The molecular expression from genomic, proteomic, and metabolomic processes ongoing in living cells is enormously complex, continuously challenging our ability to measure and understand their integrated totality. Important biological studies and advances in the understanding of the biochemistry and biology of living cells have most often been preceded by innovations in the technology used to probe cells, tissues, and animals that then facilitate new insightful observations. A great deal has been learned over the decades about individual enzymes and pathways from isolated samples and extracts from a wide variety of bacterial and animal sources and much progress has been made toward integrating these findings into a framework that describes the underlying biology. This reductionist approach has and will continue to be important; however, there exists a multitude of biological and clinical problems that will only be solved using a systems approach. The innovative and groundbreaking advances in genomic technology and the resultant information has led to a dramatic increase in our ability to measure and predict many of the molecular events involving both genotypic and proteomic processes. Today, multiplex molecular interactions and pathway connectivity can be understood through both direct measurement and predictive models in an impressive attempt to understand this totality at this point in our current knowledge. Although these efforts have greatly advanced our understanding of biological processes, the end game remains far off, especially since spatial and temporal dimensions are yet to be fully explored and integrated into this view of the intact integrated cell. Further, cells react with their environment so that even the same basic cell type will have altered expression depending on their local cellular environment. Although the overall task is daunting, investigators continue to make impressive breakthroughs in understanding this complexity and interdependence.
Understanding the spatial arrangement of biomolecules in tissues and cells remains an area of intense interest. Imaging platforms, such a microscopy, NMR, positron emission tomography, and many more, have brought valuable insight into this important field. One significant new molecular technology in this regard is imaging mass spectrometry (IMS), bringing unparalleled molecular specificity and sensitivity to create molecular image maps of molecules in tissues. In essence, one can produce a series of images, each presented at a discrete molecular weight (measured as m/z values), in multiple molecular dimensions of an entire sample ranging from a simple biopsy to a whole animal tissue section. Using a microprobe approach, ablation of spots on the target tissue encompassing an array across the field of interest provides the basic dataset. Each signal in the mass spectrum of each spot can be plotted over the entire array, giving rise to a molecular map of each of the signals. The spatial resolution of the map or image is defined by the diameter of the ablation spot and the pitch of the spots. Literally, hundreds to thousands of discrete molecular images can be generated from a single-array acquisition.
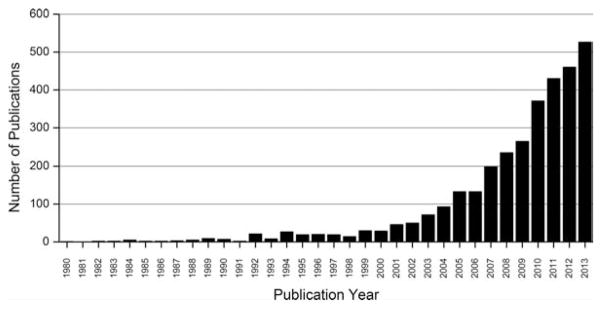
Many different ionization modes have been used for IMS and include secondary ion MS [1], laser desorption ionization [2], MALDI [3], and desorption ESI of a number of varieties and variations [4]. All have unique capabilities and limitations and have led to an astonishing rise in the number of papers published in this field, as shown in Fig. 1. Together, these technologies have included the mapping of trace elements, low molecular weight organic molecules such as endogenous metabolites and drugs, lipids, peptides, proteins, polynucleotides, and synthetic polymers, as well as other molecules.
Figure 1.
Publications reporting the development and application of IMS. Data were collected from a PubMed search of the following key words: imaging mass spectrometry or mass spectrometry imaging, and “1980” (Date—Publication): “2014” (Date—Publication). The results for 2013 were available up to December 11 and for visual comparison with other years, the number of hits for 2013 was linearly extrapolated to represent a full year.
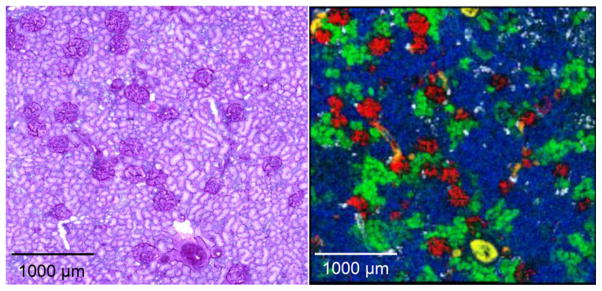
In the biological and medical arena, the most generally useful technology for imaging biomolecules is MALDI IMS. The first description of this imaging platform and its use for imaging cells and tissues was published in 1997 where it was demonstrated that signals for peptides and proteins could be obtained directly from tissues and blots of tissues [5,6]. MALDI IMS enjoys the widest applicability to biological and medical research due to the balance among critical parameters in image analysis, including spatial resolution, molecular types amenable to analysis, molecular mass range, and sensitivity. For example, the MALDI MS image shown in Fig. 2 was obtained at 10 μm spatial resolution from a section of human kidney. Specific molecular images are overlaid in different colors representing unique m/z values for glomeruli (m/z = 13 787, red), tubules (m/z = 2592, green and 1618, blue) and a blood vessel (m/z = 8415, yellow).
Figure 2.
Molecular images (right panel) of specific peptides and proteins acquired by MALDI IMS at 10 μm spatial resolution from a section of human kidney and the corresponding microscopy image (left panel) corresponding periodic acid Schiff stain of the same section after the MS image was taken. The glomeruli are shown in red and the tubules in green and blue on the MS image. See text for details. (Images courtesy: Kerri Grove, National Resource for Imaging Mass Spectrometry, Vanderbilt University.)
Perhaps one of the greatest opportunities and challenges for IMS lies in its clinical potential to aid diagnosis, prognosis, and effectiveness of therapy through the molecular assessment of biopsies obtained from patients [7]. Through the unique combination of the cell type specific sampling and the multiplex capabilities of the mass spectrometer, molecular signatures of specific diseased cells can be assayed where 10–20 or more proteins or metabolites can be measured virtually at the same time and at high sensitivity. For the first time, anatomical pathologists will have the tools to probe tissues directly without the limitations and expense of needing specific molecular probes. For clinical research, the potential for discovery is maximized since target-specific reagents such as antibodies are not required. It is clear that direct molecular mapping in disease will become increasingly important for molecular pathology in the coming years and represents a paradigm shift in this field.
Although the vision of creating a functional molecular microscope goes back many years, the technology advancements recently realized in modern MS in terms of speed, sensitivity, and ablation spot size allow such a vision to become a reality. To be certain, this magnificent journey has been made possible by the many pioneering scientists whose innovative spirits in both fundamental MS and the application of imaging technology to biological studies must be recognized and applauded. Moreover, in working with colleagues in virtually every area of molecular sciences, new discoveries will be made as a consequence of our ability to look deeper into the molecular complexity of living cells while leaving the basic biological structures intact. When taken together with other molecular analytical tools available today, it is certain that IMS will be an indispensable technology in providing specific molecular information for discovery of new and exciting biological processes and their relevancy in disease. IMS is now a newly matured technology, and it is certain that the capabilities we enjoy today will seem modest in the coming years as the technology continues to develop and research investigators push the need for higher and higher performance.
Footnotes
The authors have declared no conflict of interest.
References
- 1.Fletcher JS, Vickerman JC, Winograd N. Label free biochemical 2D and 3D imaging using secondary ion mass spectrometry. Curr Opin Chem Biol. 2011;15:733–740. doi: 10.1016/j.cbpa.2011.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McDonnell LA, Heeren RM. Imaging mass spectrometry. Mass Spectrom Rev. 2007;26:606–643. doi: 10.1002/mas.20124. [DOI] [PubMed] [Google Scholar]
- 3.Norris JL, Caprioli RM. Analysis of tissue specimens by matrix-assisted laser desorption/ionization imaging mass spectrometry in biological and clinical research. Chem Rev. 2013;113:2309–2342. doi: 10.1021/cr3004295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu C, Dill AL, Eberlin LS, Cooks RG, Ifa DR. Mass spectrometry imaging under ambient conditions. Mass Spectrom Rev. 2013;32:218–243. doi: 10.1002/mas.21360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caprioli RM, Farmer TB, Gile J. Molecular imaging of biological samples: localization of peptides and proteins using MALDI-TOF MS. Anal Chem. 1997;69:4751–4760. doi: 10.1021/ac970888i. [DOI] [PubMed] [Google Scholar]
- 6.Chaurand P, Stoeckli M, Caprioli RM. Direct profiling of proteins in biological tissue sections by MALDI mass spectrometry. Anal Chem. 1999;71:5263–5270. doi: 10.1021/ac990781q. [DOI] [PubMed] [Google Scholar]
- 7.Norris JL, Caprioli RM. Imaging mass spectrometry: a new tool for pathology in a molecular age. Proteomics Clin Appl. 2013;7:733–738. doi: 10.1002/prca.201300055. [DOI] [PMC free article] [PubMed] [Google Scholar]