LMT 2 | 0.321* | ||
| Leaf morphological traits incorporated from specific leaf area and leaf dry weight (LMT 2) | Total | −0.833*** | |
| Direct | −0.833*** | ||
| Indirect | – |
Significant effects are at P < 0.05 (*), <0.01 (**) and <0.001 (***).
Model for Narea
The model for Narea (Fig. 1b) was also a good fit with the data (Table 3), and environmental variables and leaf morphological traits (LMT 2, incorporating SLA and LDW) accounted for 83% of the variation in Narea (Tables 4 and 5, Fig. 1b). The relationships between MAP, TSK and TSP were the same as those in the model for Nmass. TSK also had direct positive effect on Narea, which was higher than that on Nmass. TSP had a direct negative effect and a positive indirect effect through LMT 2 on Narea (Table 4, Fig. 1b). LMT 2 had a negative effect on Narea. MAT, TSN and LS were not included in the Narea model because neither had a significant direct or indirect effect on Narea (Fig. 1b).
Table 5. Partitioning of explained variations of each variable.
| Response variable | Variation explained by predictors |
Total explained variation (%) | |||
|---|---|---|---|---|---|
| MAP | TSK and TSP | LMT 1 | LMT 2 | ||
| TSK | 27.8 | 27.8 | |||
| TSP | 12.8 | 12.8 | |||
| LMT 1 | 1.3 | 9.2 | 10.5 | ||
| LMT 2 | 1.9 | 12.9 | 14.8 | ||
| Nmass | 1.1 | 9.2 | 71.4 | 81.7 | |
| Narea | 6.7 | 17.7 | 58.9 | 83.3 | |
Abbreviations are explained in Table 1.
Partitioning of the explained variation of Nmass and Narea
MAP explained 1% of the variation in Nmass and 7% of the variation in Narea, and soil nutrients, which included TSK and TSP, explained 9% of the variation in Nmass and 18% of the variation in Narea (Table 5). LMT 1 explained 71% of the variation in Nmass, and LMT 2 explained 59% of the variation in Narea (Table 5). MAP, TSK, and TSP explained 24% of the variation in Narea, which was more than twice the variation explained in Nmass (10%) (Table 5).
Discussion
The relationship between leaf morphological traits and leaf N content should be emphasized. Our results revealed multiple determinants for the N content of leaves (Nmass and Narea) of Quercus wutaishanica in North China. In contrast with previous intraspecific studies that focused only on environmental influences22,23,24, we found that the variations in Nmass and Narea were more strongly associated with the morphological traits of leaves than environmental changes in climate and soil characteristics, with the latter including both direct effects and indirect effects via their influences on leaf trait variables, across the study area. This finding suggests that native ranges of morphological traits18,20,21 may be a strong determinant for foliar N of Q. wutaishanica. Future efforts, transplant studies for example26,35, are necessary to examine the genetic evidence associated with the foliar N, but study the variations and determinants across the native ranges of individuals (which reflects both plasticity and differences in genotype11) is an important first step.
SLA was strongly correlated with Nmass (R = 0.72) and Narea (R = −0.62) (Fig. 1). This finding was consistent with previous conclusions of interspecific comparisons1,4,7,29. LS included in SEM for Nmass (Fig. 1a) and LDW included in SEM for Narea (Fig. 1b) were reported here, however the important portion of variation in LS and LDW caused by genetically differences existing extensively across species11,18 lead to less attention than SLA. The negative correlation between SLA and LDW (Fig. 1b) was due to increased requirement for costly material support for a given leaf area with increasing LS31; however, there is no similar report for the relationship between LS and SLA. Nmass and LS were positively correlated in our SEM (Fig. 1a), which mean larger LS corresponding higher SLA. One of the major mechanisms by which plants adjust to resource imbalance is by allocating new biomass to the organs that acquire the most strongly limiting resources36. Larger leaves intercept more sunlight while cost more investment (positive correlation between LS and LDW, Table 1). Based on the extra mechanically support from twig37, the relative thinner or lower tissue density for the reduction of the burden for support tissue is achievable. Furthermore, compared to shrubs, larger leaves of trees are less disturbed by herbivores, which may lead to their less investment in defensive tissues. Hence the slower pace of LDW increase than LS lead to the higher SLA for larger leaves (Fig. 1a).
Both Nmass and Narea were affected by the total P and K in soils but not by the total N in soils. This finding is in contrast to the pattern reported in N addition experiments. Many planting experiments have reported that N addition in appropriate quantities may increase Nmass and Narea6. However, chronic N addition in mature sugar maple forests initially increased Nmass and Narea; notably, both indices began to decrease in the later stages of the experiment, which resulted in an insignificant effect of NO3- addition on Nmass or Narea38. Adult trees used in experiments have a far greater proportion of biomass than seedlings or young individuals for the storage nutrients, which leads to a considerably delayed response to environmental change and less dependence on environmental nutrient supplies through nutrient storage and resorption14,39. What’s more, our study species showed an N:P ratio of 21.78 (standard error = 0.54) in Q. wutaishanica (Table 1), indicating a relative N-surplus and P-limited environment40. There is most likely a correlation between the natural N supply and the level of N deposition today41. The annual bulk N deposition was 22–38 kilograms of N per hectare per year in the Loess Plateau in 201316. With the enhanced N deposition over China around the year 2000 across China, there are significant increase of plant foliar N concentrations in natural and semi-natural ecosystems but no apparent soil N and P change15. The widespread increase in plant foliar N concentrations was caused by the cumulative effects of enhanced N deposition rather than alterations in soil15. Relative surplus foliar N accumulation from atmospheric depositions in our study area15 may lead to less dependency on soil N supply, which show us non-significant correlation between foliar N and the soil resident N variations. Alternatively, although soil total N can be strongly related to available N to plants including our study tree species, available soil N may be better linked foliar N. Future work could test the strength of relationships between soil total N vs soil available N to foliar N in the environment under high atmospheric N deposition.
Soil K was shown to have direct positive effects on both Nmass and Narea, which has not been a focus in previous foliar N studies. K is a key element as an activator of the many enzymes that are essential for photosynthesis and respiration and as a contributor to the osmotic potential of cells42. A shortage of K leads to a decrease in the chlorophyll content of leaves in addition to a weakened capacity for photosynthesis43. Because the N within leaves is widely distributed in chlorophyll44, it has a direct positive effect on K within leaves in terms of both Nmass and Narea10. The loss of K from leaves through leaching is higher than for other elements; thus, additional soil resident K ensures a supply of K to leaves45,46. Furthermore, K promotes the growth and secretion of ectotrophic mycorrhiza47, which exudes chemical compounds and enzymes into the rhizosphere and enhances the uptake of N48. At last, supplementary N could be provided to trees in high soil K environments due to greater net N mineralization and nitrification12.
We found that P in soils imparted a direct negative effect on Narea, an indirect negative effect on Nmass and an indirect positive effect on Narea via leaf morphological traits. P is an essential element in photosynthesis that also improves the synthesis and transportation of photosynthetic products43. The addition of P may enhance the photosynthetic N use efficiency, leading to a negative effect on the distribution of N per unit area (Narea decreases)49. Comparing the positive effect of path “TSP → LMT 2 → Narea” with the negative effect of path “TSP → Narea”, the positive effect of TSP on Narea via LMT 2 complemented the decreased photosynthetic capacity per area caused by the negative direct impact of TSP on Narea. Morphological traits of leaves could be one strategy of N utilization for optimizing N use efficiency and photosynthetic capacity of leaves. Additionally, considered relatively low level of TSP of the Loess Plateau region in China50, the photosynthetic products in low soil P habitats (with the exception of those that are conserved for leaf consumption) are distributed to meet increased needs for root growth2, leading to high Nmass and low leaf dry weight. However, specific leaf areas and leaf size increase to intercept additional light per unit mass and thus achieve higher use efficiency of leaf dry mass28. As a consequence, leaf dry mass per unit area and Narea are both decreased.
MAP had a negative total effect on the content of N in leaves, which was consistent with previous studies8,26. The MAP effects were indirectly accomplished through soil K and P, likely because higher MAP leads to extra soil nutrient leaching5,33. Foliar N may also be affected by increased leaf leaching26,34 and altered N absorption by the canopy associated with high MAP46. Our observed MAP effects on foliar N likely indicate that the leaf N leaching loss was counteracted by the increased foliar uptake associated with ongoing N deposition enhancement in China15.
Extensive comparisons between Nmass and Narea have been made. As previously noted, Narea should be selected for the leaf light intercept, whereas Nmass is allocated for resource distribution and plant growth1,7,25. In our SEMs, Nmass was also correlated with leaf size, whereas Narea was also correlated with the dry weight of leaves. This finding suggests that Nmass and Narea were both comprehensive functional leaf traits and that they should be simultaneously considered for the elucidation of foliar N traits. We also found a higher coefficient of variation (CV) in Narea (21%) than Nmass (14%), indicating that Nmass was a more stable variable than Narea in determining N content in leaves. This result is in agreement with a conclusion for 2548 species on a global scale1. Soil resident P and K and MAP had higher direct or indirect effects on Narea than Nmass and explained 24% of the variance in Narea, which was more than twice that for Nmass (10%), indicating that Narea is a superior leaf N variable to reflect environmental variations.
Conclusion
Our study established the determinants of intraspecific foliar N of natural forests over wide climate and soil nutrient gradients. To the best of our knowledge, this study represents the first attempt to quantitatively relate the N content of leaves to climate, soil nutrients, and the morphological traits of leaves at the intraspecific level. Our SEM demonstrated that Nmass and Narea of Q. wutaishanica were more strongly correlated to with morphological traits of leaves than to climate and soil nutrients and that different morphological traits of leaves were not equally correlated to Nmass and Narea. Second, we found that soil resident K and P, but not N, exerted direct or indirect effects on Nmass and Narea of Q. wutaishanica trees. Thus, soil K and P are important and relevant nutrient variables for the management and conservation of Q. wutaishanica forests in the context of ongoing N deposition enhancement in China. Third, we found that both Nmass and Narea are critical indices for a full appreciation of foliar N within Q. wutaishanica. The lower CV of Nmass leads to a more stable indicator of the content of N in leaves than Narea, whereas Narea is more sensitive to the variations in climate and soil nutrient conditions. Our findings highlight that foliar N content is influenced by multiple environmental drivers, whose relative importance can differ strongly depending on the different indices for foliar N content.
Methods
Study area
The study was conducted in the Loess Plateau, Northern China, where Q. wutaishanica was primarily distributed across six mountains (34°03′–37°08′N; 106°41′–113°30′ E, Fig. 2). The mean annual temperature and precipitation in this region ranges from 4.1 to 10.3 °C and 554 to 880 mm, respectively, and the elevation ranges from 1252 to 2303 m (Table 1).
Sampling strategy
To ensure a wide-ranging coverage of environmental conditions, we sampled Q. wutaishanica trees according to every 100-m elevation interval from each of the six mountains. Black dots show the locations of sampling sites. Green-shaded portions are Quercus wutaishanica forest in the Loess Plateau derived from the database for China’s terrestrial ecosystems (http://www.ecosystem.csdb.cn/ecosys/index.jsp) (Fig. 2). At each elevation sample site, we randomly sampled three dominant Q. wutaishanica trees, and within each sample tree, we collected 20 canopy leaves from the sunny side. The spatial location of each sample tree (for a total of 90 trees), latitude, longitude, and elevation were determined via GPS Garmin 60CSx (Garmin International Inc., Olathe, KS, USA) (Table 1). Surrounding the locations of each sample tree, within a 400 m2 proximity, three soil samples were randomly collected from a 0–20 cm soil depth, where the bulk of the fine roots of most plants occurs, and large amounts of N, P and K accumulate due to the uplift and releasing at the surface by plants through litter falls and fine root turnover2,51. Three soil samples were combined to a composite sample for chemical analysis in the laboratory.
Functional traits
The leaf Nmass, Narea, N:P ratio, and morphological traits, i.e., SLA, LS, and LDW, were measured or calculated at the sample tree level. We first determined the average LS of each tree by scanning the fresh leaves. Subsequent to the oven drying of these leaf samples at 80 °C for 48 hours, we measured and calculated the average LDW. The dried samples from each tree were then pulverized using a plant sample mill and sieved through a 0.15-mm mesh screen. We employed an elemental analyser (Vario EL, Elementar Analyser systeme GmbH, Hanau, Germany) to determine the N concentration (Nmass, mg/g) and P concentration52. Narea was calculated as Nmass×LDW/LS. The N:P ratio was calculated as Nmass/Pmass.
Environmental variables
Each soil sample was oven-dried at 105 °C for 24 hours and then pulverized using a soil sample mill. followed by sieving through a 0.15-mm mesh sieve and analysis for TSN using an elemental analyser (Vario EL, Elementar Analyser systeme GmbH, Hanau, Germany), TSP and TSK were performed using inductively coupled plasma atomic emission spectrometry measurements (ICP-AES, SPECTRO ARCOS EOP, SPECTRO, Germany). The MAP and MAT for each corresponding sample tree were derived from http://www.worldclim.org/ based on their spatial coordinates (latitude, longitude, and elevation).
Statistical analysis
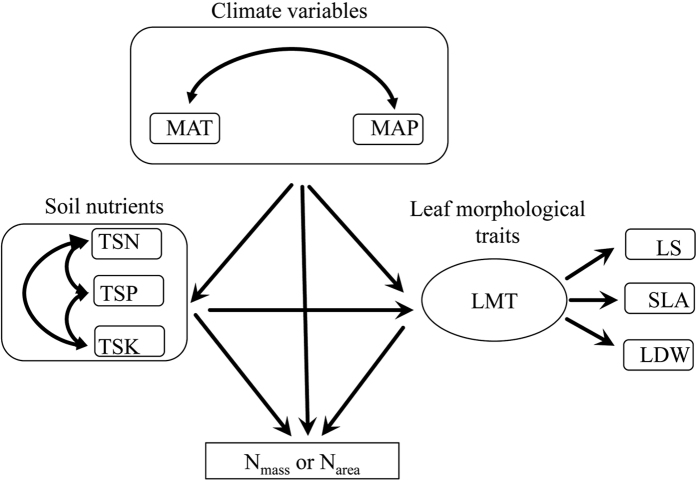
The structural equation model (SEM) is an advanced and robust multivariate statistical method that enables hypothesis testing of complex path-relation networks53,54. SEM has increasingly been used in ecology to separate direct and indirect effects between exogenous and endogenous variables55. We first examined the bivariate relationships between several key hypothesized causal paths according to previous studies of the relationships between foliar N and driving variables. We then established a prior model based on the known theoretical construct including the key variables and their paths (Fig. 3). Three closely correlated leaf morphological traits (SLA, LDW, and LS, Table 1) were incorporated into a latent variable. Three observable soil nutrient variables (TSN, TSP, and TSK) were disposed as observable variables because these elements (N, P, and K) had different effects on foliar N caused by their different roles in plant physiology and growth3,42. Two observable climate variables (MAT and MAP) were also established as observable variables for the same reason. We subsequently used stepwise procedures that were guided by Akaike information criterion (AIC) values to obtain the most parsimonious set of predictors54. We adopted several indices to evaluate the suitability of the model: the chi-square test (χ2), the root square mean error of approximation (RMSEA), the adjusted goodness of fit index (AGFI), and the comparative fit index (CFI)56. Finally, we partitioned the explained variation (R2) of each variable to establish the influence of other predictors57.
Figure 3. Illustration of all potential interaction pathways for leaf nitrogen in the study system.
MAT, mean annual temperature; MAP, mean annual precipitation; TSN, total soil nitrogen; TSK, total soil potassium; TSP, total soil phosphorus; SLA, specific leaf area; LS, leaf size; LDW, leaf dry weight; LMT, leaf morphological traits; Nmass, leaf nitrogen content per unit mass; Narea, leaf nitrogen content per unit area.
Additional Information
How to cite this article: Xing, K. et al. Determinants of the N content of Quercus wutaishanica leaves in the Loess Plateau: a structural equation modeling approach. Sci. Rep. 6, 26845; doi: 10.1038/srep26845 (2016).
Acknowledgments
The authors owe many thanks to Danhui Liu, Yu Liang, Feng Xue and Tan Liu for their assistance with field data collection and nutrient determination works. This research was supported by the National Science Foundation of China (No. 41271059), the National Key Basic Research Special Foundation of China (No. 2011FY110300), the National Natural Science Foundation of China (No. 41271549), and the Fundamental Research Funds for the Central Universities (No. 2014KJJCB33).
Footnotes
The authors declare no competing financial interests.
Author Contributions K.X., M.K. and X.D. designed the study, K.X. and M.Z. performed analyses, K.X., M.Z., G.W., Y.W. and C.C. collected data, K.X. wrote the first draft of the manuscript, and H.C. made a couple of rounds of careful revsions, and M.K., H.C., X.D. and Y.L. contributed substantially to revisions.
References
- Wright I. J. et al. The worldwide leaf economics spectrum. Nature 428, 821–827, doi: 10.1038/nature02403 (2004). [DOI] [PubMed] [Google Scholar]
- Yuan Z. & Chen H. Y. A global analysis of fine root production as affected by soil nitrogen and phosphorus. P. Roy. Soc. B-Biol. Sci. 279, 3796–3802, doi: 10.1098/rspb.2012.0955 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallardy S. G. Physiology of woody plants. 3rd Edition, 324–325 (Science Press, Beijing, 2010) (in Chinese). [Google Scholar]
- Reich P. B. & Walters M. B. Photosynthesis-Nitrogen Relations in Amazonian Tree Species. II. Variation in Nitrogen Vis-a-Vis Specific Leaf Area Influences Mass- and Area-Based Expressions. Oecologia 97, 73–81, doi: 10.1007/BF00317910 (1994). [DOI] [PubMed] [Google Scholar]
- Reich P. B. & Oleksyn J. Global patterns of plant leaf N and P in relation to temperature and latitude. P. Natl. Acad. Sci. USA 101, 11001–11006, doi: 10.1073/pnas.0403588101 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicodemus M. A., Salifu F. K. & Jacobs D. F. Growth, nutrition, and photosynthetic response of black walnut to varying nitrogen sources and rates. J. Plant Nutr. 31, 1917–1936, doi: 10.1080/01904160802402856 (2008). [DOI] [Google Scholar]
- Lloyd J., Bloomfield K., Domingues T. F. & Farquhar G. D. Photosynthetically relevant foliar traits correlating better on a mass vs an area basis: of ecophysiological relevance or just a case of mathematical imperatives and statistical quicksand? New Phytol. 199, 311–321, doi: 10.1111/nph.12281 (2013). [DOI] [PubMed] [Google Scholar]
- Cunningham S. A., Summerhayes B. & Westoby M. Evolutionary divergences in leaf structure and chemistry, comparing rainfall and soil nutrient gradients. Ecol. Monogr. 69, 569–588, doi: 10.1890/0012-9615(1999)069[0569:EDILSA]2.0.CO;2 (1999). [DOI] [Google Scholar]
- Han W., Fang J., Guo D. & Zhang Y. Leaf nitrogen and phosphorus stoichiometry across 753 terrestrial plant species in China. New Phytol. 168, 377–385, doi: 10.1111/j.1469-8137.2005.01530.x (2005). [DOI] [PubMed] [Google Scholar]
- Wright I. J. et al. Assessing the generality of global leaf trait relationships. New Phytol. 166, 485–496, doi: 10.1111/j.1469-8137.2005.01349.x (2005). [DOI] [PubMed] [Google Scholar]
- Royer D. L., McElwain J. C., Adams J. M. & Wilf P. Sensitivity of leaf size and shape to climate within Acer rubrum and Quercus kelloggii. New Phytol. 179, 808–817, doi: 10.1111/j.1469-8137.2008.02496.x (2008). [DOI] [PubMed] [Google Scholar]
- Groffman P., Hardy J., Fisk M., Fahey T. & Driscoll C. Climate Variation and Soil Carbon and Nitrogen Cycling Processes in a Northern Hardwood Forest. Ecosystems 12, 927–943, doi: 10.1007/s10021-009-9268-y (2009). [DOI] [Google Scholar]
- Han W., Fang J., Reich P. B., Ian Woodward F. & Wang Z. Biogeography and variability of eleven mineral elements in plant leaves across gradients of climate, soil and plant functional type in China. Ecol. Lett. 14, 788–796, doi: 10.1111/j.1461-0248.2011.01641.x (2011). [DOI] [PubMed] [Google Scholar]
- Ågren G. I. & Weih M. Plant stoichiometry at different scales: element concentration patterns reflect environment more than genotype. New Phytol. 194, 944–952, doi: 10.1111/j.1469-8137.2012.04114.x (2012). [DOI] [PubMed] [Google Scholar]
- Liu X. et al. Enhanced nitrogen deposition over China. Nature 494, 459–462, doi: 10.1038/nature11917 (2013). [DOI] [PubMed] [Google Scholar]
- Zhu J. et al. The composition, spatial patterns, and influencing factors of atmospheric wet nitrogen deposition in Chinese terrestrial ecosystems. Sci. Total Environ. 511, 777–785, doi: 10.1016/j.scitotenv.2014.12.038 (2015). [DOI] [PubMed] [Google Scholar]
- Albert C. H. et al. Intraspecific functional variability: extent, structure and sources of variation. J. Ecol. 98, 604–613, doi: 10.1111/j.1365-2745.2010.01651.x (2010). [DOI] [Google Scholar]
- Cordell S., Goldstein G., Mueller-Dombois D., Webb D. & Vitousek P. M. Physiological and morphological variation in Metrosideros polymorpha, a dominant Hawaiian tree species, along an altitudinal gradient: the role of phenotypic plasticity. Oecologia 113, 188–196, doi: 10.1007/s004420050367 (1998). [DOI] [PubMed] [Google Scholar]
- Fajardo A. & Piper F. I. Intraspecific trait variation and covariation in a widespread tree species (Nothofagus pumilio) in southern Chile. New Phytol. 189, 259–271, doi: 10.1111/j.1469-8137.2010.03468.x (2011). [DOI] [PubMed] [Google Scholar]
- Kichenin E., Wardle D. A., Peltzer D. A., Morse C. W. & Freschet G. T. Contrasting effects of plant inter- and intraspecific variation on community-level trait measures along an environmental gradient. Funct. Ecol. 27, 1254–1261, doi: 10.1111/1365-2435.12116 (2013). [DOI] [Google Scholar]
- Siefert A. et al. A global meta-analysis of the relative extent of intraspecific trait variation in plant communities. Ecol. Lett. 18, 1406–1419, doi: 10.1111/ele.12508 (2015). [DOI] [PubMed] [Google Scholar]
- Wu T. et al. Patterns of leaf nitrogen and phosphorus stoichiometry among Quercus acutissima provenances across China. Ecol. Complex. 17, 32–39, doi: 10.1016/j.ecocom.2013.07.003 (2014). [DOI] [Google Scholar]
- Kang H. et al. Variation in leaf nitrogen and phosphorus stoichiometry in Picea abies across Europe: An analysis based on local observations. Forest Ecol. Manag. 261, 195–202, doi: 10.1016/j.foreco.2010.10.004 (2011). [DOI] [Google Scholar]
- Wu T., Dong Y., Yu M., Geoff Wang G. & Zeng D.-H. Leaf nitrogen and phosphorus stoichiometry of Quercus species across China. Forest Ecol. Manag. 284, 116–123, doi: 10.1016/j.foreco.2012.07.025 (2012). [DOI] [Google Scholar]
- Westoby M., Reich P. B. & Wright I. J. Understanding ecological variation across species: area-based vs mass-based expression of leaf traits. New Phytol. 199, 322–323, doi: 10.1111/nph.12345 (2013). [DOI] [PubMed] [Google Scholar]
- McLean E. H. et al. Plasticity of functional traits varies clinally along a rainfall gradient in Eucalyptus tricarpa. Plant Cell Environ. 37, 1440–1451, doi: 10.1111/pce.12251 (2014). [DOI] [PubMed] [Google Scholar]
- Read Q. D., Moorhead L. C., Swenson N. G., Bailey J. K. & Sanders N. J. Convergent effects of elevation on functional leaf traits within and among species. Funct. Ecol. 28, 37–45, doi: 10.1111/1365-2435.12162 (2014). [DOI] [Google Scholar]
- Meziane D. & Shipley B. Direct and indirect relationships between specific leaf area, leaf nitrogen and leaf gas exchange. Effects of irradiance and nutrient supply. Ann. Bot-London 88, 915–927, doi: 10.1006/anbo.2001.1536 (2001). [DOI] [Google Scholar]
- Poorter H., Niinemets Ü., Poorter L., Wright I. J. & Villar R. Causes and consequences of variation in leaf mass per area (LMA): a meta‐analysis. New Phytol. 182, 565–588, doi: 10.1111/j.1469-8137.2009.02830.x (2009). [DOI] [PubMed] [Google Scholar]
- Niinemets Ü. et al. Do we Underestimate the Importance of Leaf Size in Plant Economics? Disproportional Scaling of Support Costs Within the Spectrum of Leaf Physiognomy. Ann. Bot-London 100, 283–303, doi: 10.1093/aob/mcm107 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niklas K. J. et al. “Diminishing returns” in the scaling of functional leaf traits across and within species groups. P. Natl. Acad. Sci. USA 104, 8891–8896, doi: 10.1073/pnas.0701135104 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Wu Z.-y. & Raven P. H. Flora of China (Science Press, Beijing, 1999) (In Chinese). [Google Scholar]
- Chadwick O. A., Derry L., Vitousek P. M., Huebert B. J. & Hedin L. O. Changing sources of nutrients during four million years of ecosystem development. Nature 397, 491–497, doi: 10.1038/17276 (1999). [DOI] [Google Scholar]
- Chapin F. S. III. Effects of multiple environmental stresses on nutrient availability and use. 67–88 (Academic Press, San Diego, 1991). [Google Scholar]
- Hovenden M. J. & Vander Schoor J. K. Nature vs nurture in the leaf morphology of Southern beech, Nothofagus cunninghamii (Nothofagaceae). New Phytol. 161, 585–594, doi: 10.1046/j.1469-8137.2003.00931.x (2004). [DOI] [PubMed] [Google Scholar]
- Chapin F. S., Bloom A. J., Field C. B. & Waring R. H. Plant Responses to Multiple Environmental Factors. BioScience 37, 49–57, doi: 10.2307/1310177 (1987). [DOI] [Google Scholar]
- Yang D., Niklas K. J., Xiang S. & Sun S. Size-dependent leaf area ratio in plant twigs: implication for leaf size optimization. Ann. Bot-London 105, 71–77, doi: 10.1093/aob/mcp262 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talhelm A. F., Pregitzer K. S. & Burton A. J. No evidence that chronic nitrogen additions increase photosynthesis in mature sugar maple forests. Ecol. Appl. 21, 2413–2424, doi: 10.1890/10-2076.1 (2011). [DOI] [PubMed] [Google Scholar]
- Yuan Z. & Chen H. Y. Global‐scale patterns of nutrient resorption associated with latitude, temperature and precipitation. Global Ecol. Biogeogr. 18, 11–18, doi: 10.1111/j.1466-8238.2008.00425.x (2009). [DOI] [Google Scholar]
- Güsewell S. & Bollens U. Composition of plant species mixtures grown at various N:P ratios and levels of nutrient supply. Basic Appl. Ecol. 4, 453–466, doi: 10.1078/1439-1791-00174 (2003). [DOI] [Google Scholar]
- Högberg P. What is the quantitative relation between nitrogen deposition and forest carbon sequestration? Global Change Biol. 18, 1–2, doi: 10.1111/j.1365-2486.2011.02553.x (2012). [DOI] [Google Scholar]
- Lambers H., Chapin F. S. III & Pons T. L. Plant Physiological Ecology. 2nd Edition, 590 (Springer, New York, 2008). [Google Scholar]
- Buchanan B. B., Gruissem W. & Jones R. L. Biochemistry & molecular biology of plants. Vol. 40 (American Society of Plant Physiologists Rockville, MD, 2000). [Google Scholar]
- Chapin F. S. III, Matson P. A. & Vitousek P. Principles of terrestrial ecosystem ecology. (Springer Science & Business Media, New York, 2011). [Google Scholar]
- Parker G. Throughfall and stemflow in the forest nutrient cycle. Adv. Ecol. Res. 13, 57–133 (1983). [Google Scholar]
- Carlyle-Moses D., Levia D. F., Carlyle-Moses D. & Tanaka T. Forest Hydrology and Biogeochemistry: Synthesis of Past Research and Future Directions. Vol. 216 (Springer Science & Business Media, New York, 2011). [Google Scholar]
- Munir E., Yoon J. J., Tokimatsu T., Hattori T. & Shimada M. A physiological role for oxalic acid biosynthesis in the wood-rotting basidiomycete Fomitopsis palustris. P. Natl. Acad. Sci. USA 98, 11126–11130, doi: 10.1073/pnas.191389598 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambers H., Shane M. W., Cramer M. D., Pearse S. J. & Veneklaas E. J. Root structure and functioning for efficient acquisition of phosphorus: matching morphological and physiological traits. Ann. Bot-London 98, 693–713, doi: 10.1093/aob/mcl114 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu F., Lu X. & Mo J. Phosphorus limitation on photosynthesis of two dominant understory species in a lowland tropical forest. J. Plant Ecol. 7, 526–534, doi: 10.1093/jpe/rtu001 (2014). [DOI] [Google Scholar]
- Liu Z.-P., Shao M.-A. & Wang Y.-Q. Spatial patterns of soil total nitrogen and soil total phosphorus across the entire Loess Plateau region of China. Geoderma 197, 67–78, doi: 10.1016/j.geoderma.2012.12.011 (2013). [DOI] [Google Scholar]
- Jobbágy E. G. & Jackson R. B. The uplift of soil nutrients by plants: biogeochemical consequences across scales. Ecology 85, 2380–2389, doi: 10.1890/03-0245 (2004). [DOI] [Google Scholar]
- Cornelissen J. et al. A handbook of protocols for standardised and easy measurement of plant functional traits worldwide. Aust. J. Bot. 51, 335–380, doi: 10.1071/BT02124 (2003). [DOI] [Google Scholar]
- Malaeb Z., Summers J. K. & Pugesek B. Using structural equation modeling to investigate relationships among ecological variables. Environ. Ecol. Stat. 7, 93–111, doi: 10.1023/A:1009662930292 (2000). [DOI] [Google Scholar]
- Jonsson M. & Wardle D. A. Structural equation modelling reveals plant-community drivers of carbon storage in boreal forest ecosystems. Biol. Letters 6, 116–119, doi: 10.1098/rsbl.2009.0613 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace J. B., Anderson T. M., Olff H. & Scheiner S. M. On the specification of structural equation models for ecological systems. Ecol. Monogr. 80, 67–87, doi: 10.1890/09-0464.1 (2010). [DOI] [Google Scholar]
- Grace J. B. Structural Equation Modeling and Natural Systems. (Cambridge University Press, Cambridge, 2006). [Google Scholar]
- Maruyama G. M. Basics of Structural Equation Modeling. (Sage Publications, Thousand Oaks, 1997). [Google Scholar]