Abstract
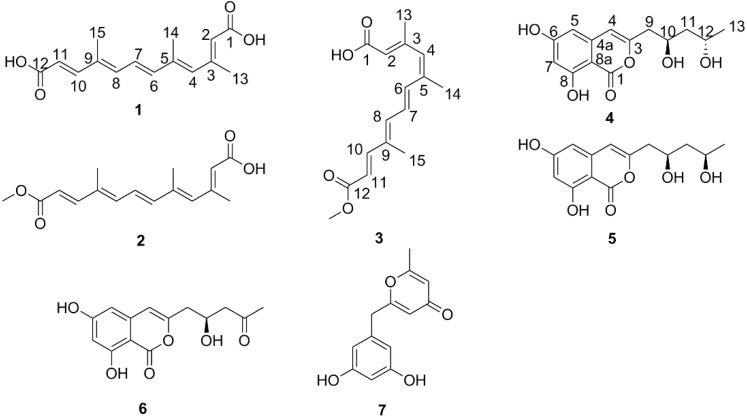
Four new polyketides: nectriacids A–C (1–3) and 12-epicitreoisocoumarinol (4), together with three known compounds: citreoisocoumarinol (5), citreoisocoumarin (6), and macrocarpon C (7) were isolated from the culture of the endophytic fungus Nectria sp. HN001, which was isolated from a fresh branch of the mangrove plant Sonneratia ovata collected from the South China Sea. Their structures were determined by the detailed analysis of NMR and mass spectroscopic data. The absolute configuration of the stereogenic carbons for compound 4 was further assigned by Mosher’s ester method. All of the isolated compounds were tested for their α-glucosidase inhibitory activity by UV absorbance at 405 nm, and new compounds 2 and 3 exhibited potent inhibitory activity with IC50 values of 23.5 and 42.3 μM, respectively, which were more potent than positive control (acarbose, IC50, 815.3 μM).
Keywords: polyketides, α-glucosidase inhibitor, Nectria sp., pentaene diacid derivatives
1. Introduction
Diabetes mellitus, one of the most common chronic metabolic diseases, occurs when the pancreas produces insufficient levels of insulin or when the body cannot use the insulin effectively [1]. In 2015, about 415 million people had diabetes worldwide, with type II diabetes accounting for about 90% of the cases [2,3]. α-Glucosidase is an important enzyme for breaking down complex carbohydrates for absorption, and α-glucosidase inhibitors such as acarbose, miglitol, and voglibose, all originating from natural products, are widely used to treat type II diabetes, indicating that natural products are an important source of anti-diabetes drugs.
Endophytic fungi can produce a diversity of natural products, which are structurally unique and possess interesting biological and pharmacological properties [4,5]. As part of our ongoing investigation into bioactive metabolites from mangrove endophytic fungi collected from the South China Sea [6,7,8,9,10,11,12], a chemical investigation of the mangrove-derived fungus Nectria sp. HN001, isolated from a fresh branch of the mangrove plant Sonneratia ovata, had led to the isolation and characterization of four new polyketides: nectriacid A (1), nectriacid B (2), nectriacid C (3), and 12-epicitreoisocoumarinol (4), as well as three known compounds: citreoisocoumarinol (5), citreoisocoumarin (6), and macrocarpon C (7) (Figure 1). Previous studies showed that linear polyene derivatives exhibited anti-inflammatory [13], antihypertensive [14], antibacterial [15], and antifungal activities [16]. In this report, compounds (1–7) from the Nectria sp. HN001 were evaluated for α-glucosidase inhibitory activity. The results showed that compounds 2 and 3 exhibited significant inhibitory activity toward α-glucosidase. Here, details of the isolation, structure elucidation, and activity against α-glucosidase of these compounds are described.
Figure 1.
Chemical constituents of Nectria sp. HN001.
2. Results
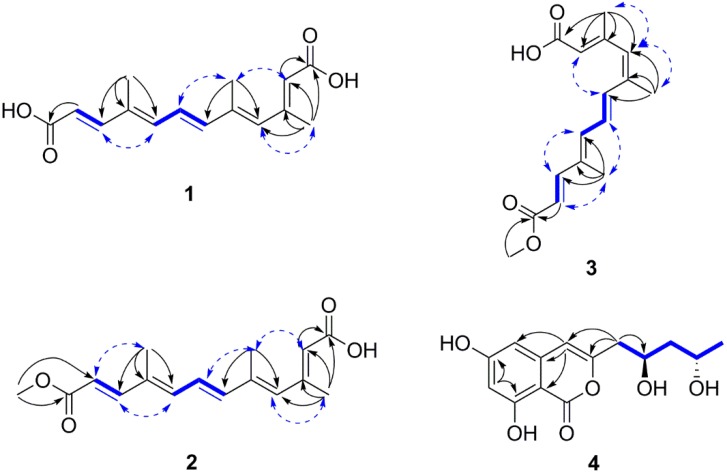
Nectriacid A (1) was obtained as yellow amorphous powder. Its molecular formula C15H18O4 was established by the (−)-HRESIMS at m/z 261.1130 [M − H]− (calcd for 261.1132), implying seven degrees of unsaturation. Its IR spectrum exhibited absorption bands for hydroxyl (3363 cm−1) and conjugated carbonyl (1684 cm−1) groups. The 1H NMR data of 1 (Table 1) showed resonances for three methyl groups [δH 1.93 (3H, s, H-15); δH 2.02 (3H, s, H-14); δH 2.24 (3H, s, H-13)], three olefinic protons [δH 6.57 (1H, d, J = 11.2 Hz, H-8), δH 6.20 (1H, s, H-4), and δH 5.76 (1H, s, H-2)], two E-configured olefinic protons [δH 7.27 (1H, d, J = 15.6 Hz, H-10) and δH 5.86 (1H, d, J = 15.6 Hz, H-11)], and another two E-configured olefinic protons [δH 6.76 (1H, dd, J = 15.1, 11.2 Hz, H-7) and δH 6.60 (1H, d, J = 15.1 Hz, H-6)]. The 13C NMR (Table 1) and DEPT data showed 15 carbon resonances corresponding to three methyl (δC 18.5, 14.2, 12.5), seven methine sp2 (δC 148.1, 141.8, 138.4, 136.1, 126.2, 120.2, 117.9), three quaternary sp2 (δC 151.6, 138.5, 134.4), and two carbonyls (δC 167.7, 167.4) carbons. The 1H and 13C NMR data of 1 were similar to those of all-E-4, 9-dimethyldodeca-2, 4, 6, 8, 10-pentaenedioic acid [17], which was isolated from the root of Mycorrhizal colonization except for the presence of an additional methyl group on C-5 (CH3-14) in 1. The key HMBC correlations from H3-14 to C-6 and C-4 demonstrated that CH3-14 (δH 2.02, s, δC 14.2) was connected to C-5. Besides, comparing 1 with the known all-E-4, 9-dimethyldodeca-2, 4, 6, 8, 10-pentaenedioic acid, CH3-13 (δH 2.24, s, δC 18.5) of 1 was linked to C-3 rather than C-4, and this was supported by the HMBC correlations (Figure 2) from H3-13 to C-4, C-3, C-2, and C-1. Furthermore, the configurations of the double bonds (E or Z) for compound 1 were determined by the coupling constant and NOESY data. The E-geometry of the double bonds at C-6 and C-10 was assigned by the vicinal coupling constants, J = 15.6 Hz, J = 15.1 Hz, respectively. In addition, the geometry of the remaining three substituted double bonds was confirmed as 2E, 4E and 8E on the basis of the NOESY correlations (Figure 2) from H3-13 to H-4, H3-14 to H-2 and H-7, H-8 to H-10, as was previously reported [18]. Thus, compound 1 was determined as 2E, 4E, 6E, 8E, 10E-3, 5, 9-trimethyldodeca-2, 4, 6, 8, 10-pentaenedioic acid, and named nectriacid A.
Table 1.
1H (500 MHz) and 13C (125 MHz) NMR data for compounds 1 (DMSO-d6), 2 (CDCl3), and 3 (CDCl3).
| Position | 1 | 2 | 3 | |||
|---|---|---|---|---|---|---|
| δH (J in Hz) | δC | δH (J in Hz) | δC | δH (J in Hz) | δC | |
| 1 | 167.7, C | 171.6, C | 170.9, C | |||
| 2 | 5.76, s | 120.2, CH | 5.78, s | 118.8, CH | 5.76, s | 118.9, CH |
| 3 | 151.6, C | 155.8, C | 155.7, C | |||
| 4 | 6.20, s | 136.1, CH | 6.08, s | 136.3, CH | 5.97, s | 134.3, CH |
| 5 | 138.5, C | 134.6, C | 137.7, C | |||
| 6 | 6.60, d (15.1) | 141.8, CH | 6.44, d (15.2) | 141.8, CH | 6.91, d (15.1) | 134.6, CH |
| 7 | 6.76, dd (15.1, 11.2) | 126.2, CH | 6.70, dd (15.2, 11.2) | 126.6, CH | 6.73, dd (15.1,11.4) | 127.8, CH |
| 8 | 6.57, d (11.2) | 138.4, CH | 6.43, d (11.2) | 138.8, CH | 6.49, d (11.4) | 139.0, CH |
| 9 | 134.4, C | 135.1, C | 135.1, C | |||
| 10 | 7.27, d (15.6) | 148.1, CH | 7.38, d (15.6) | 149.7, CH | 7.38, d (15.6) | 149.0, CH |
| 11 | 5.86, d (15.6) | 117.9, CH | 5.92, d (15.6) | 116.6, CH | 5.92, d (15.6) | 117.1, CH |
| 12 | 167.4, C | 168.0, C | 167.9, C | |||
| 13 | 2.24, s | 18.5, CH3 | 2.30, s | 20.3, CH3 | 2.26, s | 20.1, CH3 |
| 14 | 2.02, s | 14.2, CH3 | 2.04, s | 14.8, CH3 | 2.00, s | 21.4, CH3 |
| 15 | 1.93, s | 12.5, CH3 | 1.93, s | 12.8, CH3 | 1.93, s | 12.9, CH3 |
| -OCH3 | 3.74, s | 51.8, CH3 | 3.74, s | 51.7, CH3 |
Figure 2.
Selected 1H–1H COSY (bold line), HMBC (arrow), and key NOESY (dashed lines) correlations of compounds 1–4.
Nectriacid B (2) was also isolated as yellow powder, and had a molecular formula of C16H20O4 according to its (−)-HRESIMS m/z 275.1287 [M − H]−. The 1H NMR data exhibited the signals for three methyl groups [δH 1.93 (3H, s, H-15); δH 2.04 (3H, s, H-14); δH 2.30 (3H, s, H-13)], one methoxy group (δH 3.74, s), three olefinic protons [δH 5.78 (1H, s, H-2); δH 6.08 (1H, s, H-4); δH 6.43 (1H, d, J = 11.2 Hz, H-8)], and two pairs of E-configured protons [δH 6.44 (1H, d, J = 15.2 Hz, H-6), δH 6.70 (1H, dd, J = 15.2, 11.2 Hz, H-7); δH 7.38 (1H, d, J = 15.6 Hz, H-10), δH 5.92 (1H, d, J = 15.6 Hz, H-11)]. The 13C NMR (Table 1) and HSQC spectra exhibited three methyl (δC 20.3, 14.8, 12.8), one methoxy (δC 51.8), seven methine sp2 (δC 149.7, 141.8, 138.8, 136.3, 126.6, 118.8, 116.6), five quaternary sp2 (δC 171.6, 168.0, 155.8, 135.1, 134.6) carbons. The above spectral features suggested that 2 was quite similar to 1 except for the presence of one methoxy group (δH 3.74, δC 51.8). This evidence suggested that compound 2 was derived from a methyl esterification of compound 1, which was further supported by the HMBC correlations (Figure 2) from the methoxy protons to C-12 (δC 168.0). The 6E and 10E configurations were confirmed by the vicinal coupling constants between H-10 and H-11 (J = 15.6 Hz), H-6 and H-7 (J = 15.2 Hz). The configurations of 2E, 4E and 8E were assigned on the basis of the NOESY correlations (Figure 2) from H3-13 to H-4, H3-14 to H-2 and H-7, H-8 to H-10, H3-15 to H-11, as was previously reported [18]. Thus, the structure of 2 was established as 2E, 4E, 6E, 8E, 10E-12-methoxy-3, 5, 9-trimethyl-12-oxododeca-2, 4, 6, 8, 10-pentaenoic acid, and named nectriacid B.
Nectriacid C (3) was isolated as pale yellow powder, and its molecular formula of C16H20O4 was established by the (−)-HRESIMS m/z 275.1287 [M − H]−. The 13C NMR data (Table 1) of compound 3 showed 16 carbon resonances, which were classified according to DEPTs and HSQC spectra, as three methyl (δC 21.4, 20.1, 12.9), five quaternary sp2 (δC 170.9, 167.9, 155.7, 137.7, 135.1), seven methine sp2 (δC 149.0, 139.0, 134.6, 134.3, 118.9, 117.1), and one methoxy (δC 51.7) carbons. Comparison of NMR data with those of 2 suggested that compound 3 possessed the same planar structure as 2. However, the double bonds geometry was different. The 6E and 10E configurations were confirmed by the vicinal coupling constants between H-6 and H-7 (J = 15.1 Hz), H-10 and H-11 (J = 15.6 Hz), respectively. The 2E, 4Z, and 8E configurations of the double bonds were established by the NOESY correlations from H3-13 and H3-14 to H-4, from H-2 to H-6, from H3-15 to H-7 and H-11, from H-10 to H-8 (Figure 2) [18]. Consequently, 3 was determined as 2E, 4Z, 6E, 8E, 10E-12-methoxy-3, 5, 9-trimethyl-12-oxododeca-2, 4, 6, 8, 10-pentaenoic acid, and named nectriacid C.
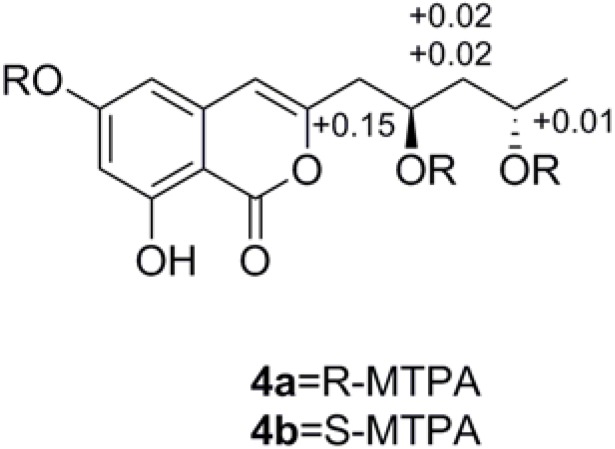
Compound 4 was obtained as colorless powder. Its molecular formula C14H16O6 was deduced by the (−)-HRESIMS m/z 279.0871 [M − H]−, corresponding to seven degrees of unsaturation. The IR spectrum displayed typical absorption bands for hydroxyl (3394 cm−1) and aromatic ring system (1684, 1629, and 1588 cm−1). The 1H NMR spectrum (Table 2) suggested the presence of one methyl group [δH 1.21 (3H, d, J = 6.3 Hz, H-13)], two methylene groups [δH 2.65 (1H, dd, J = 14.5, 4.9 Hz, H-9a), 2.59 (1H, dd, J = 14.5, 8.0 Hz, H-9b); δH 1.60 (1H, ddd, J = 14.3, 10.6, 3.4 Hz, H-11a), 1.55 (1H, ddd, J = 14.3, 10.6, 3.4 Hz, H-11b], two methine groups [δH 4.21 (1H, m, H-10), 4.02 (1H, m, H-12)], and three olefinic protons [δH 6.37 (1H, s, H-4), 6.31 (2H, overlap, H-5, H-7)]. The 13C NMR spectrum of 4 (Table 2) showed 14 carbon resonances, including one methyl (δC 24.5), six quaternary sp2 (δC 168.0, 167.5, 165.0, 156.3, 141.4, 100.0), two methylene sp3 (δC 47.1, 43.1), three methine sp2 (δC 107.2, 103.8, 102.8), and two oxygen-bearing methine sp3 (δC 67.2, 65.4) carbons. Detailed analysis of the 1H and 13C NMR data (Table 2) suggested that 4 belonged to the isocoumarin class, and its NMR data were similar to those of citreoisocoumarinol 5 [19]. Comparison of the NMR data of compounds 4 and 5 suggested that they differed only in the substituents on C-3. The absolute configuration of C-10 and C-12 of compound 4 was determined as 10R, 12S by applying Mosher’s methods to the 1, 3-anti-diol model as reported [20,21,22]. The procedure started with esterification of 4 with the two enantiomers of (R)- or (S)-MTPA chloride (4a = R or 4b = S) [23], and then the differences in chemical shift values (Δδ = δS − δR) for 4b and 4a were calculated to assign the configuration of C-10 and C-12 (Figure 3). So 4 was named 12-epicitreoisocoumarinol.
Table 2.
1H (500 MHz) and 13C (125 MHz) NMR data for compound 4 (MeOD-d4) and compound 5 (MeOD-d4).
| 4 | 5 | |||
|---|---|---|---|---|
| Position | δH (J in Hz) | δC | δH (J in Hz) | δC |
| 1 | 168.0, C | 168.0, C | ||
| 3 | 156.3, C | 156.1, C | ||
| 4 | 6.37, s | 107.2, CH | 6.37, s | 107.3, CH |
| 4a | 141.4, C | 141.4, C | ||
| 5 | 6.31, s | 103.8, CH | 6.31, s | 103.8, CH |
| 6 | 167.5, C | 167.5, C | ||
| 7 | 6.31, s | 102.8, CH | 6.31, s | 102.8, CH |
| 8 | 165.0, C | 165.0, C | ||
| 8a | 100.0, C | 100.0, C | ||
| 9 | 2.59, dd (14.5, 8.0) | 43.1, CH2 | 2.57, dd (14.5, 8.3) | 42.5, CH2 |
| 2.65, dd (14.5, 4.9) | 2.70, dd (14.5, 4.4) | |||
| 10 | 4.21, m | 67.2, CH | 4.14, m | 68.6, CH |
| 11 | 1.60, ddd (14.3, 10.6, 3.4) | 47.1, CH2 | 1.71, ddd (13.9, 8.8, 7.6) | 46.4, CH2 |
| 1.55, ddd (14.3,10.6, 3.4) | 1.61, ddd (13.9, 5.4, 4.3) | |||
| 12 | 4.02, m | 65.4, CH | 4.00, m | 67.0, CH |
| 13 | 1.21, d (6.3) | 24.5, CH3 | 1.20, d (6.2) | 23.5, CH3 |
Figure 3.
ΔδS-R values of (R)- and (S)-MTPA esters of 4.
The genus Nectria has been reported as a prolific source of bioactive secondary metabolites, such as heptaketides with antimicrobial activity [24], terpenoids with anti-acetylcholinesterase and anti-β-glucuronidase activity [25], and phytotoxin with herbicidal activity [26]. Nectriacids A–C (1–3) were the first examples of polyenes derivatives with linear C15 conjugated pentaene diacid derivatives containing three methyl groups. To date only a few examples of similar substances have been previously isolated. These include bixin and norbixin from Bixa orellana [27], crocetin from Crocus sativus [28], the monocarboxylic acid azafrin from Escobedia scabrifolia and E. linearis [29], and (3Z, 5E, 7E, 9E, 11E, 13Z, 15E, 17E)-18-methyl-19-oxoicosa-3, 5, 7, 9, 11, 13, 15, 17-octaenoic acid and (3E, 5Z, 7E, 9E, 11E, 13E, 15Z, 17E, 19E)-20-methyl-21-oxodocosa-3, 5, 7, 9, 11, 13, 15, 17, 19-nonaenoic acid from white-rotting Basidiomycete [30].
The remaining three known compounds from the fungus Nectria sp. HN001 were identified as citreoisocoumarinol (5) [19,31], citreoisocoumarin (6) [19,31], and macrocarpon C (7) [19,31], by comparison of their MS and NMR data with those reported in the literature.
All of the isolates were evaluated for in vitro α-glucosidase inhibitory activity [10]. The results showed that compounds 2 and 3 possessed stronger activity than positive control (acarbose, IC50, 815.3 μM) with IC50 values of 23.5 and 42.3 μM, respectively. However, compounds 4–6 showed moderate activity with IC50 values ranging from 300 to 600 μM (Table 3), while compound 7 did not display inhibitory activity compared to positive control. Interestingly, although compounds 1–3 possess same carbon skeleton, their α-glucosidase inhibitory activity are different. The activity against α-glucosidase for 2 (23.5 μM) and 3 (42.3 μM) was more potent than that for 1 (121.8 μM), which suggested that esterification of terminal carboxyl group (C-12) may play a key role in the inhibitory effects. Although compounds 2 and 3 possess different configuration of the C4–C5 double bonds, they exhibited the same level of activity. Meanwhile, compounds 4 and 5 exhibited relatively stronger activity when compared to 6.
Table 3.
Inhibitory effects of the isolates against α-glucosidase.
| Compounds | 1 | 2 | 3 | 4 | 5 | 6 | 7 | Acarbose a |
|---|---|---|---|---|---|---|---|---|
| IC50 (μM) b | 121.8 ± 0.4 | 23.5 ± 0.3 | 42.3 ± 0.2 | 343.7 ± 1.0 | 392.5 ± 1.7 | 538.7 ± 4.3 | >900 | 815.3 ± 3.8 |
a Positive control; b Data are shown as mean ± SD from three parallel measurements.
3. Experimental Section
3.1. General
Optical rotations were measured on a Bellingham-Stanley ADP 440+ polarimeter at 25 °C. IR data were recorded on a Nicolet 5DX-FTIR (Thermo Fisher Scientific, Inc., Hudson, NH, USA), in KBr discs. UV data were recorded on a Shimadzu UV-240 spectrophotometer (Shimadzu, Kyoto, Japan). The 1H NMR (500 MHz), 13C NMR (125 MHz), and 2D NMR spectra were obtained on a Bruker AVANCE-500 (Bruker BioSpin Corporation, Billerica, MA, USA) using TMS as an internal reference. HRESIMS were acquired on a Thermofisher LTQ Orbitrp Elite LC-MS spectrometer (Thermo Fisher Scientific, Inc., Hudson, NH, USA), and the ESIMS data were measured on a Micro Mass Q-TOF spectrometer (Waters Corporation, Milford, MA, USA). TLC analysis was carried out on silica gel plates (Marine Chemical Ltd., Qingdao, China). RP-C18 silica gel (Fuji, 40–75 μm, Fuji Silysia Chemical Ltd., Kasugai, Japan), Silica gel (200–300 mesh, Marine Chemical Ltd., Qingdao, China), High silica gel (H, Marine Chemical Ltd., Qingdao, China), and Sephadex LH-20 (GE Healthcare Bio-Sciences AB, Stockholm, Sweden) were used for column chromatography (CC). The chiral HPLC separation of compound 4 was accomplished over a S-Chiral A (column size: 4.6 × 250 mm 5 μm; Acchrom Technologies Co., Ltd., Beijing, China; flow rate: 1.0 mL/min; solvent: n-hexane-isopropanol = 9:1, tR 18.5 min). α-Glucosidase from Saccharomyces cerevisiae was purchased from Sigma-Aldrich Co. (CAS number: 9001-42-7, E.C 3.2.1.20; Buchs, Switzerland). Acarbose (>98%) was purchased from Adamas-beta Co. Ltd. (Shanghai, China).
3.2. Fungal Material
The fungal strain HN001 was isolated from the branches of the mangrove plant Sonneratia ovata collected from the South China Sea in Hainan province, China. The fungus was identified by our team as Nectria sp. HN001, Nectriaceae, according to a molecular biological protocol by DNA amplification and sequencing of the ITS region [10] (deposited in GenBank, accession No. KU359411). A voucher strain was deposited in School of Chemistry and Chemical Engineering, Sun Yat-Sen University, Guangzhou, China, with the access code, 2015-HN001.
3.3. Fermentation, Extraction, and Isolation
The fungus Nectria sp. HN001 was primary cultivated on PDA medium (20 g of glucose, 20 g of agar, and 2 g of sea salt in 1 L of potato infusion). Plugs of agar supporting mycelial growth were cut and transferred aseptically to 250 mL Erlenmeyer flasks containing 100 mL of PDB medium (20 g of glucose and 2 g of sea salt in 1 L of potato infusion). The flasks were incubated at 28 °C on a rotary shaker for three days, and then the mycelia were aseptically transferred to a solid autoclaved rice substrate medium (60 × 500 mL Erlenmeyer flasks, each containing 50 g of rice and 50 mL of 0.3% of saline water) for 28 days at 25 °C. The mycelia and solid rice medium were extracted with MeOH (3 × 15 L, 24 h each) for three times. The solvent was evaporated under reduced pressure to yield a crude extract (296 g), which was suspended in H2O and extracted with EtOAc (3 × 3 L, 45 min each) to yield a crude ethyl acetate extract (110 g). The ethyl acetate extract (110 g) was chromatographed on silica gel column (400 g, 100–200 mesh, 10 × 70 cm) eluting with a step gradient of petroleum ether-EtOAc (100:0; 9:1; 8:2; 7:3; 6:4; 5:5; 4:6; 3:7; 0:100, v/v, each 2 L) to give nine fractions (F1–F9). F8 (1.5 g) was further fractionated on another silica gel column using petroleum-EtOAc (2 L, 8:2; 7:3; 6:4; 5:5; 4:6, v/v, each 400 mL) as the mobile phase to yield five subfractions (F801 to F805). Compound 1 (28 mg) was obtained from F802 by column chromatography on silica gel (gel H, 25 × 340 mm, 30 g) eluting with CHCl3-MeOH (1 L, 95:5). Then, the F803 fraction was purified using HPLC on a semipreparative RP-HPLC column (250 × 9.4 mm, 5 μm), using with Acetonitrile-H2O (55:45, v/v, flow rate: 1.0 mL/min) as the solvent system, to obtain 2 (18 mg, tR 26.3 min) and 3 (15 mg, tR 27.8 min). F804 was subjected to RP-18 using MeOH-H2O (8:2, v/v) to give compounds 6 (35 mg) and 7 (8 mg). F805 was chromatographed on Sephadex LH-20 (110 g, 110 × 3 cm) eluting with CHCl3-MeOH (1 L, 1:1, v/v) to give the mixture of F8051 (4 and 5). F8051 was conducted by the S-Chiral A (n-hexane-isopropanol = 9:1, v/v, flow rate: 1.0 mL/min) column to afford 4 (18 mg, tR 18.5 min) and 5 (10 mg, tR 25.5 min).
Nectriacid A (1): yellow powder; UV (MeOH) λmax (log ε) 355 (4.70); IR (KBr) νmax 3363, 2955, 2922, 2852, 1684, 1616, 1592, 1268, 1186, 980, 895, 855 cm−1; 1H NMR and 13C NMR data, see Table 1; ESIMS m/z 261.1 [M − H]−; HRESIMS m/z 261.1130 [M − H]− (calcd for C15H17O4, 261.1132).
Nectriacid B (2): yellow powder; UV (MeOH) λmax (log ε) 359 (4.23); IR (KBr) νmax 3434, 2955, 2922, 2852, 1723, 1656, 1439, 1380, 1278, 1202, 1173, 984, 857 cm−1; 1H NMR and 13C NMR data, see Table 1; ESIMS m/z 275.3 [M − H]−; HRESIMS m/z 275.1287 [M − H]− (calcd for C16H19O4, 275.1288).
Nectriacid C (3): pale yellow powder; UV (MeOH) λmax (log ε) 355 (3.69); IR (KBr) νmax 3365, 2956, 2922, 2852, 1723, 1655, 1634, 1464, 1439, 1380, 1280, 1202, 1174, 1073, 984, 720 cm−1; 1H NMR and 13C NMR data, see Table 1; ESIMS m/z 275.4 [M − H]−; HRESIMS m/z 275.1287 [M − H]− (calcd for C16H19O4, 275.1288).
12-epicitreoisocoumarinol (4): colorless powder; −13.3 (c 0.3, MeOH); UV (MeOH) λmax (log ε) 245 (4.89); IR (KBr) νmax 3394, 3191, 2962, 2921, 2850, 1681, 1629, 1588, 1510, 1466, 1382, 1241, 1172, 1134, 1065, 849, 798, 694 cm−1; 1H NMR and 13C NMR data, see Table 2; ESIMS m/z 279.4 [M − H]−; HRESIMS m/z 279.0871 [M − H]− (calcd for C14H15O6, 279.0874).
3.4. Preparation of (R)- and (S)-MTPA Esters of 4
As our previous reported method [23], 12-epicitreoisocoumarinol (4) (1 mg) was dissolved in 1 mL of pyridine and stirred at room temperature for 10 min. An excess of (R)- or (S)-MTPA chloride (10 μL) was added, and the reaction was stirred overnight at room temperature. The solvent was removed in vacuo, and the crude reaction product was purified by preparative silica gel TLC using pure dichloromethane as developing solvent.
1H NMR data of (S)-MTPA ester of 4 (500 MHz, CDCl3): δH 11.0 (1H, s, 8-OH), 7.60–7.29 (15H, m, Ar-H), 6.70 (1H, d, J = 2.0 Hz, H-5), 6.42 (1H, d, J = 2.0 Hz, H-7), 5.87 (1H, s, H-4), 5.34 (1H, m, H-10), 5.07 (1H, m, H-12), 3.66-3.45 (9H, s, 3-OCH3), 2.72 (1H, dd, J = 15.1, 5.0 Hz, H-9a), 2.71 (1H, dd, J = 15.1, 6.7 Hz, H-9b), 1.99 (1H, m, H-11a), 1.96 (1H, m, H-11b), 1.27 (3H, d, J = 6.2 Hz, H-13); ESIMS m/z 927.2 [M − H]−.
1H NMR data of (R)-MTPA ester of 4 (500 MHz, CDCl3): δH 11.0 (1H, s, 8-OH), 7.61–7.29 (15H, m, Ar-H), 6.71 (1H, d, J = 2.1 Hz, H-5), 6.48 (1H, d, J = 2.1 Hz, H-7), 5.97 (1H, s, H-4), 5.18 (1H, m, H-10), 5.06 (1H, m, H-12), 3.66-3.48 (9H, s, 3-OCH3), 2.71 (1H, overlap, H-9a), 2.70 (1H, overlap, H-9b), 1.96 (1H, m, H-11a), 1.93 (1H, m, H-11b), 1.32 (3H, d, J = 6.2 Hz, H-13); ESIMS m/z 927.2 [M − H]−.
3.5. In Vitro Inhibition Studies on α-Glucosidase
An assay of α-glucosidase inhibitory activity was performed using a reported method, with slight modifications [10]. All the assays were performed using 0.01 M KH2PO4-K2HPO4 buffers, pH 7.0, and a Bio-Rad iMark microplate reader (Bio-Rad Laboratories, Inc., Kyoto, Japan). Enzyme solution was prepared to give 2.0 Units/mL in 2 mL aliquots. The assay medium contained phosphate buffer, pH 7.0 (130 μL), 10 μL of enzyme solution, 20 μL of DMSO or inhibitor (dissolved in DMSO), and 40 μL of substrate (p-nitrophenyl glycoside, 3 mg/mL). The substrate was added to the assay medium containing enzyme and buffer with inhibitor added after 15 min of incubation time at 37 °C. The activity was determined by measuring the increase in absorbance at 405 nm for a 1 min interval. Calculations were performed according to the equation:
| η (%) = [(B − S)/B] × 100% |
(B stands for the assay medium with DMSO; S stands for the assay medium with inhibitor). All measurements were done in triplicate from two independent experiments. The reported IC50 was the average value of two independent experiments.
4. Conclusions
Four new (1–4) and three known polyketides (5–7) were isolated and identified from the culture of the endophytic fungus Nectria sp. HN001. Compounds 2 and 3 exhibited stronger inhibitory activity on α-glucosidase than positive control [10]. To the best of our knowledge, this is the first report of the α-glucosidase inhibitory activity of the C15 conjugated pentaene diacid derivatives. This finding can allow us to explore structural diversity of linear polyene diacid derivatives and offer new guidance to discover α-glucosidase inhibitors.
Acknowledgments
We thank the National Natural Science Foundation of China (21472251, 41276146), the Science & Technology Plan Project of Guangdong Province of China (2013B021100011), the Science & Technology Plan Project of Guangdong Province of China (2014A020210003), Special Financial Fund of Innovative Development of Marine Economic Demonstration Project (GD2012-D01-001), China’s Marine Common Wealth Research Project (201305017), and the Fundamental Research Funds for the Central Universities (141gjc16) for generous support.
Author Contributions
Conceived and designed the experiments: Z.S., X.H., H.C., and Y.L. Performed the experiments: H.C., Y.L., Y.N., Z.Z., Z.L. Analyzed the data: Z.S., X.H., H.C., S.C., and Y.L. Contributed reagents/materials/ analysis tools: Y.L, L.H. Wrote the paper: Z.S., X.H., H.C. Read and approved the final manuscript: Z.S., X.H., H.C.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Kato A., Hayashi E., Miyauchi S., Adachi I., Imahori T., Natori Y., Yoshimura Y., Nash R.J., Shimaoka H., Nakagome I., et al. Alpha-1-C-butyl-1,4-dideoxy-1,4-imino-l-arabinitol as a second-generation iminosugar-based oral alpha-glucosidase inhibitor for improving postprandial hyperglycemia. J. Med. Chem. 2012;55:10347–10362. doi: 10.1021/jm301304e. [DOI] [PubMed] [Google Scholar]
- 2.Association A.D. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33:62–69. doi: 10.2337/dc10-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.International Diabetes Federation IDF Diabetes Atlas. 7th ed. [(accessed on 1 December 2015)]. Revision 2015. Available online: http://www.Idf.Org/diabetesatlas/
- 4.Zhang H.W., Song Y.C., Tan R.X. Biology and chemistry of endophytes. Nat. Prod. Rep. 2006;23:753–771. doi: 10.1039/b609472b. [DOI] [PubMed] [Google Scholar]
- 5.Ebel R., Rateb M.E. Secondary metabolites of fungi from marine habitats. Nat. Prod. Rep. 2011;28:290. doi: 10.1039/c0np00061b. [DOI] [PubMed] [Google Scholar]
- 6.Li H., Jiang J., Liu Z., Lin S., Xia G., Xia X., Ding B., He L., Lu Y., She Z. Peniphenones A–D from the mangrove fungus Penicillium dipodomyicola HN4–3A as inhibitors of Mycobacterium tuberculosis Phosphatase Mptpb. J. Nat. Prod. 2014;77:800–806. doi: 10.1021/np400880w. [DOI] [PubMed] [Google Scholar]
- 7.Wen L., Cai X., Xu F., She Z., Chan W.L., Vrijmoed L., Jones E.G., Lin Y. Three metabolites from the mangrove endophytic fungus Sporothrix sp.(# 4335) from the South China Sea. J. Org. Chem. 2009;74:1093–1098. doi: 10.1021/jo802096q. [DOI] [PubMed] [Google Scholar]
- 8.Xiao Z., Huang H., Shao C., Xia X., Ma L., Huang X., Lu Y., Lin Y., Long Y., She Z. Asperterpenols A and B, new sesterterpenoids isolated from a mangrove endophytic fungus Aspergillus sp. 085242. Org. Lett. 2013;15:2522–2525. doi: 10.1021/ol401005j. [DOI] [PubMed] [Google Scholar]
- 9.Huang X., Huang H., Li H., Sun X., Huang H., Lu Y., Lin Y., Long Y., She Z. Asperterpenoid A, a new sesterterpenoid as an inhibitor of Mycobacterium tuberculosis protein tyrosine phosphatase B from the culture of Aspergillus sp. 16–5c. Org. Lett. 2013;15:721–723. doi: 10.1021/ol303549c. [DOI] [PubMed] [Google Scholar]
- 10.Liu Y., Yang Q., Xia G., Huang H., Li H., Ma L., Lu Y., He L., Xia X., She Z. Polyketides with α-glucosidase inhibitory activity from a mangrove endophytic fungus Penicillium sp. Hn29–3b1. J. Nat. Prod. 2015;78:1816–1822. doi: 10.1021/np500885f. [DOI] [PubMed] [Google Scholar]
- 11.Liu Z., Xia G., Chen S., Liu Y., Li H., She Z. Eurothiocin A and B, sulfur-containing benzofurans from a soft coral-derived fungus Eurotium rubrum SH-823. Mar. Drugs. 2014;12:3669–3680. doi: 10.3390/md12063669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xiao Z.E., Lin S.E., Tan C., Lu Y., He L., Huang X., She Z. Asperlones A and B, dinaphthalenone derivatives from a mangrove endophytic fungus Aspergillus sp. 16–5C. Mar. Drugs. 2015;13:366–378. doi: 10.3390/md13010366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nam K.N., Park Y.M., Jung H.J., Lee J.Y., Min B.D., Park S.U., Jung W.S., Cho K.H., Park J.H., Kang I., et al. Anti-inflammatory effects of crocin and crocetin in rat brain microglial cells. Eur. J. Pharmacol. 2010;648:110–116. doi: 10.1016/j.ejphar.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 14.Mancini A., Serrano-Diaz J., Nava E., D’Alessandro A.M., Alonso G.L., Carmona M., Lorens S. Crocetin, a carotenoid derived from saffron (Crocus Sativus L.), improves acetylcholine-induced vascular relaxation in hypertension. J. Vasc Res. 2014;51:393–404. doi: 10.1159/000368930. [DOI] [PubMed] [Google Scholar]
- 15.Bae M., Kim H., Shin Y., Kim B.Y., Lee S.K., Oh K.B., Shin J., Oh D.C. Separacenes A–D, novel polyene polyols from the marine actinomycete, Streptomyces sp. Mar. Drugs. 2013;11:2882–2893. doi: 10.3390/md11082882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kundim B.A., Itou Y., Sakagami Y., Fudou R., Yamanaka S., Ojika M. Novel antifungal polyene amides from the myxobacterium Cystobacter fuscus: Isolation, antifungal activity and absolute structure determination. Tetrahedron. 2004;60:10217–10221. doi: 10.1016/j.tet.2004.09.013. [DOI] [Google Scholar]
- 17.Klingner A., Bothe H., Wray V., Marner F.-J. Identification of a yellow pigment formed in maize roots upon mycorrhizal colonization. Phytochemistry. 1995;38:53–55. doi: 10.1016/0031-9422(94)00538-5. [DOI] [Google Scholar]
- 18.Liu D., Li X.M., Meng L., Li C.S., Gao S.S., Shang Z., Proksch P., Huang C.G., Wang B.G. Nigerapyrones A–H, alpha-pyrone derivatives from the marine mangrove-derived endophytic fungus Aspergillus niger MA-132. J. Nat. Prod. 2011;74:1787–1791. doi: 10.1021/np200381u. [DOI] [PubMed] [Google Scholar]
- 19.Ola A., Thomy D., Lai D., Oesterhelt H., Proksch P. Inducing secondary metabolite production by the endophytic fungus Fusarium tricinctum through coculture with Bacillus subtilis. J. Nat. Prod. 2013;76:2094–2099. doi: 10.1021/np400589h. [DOI] [PubMed] [Google Scholar]
- 20.Seco J.M., Quinoá E., Riguera R. The assignment of absolute configuration by NMR. Chem. Rev. 2004;104:17–118. doi: 10.1021/cr000665j. [DOI] [PubMed] [Google Scholar]
- 21.Seco J.M., Martino M., Quiñoá E., Riguera R. Absolute configuration of 1,n-diols by NMR: The importance of the combined anisotropic effects in bis-arylmethoxyacetates. Org. Lett. 2000;2:3261–3264. doi: 10.1021/ol0062852. [DOI] [PubMed] [Google Scholar]
- 22.Li R., Chen S., Niu S., Guo L., Yin J., Che Y. Exserolides A–F, new isocoumarin derivatives from the plant endophytic fungus Exserohilum sp. Fitoterapia. 2014;96:88–94. doi: 10.1016/j.fitote.2014.04.013. [DOI] [PubMed] [Google Scholar]
- 23.Cui H., Xu B., Wu T., Xu J., Yuan Y., Gu Q. Potential antiviral lignans from the roots of Saururus chinensis with activity against Epstein–Barr virus lytic replication. J. Nat. Prod. 2014;77:100–110. doi: 10.1021/np400757k. [DOI] [PubMed] [Google Scholar]
- 24.Parisot D., Devys M., Barbier M. 6-O-demethyl-5-deoxyfusarubin and its anhydro derivative produced by a mutant of the fungus nectria haematococca blocked in fusarubin biosynthesis. J. Antibiot. 1991;44:103–107. doi: 10.7164/antibiotics.44.103. [DOI] [PubMed] [Google Scholar]
- 25.Gutiérrez M., Theoduloz C., Rodríguez J., Lolas M., Schmeda-Hirschmann G. Bioactive metabolites from the fungus Nectria galligena, the main apple canker agent in chile. J. Agric. Food Chem. 2005;53:7701–7708. doi: 10.1021/jf051021l. [DOI] [PubMed] [Google Scholar]
- 26.Irvine N.M., Yerkes C.N., Graupner P.R., Roberts R.E., Hahn D.R., Pearce C., Gerwick B.C. Synthesis and characterization of synthetic analogs of cinnacidin, a novel phytotoxin from Nectria sp. Pest. Manag. Sci. 2008;64:891–899. doi: 10.1002/ps.1579. [DOI] [PubMed] [Google Scholar]
- 27.Barber M., Hardisson A., Jackman L., Weedon B. 316. Studies in nuclear magnetic resonance. Part IV. Stereochemistry of the bixins. J. Chem. Soc. 1961:1625–1630. doi: 10.1039/jr9610001625. [DOI] [Google Scholar]
- 28.Pfander H., Wittwer F. Carotinoid-glykoside 2. Mitteilung untersuchungen zur carotinoid-zusammensetzung im safran. Helv. Chim. Acta. 1975;58:1608–1620. doi: 10.1002/hlca.19750580615. [DOI] [PubMed] [Google Scholar]
- 29.Eschenmoser W., Hans Eugster C. Absolute konfiguration von azafrin. Helv. Chim. Acta. 1975;58:1722–1727. doi: 10.1002/hlca.19750580624. [DOI] [Google Scholar]
- 30.Schwenk D., Nett M., Dahse H.M., Horn U., Blanchette R.A., Hoffmeister D. Injury-induced biosynthesis of methyl-branched polyene pigments in a white-rotting basidiomycete. J. Nat. Prod. 2014;77:2658–2663. doi: 10.1021/np500552a. [DOI] [PubMed] [Google Scholar]
- 31.Lai S., Shizuri Y., Yamamura S., Kawai K., Furukawa H. Three new phenolic metalolites from Penicillium species. Heterocycles. 1991;32:297–305. [Google Scholar]