Abstract
A conformational restriction strategy was used to design and synthesize nine TZT-1027 analogues. 3-Aryl-azetidine moiety was used to replace phenylethyl group of TZT-1027 at the C-terminus. These analogues exhibited moderate to excellent antiproliferative activities, and the most potent compound 1a showed IC50 values of 2.2 nM against A549 and 2.1 nM against HCT116 cell lines, respectively. However, 1a could not achieve effective inhibition at all the dose levels in the A549 xenograft model (up to 5 mg/kg, injection, once a day), which is only 16%–35% inhibition at the end of the experiment.
Keywords: TZT-1027, azetidine, conformation restriction, antiproliferative activity
1. Introduction
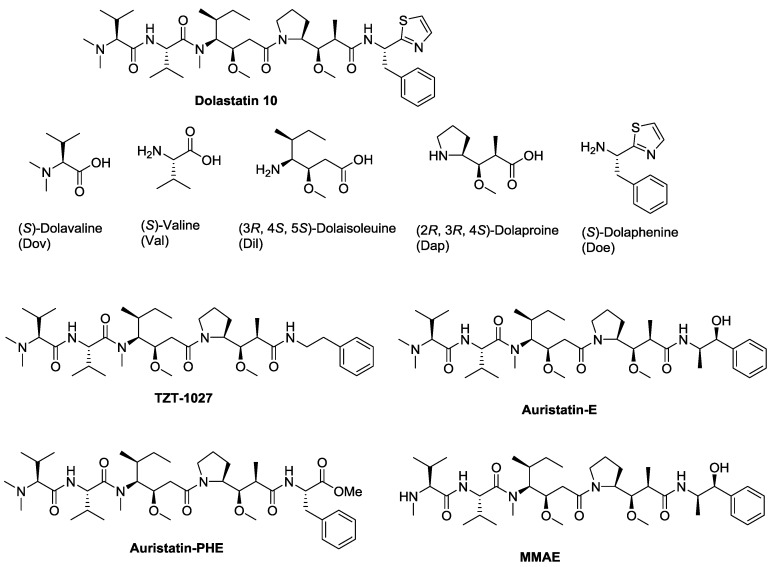
Dolastatin 10 and its natural analogues are highly-cytotoxic peptides isolated from the sea hare Dolabella auricularia from the India Ocean [1]. These compounds have been demonstrated to be effective against a broad spectrum of cancer cells [2]. The extraordinary cytotoxicity is caused by their ability to inhibit microtubule assembly and tubulin-dependent guanosine triphosphate (GTP) hydrolysis, which result in cell cycle arrest and apoptosis [3]. A large number of synthetic analogues of dolastatin 10 have been reported [4,5,6]. Some of them, such as TZT-1027, auristatin E, and auristatin PHE were advanced into clinical trials (Figure 1). However, significant side effects were observed in clinical trials at dose levels that were not sufficient to attain clinical efficacy [7,8]. MMAE, a monomethyl analog of Auristatin-E, was conjugated to monoclonal antibodies, leading to the discovery of the FDA approved ADC brentuximab vedotin (ADCETRIS) for the treatment of relapsed Hodgkin lymphoma and systemic anaplastic large cell lymphoma [9].
Figure 1.
Structures of dolastatin 10 and its representative analogues.
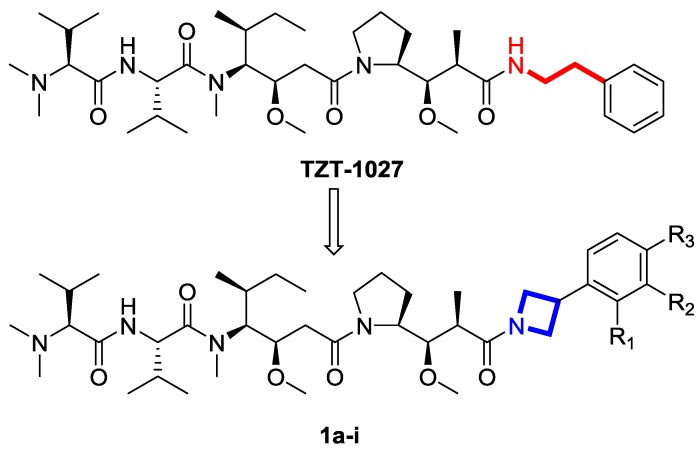
Conformational study of dolastatin 10 analogues bound to tubulin revealed a compact structure that folded around the central Val-Dil bond in its cis form, whereas the flexible C-terminus does not interact with any amino acid residue directly, indicating that its main role might be arranging the molecule’s overall orientation [10,11]. Here we introduced azetidine moiety into C-terminus of TZT-1027 to explore the effect of conformational restriction on potency (Figure 2) [12]. Thus, nine conformational restricted analogues were synthesized and evaluated for inhibitory effects.
Figure 2.
Designed target compounds.
2. Results and Discussion
2.1. Chemistry
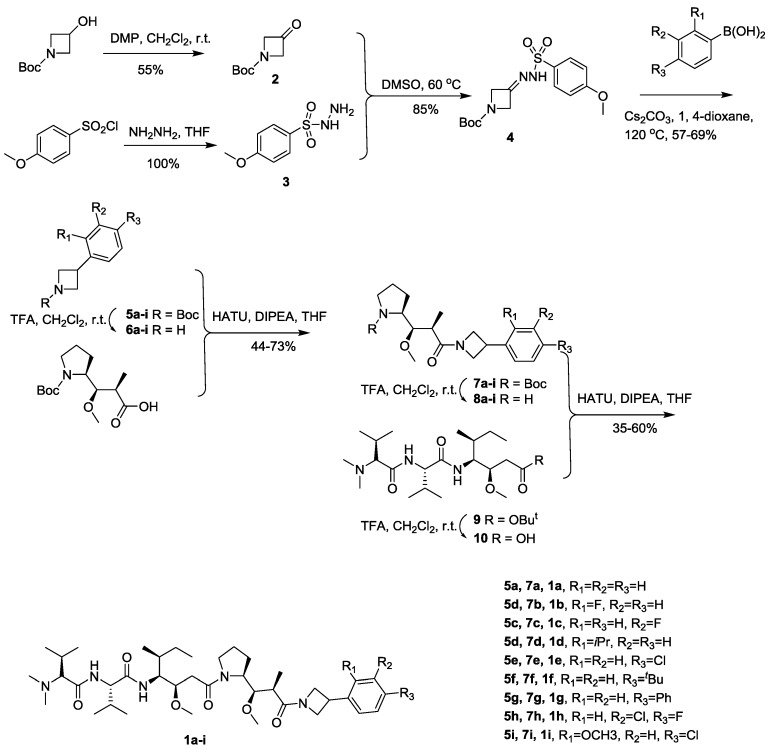
The synthetic route is outlined in Scheme 1. 3-Aryl-azetidines 5a–i were prepared according to known procedure [13]. Removal of the Boc group with trifluoroacetic acid (TFA) yielded the TFA salts 6a–i, which were coupled with N-Boc-(2R, 3R, 4S)-dolaproine (Dap) in the presence of HATU to give compounds 7a–i. Removal of the Boc group with TFA in 7a–i yielded the TFA salts 8a–i, which were coupled with Dov-Val-Dil·TFA (9) in the presence of HATU to provide the title compounds [5].
Scheme 1.
Synthetic route of target compounds.
2.2. In Vitro Antiproliferative Assay
As shown in Table 1, these analogues demonstrated moderate to excellent antiproliferative activities. Among them, compound 1a was the most potent with IC50 values of 2.2 nM against A549 cell lines and 2.1 nM against HCT116 cell lines. Structure-activity relationship could not be well illustrated due to a limited set of compounds. Basically, different substitutions on the phenyl group such as ortho-fluor (1b), meta-fluor (1c), para-chloro (1e), para-tert-butyl (1f), and para-phenyl (1g) could not improve the antiproliferative activities. These compounds resulted in about 20–30-fold loss of potency against A549 cell lines. When a bulky isopropyl group was introduced to the ortho-position of phenyl group, inhibitory activity was reduced about 60-folds (1d). All the target compounds showed weaker activity than TZT1027, indicating that conformational restriction at the C-terminus may not be beneficial to the activity. Membrane permeability can be a limiting factor for potency. The permeability data of synthesized compounds were not measured but we hypothesized that different substitutes of C-terminus could influence permeability, hence the antiproliferative activities. In addition, all the compounds showed better activity in HCT116 cell lines over A549 cell lines, demonstrating a cell selectivity. This is because HCT116 cells demonstrated a rapid proliferation rate than A549 cells and it is known that cytotoxic cancer drugs are believed to gain selectivity by targeting cells that proliferate rapidly.
Table 1.
IC50 values of compounds against A549 and HCT116 (MTT assay).
| Compounds | A549 (nM ± SD) a | HCT116(nM ± SD) a |
|---|---|---|
| 1a | 2.2 ± 4.8 | 2.1 ± 0.4 |
| 1b | 47.0 ± 9.9 | 2.3 ± 0.2 |
| 1c | 35.0 ± 0.6 | 4.6 ± 0.7 |
| 1d | 130.1 ± 48.3 | 25.5 ± 0.2 |
| 1e | 19.5 ± 1.7 | 15.5 ± 1.8 |
| 1f | 56.0 ± 3.3 | 3.7 ± 0.3 |
| 1g | 41.5 ± 13.3 | 3.1 ± 0.8 |
| 1h | 39.3 ± 2.4 | 8.3 ± 0.6 |
| 1i | 8.7 ± 3.7 | 3.5 ± 0.9 |
| TZT-1027 | 0.2 ± 0.06 | 0.3 ± 0.2 |
| Docetaxel | 23.5 ± 9.5 | 0.3 ± 0.1 |
a The data were means from at least three independent experiments.
2.3. Inhibitory Activity of Compound 1a in A549 Xenograft Model
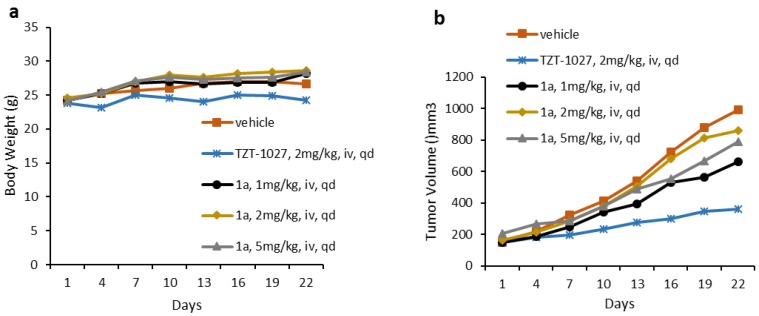
Further in vivo antitumor activities of 1a was evaluated in A549 xenograft models in mice via tail vein intravenous injection for 22 days. It is reported that a dose of 4 mg/kg of TZT-1027 seemed to be toxic [14,15]. Considering of that, the maximum dose of 1a was chosen as 5 mg/kg. After given 1a at 1 mg/kg/day, 2 mg/kg/day, and 5 mg/kg/day dosages, no overt toxicity and weight-loss were observed. However, compound 1a could not achieve effective inhibition at all the dose levels (Figure 3b). TZT-1027 (2 mg/kg/day) inhibited tumor growth by 61% over the 22-day administration schedule, however 1a only inhibited tumor growth by 16%–35% at difference dose (Supplementary Materials, Tables S1–S3). No time- and dosage-dependent inhibition were observed. Higher dosage of 1a was not explored due to its poor solubility (Supplementary Materials, Table S4). Pharmacokinetic (PK) study was not conducted because in a mouse liver microsomes metabolic stability study, compound 1a demonstrated a T1/2 of less than 2 min (Supplementary Materials, Table S5). The synthesis of analogues suitable for formulation is of considerable interest and this work will be reported in due course.
Figure 3.
Antitumor activity of 1a in A549 xenograft mice at different dosages. (a) Body weight and (b) tumor volume were measured on the indicated days after treated with vehicle or 1a once a day.
3. Experimental Section
3.1. Chemistry
3.1.1. General
All starting materials, reagents, and solvents were commercially available. All reactions were monitored by thin-layer chromatography on silica gel plates (GF-254) and visualized with UV light. All the melting points were determined on a micromelting-point apparatus and thermometer was uncorrected. 1H-NMR spectra and 13C-NMR were recorded in acetone-d6 or CDCl3 on a 400 or 600 Bruker NMR spectrometer with tetramethylsilane (TMS) as an internal reference. All chemical shifts are reported in parts per million (ppm). High-resolution exact mass measurements were performed using electrospray ionization (positive mode) on a quadrupole time-of-flight (QTOF) mass spectrometer (Maxis Q-TOF, Bruker Inc., Billerica, MA, USA).
3.1.2. General Synthesis for 3-Aryl-Azetidines 5a–i
To a solution of sulfonyl chloride (1.0 equiv) in THF (0.2 M) at 0 °C was added hydrazine hydrate (2.5 equiv) dropwise. The reaction mixture was stirred at 0 °C until complete conversion was observed by thin-layer chromatography. The mixture was diluted with EtOAc, washed with brine, dried over Na2SO4 and solvents removed in vacuo to give sulfonylhydrazides. To a solution of sulfonylhydrazones (1.0 equiv) in MeOH (0.5 M) was added ketone (1.0 equiv). The reaction mixture was stirred at room temperature until complete conversion was observed by TLC. Solvents were removed in vacuo to give sulfonylhydrazones. Sulfonylhydrazone (0.5 mmol, 1.0 equiv), boronic acid (0.75 mmol, 1.5 equiv), and cesium carbonate (0.75 mmol, 1.5 equiv) were placed in an oven-dried tube in vacuo for 30 min. The tube was backfilled with argon followed by the addition of dry degassed 1,4-dioxane (2 mL, 0.25 M). This tube was sealed and heated to 110 °C for 18 h before being cooled to room temperature, quenched with NaHCO3 (2 mL of a saturated aqueous solution), and extracted with CH2Cl2 (3 × 5 mL). The organic phase was dried over MgSO4, and solvents were removed in vacuo to give a residue, which was purified by flash column chromatography (10%−30% EtOAc/hexane) to give the title compounds.
tert-Butyl 3-phenylazetidine-1-carboxylate (5a). Colorless oil; yield 57%; 1H-NMR (400 MHz, CDCl3) δ 7.39–7.29 (m, 4H), 7.27–7.23 (m, 1H), 4.33 (t, J = 8.6 Hz, 2H), 3.98 (t, J = 8.6 Hz, 2H), 3.73 (tt, J = 8.6, 6.0 Hz, 1H), 1.47 (s, 3H); 13C-NMR (150 MHz, CDCl3) δ 156.5, 142.3, 128.8, 127.0, 126.8, 79.6, 33.6, 28.5; HRMS (ESI) calcd for C14H19NO2Na: 256.1308, found: 256.1307.
tert-Butyl 3-(2-fluorophenyl)azetidine-1-carboxylate (5b). Colorless oil, yield 69%; 1H-NMR (400 MHz, CDCl3) δ 7.31 (d, J = 7.8 Hz, 1H), 7.08 (d, J = 7.7 Hz, 1H), 7.02 (d, J = 9.9 Hz, 1H), 6.96 (t, J = 8.3 Hz, 1H), 4.33 (t, J = 8.7 Hz, 2H), 3.99–3.92 (m, 2H), 3.72–3.70 (m, 1H), 1.47 (s, 9H); 13C-NMR (150 MHz, CDCl3) δ 161.7, 160.1, 156.5, 128.8, 128.7, 128.6, 128.6, 128.0, 127.9, 124.4, 115.6, 115.4, 79.6, 29.8, 28.5, 27.6, 27.5; HRMS (ESI) calcd for C14H18NO2FNa: 274.1214, found: 256.1215.
tert-Butyl 3-(3-fluorophenyl)azetidine-1-carboxylate (5c). Colorless oil, yield 62%; 1H-NMR (400 MHz, CDCl3) δ 7.31 (td, J = 7.9, 6.1 Hz, 1H), 7.08 (d, J = 7.7 Hz, 1H), 7.03 (dt, J = 10.0, 2.1 Hz, 1H), 6.96 (td, J = 8.6, 2.9 Hz, 1H), 4.33 (t, J = 8.7 Hz, 2H), 3.96 (dd, J = 8.6, 5.9 Hz, 2H), 3.77–3.68 (m, 1H), 1.42 (s, 9H); 13C-NMR (150 MHz, CDCl3) δ 163.2, 161.6, 155.7, 144.1, 144.1, 129.7, 129.6, 129.5, 121.7, 121.7, 113.3, 113.2, 113.1, 79.1, 76.5, 76.3, 76.1, 32.6, 27.7, 27.6; HRMS (ESI) calcd for C14H18NO2FNa: 274.1214, found: 256.1215.
tert-Butyl 3-(2-isopropylphenyl)azetidine-1-carboxylate (5d). Colorless oil, yield 57%; 1H-NMR (400 MHz, CDCl3) δ 7.45–7.39 (m, 1H), 7.31–7.20 (m, 3H), 4.31 (t, J = 8.0 Hz, 2H), 4.13–4.05 (m, 1H), 4.03 (m, 2H), 1.46 (s, 10H), 1.21 (s, 3H), 1.19 (s, 3H); 13C-NMR (150 MHz, CDCl3) δ 156.6, 146.6, 138.3, 127.2, 126.3, 125.7, 125.4, 79.6, 29.8, 29.1, 28.5, 23.9; HRMS (ESI) calcd for C17H25NO2Na: 298.1778, found: 258.1777.
tert-Butyl 3-(4-chlorophenyl)azetidine-1-carboxylate (5e). Colorless oil, yield 50%; 1H-NMR (400 MHz, CDCl3) δ 7.30 (d, J = 8.1 Hz, 2H), 7.22 (d, J = 8.1 Hz, 2H), 4.31 (t, J = 8.7 Hz, 2H), 3.91 (dd, J = 8.6, 5.8 Hz, 2H), 3.72–3.63 (m, 1H), 1.46 (s, 9H); 13C-NMR (150 MHz, CDCl3) δ 161.0, 159.3, 155.7, 127.9, 127.8, 127.2, 127.2, 123.7, 123.6, 114.8, 114.7, 78.9, 27.7; HRMS (ESI) calcd for C14H18ClNO2Na: 290.0918, found: 290.0916.
tert-Butyl 3-(4-(tert-butyl)phenyl)azetidine-1-carboxylate (5f). Colorless oil, yield 53%; 1H-NMR (400 MHz,CDCl3) δ 7.38 (d, J = 8.3 Hz, 2H), 7.25 (d, J = 8.1 Hz, 2H), 4.31 (t, J = 8.6 Hz, 2H), 4.02–3.93 (m, 2H), 3.71–3.69 (m, 1H), 1.47 (s, 9H), 1.32 (s, 9H); 13C-NMR (150 MHz, CDCl3) δ 155.8, 149.2, 138.5, 125.8, 124.9, 78.8, 33.8, 32.4, 30.7, 27.8; HRMS (ESI) calcd for C18H27NO2Na: 312.1934, found: 312.1936.
tert-Butyl 3-([1,1′-biphenyl]-4-yl)azetidine-1-carboxylate (5g). Colorless oil, yield 66%; 1H-NMR (400 MHz, CDCl3) δ 7.58 (d, J = 7.8 Hz, 4H), 7.44 (t, J = 7.5 Hz, 2H), 7.39–7.32 (m, 3H), 4.35 (t, J = 8.6 Hz, 2H), 4.08–3.97 (m, 2H), 3.78–3.75 (m, 1H), 1.48 (s, 9H); 13C-NMR (150 MHz, CDCl3) δ 155.8, 140.6, 140.0, 139.3, 128.1, 128.1, 126.8, 126.6, 126.5, 126.4, 78.9, 27.8; HRMS (ESI) calcd for C20H23NO2Na: 332.1621, found: 332.1623.
tert-Butyl 3-(3-chloro-4-fluorophenyl)azetidine-1-carboxylate (5h). Colorless oil, yield 55%; 1H-NMR (400 MHz, CDCl3) δ 7.39–7.34 (m, 1H), 7.21–7.15 (m, 1H), 7.12 (t, J = 7.8 Hz, 1H), 4.33 (t, J = 8.7 Hz, 2H), 3.95–3.87 (m, 2H), 3.73–3.63 (m, 1H), 1.47 (s, 9H); 13C-NMR (150 MHz, CDCl3) δ 156.3, 139.3, 129.0, 126.5, 126.45, 121.2, 117.2, 116.8, 116.7, 114.7, 79.8, 56.5, 32.7, 28.4; HRMS (ESI) calcd for C14H17ClFNO2Na: 308.0830, found: 308.0829.
tert-Butyl 3-(4-chloro-2-methoxyphenyl)azetidine-1-carboxylate (5i). Colorless oil, yield 62%; 1H-NMR (400 MHz, CDCl3) δ 6.94 (dd, J = 8.1, 1.8 Hz, 1H), 6.83 (d, J = 1.7 Hz, 1H), 4.25 (t, J = 8.4 Hz, 2H), 4.04–3.86 (m, 3H), 3.80 (s, 3H), 1.45 (s, 9H); 13C-NMR (150 MHz, CDCl3) δ 157.2, 155.9, 132.6, 127.6, 127.1, 119.8, 110.4, 78.7, 54.9, 27.9, 27.8; HRMS (ESI) calcd for C15H20ClNO3Na: 320.1024, found: 320.1024.
3.1.3. General Synthesis for 7a–i
Compounds 5a–i (1 equiv.) were dissolved in 2 mL CH2Cl2/TFA (1:1, v/v) at 0 °C, and the mixture was stirred for 1 h at room temperature. The reaction was then concentrated in vacuum, followed by azeotroping with dichloromethane three times to obtain the trifluoroacetate salts. To a stirring solution of the Dap in dry dichloromethane at 0 °C were sequentially added HATU (1.5 equiv.). After 10 min, the previously prepared trifluoroacetate salts dissolved in dichloromethane was added to reaction mixture followed by the addition of DIPEA (3 equiv.). After stirring for 12 h at room temperature, the reaction mixture was diluted with EtOAc/CH2Cl2, washed with 1M HCl, saturated NaHCO3 solution, water and brine, dried, filtered and concentrated in vacuo. Purification by silica gel column chromatography (EtOAc/Petroleum ether, 1/1) afforded compounds 7a–i.
tert-Butyl (S)-2-((1R,2R)-1-methoxy-2-methyl-3-oxo-3-(3-phenylazetidin-1-yl)propyl)pyrrolidine-1-carboxylate (7a). Colorless oil, yield 57%; 1H-NMR (400 MHz, CDCl3) δ 7.39–7.34 (m, 2H), 7.30–7.29 (m, 3H), 4.58–4.51 (m, 1H), 4.45–4.34 (m, 1H), 4.20–4.01 (m, 2H), 3.91–3.87 (m, 1H), 3.78–3.76 (m, 2H), 3.46 (s, 3H), 3.29–3.23 (m, 1H), 2.41–2.37 (m, 1H), 2.01–1.93 (m, 2H), 1.85–1.72 (m, 1H), 4.52 (d, J = 10.6 Hz, 3H), 1.25 (s, 9H); 13C-NMR (150 MHz, CDCl3) δ 148.9, 139.4, 135.0, 128.5, 128.1, 127.7, 124.8, 124.5, 120.0, 53.5, 50.9, 41.9, 41.5, 28.1, 25.8, 19.5, 18.7, 12.1, 10.8. HRMS (ESI) calcd for C23H35N2O4: 403.2591, found: 403.2597; -35.000 (CHCl3, c = 0.50).
tert-Butyl (S)-2-((1R,2R)-3-(3-(2-fluorophenyl)azetidin-1-yl)-1-methoxy-2-methyl-3-oxopropyl)pyrrolidine-1-carboxylate (7b). Colorless oil, yield 60%; 1H-NMR (400 MHz, CDCl3) δ 7.27–7.10 (m, 1H), 6.96–6.94 (m, 1H), 6.83 (d, J = 1.7 Hz, 1H), 4.50–4.43 (m, 1H), 4.35–4.31 (m, 1H), 4.27–4.24 (m, 1H), 4.19–4.08 (m, 2H), 3.97–3.82 (m, 3H), 3.81 (s, 3H), 3.78–3.74 (m, 1H), 3.26–3.22 (m,1H), 1.95 (s, 3H), 1.92–1.68 (m, 4H), 1.52 (s, 3H), 1.44 (d, J = 7.8 Hz, 3H); 13C-NMR (150 MHz, CDCl3) δ 174.4, 154.6, 129.0, 128.1, 128.0, 124.5, 115.8, 84.2, 84.1, 82.1, 79.9, 79.1, 61.2, 60.8, 59.5, 59.3, 58.9, 56.4, 53.5, 53.3, 47.0, 46.7, 39.2, 38.4, 28.7, 28.6, 27.8, 27.4, 26.2, 26.1, 25.7, 24.7, 24.6, 24.3, 14.7, 14.6, 13.8; HRMS (ESI) calcd for C23H33N2O4FNa: 443.2317, found: 443.2318; -42.000 (CHCl3, c = 0.50).
tert-Butyl (S)-2-((1R,2R)-3-(3-(3-fluorophenyl)azetidin-1-yl)-1-methoxy-2-methyl-3-oxopropyl)pyrrolidine-1-carboxylate (7c). Colorless oil, yield 55%; 1H-NMR (400 MHz, CDCl3) 7.31–7.27 (m, 1H), 7.07 (d, J = 7.4 Hz, 1H), 7.02–6.97 (m, 2H), 4.64–4.49 (m, 2H), 4.40–4.35 (m, 1H), 4.26–4.03 (m, 1H), 4.02–3.96 (m, 1H), 3.94–3.84 (m, 1H), 3.83–3.74 (m, 1H), 3.64–3.50 (m, 1H), 3.45 (s, 3H), 3.30–3.21 (m, 2H), 1.99–1.91 (m, 3H), 1.89–1.72 (m, 2H), [1.52 (s), 1.47 (s), 1.44 (s), total 9H], 1.24 (d, J = 6.9 Hz, 3H); 13C-NMR (150 MHz, CDCl3) δ 163.9, 163.9, 162.3, 162.2, 154.5, 130.4, 122.3, 114.1, 113.6, 113.5, 84.3, 84.1, 82.6, 82.3, 79.8, 79.1, 61.0, 60.7, 60.4, 58.7, 57.4, 57.4, 54.8, 54.6, 46.9, 46.8, 38.5, 32.8, 28.6, 26.2, 24.1, 14.5, 14.2; HRMS (ESI) calcd for C23H33N2O4FNa: 443.2317, found: 443.2321; -66.200 (CHCl3, c = 0.50).
tert-Butyl (S)-2-((1R,2R)-3-(3-(2-isopropylphenyl)azetidin-1-yl)-1-methoxy-2-methyl-3-oxopropyl)pyrrolidine-1-carboxylate (7d). Colorless oil, yield 65%; 1H-NMR (400 MHz, CDCl3) 7.37–7.35 (m, 1H), 7.28–7.24 (m, 3H), 4.56–4.51 (m, 1H), 4.43–4.34 (m, 1H), 4.22–4.07 (m, 3H), 3.88–3.77 (m, 3H), 3.57–3.55 (m, 1H), [3.45 (s) and 3.44 (s), total 3H], 3.26–3.23 (m, 1H), 3.01–2.93 (m, 1H), 2.50–2.39 (m, 1H), 1.95–1.72 (m, 6H), [1.51 (s), 1.47 (s) and 1.43 (s), total 9H], 1.25–1.19 (m, 9H); 13C-NMR (150 MHz, CDCl3) δ 173.6, 173.3, 153.9, 145.9, 137.2, 126.8, 125.6, 124.8, 124.6, 124.5, 83.6, 83.4, 81.9, 81.8, 79.2, 78.4, 60.5, 58.2, 58.1, 56.3, 56.2, 53.6, 53.4, 46.3, 46.0, 38.5, 37.9, 29.0, 28.5, 28.4, 28.0, 27.9, 25.5, 23.5, 23.2, 23.1, 13.9; HRMS (ESI) calcd for C26H41N2O4: 445.3061, found: 445.3065; -46.200 (CHCl3, c = 0.50).
tert-Butyl (S)-2-((1R,2R)-3-(3-(4-chlorophenyl)azetidin-1-yl)-1-methoxy-2-methyl-3-oxopropyl)pyrrolidine-1-carboxylate (7e). Colorless oil, yield 45%; 1H-NMR (400 MHz, CDCl3) 7.33–7.32 (m, 2H), 7.24–7.22 (m, 2H), 4.58–4.36 (m, 2H), 4.17–3.75 (m, 5H), 3.60–3.55 (m, 1H), [3.45 (s) and 3.44 (s), total 3H], 3.30–3.24 (m, 1H), 2.47–2.40 (m, 1H), 1.95–1.74 (m, 6H), [1.52 (s), 1.47 (s) and 1.44 (s), total 9H], 1.23 (d, J = 7.4 Hz, 3H); 13C-NMR (150 MHz, CDCl3) δ 173.9, 173.6, 173.4, 153.8, 139.6, 139.4, 132.4, 128.3, 127.3, 83.6, 83.4, 81.9, 81.7, 79.2, 78.4, 60.4, 60.1, 58.1, 56.9, 54.2, 54.1, 46.3, 46.0, 37.9, 37.8, 31.9, 29.0, 28.0, 25.6, 23.5; HRMS (ESI) calcd for C23H33ClN2O4Na: 459.2021, found: 459.2024; -46.600 (CHCl3, c = 0.50).
tert-Butyl (S)-2-((1R,2R)-3-(3-(4-(tert-butyl)phenyl)azetidin-1-yl)-1-methoxy-2-methyl-3-oxopropyl)pyrrolidine-1-carboxylate (7f). Colorless oil, yield 73%; 1H-NMR (400 MHz, CDCl3) δ 7.39 (d, J = 6.8 Hz, 2H), 7.24 (d, J = 8.1 Hz, 2H), 4.56–4.51 (m, 1H), 4.43–4.32 (m, 1H), 4.17–4.01 (m, 3H), 3.89–3.74 (m, 3H), 3.60–3.55 (m, 1H), [3.46 (s) and 3.45 (s), total 3H], 3.30–3.25 (m, 1H), 2.47–2.39 (m, 2H), 1.98–1.74 (m, 6H), [1.52 (s), 1.47 (s) and 1.45 (s), total 9H], [1.32 (s) and 1.31 (s), total 9H], 1.24 (d, J = 7.4 Hz, 3H); 13C-NMR (150 MHz, CDCl3) δ 173.9, 173.7, 173.6, 173.3, 153.8, 149.6, 149.5, 138.1, 137.9, 125.7, 125.1, 83.6, 83.4, 81.9, 81.8, 79.2, 78.4, 60.50, 58.1, 57.1, 54.4, 54.2, 46.0, 37.8, 33.8, 32.1, 31.9, 30.6, 28.0, 27.9, 25.6, 25.5, 23.6, 13.9; HRMS (ESI) calcd for C27H43N2O4: 4059.3217, found: 459.3220; -73.200 (CHCl3, c = 1).
tert-Butyl (S)-2-((1R,2R)-3-(3-([1,1′-biphenyl]-4-yl)azetidin-1-yl)-1-methoxy-2-methyl-3-oxopropyl)pyrrolidine-1-carboxylate (7g). Colorless oil, yield 45%; 1H-NMR (400 MHz, CDCl3) δ 7.59 (d, J = 7.1 Hz, 4H), 7.45 (t, J = 7.6 Hz, 3H), 7.46–7.33 (m, 3H), 4.60–4.53 (m, 1H), 4.47–4.36 (m, 1H), 4.25–4.04 (m, 3H), 3.89–3.79 (m, 3H), 3.60–3.55 (m, 1H), 3.46 (s, 3H), 3.28–3.25 (m, 1H), 2.53–2.41 (m, 1H), 1.97–1.75 (m, 5H), [1.52 (s), 1.48 (s) and 1.44 (s), total 9H], 1.25 (d, J = 7.4 Hz, 3H); 13C-NMR (150 MHz, CDCl3) δ 173.6, 173.4, 153.8, 139.9, 139.6, 128.2, 126.9, 126.7, 126.3, 83.6, 83.4, 81.9, 81.8, 79.2, 78.5, 60.5, 58.1, 57.1, 57.0, 54.3, 46.0, 37.8, 32.2, 32.1, 28.0, 27.9, 25.6, 23.5, 14.0. HRMS (ESI) calcd for C29H38N2O4: 479.2904, found: 4479.2910; -36.500 (CHCl3, c = 0.50).
tert-Butyl (S)-2-((1R,2R)-3-(3-(3-chloro-4-fluorophenyl)azetidin-1-yl)-1-methoxy-2-methyl-3-oxopropyl)pyrrolidine-1-carboxylate (7h). Colorless oil, yield 63%; 1H-NMR (400 MHz, acetone-d6) δ 7.58–7.54 (m, 1H), 7.39–7.34 (m, 2H), 4.57–4.45 (m, 1H), 4.24–4.02 (m, 4H), 3.88–3.59 (m, 5H), 3.31 (s, 3H), 3.15–3.09 (m, 1H), 1.82–1.64 (m, 6H), 1.39 (s, 9H), 0.06 (d, J = 7.4 Hz, 3H); 13C-NMR (150 MHz, acetone-d6) δ 173.1, 172.9, 156.7, 155.1, 153.1, 140.1, 139.7, 139.7, 129.4, 128.9, 128.7, 127.4, 127.3, 127.2, 119.4, 119.3, 119.2, 116.9, 116.8, 116.7, 83.7, 83.6, 81.8, 81.7, 78.4, 78.0, 60.3, 60.1, 58.6, 58.2, 56.8, 56.4, 54.2, 46.4, 46.2, 31.3, 28.0, 25.4, 24.9, 23.9, 23.4, 14.0; HRMS (ESI) calcd for C23H32ClFN2O4Na: 477.1927, found: 477.1927; -35.000 (CHCl3, c = 0.50).
tert-Butyl (S)-2-((1R,2R)-3-(3-(4-chloro-2-methoxyphenyl)azetidin-1-yl)-1-methoxy-2-methyl-3-oxopropyl)pyrrolidine-1-carboxylate (7i). Colorless oil, yield 44%; 1H-NMR (400 MHz, CDCl3) δ 7.12–7.069 (m, 1H), 6.95–6.92 (m, 1H), 6.85–6.84 (m, 1H), 4.48–4.43 (m, 1H), 4.33–4.05 (m, 3H), 3.96–3.81 (m, 2H), 3.80 (s, 3H), 3.77–3.73 (m, 1H), 3.56–3.54 (m, 1H), [3.44 (s) and 3.43 (s), total 3H], 1.95–1.51 (m, 5H), [1.50 (s), 1.46 (s) and 1.42 (s), total 9H], [1.22 (d, J = 6.9 Hz) and 1.18 (d, J = 6.9 Hz), total 3H]; 13C-NMR (150 MHz, CDCl3) δ 173.6, 157.3, 153.8, 127.3, 127.2, 119.9, 110.6, 83.4, 83.3, 81.8, 79.2, 78.4, 60.5, 58.2, 58.1, 55.2, 54.9, 54.9, 52.0, 51.9, 46.3, 46.0, 37.9, 29.0, 28.0, 27.9, 25.5, 23.5, 14.0, 13.8; HRMS (ESI) calcd for C24H36N2O5: 467.2307, found: 467.2311; -32.300 (CHCl3, c = 0.50).
3.1.4. General Synthesis for 1a–i
Commercially available tripeptide (1 equiv.) and 7a–i (1 equiv.) was dissolved in CH2Cl2/TFA (1:1, v/v). After stirred for 2 h at room temperature, the solvent was removed in vacuum, followed by azeotroping with CH2Cl2 three times. Then the mixture was dissolved in dry CH2Cl2. DIPEA was added until reaction mixture was basic, followed by HATU (1.5 equiv.). After stirring for 12 h at room temperature, the reaction mixture was diluted with EtOAc, washed with 1M HCl, saturated NaHCO3 solution, water and brine, dried, filtered, and concentrated in vacuum. Purification by silica gel column chromatography (CH2Cl2/MeOH, 20/1) afforded the title compounds 1a–i.
(S)-2-((R)-2-(Dimethylamino)-3-methylbutanamido)-N-((3R,4S,5S)-3-methoxy-1-((S)-2-((1R,2R)-1-methoxy-2-methyl-3-oxo-3-(3-phenylazetidin-1-yl)propyl)pyrrolidin-1-yl)-5-methyl-1-oxoheptan-4-yl)-N,3-dimethylbutanamide (1a). Colorless oil, yield 45%; 1H-NMR (400 MHz, CDCl3) 7.40–7.36 (m, 2H), 7.31–7.27 (m, 3H), 6.93 (d, J = 9.1 Hz, 1H), [4.87 (t, J = 7.2 Hz) and 4.79 (t, J = 7.8 Hz), total 1H], 4.66–4.59 (m, 1H), 4.54–4.46 (m, 1H), 4.45–4.40 (m, 1H), 4.37–4.32 (m, 1H), 4.28–4.23 (m, 1H), 4.21–3.77 (m, 11H), [3.43 (s) and 3.41 (s), total 3H], [3.36 (s) and 3.35 (s), total 3H], [3.34 (s) and 3.30 (s), total 3H], 3.14 (d, J = 6.2 Hz, 1H), 3.03–3.02 (m, 2H), 2.63–0.79 (m, 36H); 13C-NMR (150 MHz, CDCl3) 174.4, 147.1, 173.8, 173.7, 173.1, 171.2, 170.2, 169.9, 161.8, 161.7, 160.1, 129.2, 129.1, 129.0, 128.8, 128.4, 128.2, 128.1, 127.9, 127.7, 124.5, 124.4, 116.0, 115.8, 115.7, 115.6, 115.5, 86.4, 82.6, 82.4, 78.3, 77.8, 76.0, 61.9, 61.8, 60.5, 60.4, 59.4, 59.2, 59.0, 58.1, 57.9, 57.8, 56.5, 56.4, 56.2, 56.0, 55.8, 53.8, 53.6, 53.4, 53.2, 47.7, 47.5, 46.6, 46.5, 42.6, 39.4, 38.8, 37.7, 37.4, 35.8, 33.2, 33.1, 32.3, 31.8, 30.9, 28.4, 27.8, 27.7, 27.3, 26.2, 26.0, 25.7, 25.0, 24.9, 24.6, 23.6, 23.5, 20.1, 19.8, 19.5, 17.9, 17.8, 15.8, 15.4, 14.8, 14.6, 13.7, 13.5, 10.8, 10.7, 10.3; HRMS (ESI) calcd for C40H68N5O6: 714.5164, found: 714.5169; -50.200 (MeOH, c = 0.50).
(S)-2-((R)-2-(Dimethylamino)-3-methylbutanamido)-N-((3R,4S,5S)-1-((S)-2-((1R,2R)-3-(3-(2-fluorophenyl)azetidin-1-yl)-1-methoxy-2-methyl-3-oxopropyl)pyrrolidin-1-yl)-3-methoxy-5-methyl-1-oxoheptan-4-yl)-N,3-dimethylbutanamide (1b). Colorless oil, yield 40%; 1H-NMR (400 MHz, acetone-d6) δ 7.45–7.38 (m, 1H), 7.36–7.31 (m 1H), 7.23–7.19 (m, 1H), 7.16–7.11 (m, 1H), 4.80–4.63 (m, 3H), 4.45–4.33 (m, 2H), 4.15–3.93 (m, 4H), 3.68–3.55 (m, 2H), [3.42 (s) and 3.33 (s), total 3H], [3.30 (s) and 3.13 (s), total 3H], 2.75–2.05 (m, 10H), 1.20–0.79 (m, 24H); 13C-NMR (150 MHz, acetone-d6) δ 174.2, 174.2, 172.4, 170.1, 161.2, 159.6, 128.5, 128.4, 128.4, 128.0, 127.9, 127.5, 127.4, 127.3, 125.5, 124.0, 114.8, 114.7, 114.6, 81.4, 81.3, 78.7, 73.9, 60.2, 59.0, 58.9, 56.2, 55.2, 53.8, 52.9, 47.1, 47.0, 41.1, 38.2, 37.2, 36.0, 31.7 30.5, 29.6, 29.1, 27.1, 27.0, 26.6, 25.0, 24.2, 23.4, 18.5, 18.1, 17.6, 17.1, 16.9, 14.8, 13.3; HRMS (ESI) calcd for C40H67FN5O6: 732.5070, found: 732.5088; -35.800 (CHCl3, c = 0.50).
(S)-2-((R)-2-(Dimethylamino)-3-methylbutanamido)-N-((3R,4S,5S)-1-((S)-2-((1R,2R)-3-(3-(3-fluorophenyl)azetidin-1-yl)-1-methoxy-2-methyl-3-oxopropyl)pyrrolidin-1-yl)-3-methoxy-5-methyl-1-oxoheptan-4-yl)-N,3-dimethylbutanamide (1c). Colorless oil, yield 54%; 1H-NMR (400 MHz, CDCl3) δ 7.29–7.23 (m, 2H), 7.19–7.11 (m, 1H), 7.06–6.97 (m, 1H), 4.85–4.74 (m, 1H), 4.60–4.54 (m, 1H), 4.52–4.73 (m, 1H), 4.41–4.34 (m, 1H), 4.23–4.17 (m, 2H), 4.12–3.85 (m, 5H), 3.48–3.45 (m, 2H), [3.41 (s) and 3.40 (s), total 3H], [3.31 (s) and 3.29 (s), total 3H], 3.05 (s, 3H), 2.64–2.38 (m, 4H), 2.37–2.27 (m, 6H), 2.18–1.77 (m, 10H), 1.37–0.76 (m, 21H); 13C-NMR (150 MHz, CDCl3) δ 174.1, 174.0, 172.4, 170.6, 169.9, 169.6, 161.2, 159.6, 159.4, 128.7, 128.3, 128.3, 127.8, 127.2, 127.1, 126.9, 124.1, 123.8, 115.0, 114.9, 114.8, 114.7, 81.3, 79.0, 75.4, 68.9, 60.6, 59.0, 58.9, 58.4, 57.5, 56.8, 56.8, 56.0, 55.6, 53.1, 52.7, 52.6, 47.2, 42.0, 42.0, 38.5, 38.3, 36.2, 32.0, 31.1, 30.8, 30.2, 29.0, 28.6, 27.3, 26.9, 25.0, 24.3, 24.2, 23.8, 23.7, 19.4, 19.3, 19.1, 18.7, 17.6, 17.0, 15.1, 13.7, 13.6; HRMS (ESI) calcd for C40H67FN5O6: 732.5070, found: 732.5078; -35.200 (MeOH, c = 0.50).
(S)-2-((R)-2-(Dimethylamino)-3-methylbutanamido)-N-((3R,4S,5S)-1-((S)-2-((1R,2R)-3-(3-(2-isopropylphenyl)azetidin-1-yl)-1-methoxy-2-methyl-3-oxopropyl)pyrrolidin-1-yl)-3-methoxy-5-methyl-1-oxoheptan-4-yl)-N,3-dimethylbutanamide (1d). Colorless oil, yield 48%; 1H-NMR (400 MHz, CDCl3) δ 7.45–7.40 (m, 1H), 7.33–7.28 (m, 2H), 7.26–7.17 (m, 1H), 5.11–4.69 (m, 3H), 4.35–4.22 (m, 4H), 4.06–4.02 (m, 3H), 3.57–3.55 (m, 2H), [3.40 (s) and 3.38 (s), total 3H), 3.39–3.01 (m, 10H), 2.66–2.45 (m, 8H), 2.20–1.95 (m, 6H), 1.88–1.71 (m, 4H), 1.41–1.35 (m, 2H), 1.21–1.13 (m, 8H), 1.02–0.76 (m, 19H); 13C-NMR (150 MHz, CDCl3) δ 173.9, 173.6, 173.1, 173.0, 172.4, 169.7, 169.6, 169.0, 167.6, 167.5, 159.9, 159.7, 148.0, 145.9, 145.8, 139.2, 137.3, 134.3, 127.3, 126.5, 126.4, 126.4, 125.5, 125.5, 124.8, 124.7, 124.6, 124.6, 124.5, 124.5, 118.8, 117.5, 115.5, 81.56 81.4, 72.6, 72.5, 60.3, 59.8, 59.0, 58.9, 58.3, 56.7, 56.6, 56.3, 56.3, 55.9, 54.0, 53.7, 53.6, 53.4, 46.9, 45.4, 45.3, 40.4, 40.4, 38.3, 38.2, 38.0, 31.8, 29.8, 29.7, 29.0, 28.9, 28.8, 26.8, 26.8, 25.4, 25.3, 25.0, 24.1, 24.0, 23.8, 23.7, 22.8, 22.6, 18.5, 18.4, 18.1, 17.6, 17.5, 17.3, 17.3, 14.8, 13.5, 13.0, 12.7, 9.4; HRMS (ESI) calcd for C43H74N5O6: 778.5453, found: 778.5453; -40.200 (CHCl3, c = 1).
(S)-N-((3R,4S,5S)-1-((S)-2-((1R,2R)-3-(3-(4-Chlorophenyl)azetidin-1-yl)-1-methoxy-2-methyl-3-oxopropyl)pyrrolidin-1-yl)-3-methoxy-5-methyl-1-oxoheptan-4-yl)-2-((R)-2-(dimethylamino)-3-methylbutanamido)-N,3-dimethylbutanamide (1e). Colorless oil, yield 66%; 1H-NMR (400 MHz, CDCl3) δ 7.44–7.37 (m, 4H), 4.83–4.67 (m, 3H), 4.40–4.13 (m, 4H), 4.02–3.83 (m, 4H), 3.60–3.55 (m, 3H), [3.40 (s) and 3.37 (s), total 3H], [3.31 (s) and 3.26 (s), total 3H], 3.22–3.09 (m, 4H), 2.68–2.39 (m, 5H), 2.28 (s, 3H), 2.27 (s, 3H), 2.14–1.80 (m, 10H), 1.20–1.13 (m, 4H), 1.02–0.79 (m, 21H); 13C-NMR (150 MHz, CDCl3) δ 173.2, 172.9, 172.7, 169.7, 169.6, 168.8, 168.7, 141.1, 140.8, 140.6, 140.5, 131.5, 131.4, 128.1, 128.0, 127.9, 127.8, 85.7, 85.5, 81.8, 77.8, 77.6, 77.2, 74.3, 74.2, 60.3, 59.1, 58.6, 58.5, 58.2, 58.1, 56.6, 56.5, 54.2, 54.1, 54.0, 53.9, 53.2, 53.1, 46.5, 46.3, 45.6, 41.0, 38.5, 38.0, 37.9, 36.8, 36.5, 35.2, 31.9, 31.7, 29.9, 28.7, 28.6, 28.5, 28.3, 28.2, 28.1, 27.9, 26.6, 25.5, 25.4, 25.0, 24.9, 24.4, 24.0, 23.7, 22.8, 18.7, 18.5, 18.2, 17.8 17.4, 17.3, 14.8, 14.5, 13.6, 13.5, 12.4, 11.9, 9.5, 9.2; HRMS (ESI) calcd for C40H67ClN5O6: 748.4774, found: 748.4778; -34.500 (CHCl3, c = 0.50).
(S)-N-((3R,4S,5S)-1-((S)-2-((1R,2R)-3-(3-(4-(tert-Butyl)phenyl)azetidin-1-yl)-1-methoxy-2-methyl-3-oxopropyl)pyrrolidin-1-yl)-3-methoxy-5-methyl-1-oxoheptan-4-yl)-2-((R)-2-(dimethylamino)-3-methylbutanamido)-N,3-dimethylbutanamide (1f). Colorless oil, yield 35%; 1H-NMR (400 MHz, CDCl3) δ 7.44–7.35 (m, 2H), 7.26–7.15 (m, 2H), 4.86–4.69 (m, 3H), 4.64–4.51 (m, 2H), 4.22–4.01 (m, 2H), 4.01–3.76 (m, 4H), 3.75–3.66 (m, 4H), 3.55–3.47 (m, 5H), [3.42 (s) and 3.39 (s), total 3H], [3.29 (s) and 3.28 (s), total 3H], 3.24–3.11 (m, 3H), 3.03–2.62 (m, 1H), 2.59–2.27 (m, 4H), 2.21–1.69 (m, 3H), 1.47–1.15 (m, 10H), 1.05–0.71 (m, 18H); 13C-NMR (150 MHz, CDCl3) δ 174.0, 173.9, 172.5, 170.1, 170.0, 149.8, 149.7, 137.5, 137.2, 125.6, 125.1, 81.2, 81.0, 78.8, 60.7, 59.2, 59.0, 57.5, 57.2, 56.8, 54.8, 54.6, 47.6, 47.4, 42.8, 41.9, 41.8, 38.5, 38.4, 33.8, 31.9, 31.5, 31.3, 30.6, 30.2, 28.6, 26.9, 25.1, 24.3, 24.2, 23.8, 19.2, 19.0, 18.4, 18.4, 17.8, 17.0, 16.6, 15.1, 14.0, 13.8, 12.0, 10.0; HRMS (ESI) calcd for C44H76N5O6: 770.5790, found: 770.5809; -36.000 (CHCl3, c = 0.50).
(S)-N-((3R,4S,5S)-1-((S)-2-((1R,2R)-3-(3-([1,1′-Biphenyl]-4-yl)azetidin-1-yl)-1-methoxy-2-methyl-3-oxopropyl)pyrrolidin-1-yl)-3-methoxy-5-methyl-1-oxoheptan-4-yl)-2-((R)-2-(dimethylamino)-3-methylbutanamido)-N,3-dimethylbutanamide (1g). Colorless oil, yield 70%; 1H-NMR (400 MHz, CDCl3) δ 7.60 (d, J = 8.4 Hz, 4H), 7.47–7.44 (m, 2H), 7.40–7.34 (m, 3H), 4.89–4.79 (m, 3H), 4.68–4.65 (m, 1H), 4.58–4.47 (m, 1H), 4.45–4.39 (m, 1H), 4.23–3.85 (m, 10H), [3.45 (s) and 3.42 (s), total 3H], [3.37 (s) and 3.32 (s), total 3H], 3.16–3.03 (m, 4H), 2.80–2.02 (m, 10H), 1.26–0.82 (m, 23H); 13C-NMR (150 MHz, CDCl3) δ 173.8, 173.5, 169.6, 169.2, 139.8, 139.5, 128.1, 127.0, 126.9, 126.7, 126.5, 126.4, 126.3, 126.2, 81.9, 75.7,58.5, 58.4, 57.3, 57.2, 57.0, 56.9, 54.4, 53.1, 42.1, 38.2, 32.6, 32.5, 32.4, 32.2, 32.1, 31.2, 30.3, 29.0, 28.7, 27.0, 25.7, 25.1, 24.5, 24.4, 24.1, 23.0, 22.0, 19.4, 19.2, 18.9, 17.1, 15.2, 14.1, 13.4, 13.0, 10.1, 9.7; HRMS (ESI) calcd for C46H72N5O6: 790.5477, found: 790.5475; -20.900 (CHCl3, c = 1).
(S)-N-((3R,4S,5S)-1-((S)-2-((1R,2R)-3-(3-(3-Chloro-4-fluorophenyl)azetidin-1-yl)-1-methoxy-2-methyl-3-oxopropyl)pyrrolidin-1-yl)-3-methoxy-5-methyl-1-oxoheptan-4-yl)-2-((R)-2-(dimethylamino)-3-methylbutanamido)-N,3-dimethylbutanamide (1h). Colorless oil, yield 44%; 1H-NMR (400 MHz, CDCl3) δ 7.37–7.35 (m, 1H), 7.16–7.09 (m, 2H), 4.90–4.77 (m, 2H), 4.64–4.60 (m, 1H), 4.52–4.40 (m, 2H), 4.18–4.00 (m, 4H), 3.80–3.76 (m, 3H), [3.43 (s) and 3.41 (s), total 3H], [3.38 (s) and 3.36 (s), total 3H], 3.34–3.30 (m, 4H), 3.18–3.02 (m, 4H), 2.37–2.00 (m, 7H), 1.94–1.70 (m, 10H), 1.42–0.80 (m, 13H); 13C-NMR (150 MHz, CDCl3) δ 174.1, 174.0, 172.4, 170.6, 169.6, 161.2, 161.0, 159.6, 159.4, 128.3, 127.8, 127.1, 124.1, 123.8, 115.0, 114.9, 114.8, 114.7, 81.3, 79.0, 68.9, 60.6, 59.0, 58.9, 58.4, 57.5, 56.8, 56.0, 55.6, 53.1, 52.7, 52.6, 47.2, 42.0, 42.0, 38.5, 38.3, 36.2, 32.0, 31.1, 30.8, 30.2, 29.0, 28.6, 27.3, 26.9, 25.0, 24.3, 24.2, 23.8, 23.7, 19.4, 19.3, 19.1, 18.7, 17.6, 17.0, 15.1, 13.7, 13.6, 10.0; HRMS (ESI) calcd for C40H66ClFN5O6: 766.4680, found: 766.4685; -41.400 (MeOH, c = 0.50).
(S)-N-((3R,4S,5S)-1-((S)-2-((1R,2R)-3-(3-(4-Chloro-2-methoxyphenyl)azetidin-1-yl)-1-methoxy-2-methyl-3-oxopropyl)pyrrolidin-1-yl)-3-methoxy-5-methyl-1-oxoheptan-4-yl)-2-((R)-2-(dimethylamino)-3-methylbutanamido)-N,3-dimethylbutanamide (1i). Colorless oil, yield 45%; 1H-NMR (400 MHz, CDCl3) δ 7.15–7.10 (m, 1H), 7.01–6.92 (m, 1H), 6.87–6.84 (m, 1H), 4.88–4.73 (m, 3H), 4.56–4.50 (m, 1H), 4.45–4.41 (m, 1H), 4.37–4.06 (m, 4H), 3.99–3.91 (m, 2H), [3.83 (s) and 3.80 (s), total 3H], [3.44 (s) and 3.42 (s), total 3H], 3.39–3.29 (m, 5H), 3.14–3.01 (m, 4H), 2.82–2.76 (m, 1H), 2.63–2.24 (m, 7H), 2.31 (s, 3H), 2.29 (s, 3H), 2.18–1.77 (m, 9H), 1.37–1.13 (m, 10H), 1.05–0.79 (m, 9H); 13C-NMR (150 MHz, CDCl3) δ 174.4, 173.7, 173.5, 173.2, 171.2, 170.2, 157.9, 133.9, 133.6, 127.9, 127.5, 127.0, 120.6, 111.3, 86.2, 78.5, 78.0, 61.8, 59.4, 58.1, 57.8, 55.8, 53.8, 52.9, 52.6, 47.8, 46.6, 42.7, 39.3, 38.8, 37.4, 35.8, 32.9, 31.9, 28.8, 28.5, 26.0, 25.7, 24.9, 24.4, 23.6, 19.8, 19.4, 18.0, 17.8, 15.8, 13.9, 10.9; HRMS (ESI) calcd for C41H69ClN5O7: 778.4880, found: 778.4879; -33.400 (MeOH, c = 0.50).
3.2. Biological Evaluation Methodology
3.2.1. Cancer Cell Proliferation Inhibition Assay
The following cell lines were used for the screening stage, obtained from American Type Culture Collection (ATCC, Manassas, VA, USA); HCT116 human colon cancer cells and A549 human lung carcinoma cell lines were cultured in RPMI 1640 medium supplemented with 10% FBS. Cell cultures were maintained in a humidified atmosphere of 5% CO2 at 37 °C. Cells were seeded at 2000 cells per well in 96-well plates in a volume of 200 μL per well. The test compounds were dissolved in DMSO and diluted with culture medium to different concentrations. After seeding for 24 h, the medium was removed, and 500 μL of the test compound solution was added in duplicates and incubation continued for 72 h at 37 °C in a humidified atmosphere containing 5% CO2. Control cells were treated with vehicle alone. During the last 4 h of incubation, the cells were exposed to tetrazolium dye (MTT) solution (5 mg/mL, 20 mL per well). The generated formazan crystals were dissolved in 100 mL of dimethyl sulfoxide (DMSO), and the absorbance was read spectrophotometrically at 570 nm using an enzyme-linked immunosorbent assay plate reader. The data was calculated using Graph Pad Prism version 5.0 (GraphPad Softwrae, La Jolla, CA, USA). The IC50s were fitted using a non-linear regression model with a sigmoidal dose response.
3.2.2. In Vivo Efficacy Study
Pathogen-free, 4–6 week-old, female BALB/c athymic mice (Shanghai SCXK Laboratory Animal Technology Co. Ltd., Shanghai, China) were housed under sterile conditions. Human A549 xenograft was established in the right flanks of athymic mice according to the protocol of the National Cancer Institute. When the tumor reached a volume of 100 mm3, the mice were randomly assigned into control (n = 6 per group) and treatment groups (n = 6 per group). Control group were given lactate buffer, and treatment groups were iv administered with tested compounds. The size of tumor was measured individually on the indicated days. Tumor volume (V) was calculated as V = (length × width2)/2. The individual relative tumor volume (RTV) was calculated as follows: RTV = Vt/V0, where Vt represented the last tumor size measurement and V0 represented the pre-dosing tumor size measurement. The animal experimental protocols were approved by the Animal Ethics Committee of School of Pharmacy, Fudan University and the mice were treated in accordance with international animal ethics guidelines.
4. Conclusions
In summary, we described the design and synthesis of nine TZT-1027 analogues based on the conformation restriction strategy. 3-Aryl-zetidines were used to replace the phenylethyl group at C-terminus. Two human cancer cell lines (A549, HCT116) were used to evaluate the potency of the synthesized compounds. Compound 1a showed the strongest cytotoxic activities against A549 and HCT 116 cell lines (IC50 values were 2.2 nM and 2.1 nM, respectively). Compound 1a could not achieve effective inhibition in A549 xenograft models at different dose levels. The poor solubility of 1a limited a further exploration of in vivo activity at higher dosage. Our objective was to discover potent antitumor agents with novel scaffold. In this study, compound 1a did not show any severe toxicity up to 5 mg/kg, showing a better safety potential than TZT-1027 (a dose of 4 mg/kg seemed to be toxic as reported).
Acknowledgments
This work was financially supported by the National High-tech R & D Program of China (Grant No. 2013AA092903), the National Natural Science Foundation of China (Grant No. 81573340), and “Zhuo Xue” Talent Plan of Fudan University.
Abbreviations
The following abbreviations are used in this manuscript:
| Dap | Dolaproine |
| Boc | t-Butyloxy carbonyl |
| HATU | 2-(7-Aza-1H-benzotriazole-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate |
| DIPEA | N,N-Diisopropylethylamine |
| MTT | 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
Supplementary Materials
The following are available online at www.mdpi.com/1660-3397/14/5/85/s1, Table S1: Tumor Volume, Table S2: Relative Tumor Volume, Table S3: Tumor Growth Inhibition, Table S4: Solubility of 1a.
Author Contributions
Wei Zhang and Yingxia Li participated in the design of the research and Qi Yan wrote the manuscript. Qi Yan and Yujie Wang performed some of the experimental studies and analyzed the data. All authors read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Pettit G.R., Kamano Y., Herald C.L., Tuinman A.A., Boettner F.E., Kizu H., Schmidt J.M., Baczynskyj L., Tomer K.B., Bontems R.J. The isolation and structure of a remarkable marine animal antineoplastic constituent: Dolastatin 10. J. Am. Chem. Soc. 1987;109:6883–6885. doi: 10.1021/ja00256a070. [DOI] [Google Scholar]
- 2.Watanabe J., Minami M., Kobayashi M. Antitumor activity of TZT-1027 (soblidotin) Anticancer Res. 2006;26:1973–1981. [PubMed] [Google Scholar]
- 3.Bai R., Petit G.R., Hamel E. Dolastatin 10, a powerful cytostatic peptide derived from a marine animal: Inhibition of tubulin polymerization mediated through the vinca alkaloid binding domain. Biochem. Pharmacol. 1990;39:1941–1949. doi: 10.1016/0006-2952(90)90613-P. [DOI] [PubMed] [Google Scholar]
- 4.Maderna A., Leverett C.A. Recent advances in the development of new auristatins: Structural modifications and application in antibody drug conjugates. Mol. Pharm. 2015;12:1798–1812. doi: 10.1021/mp500762u. [DOI] [PubMed] [Google Scholar]
- 5.Pettit G.R., Singh S.B., Hogan F., Lloyd-Williams P., Herald D.L., Burkett D.D., Clewlow P.J. Antineoplastic agents. Part 189. The absolute configuration and synthesis of natural (−)-dolastatin 10. J. Am. Chem. Soc. 1989;111:5463–5465. doi: 10.1021/ja00196a061. [DOI] [Google Scholar]
- 6.Miyazaki K., Kobayashi M., Natsume T., Gondo M., Mikami T., Sakakibara K., Tsukagoshi S. Synthesis and antitumor activity of novel dolastatin 10 analogs. Chem. Pharm. Bull. 1995;43:1706–1718. doi: 10.1248/cpb.43.1706. [DOI] [PubMed] [Google Scholar]
- 7.Yamamoto N., Andoh M., Kawahara M., Fukuoka M., Niitani H. Phase I study of TZT-1027, a novel synthetic dolastatin 10 derivative and inhibitor of tubulin polymerization, given weekly to advanced solid tumor patients for 3 weeks. Cancer Sci. 2009;100:316–321. doi: 10.1111/j.1349-7006.2008.01023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horti J., Juhasz E., Monostori Z., Maeda K., Eckhardt S., Bodrogi I. Phase I study of TZT-1027, a novel synthetic dolastatin 10 derivative, for the treatment of patients with non-small cell lung cancer. Cancer Chemother. Pharmacol. 2008;62:173–180. doi: 10.1007/s00280-007-0665-7. [DOI] [PubMed] [Google Scholar]
- 9.Senter P.D., Sievers E.L. The discovery and development of brentuximab vedotin for use in relapsed hodgkin lymphoma and systemic anaplastic large cell lymphoma. Nat. Biotechnol. 2012;30:631–637. doi: 10.1038/nbt.2289. [DOI] [PubMed] [Google Scholar]
- 10.Maderna A., Matthew D., Chakrapani S., Alexander P., Leverett C.A., Vetelino B.C., Zecheng C., Hud R., Kevin P., Jayvardhan P. Discovery of cytotoxic dolastatin 10 analogues with N-terminal modifications. J. Med. Chem. 2014;57:10527–10543. doi: 10.1021/jm501649k. [DOI] [PubMed] [Google Scholar]
- 11.Cormier A., Marchand M., Ravelli R.B.G., Knossow M., Gigant B. Structural insight into the inhibition of tubulin by vinca domain peptide ligands. EMBO Rep. 2008;9:1101–1106. doi: 10.1038/embor.2008.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Evans G.B., Furneaux R.H., Greatrex B., Murkin A.S., Schramm V.L., Tyler P.C. Azetidine based transition state analogue inhibitors of N-ribosyl hydrolases and phosphorylases. J. Med. Chem. 2008;51:948–956. doi: 10.1021/jm701265n. [DOI] [PubMed] [Google Scholar]
- 13.Allwood D.M., Blakemore D.C., Brown A.D., Ley S.V. Metal-free coupling of saturated heterocyclic sulfonylhydrazones with boronic acids. J. Org. Chem. 2014;79:328–338. doi: 10.1021/jo402526z. [DOI] [PubMed] [Google Scholar]
- 14.Kobayashi M., Natsume T., Tamaoki S., Watanabe J.-I., Asano H., Mikami T., Miyasaka K., Miyazaki K., Gondo M., Sakakibara K., et al. Antitumor activity of TZT-1027, a novel doiastatin 10 derivative. Jpn. J. Cancer Res. 1997;88:316–327. doi: 10.1111/j.1349-7006.1997.tb00383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Watanabe J., Natsume T., Kobayashi M. Antivascular effects of TZT-1027 (Soblidotin) on murine colon26 adenocarcinoma. Cancer Sci. 2006;97:1410–1416. doi: 10.1111/j.1349-7006.2006.00330.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.