Abstract
The lipopolysaccharide binding domain (LBD) in anti-lipopolysaccharide factor (ALF) is the main functional element of ALF, which exhibits antimicrobial activities. Our previous studies show that the peptide LBDv, synthesized based on the modified sequence of LBD (named LBD2) from FcALF2, exhibited an apparently enhanced antimicrobial activity. To learn the prospect of LBDv application, the characteristics of LBDv were analyzed in the present study. The LBDv peptide showed higher antimicrobial and bactericidal activities compared with LBD2. These activities of the LBDv peptide were stable after heat treatment. LBDv could also exhibit in vivo antimicrobial activity to Vibrio harveyi. The LBDv peptide was found to bind bacteria, quickly cause bacterial agglutination, and kill bacteria by damaging their membrane integrity. Structure analysis showed that both LBDv and LBD2 held the β-sheet structure, and the positive net charge and amphipathicity characteristic were speculated as two important components for their antimicrobial activity. The cytotoxicity of LBDv was evaluated in cultured Spodoptera frugiperda (Sf9) cells and Cherax quadricarinatus hemocytes. More than 80% cells could survive with the LBDv concentration up to 16 μM. Collectively, these findings highlighted the potential antimicrobial mechanism of LBD peptides, and provided important information for the commercial use of LBDv in the future.
Keywords: LPS binding domain (LBD), antimicrobial, heat stability, structure, cytotoxicity
1. Introduction
Antimicrobial peptides (AMPs) are evolutionarily conserved small molecules consisting of less than 50 amino acid residues with positive charges and strong amphipathy [1]. They play a major role in the innate immune system as the first line of defense against invading microbes among most multi-cellular organisms [2,3]. These peptides possess a broad spectrum of antimicrobial activity against both Gram-positive and Gram-negative bacteria, fungi, protozoan parasites and some enveloped virus [4,5,6]. AMPs could modulate the innate immune response through a range of activities [7]. The action mechanism of cationic AMPs has been proposed [1]. Usually, the cationic AMPs bind to the negatively charged lipopolysaccharide (LPS) or lipoteichoic acid (LA) of bacteria and then lead to the perforation of their cell walls. Therefore, the cell walls of the bacteria are regarded as the main targets of AMPs [8,9]. Different from traditional antibiotics, AMPs could hardly lead to the bacterial resistance [10]. Therefore, AMPs are regarded as potential candidates of novel antibiotics.
Till now, more and more diverse sequences and structures of AMPs (i.e., α-helical, β-sheet, extended) have been reported in different species. Although diverse sequential and structural variances of AMPs have been documented, most AMPs share some universal physicochemical features [11]. Net cationic charge, hydrophobicity and amphipathicity are the basic characters of active AMPs [12,13]. Based on clarifying the characters of AMPs, more attentions are focusing on the rational design of novel AMPs with the enhanced antimicrobial activity and decreased toxicity against eukaryotic cells by modifying the chain length [14], net charge [15], hydrophobicity [16] and structure [17]. Several novel designed AMPs have been reported through substitution of some amino acids [18,19,20]. The sequence-based approach that correlates antimicrobial activity to the presence of specific amino acid/fragment at specific position is a direct and efficient method among various strategies in designing novel analogues of AMPs [21].
Anti-lipopolysaccharide factors (ALFs) have been regarded as important effectors of the innate immune system in crustaceans [22]. The LPS binding domain (LBD) is considered as the functional domain for antimicrobial and antiviral activities [23,24]. Multiple isoforms of LBDs exhibit different antimicrobial activities against either Gram-positive or Gram-negative bacteria [25,26]. The LBD motif of rALF-Pm3 shows a β-hairpin structure with two anti-parallel β-strands linking by a disulfide bond [27]. Some synthetic cationic LBDs peptides exhibit antimicrobial activity with high-efficiency by disrupting the bacterial cell wall [28].
Our previous study shows that the synthetic LBD peptide of FcALF2 (named LBD2) from Fenneropenaeus chinensis exhibits high inhibition activity to bacteria and white spot syndrome virus (WSSV) [25]. The modified synthetic LBD peptide (designated LBDv) of FcALF2 through substitution of some amino acids shows an apparently enhanced antibacterial activity [29]. In order to learn the prospect of LBDv application in the future, the characters of LBDv were evaluated, and its structure was analyzed to learn the mechanism of increased antimicrobial activity. These data will provide useful information for the development of antimicrobial drugs with high activity.
2. Results
2.1. Antibacterial and Bactericidal Activity of LBDv Peptides
The minimal growth inhibition concentration (MIC) and the minimal bactericidal concentration (MBC) of LBDv are shown in Table 1. The MIC and MBC of LBDv peptides against both G+ and G− bacteria are much lower than those of LBD2, which indicates that the antibacterial activity of the modified peptide LBDv is enhanced and the antibacterial spectrum is broadened compared with LBD2 peptide without modification.
Table 1.
Antimicrobial activities of the engineered LPS-binding domain (LBD) peptides after incubation with different kinds of bacteria
| Microorganisms | LBD2 (μM) | LBDv (μM) | Heated LBDv (μM) | |||
|---|---|---|---|---|---|---|
| MIC a | MBC b | MIC | MBC | MIC | MBC | |
| Gram negative bacteria (G−): | ||||||
| Escherichia coli | >64 | >64 | 0.5–1 | 4–8 | 1–2 | 4–8 |
| Vibrio alginolyticus | 2–4 | 16–32 | 1–2 | 8–16 | 2–4 | 8–16 |
| Vibrio harveyi | 2–4 | 16–32 | 1–2 | 2–4 | 2–4 | 2–4 |
| Vibrio Parahaemolyticus | 32–64 | 32–64 | 1–2 | 4–8 | 2–4 | 4–8 |
| Gram positive bacteria (G+): | ||||||
| Bacillus licheniformis | 16–32 | >64 | 8–16 | 32–64 | 16–32 | 32–64 |
| Staphylococcus epidermidis | 32–64 | >64 | 4–8 | 16–32 | 4–8 | 16–32 |
| Micrococcus luteus | 2–4 | >64 | 4–8 | 16–32 | 4–8 | 16–32 |
a MIC, minimal inhibitory concentration; b MBC, minimal bactericidal concentration.
The MIC and MBC of heat treated LBDv against different bacteria are also shown in Table 1. The MIC and MBC of treated LBDv show no significant difference compared with LBDv without any treatment, suggesting that LBDv shows strong heat stability.
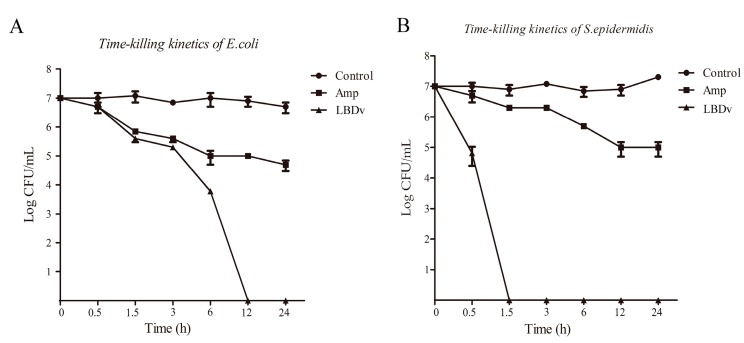
The time-killing curves of LBDv against Escherichia coli and Staphylococcus epidermidis are shown in Figure 1. After incubation with 64 μM of LBDv, E. coli is completely killed within 12 h, and S. epidermidis is killed within 1.5 h. Compared to ampicillin, 64 μM LBDv could kill the target bacteria in much shorter time than ampicillin with same concentration.
Figure 1.
Time-kill experiment of LBDv for Escherichia coli and Staphylococcus epidermidis. Y-axis represents the logarithm of colony forming units (CFU) determined by serial dilution on LB agar. X-axis represents the time point after incubation with 64 μM LBDv peptides. The 64 μM ampicillin and pGFP peptides are used as positive and negative control. Data are shown by the means ± S.E. Three replicate experiments are performed.
2.2. In Vivo Detection of the Antimicrobial Activity of LBDv Peptide
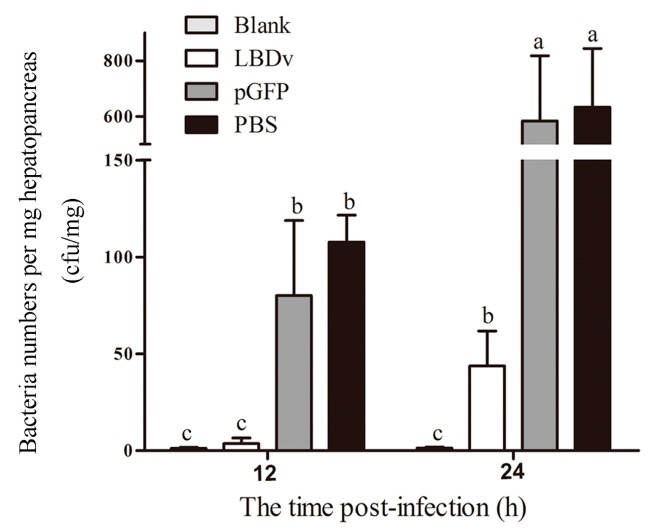
The colony numbers of Vibrio harveyi in the hepatopancreas of shrimp at 12 h and 24 h after injection are shown in Figure 2. The colony numbers of V. harveyi in the hepatopancreas of shrimp from LBDv peptide group are significantly lower (p < 0.05) than those in pGFP and PBS group at both 12 h and 24 h after injection.
Figure 2.
In vivo effect of LBDv on bacterial infections. Data are shown as the means ± S.E. Colony forming units of Vibrio harveyi are assessed after plating samples of the hepatopancreas onto TCBS agar plates. Lowercase letters (a, b, and c) represent significant difference among treatments at p < 0.05. The data are analyzed based on ANOVA with post hoc.
2.3. The Binding and Agglutination Activity of LBDv
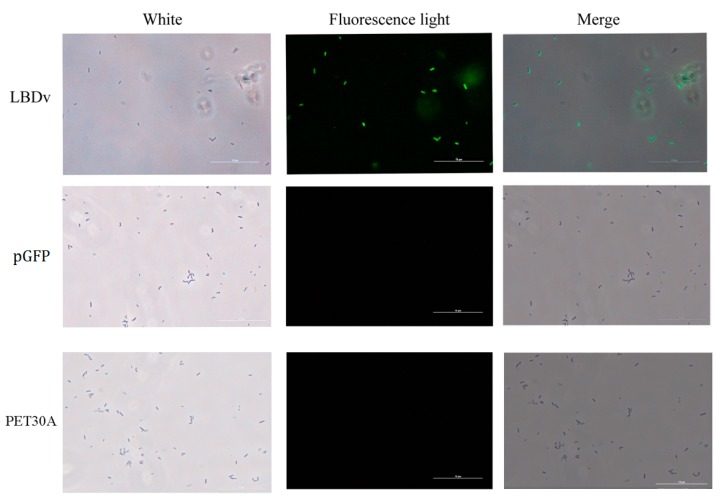
The binding detection of LBDv-His peptide to E. coli is shown in Figure 3. Compared with the control, apparent fluorescence is observed for E. coli after incubation with the LBDv-His peptide, whereas no fluorescence is detected in the controls (pGFP and PET30A treatments).
Figure 3.
Interaction of LBDv-His with E. coli bacterial cells. The signal is detected by immunocytochemistry with anti-His polyclonal antibody as the primary antibody. The same concentration of pGFP peptide and PET30A are used as negative controls. Scale bar is 10 μm.
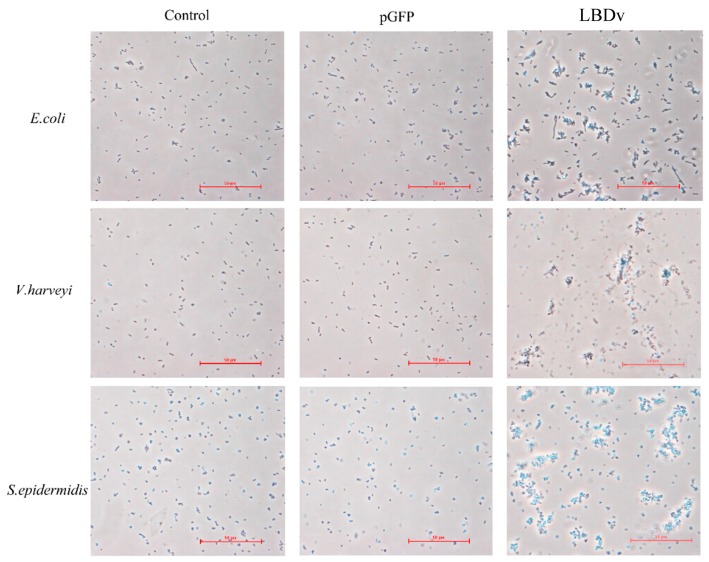
Agglutination detection shows that LBDv peptide could lead to apparent agglutination of the bacteria E. coli, V. harveyi, and S. epidermidis (Figure 4).
Figure 4.
Detection of bacterial agglutination experiment. Three kinds of bacteria are selected, including E. coli, V. harveyi and S. epidermidis. The 105 cfu/mL different bacteria are incubated with 64 μM LBDv and pGFP peptides within 30 min, respectively, and observed under optical microscope. Scale bar is 50 μm.
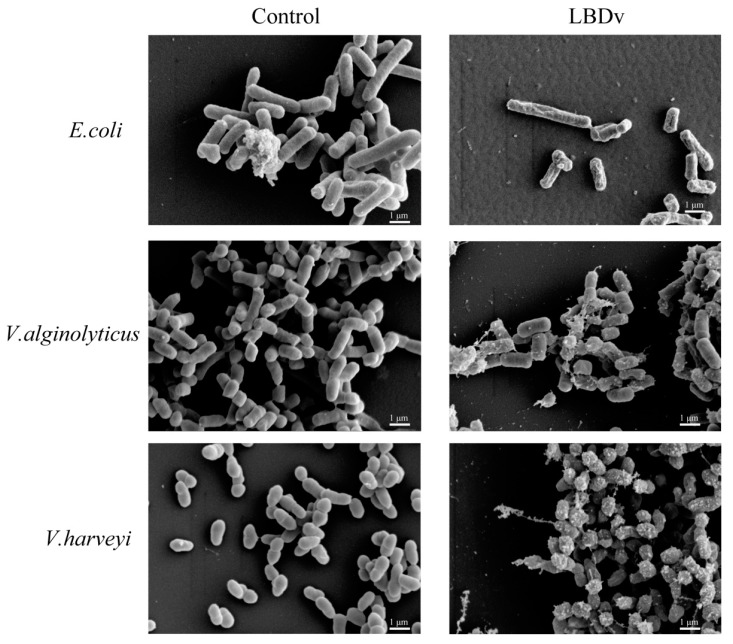
When we observe the bacteria after incubation with LBDv peptide under scanning electronic microscope, we find that the surface of bacteria treated with LBDv peptide is severely damaged with leakage of some cytoplasm, while the bacteria in control group show a relatively smooth surface (Figure 5).
Figure 5.
Morphology of the bacteria after treatments by LBDv peptide. The 108 cfu/mL different bacteria are incubated with 64 μM LBDv peptide for 2 h. The bacteria treated with same concentration pGFP peptide are used as control. Scale bar is 1 μm.
2.4. Structure Analysis of LBDv Peptide
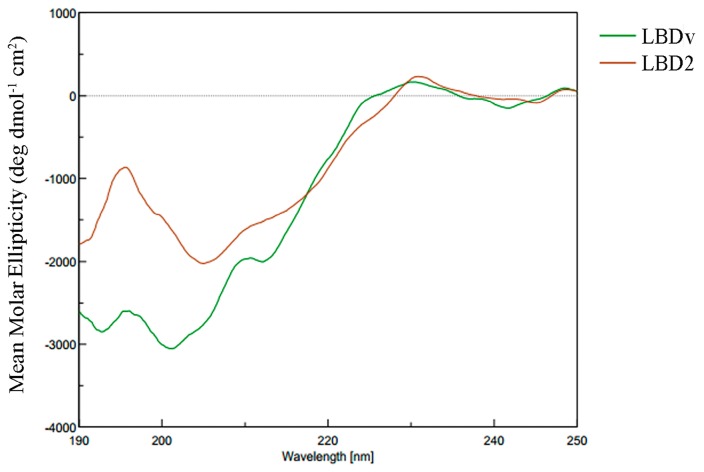
CD spectroscopy is used to measure the secondary structure of LBD2 and LBDv peptides in 20 mM phosphate buffer (pH 7.0). The CD spectra of the peptides are shown in Figure 6, which indicates that LBDv and LBD2 show a very similar secondary structure. Further analysis by Protein SSE software shows that the spectra of LBD2 and LBDv are characteristic of β-sheet conformations. The structure components of LBDv and LBD2 are shown in Table 2. LBD2 consists of 79.7% β-sheet and 20.3% random coil, while LBDv consists of 71.5% β-sheet, 4.1% β-turn and 24.4% random coil.
Figure 6.
The CD spectra of LBD2 and LBDv peptide. The peptide concentration is 0.2 mg/mL.
Table 2.
Secondary content structure of LBD2 and LBDv peptides calculated using Protein SSE software based on the CD spectra.
| Peptide | Structure Content (%) | |||
|---|---|---|---|---|
| α-helical | β-sheet | β-turn | Random coil | |
| LBD2 | 0 | 79.7 | 0 | 20.3 |
| LBDv | 0 | 71.5 | 4.1 | 24.4 |
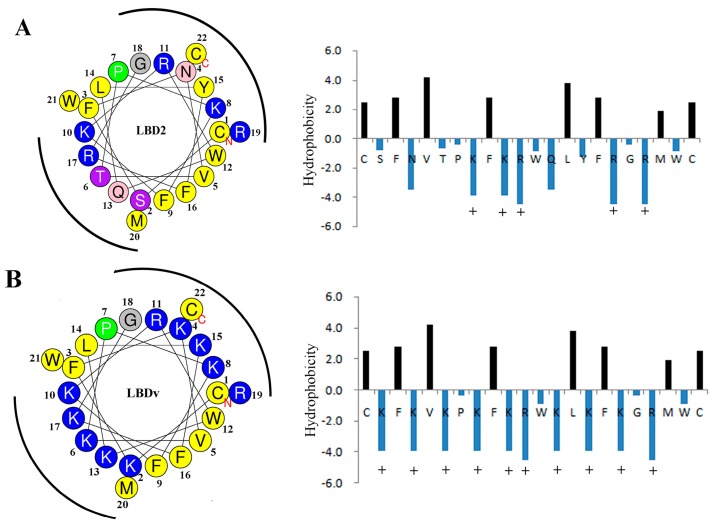
The physicochemical properties and amphipathic characters of LBDv and LBD2 are further analyzed by ProtParam tool and HeliQuest analysis. The wheel diagram and β-strand diagrams (Figure 7) show that the positively charged hydrophilic amino acid residues of LBDv are located on two sides, whereas the hydrophobic residues are on the other two sides, presenting a perfect amphipathic structure for both LBD2 peptide and LBDv. The physiochemical parameters of LBD2 and LBDv are shown in Table 3. The positive net charge and hydrophilicity of LBDv are significantly higher than those of LBD2 peptide.
Figure 7.
Helical wheel prediction and β-strand diagrams showing the distribution of amino acid side chains: (A) LBD2; and (B) LBDv. + means Lysine or Arginine. The blue color represents basic amino acids, yellow color represents the hydrophobic residues, and the other colors present the hydrophilic residues with different hydrophilic abilities.
Table 3.
Key physicochemical parameters of LBD2, LBDv and pGFP peptides.
| Peptide | Sequence | NetC a | H b | HR (%) c | GRAVY d | μH e |
|---|---|---|---|---|---|---|
| LBD2 | Ac-Y(CSFNVTPKFKRWQLYFRGRMWC)P-NH2 | 5 | 0.619 | 41 | −0.6042 | 0.103 |
| LBDv | Ac-Y(CKFKVKPKFKRWKLKFKGRMWC)P-NH2 | 10 | 0.398 | 41 | −0.9409 | 0.072 |
| pGFP | Ac-TTGKLPVPWPTLVTTFSYGVQCFS-NH2 | 1 | 0.732 | 37 | 0.3333 | 0.314 |
a NetC means net charge. Lys (K), Arg (R), His (H), and C-terminal amidation were assigned with +1 charge. Asp (D) and Glu (E) were assigned with −1 charge; b H represents mean hydrophobicity that is derived from dividing total hydrophobicity (sum of hydrophobicity indices of all residue) by the number of residues; c HR means hydrophobic ratio; d GRAVY means the Grand Average hydropathy value of the peptide; e μH represents mean hydrophobic moment.
2.5. Evaluation on the Cytotoxicity of LBDv Peptide
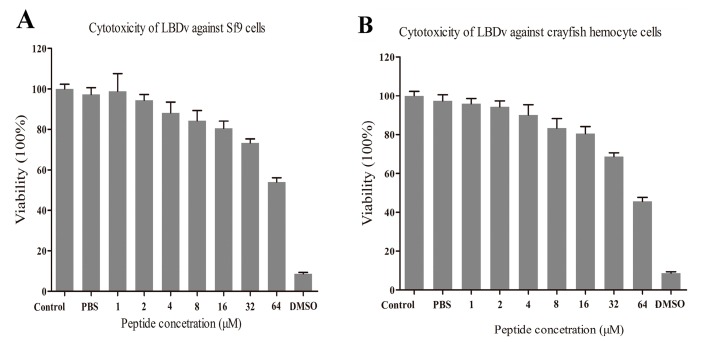
Sf9 cells and primary cultured cells of Cherax quadricarinatus hemocytesare used for the cytotoxicity detection and the cell viability is detected after addition of different concentration of peptides. From the results (shown in Figure 8), more than 80% of Sf9 cells and C. quadricarinatus hemocytes could survive in the LBDv solution with a concentration up to 16 μM. High concentration LBDv peptides solution has toxic effects on both Sf9 cells and hemocytes. Above 32 μM, LBDv exhibits significant toxicity on both cells (with mortalities of ~30% at 32 μM and ~50% at 64 μM).
Figure 8.
MTT assay of Sf9 cells and crayfish hemocytes at different LBDv concentrations: (A) cytotoxicity of LBDv against Sf9 cells; and (B) cytotoxicity of LBDv against crayfish hemocytes.
3. Discussion
Antibiotic resistance is one of the main problems concerning public health or clinical practice. AMPs have attracted a great deal of attention as the alternative antibiotic candidates [30]. The LBD peptides of ALF with 22 amino acid residues and a disulfide bond share some common traits with other AMPs [25,31]. In our previous study, a modified peptide (LBDv) derived from the LBD domain of ALF shows an enhanced antimicrobial activity, but its biological characteristics are not clear. In the present study, the biological characteristics of LBDv were analyzed. LBDv peptide could kill both Gram-negative bacteria E. coli and Gram-positive bacteria S. epidermidis at short time as other AMPs like defensin [32]. The LBDv peptide could bind to bacteria directly, which is in accordance with previous reports that LBD peptide of ALF shows a high affinity to lipopolysaccharide (LPS) and lipoteichoic acid (LA) [33]. It is interesting to note that both Gram-negative and Gram-positive bacteria could be agglutinated quickly by LBDv peptide. This phenomenon is rarely reported for AMPs, except for thanatin from insects [34]. In general, the binding of cationic peptide could reduce the surface charge density and thus reduce the electrostatic repulsion between bacteria, allowing their aggregation. Through observation on the surface of the bacteria, we found that LBDv peptide could disrupt the bacteria membrane and then cause the leakage of cytoplasm from the SEM detection. This might be the reason that LBDv peptide functions to kill bacteria. Till present, a variety of models for antimicrobial mechanism have been proposed, including the carpet, barrel-stave pore, toroidal pore, and aggregate models [35,36,37]. The proper model suitable for LBDv still needs further investigation.
Even though many naturaland modified AMPs have been studied with considerable advantages for therapeutic applications, there are still some limitations for drug development. Many AMPs generally have low stability in vivo due to the complex surrounding environments, such as the presence of protease, pH change, and physiological salt and serum conditions [38,39,40]. In the present study, the engineered LBDv peptide exhibits apparent in vivo antimicrobial activity to V. harveyi. V. harveyi has been considered as a pathogenic agent causing massive mortality in shrimp industry [41]. The effect of inhibition to this pathogenic microbe in vivo by LBDv peptide could provide some new strategies for shrimp disease control.
The secondary structure of LBDv and LBD2 was compared by CD spectra. LBDv and LBD2 sharevery similar structure such as a β-sheet. Therefore, we propose that the higher antimicrobial activity of LBDv than LBD2 to bacteria might be caused by variation of other elements. Evidence have supported that physicochemical properties of AMPs, rather than any specific amino acid sequence, are responsible for their microbiological activities [42]. Through analyzing the positive net charge and hydrophilicity of LBDv and LBD2, we found that LBDv had higher positive net charge and hydrophilicity than LBD2. The net charge of cationic AMPs can modulate antimicrobial specificity and efficacy of these peptides [43]. The cationicity of AMPs is considered to be beneficial for the initial electrostatic interactions between the AMPs peptides and negatively charged bacterial membrane components [44,45]. Increasing the net charge from +4 to +5 for magainin 2 could increaseits antimicrobial activity [15]. Giangaspero et al. shows that decreasing the net charge could reduce the antimicrobial activity of AMPs [46]. Besides the net charge, it is well accepted that the amphipathicity of AMPs is necessary for their mechanism of action [47,48]. Amphipathicity with the positively charged polar face and the non-polar face is required for insertion into the membrane interface, causing increased permeability and loss of barrier function of target cells [49]. The rational distribution of hydrophilic and hydrophobic residues for the LBDv peptides might be the reason for the enhanced antimicrobial activity.
4. Materials and Methods
4.1. Peptides Synthesis
According to our previous study, we synthesized a modified peptide (LBDv) with sequence of Ac-YCKFKVKPKFKRWKLKFKGRMWCP-NH2 based on the LBD of FcALF2 with sequence of Ac-YCSFNVTPKFKRWQLYFRGRMWCP-NH2 (accession number: JX853775) [29]. The LBDv peptide was modified with increasing the number of basic amino acids by replacing six residues (S, N, T, Q, Y and R) with lysine (K) residues. A pGFP peptide from Green Fluorescent Protein with sequence of Ac-TTGKLPVPWPTLVTTFSYGVQCFS-NH2 was also synthesized as negative control. Different peptides including LBDv, LBD and pGFP were produced by solid-phase synthesis by Ziyu Biotechnology Company (Shanghai, China). Synthetic peptides were characterized and purified by reversed-phase high-pressure liquid chromatography with >95% purity grade.
4.2. Antibacterial Activity Test
The antimicrobial activity of peptides was determined using the minimal growth inhibition concentration (MIC) assay and minimal bactericidal concentration (MBC) assay against Gram-positive and Gram-negative bacteria as described previously [25,26]. Bacteria strains including four Gram-negative bacteria, Escherichia coli, Vibrio alginolyticus, Vibrio harveyi, and Vibrio parahaemolyticus, and three Gram-positive bacteria, Bacillus licheniformis, Staphylococcus epidermidis and Micrococcus luteus, were used for detection. Briefly, bacteria cells at the mid-logarithmic-phase were diluted to 1 × 105 cfu/mL in PBS buffer (137 mmol/L NaCl, 2.7 mmol/L KCl, 10 mmol/L Na2HPO4, 1.8 mmol/L KH2PO4, pH 7.4). In sterile 96-well plates (Corning Inc., Corning, NY, USA), 15 μL peptide solution in 1/2-fold serial dilutions with PBS (pH 7.4) were added into each well. The final concentration of peptide in the medium was 64 μM, 32 μM, 16 μM, 8 μM, 4 μM, 2 μM, 1 μM and 0.5 μM. Fifteen microliters PBS and 15 μL pGFP solution were used as blank group and negative control. Then, 133 μL growth medium were added into the 96-well plates, and the mixtures were allowed to culture for 6 to 8 h depending on different bacterial strains. Absorbance at 600 nm for Gram-positive bacteria or 560 nm for Gram-negative bacteria was determined using a precision micro-plate reader (TECAN infinite M200 PRO, Salzburg, Austria). The assay was performed in triplicates. The MICs were defined as the lowest concentration of the compounds to inhibit the growth of microorganisms based on the spectroscopic absorbance readings. MBCs were determined by plotting 5 μL samples from the wells onto nutrient agar plate. MBC was the concentration at which there was no microbial growth [50]. Because only 8 peptides concentration gradients were set, the MICs and MBCs value observed at the concentration point were higher than the actual values. Thus, the actual and accurate MICs and MBCs value should between no inhibition (or bactericidal) concentrations and the observed inhibition (bactericidal) concentrations.
In order to know the heat tolerance of the peptide, the peptide LBDv was treated at 100 °C for 10 min, and its MIC and MBC value was measured as described above. The assay was performed in triplicates.
4.3. The Time-Killing Curve against Bacteria
The procedure used for the kinetics of bacterial killing assays was modified as previously described [51] to determine the rate of bacterial killing by LBDv peptide. Bacteria E. coli and S. epidermidis were chosen for the experiment. E. coli and S. epidermidis cells were grown in LB medium to exponential phase, harvested by centrifugation and washed with PBS, then bacteria was resuspended in PBS buffer. Approximately 1 × 108 cells were incubated at 37 °C with 64 μM peptide. Aliquots of 20 μL suspensions were withdrawn, serially diluted and plated on LB agar to determine the bacterial counts at 0, 0.5, 1.5, 3, 6, 12 and 24 h. After culturing at 37 °C, the number of bacteria was recorded. The time-killing curves were plotted with the log cfu/mL against time. The 64 μM ampicillin and pGFP peptides were used as positive and negative control. Triple independent experiments were performed for time-killing kinetics.
4.4. Effects of the Peptides on Bacterial Infections
For bacterial infection, V. harveyi was cultured in TSB medium at 28 °C overnight. Then bacteria cells were resuspended in sterile PBS to 5 × 107 cfu/mL. pGFP peptide and LBDv peptide were added to the cells to the final concentration of 32 μM. The bacteria diluted by PBS were used as the negative control. Exopalaemon carinicauda with body weight of 0.68 ± 0.17 g were used as the experimental animals and divided randomly into four groups: Blank, LBDv, pGFP and PBS group. The three experiment groups were injected intramuscularly with 10 μL V. harveyi, V. harveyi plus LBDv, or V. harveyi plus pGFP, respectively. Hepatopancreas was taken from shrimp at 12 h and 24 h post-infection. Three samples were collected from each group at each time point, and three individuals were put togetheras one sample. The tissues were homogenized in sterile PBS, serial diluted and plated in triplicate on TCBS agar plates. After incubation at 28 °C for 24 h, the number of colonies on the plates was recorded.
4.5. Bacteria Binding Assay
To analyze the binding activity of LBDv peptide to bacteria, the peptides with a 6× His tag have been synthesized as described previously. The E. coli cells were cultured in LB medium overnight at 37 °C. The bacteria were then collected by centrifugation, washed in PBS buffer (pH 7.4), and resuspended in PBS buffer to 108 cfu/mL. Then, 200 μL bacteria cells were incubated with 64 μM LBDv-His protein for 2 h at room temperature, then bacteria suspension was dropped onto a glass slide and fixed with 4% paraformaldehyde for 15 min, followed by washing with PBS. After treatment in blocking solution (0.5% BSA in PBS), mouse anti-His antibody (Tiangen, Beijing, China) (1/1000 dilution) was added to the slide.The slide was incubated for 1 h at room temperature and washed with PBS. Fluorescein isothiocyanate (FITC)-labeled goat anti-mouse IgG (1/1000 dilution) was added to the slide. The slide was incubated and washed as above, and observed under a fluorescence microscope (Nikon E800, Tokyo, Japan). pGFP peptide was used as control. At the same time, to exclude His tag’s influence on the bacteria binding, we also add the protein with 6×His tag produced by empty vector pET30a in E. coli (named PET30A protein) as negative control.
4.6. Bacterial Agglutination Experiment
The Gram-negative bacteria (E. coli and V. harveyi), and Gram-positive bacteria (S. epidermidis) were cultured overnight. Then, bacteria cells were diluted to 105 cfu/mL. After that, 64 μM LBDv peptide solution was incubated with the bacteria at room temperature within 30 min. The PBS and 64 μM pGFP peptide solution were used as negative control. The cells were then added to the glass slide and observed under optical Nikon TS100 microscope (Nikon Corporation, Tokyo, Japan).
4.7. Morphology of the Bacteria Treated with LBDv
The bacteria E. coli, V. alginolyticus and V. harveyi were grown to an exponential phase with shaking at 220 rpm. The cell pellets were harvested by centrifugation at 1000× g for 10 min and resuspended in PBS at 108 cfu/mL. Bacteria were incubated with 64 μM different peptides at 37 °C for 2 h. The collected bacteria were subjected to fixation with 2.5% (v/v) glutaraldehyde in 0.1 M phosphate buffer (pH 7.4) for 1 h and dehydrated for 15 min in a graded ethanol series (30%, 50%, 70%, 80%, 95% and 100%), and a mixture (1:1) of 100% ethanol and isopentyl acetate for 20 min. After critical-point drying and gold coating, the samples were visualized by Hitachi S-3400N Scanning Electron Microscope (Hitachi, Tokyo, Japan).
4.8. Structure Analysis on the Peptide LBDv and LBD2
The secondary structure content of LBD2 and LBDv were measured by circular dichroism spectroscopy (JASCO 715, Tokyo, Japan) in far UV. Spectra were recorded at room temperature, over the range of 190 to 250 nm. The peptide concentration was 0.2 mg/mL in 20 mM phosphate buffer pH 7.0. We used a scan speed of 50 nm/min, a 0.1 cm cell path length, a response time of 4 s and a band width of 1 nm. A blank spectrum containing all components except the peptide was subtracted from individual spectra to account for the baseline. Finally, the data were smoothed using Jwstda32 software. The acquired CD spectra were then converted to the mean molar ellipticity (deg·dmol−1·cm2). The secondary structural elements of the peptides, including α helix, β-sheet, β-turn and random coil, were calculated based on the CD spectra using Protein SSE software according to a standard reference spectrum.
Different physicochemical properties of peptides (i.e., net charge and hydrophobic percentage) were calculated using the online program Antimicrobial Peptide Database (http://aps.unmc.edu/AP/main.php) and ProtParam tool (http://web.expasy.org/protparam/). The amphipathic characters of these peptides were predicted using the online program HeliQuest analysis (http://heliquest.ipmc.cnrs.fr/cgi-bin/ComputParams.py).
4.9. Cytotoxicity Assays
Cytotoxicity against Spodoptera frugiperda (Sf9) cell lines and Cherax quadricarinatus hemocytes were examined by a tetrazolium-based (MTT) assay. The Sf9 cell lines were routinely maintained at 27 °C in sterile 48-well plates (Corning Inc., Corning, NY, USA) containing 200 μL Grace’s insect cell culture medium (Gibco Inc., Grand Island, NY, USA). Hemolymph was taken from C. quadricarinatus with equal volume of anticoagulant solution (26 mM sodium citrate, 30 mM citric acid, 100 mM glucose, 140 mM NaCl, pH 5.8). Then hemocytes were collected by centrifugation at 800 g for 5 min and resuspended in L15 medium (Gibco Inc., Grand Island, NY, USA) supplemented with 15% fetal bovine serum. The hemocytes were seeded in sterile 48-well plates (Corning Inc., Corning, NY, USA) with a density of 1 × 105 cells/well and maintained at 27 °C. For the MTT assay, sterile peptide solution with PBS (pH 7.4) were added into each well to the final concentration of 1, 2, 4, 8, 16, 32, 64 μM. DMSO (0.5%) was added into the medium as the positive control. Triple independent experiments were performed. After 24 h, the cells were washed with PBS and treated with MTT formazan at a concentration of 0.5 mg/mL. After 6 h, crystal was centrifuged and dissolved in dimethyl sulfoxide, and the absorbance was determined at 570 nm using a precision micro-plate reader (TECAN infinite M200 PRO, Salzburg, Austria) to determine the percent cytotoxicity.
4.10. Statistical Analysis
All the data were given in the form of mean ± S.E. and analyzed with one-way analysis of variance (ANOVA) and Duncan’s Multiple Comparisons. Differences between treatments and controls were considered significant at p < 0.05.
5. Conclusions
In conclusion, the engineered peptide LBDv exhibits broad-spectrum antimicrobial and antiviral activities in vitro and in vivo. We propose that net positive charge and amphipathicity characteristic are the important components for antimicrobial activity of different LBDs. Further cytotoxicity analysis of LBDv peptide suggests that the peptide has application prospect. These data provide useful information to develop new AMPs drugs for potential therapeutic application in medical areasas well as in control of disease in aquaculture.
Acknowledgments
This work was financially supported by the China Agriculture Research system-47 (CARS-47), National High Technology Research and Development Program of China (863 Program, No. 2014AA093501), Major State Basic Research Development Program of China (973 program) (2012CB114403), General Program of National Natural Science Foundation of China (31272683) to Fuhua Li, and the Scientific and Technological Innovation Project Financially Supported by Qingdao National Laboratory for Marine Science and Technology (No.2015ASKJ02). This work was also supported by the Hangzhou Qianjiang Distinguished Expert Program.
Author Contributions
Conceived and designed the experiments: S.L., F.L., and J.X. Performed the experiments: H.Y. Analyzed and discussed the data: H.Y., S.L., and F.L. Wrote the paper: H.Y., F.L., and S.L.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Powers J.-P.S., Hancock R.E. The relationship between peptide structure and antibacterial activity. Peptides. 2003;24:1681–1691. doi: 10.1016/j.peptides.2003.08.023. [DOI] [PubMed] [Google Scholar]
- 2.Boman H.G. Peptide antibiotics and their role in innate immunity. Annu. Rev. Immonul. 1995;13:61–92. doi: 10.1146/annurev.iy.13.040195.000425. [DOI] [PubMed] [Google Scholar]
- 3.Zasloff M. Antimicrobial peptides of multicellular organisms. Nature. 2002;415:389–395. doi: 10.1038/415389a. [DOI] [PubMed] [Google Scholar]
- 4.Aley S.B., Zimmerman M., Hetsko M., Selsted M.E., Gillin F.D. Killing of Giardia lamblia by cryptdins and cationic neutrophil peptides. Infect. Immun. 1994;62:5397–5403. doi: 10.1128/iai.62.12.5397-5403.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mohammad F.V., Noorwala M., Ahmad V.U., Sener B. Bidesmosidic triterpenoidal saponins from the roots of Symphytum officinale. Planta Med. 1995;61:94. doi: 10.1055/s-2006-958017. [DOI] [PubMed] [Google Scholar]
- 6.Nicolas P., Mor A. Peptides as weapons against microorganisms in the chemical defense system of vertebrates. Annu. Rev. Immonul. 1995;49:277–304. doi: 10.1146/annurev.mi.49.100195.001425. [DOI] [PubMed] [Google Scholar]
- 7.Boman H. Antibacterial peptides: Basic facts and emerging concepts. J. Intern. Med. 2003;254:197–215. doi: 10.1046/j.1365-2796.2003.01228.x. [DOI] [PubMed] [Google Scholar]
- 8.Dimarcq J.L., Bulet P., Hetru C., Hoffmann J. Cysteine-rich antimicrobial peptides in invertebrates. Pept. Sci. 1998;47:465–477. doi: 10.1002/(SICI)1097-0282(1998)47:6<465::AID-BIP5>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 9.Hancock R.E. Host Defence (bdCationic) Peptides. Drugs. 1999;57:469–473. doi: 10.2165/00003495-199957040-00002. [DOI] [PubMed] [Google Scholar]
- 10.Nizet V. Antimicrobial peptide resistance mechanisms of human bacterial pathogens. Curr. Issues Mol. Biol. 2006;8:11–26. [PubMed] [Google Scholar]
- 11.Yount N.Y., Yeaman M.R. Multidimensional signatures in antimicrobial peptides. Proc. Natl. Acad. Sci. USA. 2004;101:7363–7368. doi: 10.1073/pnas.0401567101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yin L.M., Edwards M.A., Li J., Yip C.M., Deber C.M. Roles of hydrophobicity and charge distribution of cationic antimicrobial peptides in peptide-membrane interactions. J. Biol. Chem. 2012;287:7738–7745. doi: 10.1074/jbc.M111.303602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen Y., Guarnieri M.T., Vasil A.I., Vasil M.L., Mant C.T., Hodges R.S. Role of peptide hydrophobicity in the mechanism of action of α-helical antimicrobial peptides. Antimicrob. Agents Chemother. 2007;51:1398–1406. doi: 10.1128/AAC.00925-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shin S.Y., Lee M.K., Kim K.L., Hahm K.S. Structure-antitumor and hemolytic activity relationships of synthetic peptides derived from cecropin A-magainin 2 and cecropin A-melittin hybrid peptides. J. Pept. Sci. 1997;50:279–285. doi: 10.1111/j.1399-3011.1997.tb01469.x. [DOI] [PubMed] [Google Scholar]
- 15.Dathe M., Nikolenko H., Meyer J., Beyermann M., Bienert M. Optimization of the antimicrobial activity of magainin peptides by modification of charge. Febs. Lett. 2001;501:146–150. doi: 10.1016/S0014-5793(01)02648-5. [DOI] [PubMed] [Google Scholar]
- 16.Lazarovici P., Primor N., Loew L.M. Purification and pore-forming activity of two hydrophobic polypeptides from the secretion of the Red Sea Moses sole (Pardachirus marmoratus) J. Biol. Chem. 1986;261:16704–16713. [PubMed] [Google Scholar]
- 17.Maeng C.-Y., Oh M.S., Park I.H., Hong H.J. Purification and structural analysis of the hepatitis B virus preS1 expressed from Escherichia coli. Biochem. Biophys. Res. Commun. 2001;282:787–792. doi: 10.1006/bbrc.2001.4641. [DOI] [PubMed] [Google Scholar]
- 18.Deslouches B., Steckbeck J.D., Craigo J.K., Doi Y., Mietzner T.A., Montelaro R.C. Rational design of engineered cationic antimicrobial peptides consisting exclusively of arginine and tryptophan, and their activity against multidrug-resistant pathogens. Antimicrob. Agents Chemother. 2013;57:2511–2521. doi: 10.1128/AAC.02218-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ueno S., Minaba M., Nishiuchi Y., Taichi M., Tamada Y., Yamazaki T., Kato Y. Generation of novel cationic antimicrobial peptides from natural non-antimicrobial sequences by acid-amide substitution. Ann. Clin. Microbiol. Antimicrob. 2011;10:11–14. doi: 10.1186/1476-0711-10-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frecer V., Ho B., Ding J.L. Interpretation of biological activity data of bacterial endotoxins by simple molecular models of mechanism of action. Eur. J. Biochem. 2000;267:837–852. doi: 10.1046/j.1432-1327.2000.01069.x. [DOI] [PubMed] [Google Scholar]
- 21.Le C.-F., Yusof M.Y. M., Hassan H., Sekaran S.D. In vitro properties of designed antimicrobial peptides that exhibit potent antipneumococcal activity and produces synergism in combination with penicillin. Sci. Rep. 2015;5:9761. doi: 10.1038/srep09761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tanaka S., Nakamura T., Morita T., Iwanaga S. Limulus anti-LPS factor: An anticoagulant which inhibits the endotoxin-mediated activation of Limulus coagulation system. Biochem. Biophys. Res. Commun. 1982;105:717–723. doi: 10.1016/0006-291X(82)91493-0. [DOI] [PubMed] [Google Scholar]
- 23.Hoess R.H. Phage display of peptides and protein domains: Current opinion in structural biology. Curr. Opin. Struct. Biol. 1993;3:572–579. doi: 10.1016/0959-440X(93)90085-Y. [DOI] [Google Scholar]
- 24.Supungul P., Klinbunga S., Pichyangkura R., Hirono I., Aoki T., Tassanakajon A. Antimicrobial peptides discovered in the black tiger shrimp Penaeus monodon using the EST approach. Dis. Aquat. Organ. 2004;61:123–135. doi: 10.3354/dao061123. [DOI] [PubMed] [Google Scholar]
- 25.Li S., Guo S., Li F., Xiang J. Functional Diversity of Anti-Lipopolysaccharide Factor Isoforms in Shrimp and Their Characters Related to Antiviral Activity. Mar. Drugs. 2015;13:2602–2616. doi: 10.3390/md13052602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang H., Li S., Li F., Lv X., Xiang J. Recombinant expression and functional analysis of an isoform of anti-lipopolysaccharide factors (FcALF5) from Chinese shrimp Fenneropenaeus chinensis. Dev. Comp. Immunol. 2015;53:47–54. doi: 10.1016/j.dci.2015.06.015. [DOI] [PubMed] [Google Scholar]
- 27.Yang Y., Boze H., Chemardin P., Padilla A., Moulin G., Tassanakajon A., Pugnière M., Roquet F., Destoumieux-Garzón D., Gueguen Y. NMR structure of Ralf-Pm3, an anti-lipopolysaccharide factor from shrimp: Model of the possible lipid A-binding site. Biopolymers. 2009;91:207–220. doi: 10.1002/bip.21119. [DOI] [PubMed] [Google Scholar]
- 28.Jaree P., Tassanakajon A., Somboonwiwat K. Effect of the anti-lipopolysaccharide factor isoform 3 (ALFPm3) from Penaeus monodon on Vibrio harveyi cells. Dev. Comp. Immunol. 2012;38:554–560. doi: 10.1016/j.dci.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 29.Li S., Guo S., Li F., Xiang J. Characterization and function analysis of an anti-lipopolysaccharide factor (ALF) from the Chinese shrimp Fenneropenaeus chinensis. Dev. Comp. Immunol. 2014;46:349–355. doi: 10.1016/j.dci.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 30.Maróti G., Kereszt A., Kondorosi E., Mergaert P. Natural roles of antimicrobial peptides in microbes, plants and animals. Res. Microbiol. 2011;162:363–374. doi: 10.1016/j.resmic.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 31.Dathe M., Wieprecht T., Nikolenko H., Handel L., Maloy W.L., MacDonald D.L., Beyermann M., Bienert M. Hydrophobicity, hydrophobic moment and angle subtended by charged residues modulate antibacterial and haemolytic activity of amphipathic helical peptides. FEBS Lett. 1997;403:208–212. doi: 10.1016/S0014-5793(97)00055-0. [DOI] [PubMed] [Google Scholar]
- 32.Zhang L., Yang D., Wang Q., Yuan Z., Wu H., Pei D., Cong M., Li F., Ji C., Zhao J. A defensin from clam Venerupis philippinarum: Molecular characterization, localization, antibacterial activity, and mechanism of action. Dev. Comp. Immunol. 2015;51:29–38. doi: 10.1016/j.dci.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 33.Somboonwiwat K., Bachère E., Rimphanitchayakit V., Tassanakajon A. Localization of anti-lipopolysaccharide factor (ALFPm3) in tissues of the black tiger shrimp, Penaeus monodon, and characterization of its binding properties. Dev. Comp. Immunol. 2008;32:1170–1176. doi: 10.1016/j.dci.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 34.Fehlbaum P., Bulet P., Chernysh S., Briand J.-P., Roussel J.-P., Letellier L., Hetru C., Hoffmann J.A. Structure-activity analysis of thanatin, a 21-residue inducible insect defense peptide with sequence homology to frog skin antimicrobial peptides. Proc. Natl. Acad. Sci. USA. 1996;93:1221–1225. doi: 10.1073/pnas.93.3.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shai Y. Mode of action of membrane active antimicrobial peptides. Pept. Sci. 2002;66:236–248. doi: 10.1002/bip.10260. [DOI] [PubMed] [Google Scholar]
- 36.Matsuzaki K. Magainins as paradigm for the mode of action of pore forming polypeptides. Biochim. Biophys. Acta. 1998;1376:391–400. doi: 10.1016/S0304-4157(98)00014-8. [DOI] [PubMed] [Google Scholar]
- 37.Wu M., Maier E., Benz R., Hancock R.E. Mechanism of interaction of different classes of cationic antimicrobial peptides with planar bilayers and with the cytoplasmic membrane of Escherichia coli. Biochemistry. 1999;38:7235–7242. doi: 10.1021/bi9826299. [DOI] [PubMed] [Google Scholar]
- 38.Rozek A., Powers J.-P.S., Friedrich C.L., Hancock R.E. Structure-based design of an indolicidin peptide analogue with increased protease stability. Biochemistry. 2003;42:14130–14138. doi: 10.1021/bi035643g. [DOI] [PubMed] [Google Scholar]
- 39.Lee I.H., Cho Y., Lehrer R.I. Effects of pH and salinity on the antimicrobial properties of clavanins. Infect. Immun. 1997;65:2898–2903. doi: 10.1128/iai.65.7.2898-2903.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maisetta G., Di Luca M., Esin S., Florio W., Brancatisano F.L., Bottai D., Campa M., Batoni G. Evaluation of the inhibitory effects of human serum components on bactericidal activity of human beta defensin 3. Peptides. 2008;29:1–6. doi: 10.1016/j.peptides.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 41.Karunasagar I., Pai R., Malathi G., Karunasagar I. Mass mortality of Penaeus monodon larvae due to antibiotic-resistant Vibrio harveyi infection. Aquaculture. 1994;128:203–209. doi: 10.1016/0044-8486(94)90309-3. [DOI] [Google Scholar]
- 42.Findlay B., Zhanel G.G., Schweizer F. Cationic amphiphiles, a new generation of antimicrobials inspired by the natural antimicrobial peptide scaffold. Antimicrob. Agents Chemother. 2010;54:4049–4058. doi: 10.1128/AAC.00530-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tossi A., Sandri L., Giangaspero A. Amphipathic, α-helical antimicrobial peptides. Pept. Sci. 2000;55:4–30. doi: 10.1002/1097-0282(2000)55:1<4::AID-BIP30>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 44.Takahashi D., Shukla S.K., Prakash O., Zhang G. Structural determinants of host defense peptides for antimicrobial activity and target cell selectivity. Biochimie. 2010;92:1236–1241. doi: 10.1016/j.biochi.2010.02.023. [DOI] [PubMed] [Google Scholar]
- 45.Matsuzaki K. Control of cell selectivity of antimicrobial peptides. Biochim. Biophys. Acta. 2009;1788:1687–1692. doi: 10.1016/j.bbamem.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 46.Giangaspero A., Sandri L., Tossi A. Amphipathic α helical antimicrobial peptides. Eur. J. Biochem. 2001;268:5589–5600. doi: 10.1046/j.1432-1033.2001.02494.x. [DOI] [PubMed] [Google Scholar]
- 47.Wiradharma N., Sng M., Khan M., Ong Z.Y., Yang Y.Y. Rationally Designedα-Helical Broad-Spectrum Antimicrobial Peptides with Idealized Facial Amphiphilicity. Macromol. Rapid. Commun. 2013;34:74–80. doi: 10.1002/marc.201200534. [DOI] [PubMed] [Google Scholar]
- 48.Nell M.J., Tjabringa G.S., Wafelman A.R., Verrijk R., Hiemstra P.S., Drijfhout J.W., Grote J.J. Development of novel LL-37 derived antimicrobial peptides with LPS and LTA neutralizing and antimicrobial activities for therapeutic application. Peptides. 2006;27:649–660. doi: 10.1016/j.peptides.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 49.Hancock R.E., Lehrer R. Cationic peptides: A new source of antibiotics. Trends. Biotechnol. 1998;16:82–88. doi: 10.1016/S0167-7799(97)01156-6. [DOI] [PubMed] [Google Scholar]
- 50.Usman H., Osuji J.C. Phytochemical and in vitro antimicrobial assay of the leaf extract of Newbouldia laevis. Afr. J. Tradit. Complem. 2008;4:476–480. doi: 10.4314/ajtcam.v4i4.31240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Deslouches B., Islam K., Craigo J.K., Paranjape S.M., Montelaro R.C., Mietzner T.A. Activity of the de novo engineered antimicrobial peptide WLBU2 against Pseudomonas aeruginosa in human serum and whole blood: Implications for systemic applications. Antimicrob. Agents Chemother. 2005;49:3208–3216. doi: 10.1128/AAC.49.8.3208-3216.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]