Abstract
Selective norepinephrine reuptake inhibitors (sNRIs) provide an effective class of approved antipsychotics, whose inhibitory mechanism could facilitate the discovery of privileged scaffolds with enhanced drug efficacy. However, the crystal structure of human norepinephrine transporter (hNET) has not been determined yet and the inhibitory mechanism of sNRIs remains elusive. In this work, multiple computational methods were integrated to explore the inhibitory mechanism of approved sNRIs (atomoxetine, maprotiline, reboxetine and viloxazine), and 3 lines of evidences were provided to verify the calculation results. Consequently, a binding mode defined by interactions between three chemical moieties in sNRIs and eleven residues in hNET was identified as shared by approved sNRIs. In the meantime, binding modes of reboxetine’s enantiomers with hNET were compared. 6 key residues favoring the binding of (S, S)-reboxetine over that of (R, R)-reboxetine were discovered. This is the first study reporting that those 11 residues are the common determinants for the binding of approved sNRIs. The identified binding mode shed light on the inhibitory mechanism of approved sNRIs, which could help identify novel scaffolds with improved drug efficacy.
Norepinephrine reuptake inhibitors (NRIs) are psychostimulant which is commonly used for mood and behavioral disorders1. Typical NRIs include the selective norepinephrine reuptake inhibitors (sNRIs)2, serotonin-norepinephrine reuptake inhibitor3 and others4. Currently, 4 sNRIs (atomoxetine, maprotiline, reboxetine and viloxazine) have been approved and marketed by either the U. S. Food and Drug Administration (FDA) or the European Medicines Agency for treating attention deficit hyperactivity disorder5 and depression6 (Fig. 1). Amongst these 4 sNRIs, reboxetine is a racemic mixture of (R, R)- and (S, S)- enantiomers. (S, S)-reboxetine showed 130-fold higher affinity to hNET than (R, R)-reboxetine, and was reported as the predominant influence on reboxetine’s steady state pharmacological property7. Due to the existing deficiencies of currently marketed sNRIs (their delayed onset of action8 and non- or partial-response9), new strategies were applied to enhance drug efficacy by improving their metabolic and pharmacological properties10,11 or by developing dual- and triple-acting antidepressants12. The binding mode shared by all approved and marketed sNRIs could contribute to the discovery of drug-like scaffold with enhanced efficacy13,14.
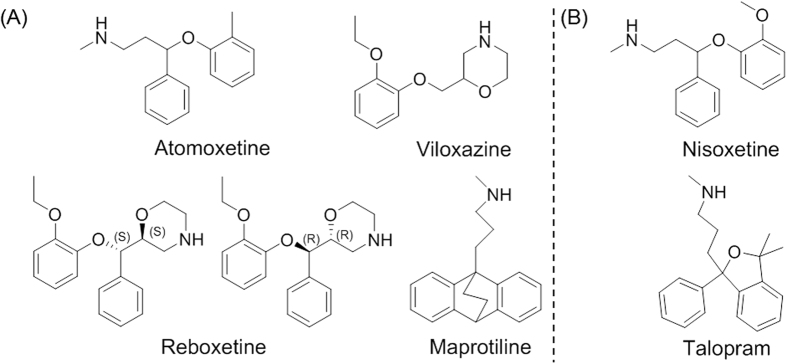
Figure 1. Structures of 6 sNRIs studied in this work.
(A) 4 currently marketed sNRIs approved by either the U. S. FDA (atomoxetine and reboxetine) or the European Medicines Agency (maprotiline and viloxazine); (B) 2 standard sNRIs (nisoxetine and talopram) widely used in scientific research.
Human norepinephrine transporter (hNET), the drug target of sNRIs15, was reported to be closely relevant to various mood and behavioral disorders16,17 by facilitating the reuptake of norepinephrine from the synaptic cleft. Current understanding of hNET was based on the X-ray crystal structures of bacterial and invertebrate homologs, including the bacterial leucine transporter LeuT18,19,20,21, the bacillus neurotransmitter/sodium symporter MhsT22 and the drosophila dopamine transporter (dDAT)23,24. As the most recently determined template, dDAT’s X-ray crystal structure of high resolution revealed the binding of sNRIs (reboxetine and nisoxetine)23 and tricyclic antidepressant (nortriptyline)24. These co-crystallized structures showed a competitive binding of inhibitors to the S1 binding site by locking hNET in an outward-open conformation23,24. As shown in SI, Fig. S1, dDAT demonstrated the highest sequence identity among those hNET’s homologs, making it a new platform for constructing reliable models of sNRIs’ binding in hNET.
Many mutational and biomedical studies have been conducted to clarify the binding mode of sNRIs with hNET and identify key residues defining their recognition25,26,27. It was found that residue Asp75 in hNET was crucial for the interaction between sNRIs and hNET27. Moreover, 2 residues (Phe323 and Ser419) were identified as sensitive (with ≥5 fold-change in the loss- or gain-of-potency) to 3 sNRIs (atomoxetine, nisoxetine and maprotiline)25. Based on the X-ray crystal structure of hNET’s bacterial and invertebrate homologs18,23, 7 residues (Phe72, Asp75, Val148, Tyr152, Phe317, Phe323, Ser420) were also suggested as critical for some sNRIs (e.g. reboxetine) by visualizing the interaction distance between ligands and the target23. In the meantime, computational methods have been proposed and frequently used to elaborate the binding mode between sNRIs and hNET with great efficiency and accuracy28. These methods were applied (1) to elucidate binding mechanisms of substrates and inhibitors to monoamine transporter (MAT)29,30,31,32,33,34,35,36 (2) to discover novel scaffolds of MAT inhibitors by virtual screening37,38,39, and (3) to distinguish various molecular mechanisms of enantiomers binding to MAT40,41. As one of these powerful computational methods, the molecular dynamics (MD) providing atomic description of protein dynamics and flexibility42,43,44,45 was employed to simulate the large scale motions of MAT27,46,47. However, MD simulation has not yet been carried out to explore the binding of sNRIs to hNET. Moreover, the variation on binding modes behind the affinity discrepancy of reboxetine’s enantiomers remains elusive. Thus, there is an urgent need to reveal the mechanism underlying sNRIs’ pharmacodynamics and target recognition23,25.
In this work, multiple computational methods were integrated to explore the inhibitory mechanism of approved sNRIs (atomoxetine, maprotiline, reboxetine and viloxazine). First, a recently reported co-crystal structure of drosophila dopamine transporter (dDAT) in complex with reboxetine23 was used as a template to construct the homology model of hNET. Then, 4 studied sNRIs were docked into hNET for MD simulation, and 3 lines of evidences were provided to verify the simulation results. Consequently, a binding mode shared by approved sNRIs was discovered by clustering the binding free energies of residues. Moreover, the binding modes of 2 reboxetine enantiomers with hNET were compared, and residues favoring the binding of (S, S)-reboxetine over that of (R, R)-reboxetine were discovered. The identified binding mode shed light on the inhibitory mechanism of approved sNRIs, which could help identify novel scaffolds with improved drug efficacy13.
Materials and Methods
Homology Modeling
The homology model of hNET was constructed by using the automated mode in SWISS-MODEL48, based on a recently determined dDAT’s X-ray crystal structure23 of 3.0 Å resolution (PDB entry: 4XNX from Arg25 to Pro596). As shown in SI, Fig. S1, dDAT23 demonstrated 61% sequence identity to hNET, which was much higher than that (23%) of LeuT18. The sequence coverage of the constructed hNET model was between Arg56 and Pro594 covering hNET’s all transmembrane (TM) regions and corresponding extracellular loops. To validate the constructed homology model, the Ramachandran plot in PROCHECK49 was further exploited. Finally, two functional Na+ in dDAT (PDB entry: 4XNX23) were added to their interacting sites in hNET by structural superimposition via PyMOL50.
Molecular Docking
Initial binding conformations of sNRIs in hNET were obtained by molecular docking using Glide31 of standard precision. The docking grid box was defined by centering (R, R)-reboxetine (PDB entry: 4XNX23) in the modeled hNET using the Receptor Grid Generation tool in Glide. Docking poses of sNRIs, with the most similar conformations or orientations as (R, R)-reboxetine in dDAT23, were chosen for MD simulation. Furthermore, a cross-docking approach was applied in this work to validate the docking method. Detailed supporting information was provided in SI, Methods.
System Setup of the Protein-Ligand and Membrane
The Membrane Builder in CHARMM-GUI51 was used to embed the sNRIs-hNET complexes into the explicit POPC lipid bilayer. TIP3P water52 was then positioned above and below the constructed bilayer (20 Å thickness). Na+ and Cl− were added to keep the environmental salt concentration at 0.15 M. As a result, each periodic cell of the entire system (83 Å × 83 Å × 127 Å) contained ~96,400 atoms. Detailed supporting information was provided in SI, Methods.
MD Simulation
AMBER1453 using GPU-accelerated PMEMD was applied to carry out MD simulation. The force field ff14SB54 was used for protein, and Lipid1455 were utilized for lipid. TIP3P water’s ions parameters were directly adopted from previous publication56. sNRIs’ parameters were generated using the General AMBER force field57 and the charges of sNRIs’ atoms were derived by the Restrained Electrostatic Potential partial charges58 in Antechamber59. Gaussian0960 was applied to optimize the geometry and calculate the electrostatic potential at the HF/6–31G* level. In all simulations, a sequential process was executed (minimization, heating and equilibration). After this, 150 ns production MD simulation at 310 K and 1 atm was conducted in NPT ensemble with the periodic boundary conditions. Meanwhile, the long-range electrostatic interactions (cutoff = 10.0 Å) was treated using Particle-Mesh Ewald method61. The SHAKE algorithm62 was applied to constrain the bond lengths involving bond to hydrogen atoms, and in simulation the integration time step was set as 2 fs. Supporting data were provided in the SI, Methods.
Calculation of the Binding Free Energies
The energies of each sNRI binding to hNET (ΔGMM/GBSA) without the entropic effect were analyzed by the MM/GBSA approach with single-trajectory63,64. The last 50 ns of simulation (500 snapshots) was used for binding free energy calculation. For each snapshot, the energy of sNRI binding to hNET was computed by:
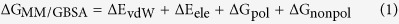 |
In Eq. (1), ΔEvdW indicates the van der Waals energy, and ΔEele denotes the electrostatic energy. ΔGpol is the polar solvent interaction energy calculated by solving the GB equation. ΔGnonpol is the non-polar solvation contribution and estimated as 0.0072 × ΔSASA using the LCPO method65, where the SASA denotes the solvent accessible area. Supporting data were provided in the SI, Methods.
Analysis of the Per-residue MM/GBSA Free Energy Decomposition
In order to analyze the per-residue contribution to sNRIs’ binding to hNET, energy was calculated by the approach of MM/GBSA decomposition using mmpbsa.pl plugin in AMBER1453. The per-residue energy(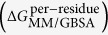 without entropic effect could be computed by:
without entropic effect could be computed by:
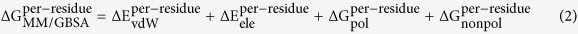 |
Most of the terms in Eq. (2) are defined in the same way as that in Eq. (1), but the energy of the non-polar solvent interaction 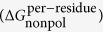 was computed by recursively approximating a sphere around an atom from an icosahedron53. Supporting data were provided in the SI, Methods.
was computed by recursively approximating a sphere around an atom from an icosahedron53. Supporting data were provided in the SI, Methods.
Hierarchical Clustering of Residues Based on Their Energy Contributions
Energy contributions of certain residue to 4 approved sNRIs (atomoxetine, maprotiline, reboxetine and viloxazine) rendered a 4 dimensional vector. The clustering tree of residues contributing to at least one studied sNRI (≠0 kcal/mol) in hNET’s binding was constructed by the statistical analyzing package R66. The Manhattan distance was selected to calculate similarities among vectors:
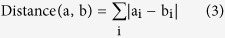 |
where i indicates the dimension of the residue’s energy contribution a and b. Ward’s minimum variance module67 in R66 for hierarchical clustering was adopted for minimizing the total variance within cluster. The hierarchical tree was depicted by the latest version of iTOL68. The residues favoring sNRI’s binding are colored in red (the one with the highest contribution was colored as red and the lower contribution one was set to fade gradually to white). In the meantime, the residues hampering sNRI’s binding are displayed in blue (the highest one was colored as blue and the lower one was set to fade gradually to white). The white color here denotes residue with no contribution to sNRIs’ binding.
Results and Discussion
Construction of the sNRIs-hNET Complexes
The homology model of the hNET was built on a 3.0 Å high-resolution crystal structure of the dDAT (PDB entry: 4XNX23). The sequence identity between the modeled fraction of hNET and dDAT was 61% (SI, Fig. S1). As shown in SI, Fig. S2, the modeled hNET contained the whole TM helices and the S1 binding site, and showed high degree of homology to dDAT’s crystal structure. Ramachandran plot49 showed a reasonable homology model, which identified 99.4% residues in the “allowed region” (SI, Fig. S3).
Then, the co-crystallized and the cross docking poses of 2 sNRIs (R, R)-reboxetine and nisoxetine (PDB entries: 4XNX and 4XUN23) were superimposed to assess the reliability of the docking methods applied in this work. As a result, these 2 poses were consensus with each other (SI, Fig. S4), indicating the correct initial binding poses generated here. 4 approved sNRIs (atomoxetine, maprotiline, reboxetine and viloxazine) and 2 standard sNRIs (nisoxetine and talopram) widely used in scientific research were included in this study for MD simulation to overcome the probable over-fitting result. Although no co-crystallized structures of atomoxetine, maprotiline, (S, S)-reboxetine, talopram or viloxazine was reported, their resulting docking poses resembled in orientation as the co-crystallized pose of (R, R)-reboxetine (SI, Fig. S5).
Assessment of the Binding Mode of sNRIs-hNET
Evaluation of the Simulation Stability
The dynamic stabilities of the 6 systems (atomoxetine, maprotiline, nisoxetine, reboxetine, talopram and viloxazine) along MD simulation were measured by their average distance from the initial structures in terms of RMSD. The RMSD values of protein backbone atoms, ligand heavy atoms and binding site residue atoms (around 5 Å of ligand) in the entire MD simulation trajectories were illustrated in SI, Fig. S6. All 6 simulated systems reached equilibration state after 100 ns with only little fluctuation in the monitored RMSD.
Analysis of the Binding Free Energy
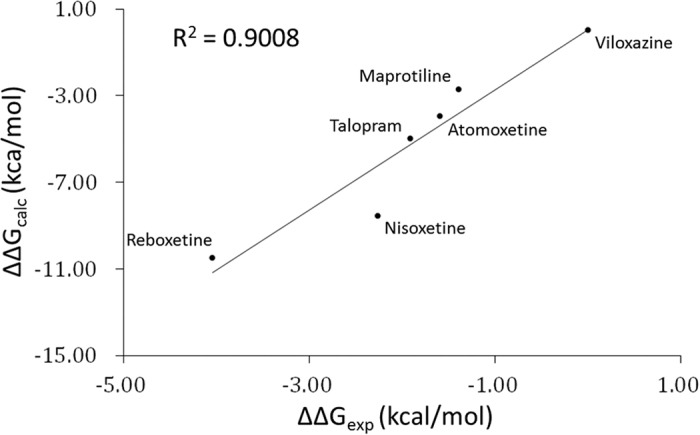
The MM/GBSA calculations were performed to quantitatively analyze the binding free energies of hNET in complex with sNRIs (Table 1). The predicted binding free energy (ΔGMM/GBSA) for hNET with atomoxetine, maprotiline, nisoxetine, talopram, viloxazine and (S, S)-reboxetine were −41.42, −40.21, −46.05, −42.47, −37.48 and −47.67 kcal/mol, respectively. Meanwhile, ΔGexp = RTln(Ki) was used to convert the experimental K values7,27,69,70,71 to the binding free energies (ΔGexp). As shown in Table 1, binding free energies for each system were overestimated compared to the experimental results. If one is only interested in the relative order of binding affinities of structurally similar ligands of resemble binding modes, the entropy effects could be omitted72. Therefore, the energy differences (Table 1) calculated (ΔΔGMM/GBSA) and estimated by experimental data (ΔΔGexp) among 4 sNRIs could tell whether these overestimations were originated from the omission of entropy effects. ΔΔGMM/GBSA was shown in Fig. 2 to correlate well with ΔΔGexp (R2 = 0.9008). In spite of an overestimation of ΔGMM/GBSA comparing to the ΔGexp7,69,70, the ascending trend of ΔGexp was reproduced well by ΔGMM/GBSA. The overestimated energy in our study was in agreement with the reported over-evaluation using the approach of MM/GBSA73,74,75. Each component of energy in Eq. (1) was illustrated in SI, Table S1. In particular, ΔEvdW and ΔEele mainly contributed to the binding of sNRIs with hNET, while the polar solvent energy (ΔGpol) impeded the binding.
Table 1. Comparison of binding free energies between the calculated results and the experimental data of 6 sNRIs of this study binding to hNET.
| sNRIs studied | Kia | ΔGexpb | ΔΔGexpc | ΔGMM/GBSAd | ΔΔGcalcc |
|---|---|---|---|---|---|
| Atomoxetine | 5.00 | −11.33 | −1.60 | −41.42 ± 0.13 | −3.94 |
| Maprotiline | 7.00 | −11.13 | −1.39 | −40.21 ± 0.09 | −2.73 |
| Nisoxetine | 1.60 | −12.00 | −2.26 | −46.05 ± 0.13 | −8.57 |
| Talopram | 2.90 | −11.65 | −1.91 | −42.47 ± 0.12 | −4.99 |
| (S, S)-reboxetine | 0.08 | −13.78 | −4.04 | −47.67 ± 0.12 | −10.19 |
| Viloxazine | 73.00 | −9.74 | 0.00 | −37.48 ± 0.12 | 0.00 |
ΔG is in kcal/mol and Ki value is in nM.
bEstimated binding free energy based on Ki values by the equation ΔGexp = RTln (Ki).
cΔΔG is defined as the change of binding free energy (ΔG) using viloxazine as a reference.
dCalculated MM/GBSA binding free energies with the standard error of the mean (the standard deviation divided by the square root of the number of snapshots) in this work.
Figure 2. Correlation between energy difference of 6 studied sNRIs calculated in this work (ΔΔGcalc) and that estimated based on experiments7,27,69,70,71 (ΔΔGexp).
Verifying the Resulting Models of MD Simulation
Besides the good correlation between the results of simulation and experiments in previous section, 3 lines of evidence further verified our resulting model of MD simulation. The first line of evidence was from the recently identified key residues that control sNRIs’ selectivity in hNET76. As reported, a mutational analysis of 6 diverging residues (Ala145, Tyr151, Ile315, Phe316, Ser420 and Ala426) in the central binding site (S1 site) of hNET to the complementary residues in the human dopamine transporter (hDAT) transferred a hDAT-like pharmacology to hNET, showing that those 6 residues were collectively key residues for sNRIs’ selectivity76. To investigate the influence on the binding of 6 studied sNRIs to the hDAT-like hNET76, in silico mutations on these 6 residues in hNET to the identity of the corresponding residues in hDAT (A145S-Y151F-I315V-F316C-S420A-A426S) were conducted in this work. As illustrated in SI, Fig. S7, 6 studied sNRIs (atomoxetine, maprotiline, nisoxetine, talopram, viloxazine and (S, S)-reboxetine) in complex with the hDAT-like hNET were analyzed by adding 20 ns simulation to the models of the wild type hNET constructed in this work. The resulting binding free energy of 6 sNRIs was calculated, and the fold-changes in their binding affinity induced by hDAT-like hNET mutations from Andersen’s experimental study76 were listed in Table 2 (detail information of each energy term can also be found in SI, Table S3). As shown, the fold-changes in binding affinities of 4 sNRIs (atomoxetine, nisoxetine, reboxetine and talopram) reported in Andersen’s study76 were reproduced well by our calculated ΔΔGMM/GBSA (ΔΔGcalc). In particular, the ΔΔGcalc were 0.47, 1.15. 2.89 and 3.70 kcal/mol for reboxetine, atomoxetine, nisoxetine and talopram, respectively, which were comparable and followed the same trends as the reported increases in binding free energies (ΔΔGexp) induced by hDAT-like hNET mutations (1.38, 1.97, 3.04 and 3.16 kcal/mol for reboxetine, atomoxetine, nisoxetine and talopram)76. Since those additional 20 ns simulations were all based on the resulting hNET models constructed in this work, this reproduction of experiments by simulation could act as one line of evidence for verifying our resulting simulation models. In the meantime, the binding free energies of 2 sNRIs (maprotiline and viloxazine) not included in Andersen’s study76 were also calculated and listed in Table 2 and SI, Table S3. The bindings of both sNRIs were affected significantly by hDAT-like hNET mutations. These results supported Andersen’s findings that 6 residues in the S1 site of hNET controlled sNRIs’ selectivity76. SI, Fig. S8 illustrated changes in the conformation and orientation of both hNET’s binding site and those 6 studied sNRIs within it.
Table 2. Comparison of binding free energies between the calculated results and the experimental data of 6 sNRIs-hNET complexes before and after hDAT-like mutations in hNET76 (ΔG is in kcal/mol).
| sNRIs studied | hDAT-like mutations in the S1 site76 | Calculated values |
Experimental values76 |
||
|---|---|---|---|---|---|
| ΔΔGcalca | Fold-change of potencyb | Fold-change of potencyc | ΔΔGexpd | ||
| Talopram | S145S-Y151F-I315V-F316C-S420A-A426S | 3.70 | 514.09 | 207.00 (151.00~284.00) | 3.16 (2.98~3.35) |
| Nisoxetine | 2.89 | 131.08 | 168.42 (107.73~251.88) | 3.04 (2.78~3.28) | |
| Atomoxetine | 1.15 | 6.96 | 27.87 (18.47~40.15) | 1.97 (1.73~2.19) | |
| Reboxetine | 0.47 | 2.70 | 10.27 (7.18~14.31) | 1.38 (1.17~1.58) | |
| Maprotiline | 2.45e | 62.39e | –e | –e | |
| Viloxazine | 1.09e | 6.29e | –e | –e | |
Detail information of each energy term can be found in SI, Table S3.
aΔΔGcalc = ΔGmutation − ΔGwild type.
bFold-changes of potency were derived from ΔΔGcalc by the equation ΔΔGcalc = RTln (FCpotency).
cFold-changes of potency measured by Ki values (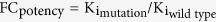 )76. Numbers out of the bracket indicated the fold-changes derived from the medium experimental values of both
)76. Numbers out of the bracket indicated the fold-changes derived from the medium experimental values of both  and
and  . The first number in the bracket indicated the minimum fold-changes, while the second one indicated the maximum fold-changes.
. The first number in the bracket indicated the minimum fold-changes, while the second one indicated the maximum fold-changes.
dΔΔGexp were derived from the FCpotency by the equation ΔΔGexp = RTln (FCpotency).
eNot included in Andersen’s experimental study, but simulated in this work76.
The second line of evidence, coming from the reported mutagenesis experiments on the sensitivity profiles of hNET’s residues, indicated that our resulting models of MD simulation were capable of distinguishing sensitive residues from non-sensitive ones. As reported, the sensitivity profiles could shed light on the binding mode of sNRIs25. The residues’ sensitivity to the binding of sNRIs’ could be estimated by the variation in the binding free energy before and after the in silico mutation. In this work, 2 sensitive mutations (S419T and F323Y with ≥5-fold changes in binding affinity) and 2 non-sensitive mutations (F72Y and N153S without markedly change in potency) of 2 sNRIs (atomoxetine and maprotiline) identified by previous experiments25 were selected, and their sensitivities were explored by in silico mutation study. Particularly, hNET of single-point mutations (S419T, F323Y, F72Y and N153S) in complex with those 2 sNRIs were analyzed by additional 20 ns simulation based on the models of the wild type hNET constructed in this work (SI, Fig. S9). The calculated binding free energies of 2 sNRIs and the fold-changes in their binding affinity from Sorensen’s experiments25 induced by those single-point mutations were shown in Table 3, and information of each energy term was listed in SI, Table S4. As shown, the sensitivity profiles of 4 mutations reported in Sorensen’s work25 were successfully discovered by the calculated ΔΔGcal in this work. Particularly, our simulation discovered F72Y and N153S as non-sensitive mutations to both sNRIs. In Table 3, the ΔΔGcalc were between −0.31 and 0.05 kcal/mol. The corresponding range of fold-changes in potency (FCpotency) could thus be estimated as from 0.59 to 1.09 by the equation ΔΔGcalc = RTln (FCpotency), which were comparable to the experimentally estimated non-sensitive FCpotency (from 0.59 to 2.34)25. Meanwhile, S419T and F323Y were identified as sensitive to both sNRIs. Their ΔΔGcalc were between 0.96 and 1.93 kcal/mol. The corresponding range of FCpotency were estimated as from 5.05 to 25.95, which were also comparable to those experimentally estimated sensitive FCpotency (from 3.89 to 12.67)25. The distinct difference in FCpotency between sensitive and non-sensitive mutations indicated that our resulting models were capable of distinguishing the sensitive mutations (S419T and F323Y) from the non-sensitive ones (F72Y and N153S). As those in silico mutational studies were based on models constructed in this work, their ability to identify the sensitivity profiles of hNET’s residues could be considered as another line of evidence to verify our resulting models. SI, Fig. S10 illustrated the changes in the conformation and orientation of both hNET’s binding site and 2 sNRIs within it.
Table 3. The calculated and experimental changes in binding free energies of 8 sNRIs-hNET complexes (2 sNRIs against 4 single-point mutations) before and after those mutations in hNET25 (ΔG is in kcal/mol).
| sNRIs studied | Single point mutations in hNET | Calculated value |
Experimental value25 |
||
|---|---|---|---|---|---|
| ΔΔGcalca | Fold-change of potencyb | Fold-change of potencyc | ΔΔGexpd | ||
| Atomoxetine | F72Y | −0.03 | 0.95 | 0.78 (0.55~1.14) | −0.15 (−0.35~0.08) |
| N153S | −0.31 | 0.59 | 2.33 (1.73~3.29) | 0.50 (0.33~0.71) | |
| F323Y | 1.93 | 25.95 | 3.89 (2.45~6.14) | 0.81 (0.53~1.08) | |
| S419T | 1.27 | 8.52 | 12.67 (8.73~18.86) | 1.51 (1.28~1.74) | |
| Maprotiline | F72Y | −0.31 | 0.59 | 0.59 (0.34~0.99) | −0.31 (−0.64~−0.01) |
| N153S | 0.05 | 1.09 | 2.34 (1.44~3.75) | 0.50 (0.22~0.78) | |
| F323Y | 0.96 | 5.05 | 4.97 (3.21~7,75) | 0.95 (0.69~1.21) | |
| S419T | 1.36 | 9.92 | 5.88 (3.61~9.43) | 1.05 (0.76~1.33) | |
Detail information of each energy term can be found in SI, Table S4.
aΔΔGcalc = Gmutation − ΔGwild type.
bFold-changes of potency were derived from ΔΔGcalc by the equation ΔΔGcalc = RTln (FCpotency).
cFold-changes of potency measured by Ki values 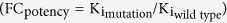 25. Numbers out of the bracket indicated the fold-changes derived from the medium experimental values of both
25. Numbers out of the bracket indicated the fold-changes derived from the medium experimental values of both  and
and  . The first number in the bracket indicated the minimum fold-changes, while the second one indicated the maximum fold-changes.
. The first number in the bracket indicated the minimum fold-changes, while the second one indicated the maximum fold-changes.
dΔΔGexp were derived from the FCpotency by the equation ΔΔGexp = RTln (FCpontency).
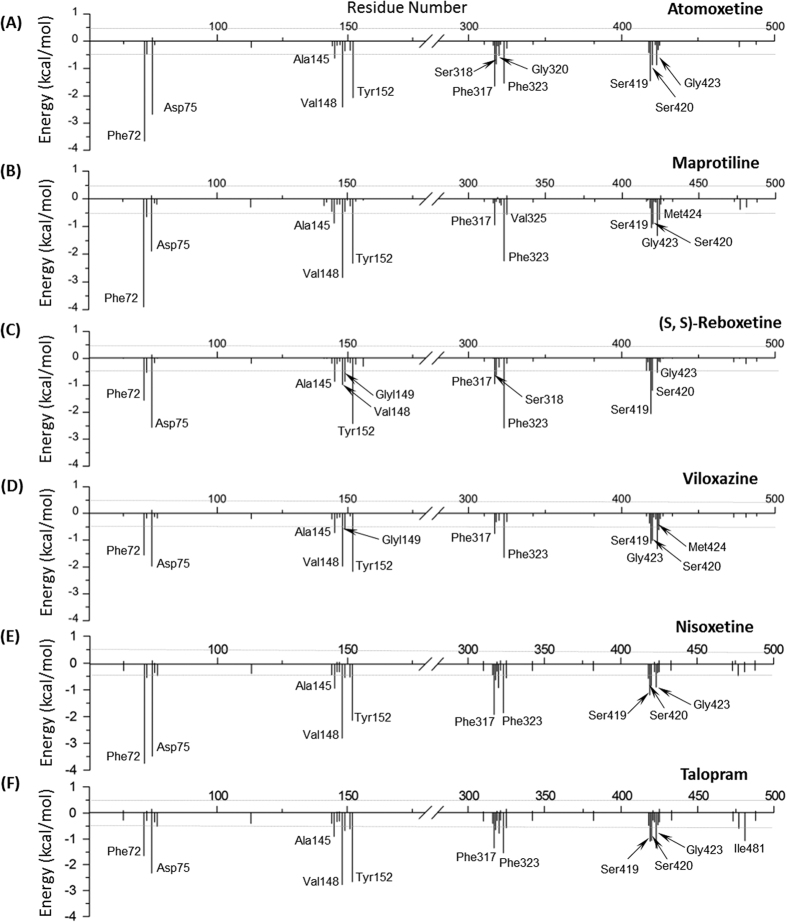
The above evidence was supported further by the third line of evidence from the crystallography study, which reported co-crystalized structures of ligands (nisoxetine and reboxetine) with dDAT23. Based on their structures, these 2 sNRIs appeared to have very similar modes of binding and action. Of these 2 complexes, the amino groups of both ligands interact with the residues Phe43 and Asp46 (the corresponding residues Phe72 and Asp75 in hNET)23. Moreover, the cavity formed by residues Phe43, Ala44, Phe319 and Ser320 (the corresponding residues Phe72, Ala73, Phe317 and Ser318 in hNET) was occupied by the amine group of nisoxetine and the morpholine nitrogen of reboxetine23. In addition, two aromatic rings of both sNRIs were involved in the hydrophobic cleft bordered by residues Val120, Tyr123, Tyr124, Phe319, Phe325 and Ser422 (the corresponding residues Val148, Tyr151, Tyr152, Phe317, Phe323 and Ser420 in hNET), which further stabilized both sNRIs in the S1 binding site23. In this study, all of those residues contributed significantly (≥0.5 kcal/mol) to the binding of sNRIs (illustrated in Fig. 3), which could be the third line of evidence verifying our resulting simulation models.
Figure 3. Per-residue binding free energy decomposition of 6 studied sNRIs-hNET complexes.
Residues with high energy contribution (the absolute energy contribution ≥0.5 kcal/mol) were labeled.
Analysis of the sNRIs’ Binding Mode in hNET
The representative structures of 6 sNRIs extracted from the equilibrated simulation trajectory slightly shifted in conformation comparing to their corresponding docking poses (SI, Fig. S11), and key interactions between the ligands and Asp75 of hNET were retained. Moreover, per-residue free energy decomposition could help identify key residues in the binding of sNRIs to hNET. As shown in Fig. 3, 12, 13, 10, 11, 10 and 12 residues were recognized as high contribution ones (≥0.5 kcal/mol) for the binding of atomoxetine, maprotiline, nisoxetine, talopram, viloxazine and (S, S)-reboxetine, respectively. On one hand, it is clear to see that energies of different residues to the same sNRI differ significantly (from −0.53 kcal/mol for Gly320 to −3.67 kcal/mol for Phe72 in atomoxetine’s binding), and energies of the same residue to different sNRIs also vary greatly (the contributions of Asp75 are from −1.97 kcal/mol for viloxazine to −3.29 kcal/mol for nisoxetine). On the other hand, Fig. 3 also reflects similarity among sNRIs to some extent. As reported, the shared binding mode of approved sNRIs are very helpful in discovering hits or lead compounds with improved efficacy13,14, which inspires us to further explore the binding mode shared by approved sNRIs.
Identification of the Shared Binding Mode of Approved sNRIs
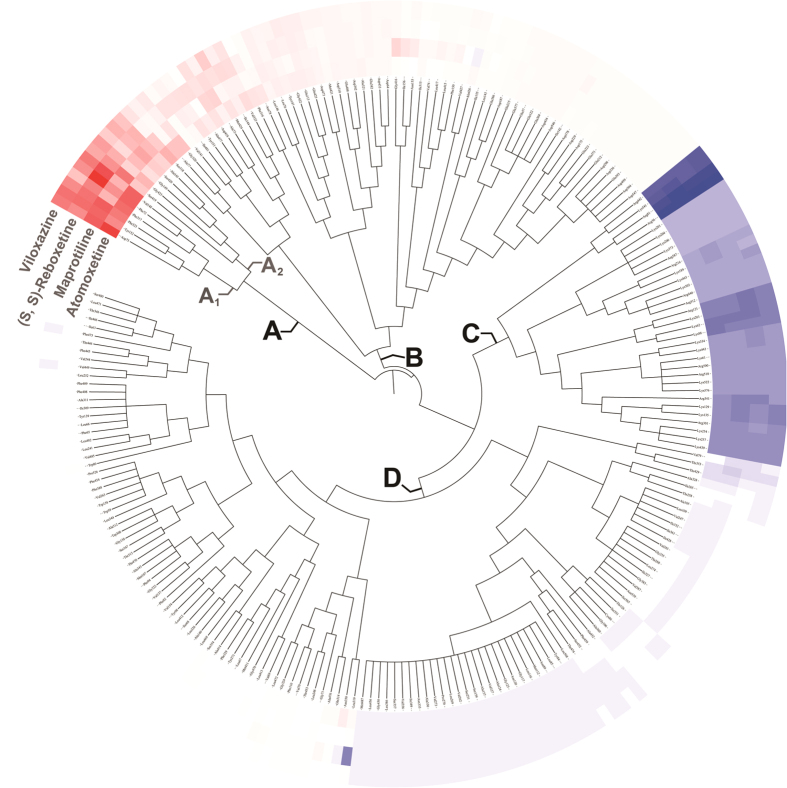
Among those 538 residues in hNET, 238 were with energy contribution to at least 1 approved sNRI. To characterize the most favorable binding mode shared by approved sNRIs, hierarchical clustering with ward algorithm67 was exploited to identify hot spots from those 238 residues based on their energies. In Fig. 4, 4 groups of residues (A, B, C and D) were discovered. The residues favoring sNRI’s binding were colored in red. The residue with the highest contribution (−3.91 kcal/mol) was colored as standard red. The color of the lower contribution one was set to fade gradually towards white (no contribution). In the meantime, the residues hampering sNRI’s binding were displayed in blue. The highest one was colored as standard blue (0.20 kcal/mol) and the color of the lower one was set to fade gradually towards white. Importantly, it should be noticed that the highest energy contribution of residue favoring sNRI’s binding is much larger (about 19 times) than that hampering the binding.
Figure 4. Tree of 238 residues with contribution to at least one studied sNRI in binding hNET by hierarchically clustering their energies.
The residues favoring sNRI’s binding are colored in red (the one with the highest contribution was colored as standard red and the lower contribution one was set to fade gradually to white). In the meantime, the residues hampering sNRI’s binding are displayed in blue (the highest one was colored as standard blue and the lower one was set to fade gradually to white). The white color here denotes residue with no contribution to sNRIs’ binding.
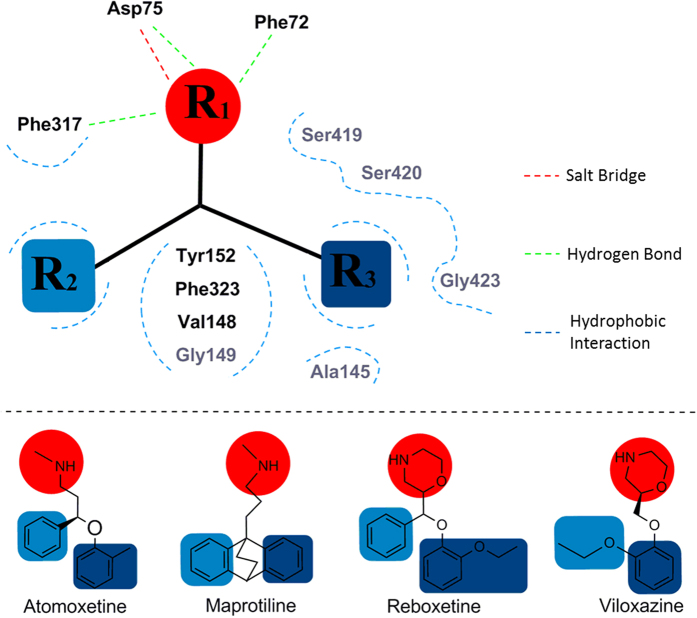
As shown in Fig. 4, energy contribution of group A (Phe72, Asp75, Ala145, Val148, Gly149, Tyr152, Phe317, Phe323, Ser419, Ser420, Gly423) were consistently higher across all approved sNRIs than that of group B, C and D, indicating a crucial role played by group A in sNRIs’ binding. In particular, the sum of group A’s energy contributions accounted for the major part of the total energy (77.80% for atomoxetine, 78.06% for maprotiline, 78.95% for (S, S)-reboxetine and 75.27% for viloxazine). Those 11 residues revealed a similar pattern in drug binding in spite of their distant chemical scaffolds, and therefore were identified as hot spots for sNRIs’ binding. Moreover, residues in subgroup A1 (Phe72, Asp75, Val148, Tyr152, Phe317 and Phe323) contributed much higher to sNRIs’ binding than those in subgroup A2 (Ala145, Gly149, Ser419, Ser420 and Gly423). The sum of subgroup A1’s energy contributions constituted 60.15%, 58.87%, 53.54% and 50.27% of the total energies for atomoxetine, maprotiline (S, S)-reboxetine and viloxazine, respectively.
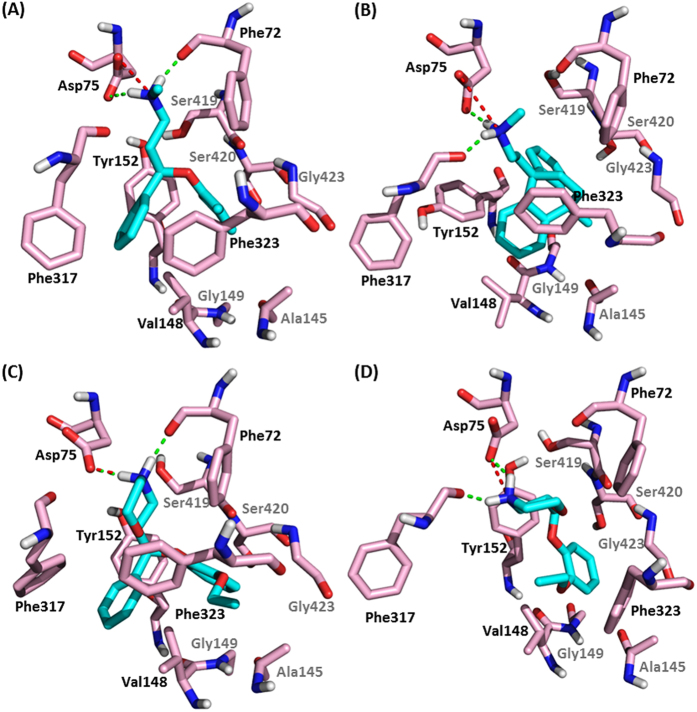
As shown in Fig. 5, the amino group of all 4 sNRIs pointed to the residue Asp75, and the rest of hot spot residues formed the hydrophobic part of the binding pockets. To measure the conformational shift among the binding pockets of 4 sNRIs, the RMSDs of the 11 hot spot residues were calculated. The resulting RMSDs of different sNRIs’ binding pockets were all <3.0 Å with the highest one of 2.8 Å and the lowest one of 1.7 Å. In addition, in order to compare the orientations of these sNRIs, the superimposition of all sNRIs were shown in SI, Fig. S12, which gave a resembled orientation among them. Thus, the generalized binding mode of sNRIs with hNET was schematically displayed in Fig. 6. As illustrated, the binding mode was represented by the interactions of salt bridge, hydrogen bond and hydrophobic contact between three chemical moieties and eleven hot spot residues (Phe72, Asp75, Ala145, Val148, Gly149, Tyr152, Phe317, Phe323, Ser419, Ser420, Gly423). In Fig. 6, these three chemical moieties were illustrated by the color of red (R1), light blue (R2) and blue (R3). Residues with strong (subgroup A1) energies were marked in black, and residues with relatively strong (subgroup A2) energies were shown in gray (Figs 5 and 6). Particularly, the moiety R1 mainly engaged in the formation of salt bridge interaction and the hydrogen bond with Asp75 and Phe72 or Phe317; R2 formed hydrophobic interactions with Val148, Gly149, Tyr152 and Phe323 and also contacted with Phe317; R3 contacted hydrophobically with Ala145, Ser419, Ser420 and Gly423.
Figure 5.
Binding modes of approved sNRIs (A) atomoxetine (B) maprotiline (C) (S, S)-reboxetine and (D) viloxazine with hNET identified in this work. The ligands were displayed in cyan and hot spot residues were depicted in light pink. The salt bridge interaction was displayed in red dashed line and the hydrogen bond interaction was in green. Hot spot residues in subgroup A1 (strong interaction) and A2 (relatively strong interaction) shown in Fig. 5 were labeled in black and gray, respectively.
Figure 6. The binding mode shared by approved sNRIs with hNET.
The identified salt bridges, hydrogen bonds and hydrophobic interactions were depicted in red, green and blue dashed lines, respectively. The red color (R1) indicated the chemical moiety with salt bridge and hydrogen bond interaction with residues in the vicinity, while the light blue (R2) and dark blue (R3) represented the chemical moiety with only hydrophobic interaction with its nearby residues. Each chemical moiety was generalized by the superimposition of 4 sNRIs in the S1 pocket. The residues in dark (Phe72, Asp75, Val148, Tyr152, Phe317, and Phe323) belonged to the subgroup A1 (Fig. 5), and the residues in gray (Ala145, Gly149, Ser419, Ser420 and Gly423) were clustered into subgroup A2 (Fig. 5).
Among those 11 identified hot spot residues, 5 (Phe72, Val148, Gly149, Phe323 and Ser419) were studied in Sorensen’s work25. By measuring the changes on the inhibitory potencies before and after the mutation, Sorensen et al. found 2 sensitive mutations (S419T and F323Y, ≥5 -fold changes for atomoxetine and maprotiline) and 2 mutations (F72Y and N153S) without markedly decrease in potency of both sNRIs. Moreover, recently determined co-crystallized structures of sNRIs in dDAT (a homologous structure of hNET) could shade light on their binding mechanism. However, besides (R, R)-reboxetine and nisoxetine23, no structure of any sNRIs complexed with hNET or its homologous structure was reported. According to (R, R)-reboxetine’s co-crystallized structure23, 7 residues (Phe72, Asp75, Val148, Tyr152, Phe317, Phe323, Ser420) out of those 11 hot spots were suggested as critical residues by visualizing interaction distances between ligand and dDAT23. In addition, crystal structures of LeuBAT (engineered LeuT) in complex with 4 selective serotonin reuptake inhibitors (SSRIs), 2 serotonin–norepinephrine reuptake inhibitors (SNRIs) and 1 tricyclic antidepressant (TCA) were determined and reported to harbor a human monoamine transporter-like pharmacology77. Analysis of these SSRIs, SNRIs and TCA ligands complexed with LeuBAT helped to identify the binding pocket defined by Tyr21, Asp24, Val104, Ala105, Tyr108, Phe253, Gly256, Phe259, Ser355, Gly359, Asp404 and Thr408 (corresponding residues in hNET were Phe72, Asp75, Val148, Gly149, Tyr151, Phe317, Gly320, Phe323, Ser419, Gly423, Asp473 and Ala477). Among these corresponding residues, 8 (Phe72, Asp75, Val148, Gly149, Phe317, Phe323, Ser419, Gly423) were overlapped with those 11 hot spots identified by this study. Overall, these 11 residues were reported for the first time as the common determinants for the binding of all approved sNRIs.
Further analysis on energy contributions of sNRIs’ different chemical moieties reveals a vital role of chemical moiety R1 in sNRIs-hNET recognition25. R1 forms salt bridge with Asp75 and hydrogen bonds with Asp75, Phe72 and Phe317, which in total consists of 34.29%, 23.04%, 28.04% and 22.70% of binding free energies for atomoxetine, maprotiline, (S, S)-reboxetine and viloxazine, respectively. To further understand these interactions anchoring different sNRIs into the binding site, salt bridge and hydrogen bond were monitored along the entire MD simulation. Detail information can be found in SI, Results and Discussion.
Two Reboxetine Enantiomers Distinguished by Their Binding Modes
(S, S)-reboxetine showed 130-fold higher affinity to hNET than its (R, R) enantiomer, and was reported as the predominant influence on reboxetine’s steady state pharmacological properties7. However, the variation on binding modes of 2 enantiomers with hNET remains elusive. In this work, a collective computational method was applied to identify binding modes of 2 enantiomers and distinguish their conformational variations in hNET. As shown in SI, Fig. S13, interactions of salt bridge, hydrogen bond and hydrophobic contact between sNRIs and 11 hot spot residues were essential for both enantiomers in hNET’s recognition, which could be further analyzed by the energy decomposition of those 2 enantiomers (SI, Fig. S14).
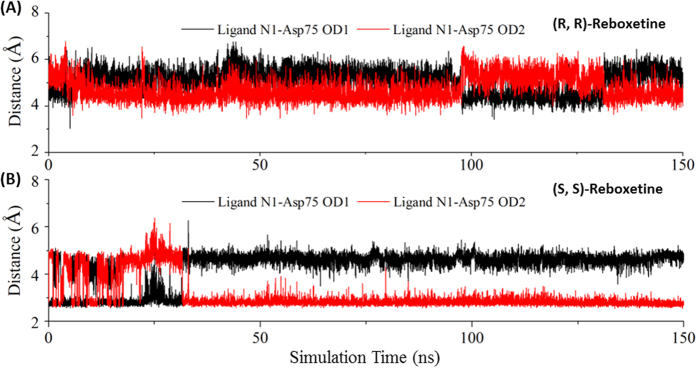
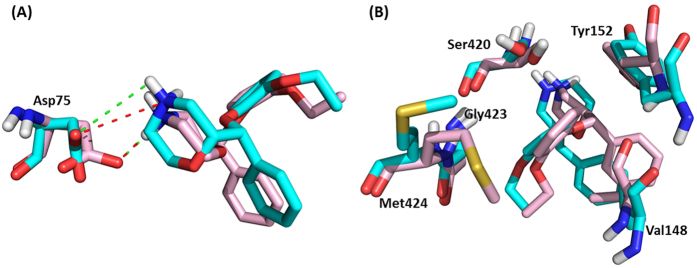
However, the calculated binding free energies of (R, R)-reboxetine and (S, S)-reboxetine were −34.69 and −47.67 kcal/mol, respectively, which were consistent with the experimental results7. To understand the difference in binding affinities of 2 enantiomers, per-residue energy contribution analysis was performed to find out residues with significant variation in energy contribution (absolute variation ≥0.5 kcal/mol) between 2 enantiomers. In this work, 6 residues (Asp75, Val148, Tyr152, Ser420, Gly423 and Met424) favoring the binding of (S, S)-reboxetine over (R, R)-reboxetine were identified. For the residue Asp75, the average interaction distance between the polar nitrogen of (S, S)-reboxetine and the carboxyloxygen of Asp75 was 2.8 Å, while the distance was 4.2 Å for (R, R)-reboxetine (Fig. 7). A shorter interaction distance for (S, S)-reboxetine guaranteed a much stronger salt bridge and hydrogen bond interaction than (R, R)-reboxetine. Meanwhile (S, S)-reboxetine formed strong hydrophobic interactions with other 5 residues (Val148, Tyr152, Ser420, Gly423 and Met424). Comparing to (S, S)-reboxetine’s binding, (R, R)-reboxetine and those 5 residues suffered from obvious conformation shifts (Fig. 8), which significantly increased the hydrophobic interaction distance between the ligand and residues.
Figure 7.
Distance between the ligand N1 and Asp75 OD in (A) (R, R)-reboxetine and (B) (S, S)-reboxetine binding to hNET during the entire 150 ns MD simulation.
Figure 8. Superimposition of and conformation variation between (R, R)-reboxetine (in cyan) and (S, S)-reboxetine (in light pink) together with their interacting residues (in corresponding color).
(A) Conformational changes of ligands and residue Asp75 were displayed; (B) conformational changes of ligands and residues Val148, Tyr152, Ser420, Gly423 and Met424 were illustrated. The salt bridge interaction was displayed in red dashed line and the hydrogen bond interaction was in green.
Conclusion
In this study, multiple computational methods were integrated to explore the inhibitory mechanism of approved sNRIs (atomoxetine, maprotiline, reboxetine and viloxazine). First, a recently reported co-crystal structure of drosophila dopamine transporter (dDAT) in complex with reboxetine was utilized to construct the homology model of hNET. Then, those studied sNRIs were docked into hNET for MD simulation. 3 lines of evidences were further provided to verify the simulation results. Consequently, a binding mode shared by approved sNRIs was discovered by clustering the binding free energies of residues. Eleven residues (Phe72, Asp75, Ala145, Val148, Gly149, Tyr152, Phe317, Phe323, Ser419, Ser420, Gly423) in hNET were identified as crucial for revealing the binding mode of sNRIs-hNET complex. Moreover, the binding modes of reboxetine’s enantiomers with hNET were compared, and 6 residues (Asp75, Val148, Tyr152, Ser420, Gly423 and Met424) favoring the binding of (S, S)-reboxetine over that of (R, R)-reboxetine were identified. This is the first study reporting that those 11 residues are the common determinants for the binding of approved sNRIs. The identified binding mode shed light on the binding mechanism of approved sNRIs, and might therefore help identify novel scaffolds with improved drug efficacy.
Additional Information
How to cite this article: Zheng, G. et al. Exploring the Inhibitory Mechanism of Approved Selective Norepinephrine Reuptake Inhibitors and Reboxetine Enantiomers by Molecular Dynamics Study. Sci. Rep. 6, 26883; doi: 10.1038/srep26883 (2016).
Supplementary Material
Acknowledgments
This work was funded by the research support of National Natural Science Foundation of China (81202459, 21505009), by Chongqing Natural Science Foundation (cstc2012jjA10116) and by Fundamental Research Funds for the Central Universities (CQDXWL-2012-Z003, CDJZR14468801, CDJKXB14011, 2015CDJXY).
Footnotes
Author Contributions W.X. and F.Z. designed research. G.Z., W.X. and F.Z. performed research. G.Z., W.X., P.W., F.Y., B.L., X.L., Y.L., X.Y. and F.Z. analyzed the data. G.Z., W.X. and F.Z. wrote the manuscript. All authors reviewed the manuscript.
References
- Weintraub D. et al. Atomoxetine for depression and other neuropsychiatric symptoms in Parkinson disease. Neurology 75, 448–455 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajos M. et al. The selective norepinephrine reuptake inhibitor antidepressant reboxetine: pharmacological and clinical profile. CNS. Drug. Rev. 10, 23–44 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanderWeide L. A., Smith S. M. & Trinkley K. E. A systematic review of the efficacy of venlafaxine for the treatment of fibromyalgia. J. Clin. Pharm. Ther. 40, 1–6 (2015). [DOI] [PubMed] [Google Scholar]
- Shao L. et al. Triple reuptake inhibitors: a patent review (2006-2012). Expert Opin. Ther. Pat. 24, 131–154 (2014). [DOI] [PubMed] [Google Scholar]
- Thapar A. & Cooper M. Attention deficit hyperactivity disorder. Lancet 387, 1240–1250 (2016). [DOI] [PubMed] [Google Scholar]
- Hannestad J. et al. Clinically relevant doses of methylphenidate significantly occupy norepinephrine transporters in humans in vivo. Biol. Psychiatry. 68, 854–860 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson N. et al. Estimation of binding rate constants using a simultaneous mixed-effects method: application to monoamine transporter reuptake inhibitor reboxetine. Br. J. Pharmacol. 160, 389–398 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artigas F. Future directions for serotonin and antidepressants. ACS. Chem. Neurosci. 4, 5–8 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papakostas G. I. Managing partial response or nonresponse: switching, augmentation, and combination strategies for major depressive disorder. J. Clin. Psychiatry. 70 Suppl 6, 16–25 (2009). [DOI] [PubMed] [Google Scholar]
- Vu A. T. et al. 1-(Indolin-1-yl)-1-phenyl-3-propan-2-olamines as potent and selective norepinephrine reuptake inhibitors. J. Med. Chem. 53, 2051–2062 (2010). [DOI] [PubMed] [Google Scholar]
- Zhang P. et al. 1- or 3-(3-Amino-2-hydroxy-1-phenyl propyl)-1,3-dihydro-2H-benzimidazol-2-ones: potent, selective, and orally efficacious norepinephrine reuptake inhibitors. J. Med. Chem. 52, 5703–5711 (2009). [DOI] [PubMed] [Google Scholar]
- Millan M. J. Dual- and triple-acting agents for treating core and co-morbid symptoms of major depression: novel concepts, new drugs. Neurotherapeutics. 6, 53–77 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan A. et al. Aurora-A kinase inhibitor scaffolds and binding modes. Drug Discov. Today 16, 260–269 (2011). [DOI] [PubMed] [Google Scholar]
- Krishnan V. & Nestler E. J. The molecular neurobiology of depression. Nature 455, 894–902 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H. et al. Therapeutic target database update 2016: enriched resource for bench to clinical drug target and targeted pathway information. Nucleic. Acids. Res. 44, D1069–1074 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J. et al. Corticotropin releasing factor up-regulates the expression and function of norepinephrine transporter in SK-N-BE (2) M17 cells. J. Neurochem. 135, 38–49 (2015). [DOI] [PubMed] [Google Scholar]
- Zhu F. et al. Update of TTD: Therapeutic Target Database. Nucleic. Acids. Res. 38, D787–791 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita A. et al. Crystal structure of a bacterial homologue of Na+/Cl-dependent neurotransmitter transporters. Nature 437, 215–223 (2005). [DOI] [PubMed] [Google Scholar]
- Singh S. K. et al. A competitive inhibitor traps LeuT in an open-to-out conformation. Science 322, 1655–1661 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamurthy H. & Gouaux E. X-ray structures of LeuT in substrate-free outward-open and apo inward-open states. Nature 481, 469–474 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S. K., Yamashita A. & Gouaux E. Antidepressant binding site in a bacterial homologue of neurotransmitter transporters. Nature 448, 952–956 (2007). [DOI] [PubMed] [Google Scholar]
- Malinauskaite L. et al. A mechanism for intracellular release of Na+ by neurotransmitter/sodium symporters. Nat. Struct. Mol. Biol. 21, 1006–1012 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penmatsa A., Wang K. H. & Gouaux E. X-ray structures of Drosophila dopamine transporter in complex with nisoxetine and reboxetine. Nat. Struct. Mol. Biol. 22, 506–508 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penmatsa A., Wang K. H. & Gouaux E. X-ray structure of dopamine transporter elucidates antidepressant mechanism. Nature 503, 85–90 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen L. et al. Interaction of antidepressants with the serotonin and norepinephrine transporters: mutational studies of the S1 substrate binding pocket. J. Biol. Chem. 287, 43694–43707 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen J. et al. Recent advances in the understanding of the interaction of antidepressant drugs with serotonin and norepinephrine transporters. Chem. Commun. 25, 3677–3692 (2009). [DOI] [PubMed] [Google Scholar]
- Andersen J. et al. Molecular determinants for selective recognition of antidepressants in the human serotonin and norepinephrine transporters. Proc. Natl. Acad. Sci. USA 108, 12137–12142 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koldso H., Grouleff J. & Schiott B. Insights to ligand binding to the monoamine transporters-from homology modeling to LeuBAT and dDAT. Front. Pharmacol. 6, 208 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen J. et al. Binding of the multimodal antidepressant drug vortioxetine to the human serotonin transporter. ACS. Chem. Neurosci. 6, 1892–1900 (2015). [DOI] [PubMed] [Google Scholar]
- Zhou Z. L. et al. Combining structure-based pharmacophore and in silico approaches to discover novel selective serotonin reuptake inhibitors. Chem. Biol. Drug Des. 82, 705–717 (2013). [DOI] [PubMed] [Google Scholar]
- Severinsen K. et al. Binding of the amphetamine-like 1-phenyl-piperazine to monoamine transporters. ACS. Chem. Neurosci. 3, 693–705 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinning S. et al. Binding and orientation of tricyclic antidepressants within the central substrate site of the human serotonin transporter. J. Biol. Chem. 285, 8363–8374 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X., Gu H. H. & Zhan C. G. Mechanism for cocaine blocking the transport of dopamine: insights from molecular modeling and dynamics simulations. J. Phys. Chem. B. 113, 15057–15066 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrest L. R. et al. Mechanism for alternating access in neurotransmitter transporters. Proc. Natl. Acad. Sci. USA 105, 10338–10343 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celik L. et al. Binding of serotonin to the human serotonin transporter. Molecular modeling and experimental validation. J. Am. Chem. Soc. 130, 3853–3865 (2008). [DOI] [PubMed] [Google Scholar]
- Jorgensen A. M. et al. Molecular dynamics simulations of Na+/Cl--dependent neurotransmitter transporters in a membrane-aqueous system. ChemMedChem. 2, 827–840 (2007). [DOI] [PubMed] [Google Scholar]
- Gabrielsen M. et al. Identification of novel serotonin transporter compounds by virtual screening. J. Chem. Inf. Model. 54, 933–943 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlessinger A. et al. Structure-based discovery of prescription drugs that interact with the norepinephrine transporter, NET. Proc. Natl. Acad. Sci. USA 108, 15810–15815 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P. et al. Identification of dual active agents targeting 5-HT1A and SERT by combinatorial virtual screening methods. Biomed. Mater. Eng. 26 Suppl 1, S2233–2239 (2015). [DOI] [PubMed] [Google Scholar]
- Andersen J. et al. Molecular basis for selective serotonin reuptake inhibition by the antidepressant agent fluoxetine (Prozac). Mol. Pharmacol. 85, 703–714 (2014). [DOI] [PubMed] [Google Scholar]
- Koldso H. et al. The two enantiomers of citalopram bind to the human serotonin transporter in reversed orientations. J. Am. Chem. Soc. 132, 1311–1322 (2010). [DOI] [PubMed] [Google Scholar]
- Ahlqvist M. et al. Software-aided structural elucidation in drug discovery. Rapid Commun. Mass. Spectrom. 29, 2083–2089 (2015). [DOI] [PubMed] [Google Scholar]
- Mallipeddi P. L. et al. Recent advances in computer-aided drug design as applied to anti-influenza drug discovery. Curr. Top. Med. Chem. 14, 1875–1889 (2014). [DOI] [PubMed] [Google Scholar]
- Xue W., Liu H. & Yao X. Molecular modeling study on the allosteric inhibition mechanism of HIV-1 integrase by LEDGF/p75 binding site inhibitors. PLos One 9, e90799 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue W. et al. Identification of the inhibitory mechanism of FDA approved selective serotonin reuptake inhibitors: an insight from molecular dynamics simulation study. Phys. Chem. Chem. Phys. 18, 3260–3271 (2016). [DOI] [PubMed] [Google Scholar]
- Gabrielsen M. et al. Substrate binding and translocation of the serotonin transporter studied by docking and molecular dynamics simulations. J. Mol. Model. 18, 1073–1085 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grouleff J. et al. Monoamine transporters: insights from molecular dynamics simulations. Front. Pharmacol. 6, 235 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold K. et al. The SWISS-MODEL workspace: a web-based environment for protein structure homology modelling. Bioinformatics. 22, 195–201 (2006). [DOI] [PubMed] [Google Scholar]
- Laskowski R. A. et al. PROCHECK: a program to check the stereochemical quality of protein structures. J. Appl. Crystallogr. 26, 283–291 (1993). [Google Scholar]
- The PyMOL Molecular Graphics System v. 1.3 (Schrödinger, LLC, New York, NY, 2010). URL: http://www.pymol.org/.
- Jo S. et al. CHARMM-GUI Membrane Builder for mixed bilayers and its application to yeast membranes. Biophys. J. 97, 50–58 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen W. L. et al. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 79, 926–935 (1983). [Google Scholar]
- AMBER v. 14 (University of California, San Francisco, 2014). URL: http://ambermd.org/.
- Hornak V. et al. Comparison of multiple Amber force fields and development of improved protein backbone parameters. Proteins 65, 712–725 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson C. J. et al. Lipid14: the amber lipid force field. J. Chem. Theory Comput. 10, 865–879 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joung I. S. & Cheatham T. E. Determination of alkali and halide monovalent ion parameters for use in explicitly solvated biomolecular simulations. J. Phys. Chem. B. 112, 9020–9041 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. et al. Development and testing of a general amber force field. J. Comput. Chem. 25, 1157–1174 (2004). [DOI] [PubMed] [Google Scholar]
- Bayly C. I. et al. A well-behaved electrostatic potential based method using charge restraints for deriving atomic charges: the RESP model. J. Phys. Chem. 97, 10269–10280 (1993). [Google Scholar]
- Wang J. et al. Automatic atom type and bond type perception in molecular mechanical calculations. J. Mol. Graph. Model. 25, 247–260 (2006). [DOI] [PubMed] [Google Scholar]
- Gaussian 09v. D.01 (Gaussian, Inc., Wallingford CT, 2009). URL: http://www.gaussian.com/index.htm/.
- Hara Y. & Murayama S. Effects of analgesic-antipyretics on the spinal reflex potentials in cats: an analysis of the excitatory action of aminopyrine. Nihon Yakurigaku Zasshi. 100, 383–390 (1992). [DOI] [PubMed] [Google Scholar]
- Springborg M. & Kirtman B. Efficient vector potential method for calculating electronic and nuclear response of infinite periodic systems to finite electric fields. J. Chem. Phys. 126, 104107 (2007). [DOI] [PubMed] [Google Scholar]
- Kollman P. A. et al. Calculating structures and free energies of complex molecules: combining molecular mechanics and continuum models. Acc. Chem. Res. 33, 889–897 (2000). [DOI] [PubMed] [Google Scholar]
- Massova I. & Kollman P. Combined molecular mechanical and continuum solvent approach (MM-PBSA/GBSA) to predict ligand binding. Perspect. Drug Discov. Des. 18, 113–135 (2000). [Google Scholar]
- Weiser J., Shenkin P. S. & Still W. C. Approximate atomic surfaces from linear combinations of pairwise overlaps (LCPO). J. Comput. Chem. 20, 217–230 (1999). [Google Scholar]
- Tippmann S. Programming tools: adventures with R. Nature 517, 109–110 (2015). URL: http://www.R-project.org/. [DOI] [PubMed] [Google Scholar]
- Barer M. R. & Harwood C. R. Bacterial viability and culturability. Adv. Microb. Physiol. 41, 93–137 (1999). [DOI] [PubMed] [Google Scholar]
- Letunic I. & Bork P. Interactive Tree Of Life (iTOL): an online tool for phylogenetic tree display and annotation. Bioinformatics 23, 127–128 (2007). URL: http://itol.embl.de/. [DOI] [PubMed] [Google Scholar]
- Bymaster F. P. et al. Atomoxetine increases extracellular levels of norepinephrine and dopamine in prefrontal cortex of rat: a potential mechanism for efficacy in attention deficit/hyperactivity disorder. Neuropsychopharmacology 27, 699–711 (2002). [DOI] [PubMed] [Google Scholar]
- Tatsumi M. et al. Pharmacological profile of antidepressants and related compounds at human monoamine transporters. Eur. J. Pharmacol. 340, 249–258 (1997). [DOI] [PubMed] [Google Scholar]
- Kung H. F. et al. 2-(2-(dimethylaminomethyl)phenoxy)-5-iodophenylamine: an improved serotonin transporter imaging agent. J. Med. Chem. 47, 5258–5264 (2004). [DOI] [PubMed] [Google Scholar]
- Wang J. et al. Use of MM-PBSA in reproducing the binding free energies to HIV-1 RT of TIBO derivatives and predicting the binding mode to HIV-1 RT of efavirenz by docking and MM-PBSA. J. Am. Chem. Soc. 123, 5221–5230 (2001). [DOI] [PubMed] [Google Scholar]
- Genheden S. & Ryde U. The MM/PBSA and MM/GBSA methods to estimate ligand-binding affinities. Expert Opin. Drug Discov. 10, 449–461 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou T. et al. Assessing the performance of the MM/PBSA and MM/GBSA methods. 1. The accuracy of binding free energy calculations based on molecular dynamics simulations. J. Chem. Inf. Model. 51, 69–82 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes C. M. & Kollman P. A. Structure and thermodynamics of RNA-protein binding: using molecular dynamics and free energy analyses to calculate the free energies of binding and conformational change. J. Mol. Biol. 297, 1145–1158 (2000). [DOI] [PubMed] [Google Scholar]
- Andersen J. et al. Binding site residues control inhibitor selectivity in the human norepinephrine transporter but not in the human dopamine transporter. Sci. Rep. 5, 15650 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H. et al. Structural basis for action by diverse antidepressants on biogenic amine transporters. Nature 503, 141–145 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.