Abstract
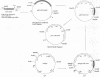
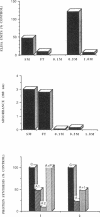
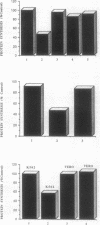
The construction and expression of a chimeric gene encoding a mouse/human antibody to the human transferrin receptor fused to the gene for angiogenin, a human homolog of pancreatic RNase, are described. F(ab')2-like antibody-enzyme fusions were prepared by linking the gene for human angiogenin to a chimeric anti-transferrin receptor heavy chain gene. The antibody-enzyme fusion gene was introduced into a transfectoma that secretes the chimeric light chain of the same antibody, and cell lines were cloned that synthesize and secrete the antibody-enzyme fusion protein of the expected size at a concentration of 1-5 ng/ml. Culture supernatants from clones secreting the fusion protein caused inhibition of growth and protein synthesis of K562 cells that express the human transferrin receptor but not toward a non-human-derived cell line that lacks this receptor. Whereas excess antibody to the same receptor did not itself inhibit protein synthesis, it was able to completely prevent the protein synthesis inhibition caused by the fusion protein. These results indicate that the cytotoxicity is due to a transferrin receptor-mediated mechanism involving the angiogenin portion of the fusion protein and demonstrate the feasibility of constructing recombinant antibody-RNase molecules capable of killing tumor cells bearing the transferrin receptor. The significance of the acquired cytotoxicity of a mouse/human chimeric antibody linked to a human protein may bear importantly in human therapeutic strategies that use mouse antibodies linked to toxins from plants or bacteria to target tumor cells. It is expected that the humanization of immunotoxins will lead to less toxicity and immunogenicity than currently available reagents.
Full text
PDF
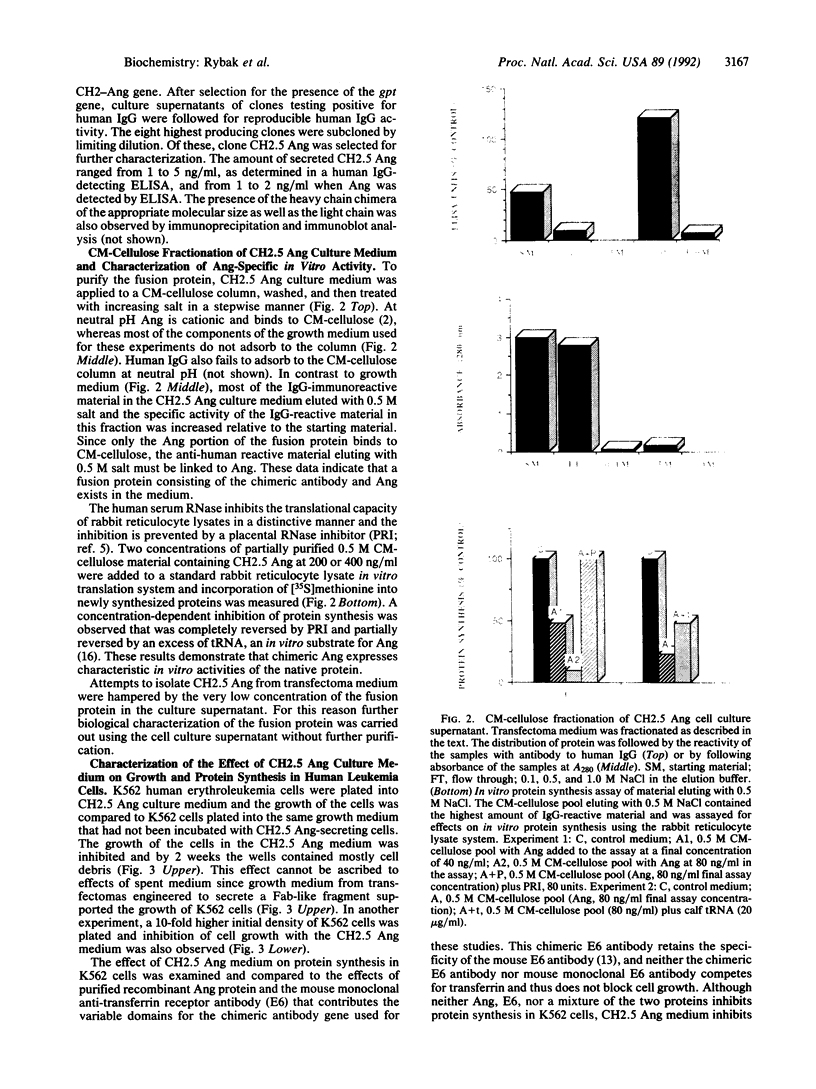
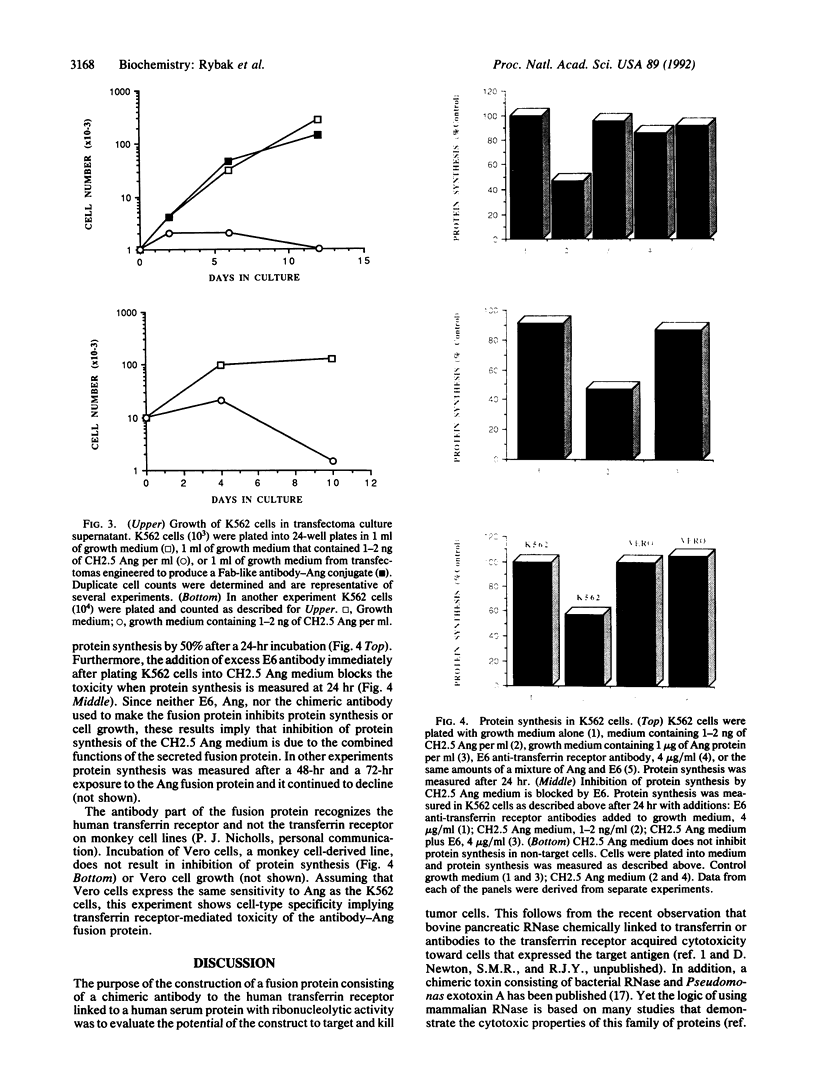

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blank A., Dekker C., Schieven G., Sugiyama R., Thelen M. Human body fluid ribonucleases: detection, interrelationships and significance. Nucleic Acids Symp Ser. 1981;(10):203–209. [PubMed] [Google Scholar]
- Casadei J., Powell M. J., Kenten J. H. Expression and secretion of aequorin as a chimeric antibody by means of a mammalian expression vector. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2047–2051. doi: 10.1073/pnas.87.6.2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fett J. W., Strydom D. J., Lobb R. R., Alderman E. M., Bethune J. L., Riordan J. F., Vallee B. L. Isolation and characterization of angiogenin, an angiogenic protein from human carcinoma cells. Biochemistry. 1985 Sep 24;24(20):5480–5486. doi: 10.1021/bi00341a030. [DOI] [PubMed] [Google Scholar]
- Gleich G. J., Loegering D. A., Bell M. P., Checkel J. L., Ackerman S. J., McKean D. J. Biochemical and functional similarities between human eosinophil-derived neurotoxin and eosinophil cationic protein: homology with ribonuclease. Proc Natl Acad Sci U S A. 1986 May;83(10):3146–3150. doi: 10.1073/pnas.83.10.3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogenboom H. R., Raus J. C., Volckaert G. Cloning and expression of a chimeric antibody directed against the human transferrin receptor. J Immunol. 1990 Apr 15;144(8):3211–3217. [PubMed] [Google Scholar]
- Hoogenboom H. R., Raus J. C., Volckaert G. Targeting of tumor necrosis factor to tumor cells: secretion by myeloma cells of a genetically engineered antibody-tumor necrosis factor hybrid molecule. Biochim Biophys Acta. 1991 Jun 5;1096(4):345–354. doi: 10.1016/0925-4439(91)90071-g. [DOI] [PubMed] [Google Scholar]
- Hoogenboom H. R., Volckaert G., Raus J. C. Construction and expression of antibody-tumor necrosis factor fusion proteins. Mol Immunol. 1991 Sep;28(9):1027–1037. doi: 10.1016/0161-5890(91)90189-q. [DOI] [PubMed] [Google Scholar]
- Kurachi K., Davie E. W., Strydom D. J., Riordan J. F., Vallee B. L. Sequence of the cDNA and gene for angiogenin, a human angiogenesis factor. Biochemistry. 1985 Sep 24;24(20):5494–5499. doi: 10.1021/bi00341a032. [DOI] [PubMed] [Google Scholar]
- Lee F. S., Vallee B. L. Characterization of ribonucleolytic activity of angiogenin towards tRNA. Biochem Biophys Res Commun. 1989 May 30;161(1):121–126. doi: 10.1016/0006-291x(89)91569-6. [DOI] [PubMed] [Google Scholar]
- Neuberger M. S. Expression and regulation of immunoglobulin heavy chain gene transfected into lymphoid cells. EMBO J. 1983;2(8):1373–1378. doi: 10.1002/j.1460-2075.1983.tb01594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuberger M. S., Williams G. T., Fox R. O. Recombinant antibodies possessing novel effector functions. Nature. 1984 Dec 13;312(5995):604–608. doi: 10.1038/312604a0. [DOI] [PubMed] [Google Scholar]
- Prior T. I., FitzGerald D. J., Pastan I. Barnase toxin: a new chimeric toxin composed of pseudomonas exotoxin A and barnase. Cell. 1991 Mar 8;64(5):1017–1023. doi: 10.1016/0092-8674(91)90325-s. [DOI] [PubMed] [Google Scholar]
- Reddi K. K. Nature and possible origin of human serum ribonuclease. Biochem Biophys Res Commun. 1975 Nov 3;67(1):110–118. doi: 10.1016/0006-291x(75)90290-9. [DOI] [PubMed] [Google Scholar]
- Rybak S. M., Fett J. W., Yao Q. Z., Vallee B. L. Angiogenin mRNA in human tumor and normal cells. Biochem Biophys Res Commun. 1987 Aug 14;146(3):1240–1248. doi: 10.1016/0006-291x(87)90781-9. [DOI] [PubMed] [Google Scholar]
- Rybak S. M., Saxena S. K., Ackerman E. J., Youle R. J. Cytotoxic potential of ribonuclease and ribonuclease hybrid proteins. J Biol Chem. 1991 Nov 5;266(31):21202–21207. [PubMed] [Google Scholar]
- Rybak S. M., Vallee B. L. Base cleavage specificity of angiogenin with Saccharomyces cerevisiae and Escherichia coli 5S RNAs. Biochemistry. 1988 Apr 5;27(7):2288–2294. doi: 10.1021/bi00407a007. [DOI] [PubMed] [Google Scholar]
- Saxena S. K., Rybak S. M., Winkler G., Meade H. M., McGray P., Youle R. J., Ackerman E. J. Comparison of RNases and toxins upon injection into Xenopus oocytes. J Biol Chem. 1991 Nov 5;266(31):21208–21214. [PubMed] [Google Scholar]
- Shapiro R., Riordan J. F., Vallee B. L. Characteristic ribonucleolytic activity of human angiogenin. Biochemistry. 1986 Jun 17;25(12):3527–3532. doi: 10.1021/bi00360a008. [DOI] [PubMed] [Google Scholar]
- Shapiro R., Strydom D. J., Olson K. A., Vallee B. L. Isolation of angiogenin from normal human plasma. Biochemistry. 1987 Aug 11;26(16):5141–5146. doi: 10.1021/bi00390a037. [DOI] [PubMed] [Google Scholar]
- Slifman N. R., Loegering D. A., McKean D. J., Gleich G. J. Ribonuclease activity associated with human eosinophil-derived neurotoxin and eosinophil cationic protein. J Immunol. 1986 Nov 1;137(9):2913–2917. [PubMed] [Google Scholar]
- St Clair D. K., Rybak S. M., Riordan J. F., Vallee B. L. Angiogenin abolishes cell-free protein synthesis by specific ribonucleolytic inactivation of ribosomes. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8330–8334. doi: 10.1073/pnas.84.23.8330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strydom D. J., Fett J. W., Lobb R. R., Alderman E. M., Bethune J. L., Riordan J. F., Vallee B. L. Amino acid sequence of human tumor derived angiogenin. Biochemistry. 1985 Sep 24;24(20):5486–5494. doi: 10.1021/bi00341a031. [DOI] [PubMed] [Google Scholar]
- Weiner H. L., Weiner L. H., Swain J. L. Tissue distribution and developmental expression of the messenger RNA encoding angiogenin. Science. 1987 Jul 17;237(4812):280–282. doi: 10.1126/science.2440105. [DOI] [PubMed] [Google Scholar]
- Williams G. T., Neuberger M. S. Production of antibody-tagged enzymes by myeloma cells: application to DNA polymerase I Klenow fragment. Gene. 1986;43(3):319–324. doi: 10.1016/0378-1119(86)90223-4. [DOI] [PubMed] [Google Scholar]