Abstract
CatalyCEST MRI can detect enzyme activity by monitoring the change in chemical exchange with water after a contrast agent is cleaved by an enzyme. Often these molecules use paramagnetic metals and are delivered with an additional non-responsive reference molecule. To improve this approach for molecular imaging, a single diamagnetic agent with enzyme-responsive and enzyme-unresponsive CEST signals was synthesized and characterized. The CEST signal from the aryl amide disappeared after cleavage of a dipeptidyl ligand with cathepsin B, while a salicylic acid moiety was largely unresponsive to enzyme activity. The ratiometric comparison of the two CEST signals from the same agent allowed for concentration independent measurements of enzyme activity. The chemical exchange rate of the salicylic acid moiety was unchanged after enzyme catalysis, which further validated that this moiety was enzyme-unresponsive. The temperature dependence of the chemical exchange rate of the salicylic acid moiety was non-Arrhenius, suggesting a two-step chemical exchange mechanism for salicylic acid. The good detection sensitivity at low saturation power facilitates clinical translation, along with the potentially low toxicity of a non-metallic MRI contrast agent. The modular design of the agent constitutes a platform technology that expands the variety of agents that may be employed by catalyCEST MRI for molecular imaging.
Keywords: CEST MRI, enzyme activity, cathepsin B, ratiometric analysis
1. INTRODUCTION
Chemical exchange saturation transfer (CEST) MRI is a new type of molecular imaging method that can detect enzyme activity within in vivo tumour tissues (1). CEST MRI is initiated by selectively saturating a proton of the agent, which eliminates the net coherent magnetization of the proton. Subsequent chemical exchange of the proton from the agent to water transfers the saturation to water, which decreases the MRI signal from water (Fig. 1A). A CEST agent can undergo a change in chemical exchange rate after enzyme catalysis, which is known as catalyCEST MRI (Fig. 1B). To date, CEST agents for catalyCEST MRI have been developed that detect the activity of caspase-3 (2), urokinase Plasminogen Activator (1,3), cathepsin D (4), transglutaminase (5), β-galactosidase (6), esterase (7), protein kinase A (8), and cytosine deaminase enzymes (9).
Figure 1.
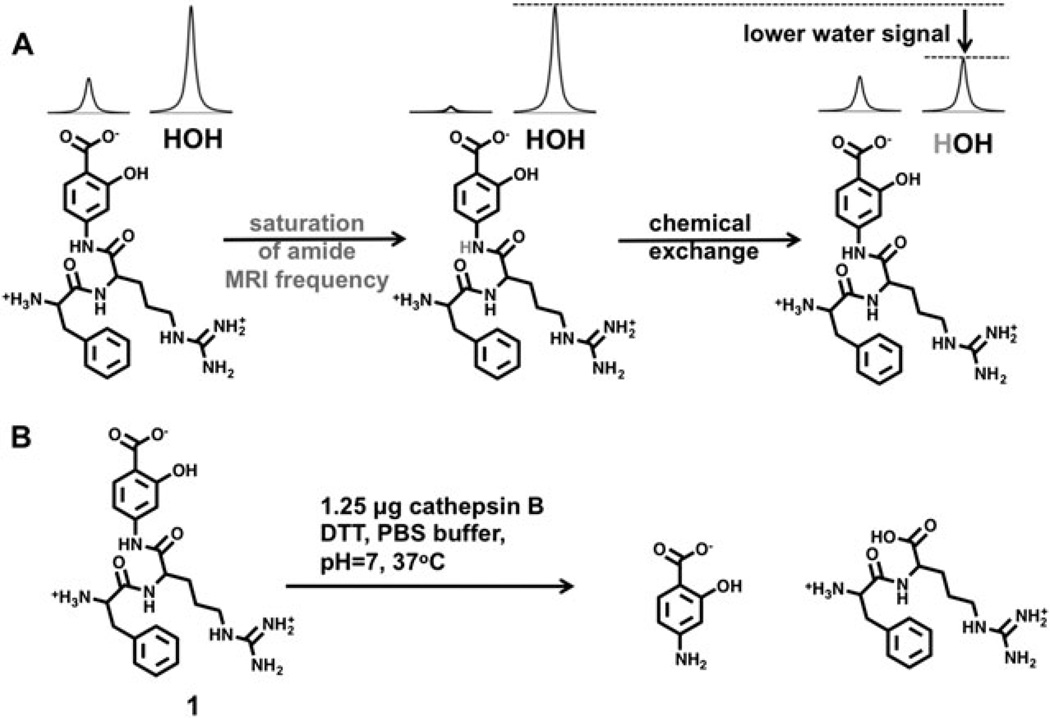
The mechanism of catalyCEST MRI. A) Saturation of the amide frequency of the agent followed by chemical exchange with water, transfers the MR saturation to water. B) Cleavage of the peptidyl ligand of 1 by cathepsin B converts the aryl amide to an aryl amine, which causes a loss of CEST signal. Saturated hydrogen atoms of the amide and water are shown in light gray.
CatalyCEST MRI has primarily employed paramagnetic CEST (paraCEST) agents that contain a lanthanide ion (1–7). This paramagnetic ion causes the protons of the agent to have large chemical shifts relative to water. This facilitates selective excitation of the exchangeable proton that generates CEST without also directly saturating the water resonance. Furthermore, the larger chemical shift range facilitates the selective detection of two CEST agents during the same catalyCEST MRI study. The comparison of an enzyme-responsive CEST agent with an unresponsive CEST agent at the same concentration can provide a concentration-independent assessment of enzyme activity, which greatly improves the specificity for detecting enzyme activity (1).
However, lanthanide-based paraCEST agents are potentially toxic especially at concentrations needed for CEST MRI detection (10). To avoid this pitfall, diamagnetic CEST (diaCEST) agents have been developed that do not contain a metal ion (8,9). Unfortunately, the lack of paramagnetism typically limits the range of chemical shifts to 0–5 ppm from the chemical shift of water (which is defined as 0 ppm in MRI). This compromises the ability to selectively detect the agent without also directly saturating water, and limits the ability to detect two CEST agents during the same catalyCEST MRI study.
To address these limitations, we proposed to design an enzyme-responsive diaCEST agent with two chemical shifts that are greater than 5 ppm from the chemical shift of water. Furthermore, we proposed to design a single diaCEST agent with enzyme-responsive and enzyme-unresponsive CEST signals to detect enzyme activity in a concentration-independent manner. To meet these two objectives, we designed a diaCEST agent based on salicylic acid, which has recently been shown to have a CEST effect at approximately 9.6 ppm (compound 1 in Fig. 1B and Fig. 2) (11). This unusually high chemical shift is caused by intramolecular hydrogen bonding between the carboxylic acid and hydroxyl group on the aromatic ring. Our proposed diaCEST agent, (Phe-Arg)-4-amino-2-hydroxybenzoic acid, has a Phe-Arg peptidyl ligand that is preferentially cleaved by cathepsin B (12). In addition, the aryl amide proton should have a high chemical shift. We hypothesized that the cleavage of the ligand would convert the amide to an amine that would cause a loss of detectable CEST from the amide, while the salicylic acid moiety would be unresponsive to the enzyme and retain its CEST signal.
Figure 2.
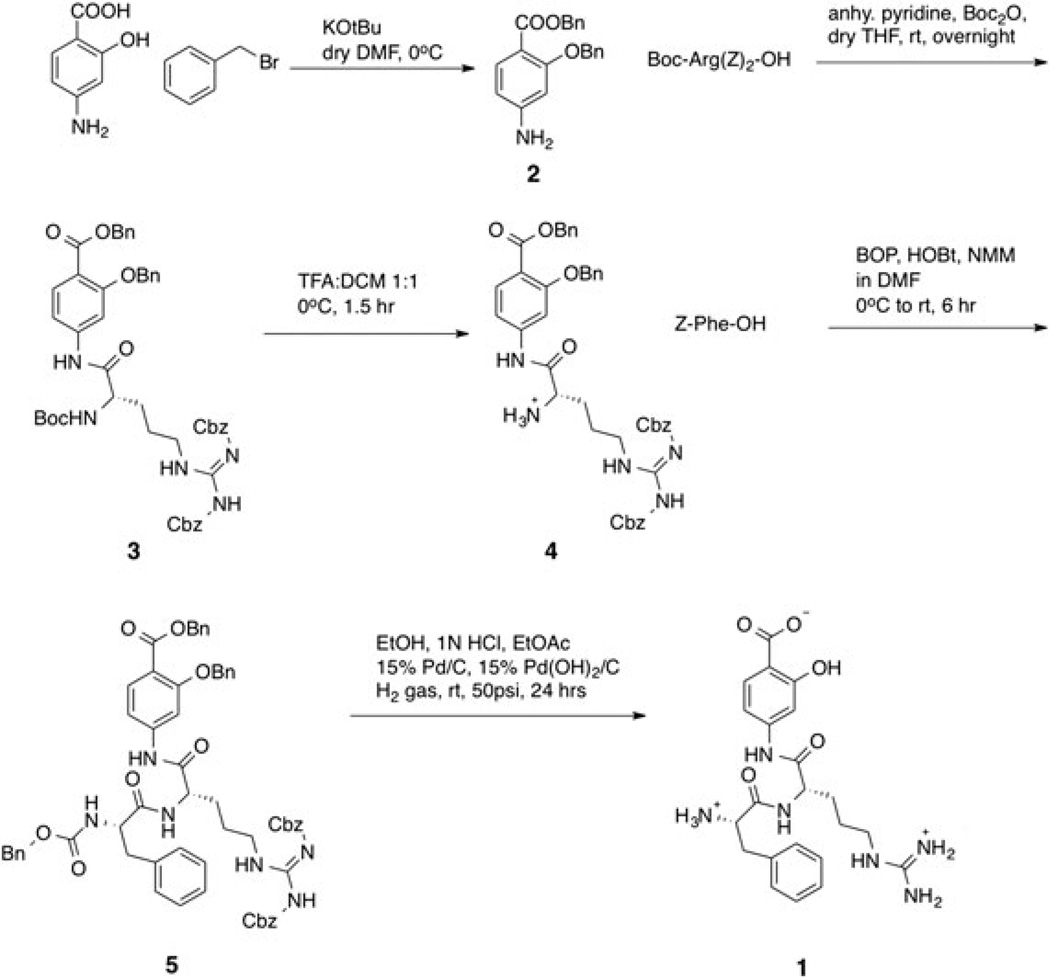
Schematic of the steps for synthesizing (Phe-Arg)-4-amino-2-hydroxybenzoate (1).
2. RESULTS
2.1. CatalyCEST MRI studies
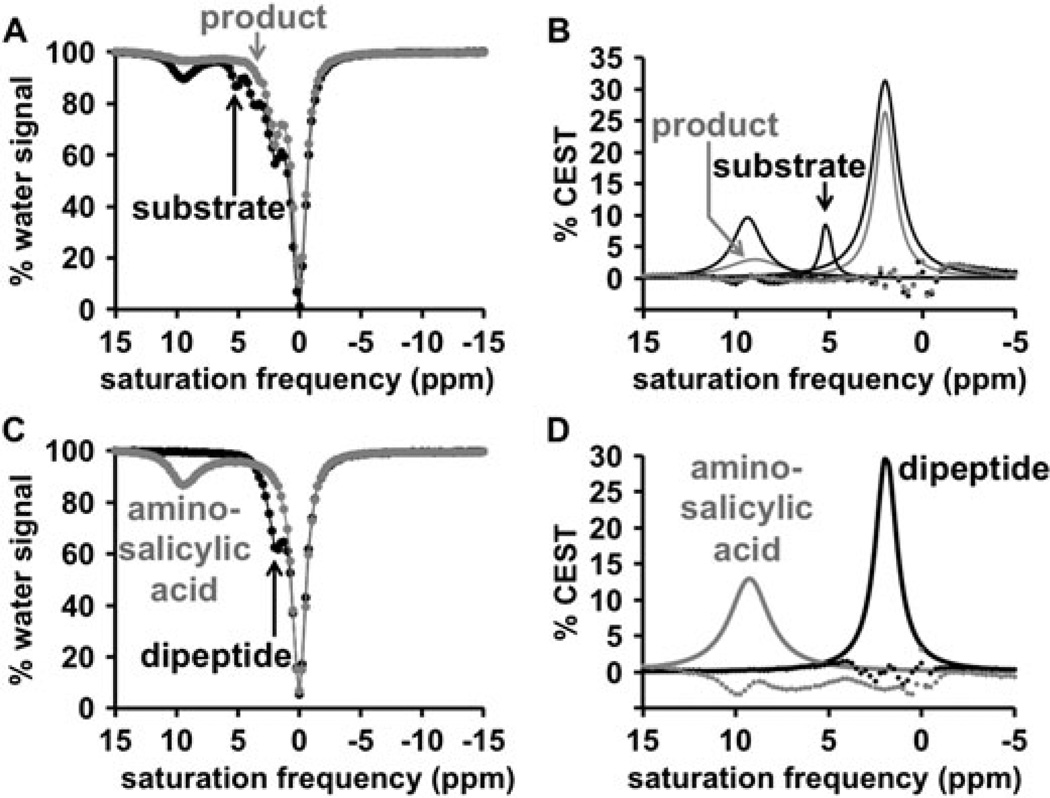
We synthesized compound 1 through a 5-step process with an overall yield of 9% (Fig. 2). The CEST spectra obtained prior to enzyme reaction showed three CEST signals at 9.5 ppm, 5.2 ppm and 2.2 ppm (Fig. 3A,B). This range of MR frequencies for the CEST signals facilitated detection of three CEST signals from the same molecule, and validated our design of a diaCEST agent with two CEST signals that had chemical shifts greater than 5 ppm. The low residuals of the Lorentzian line shape fittings showed that the fitting process could quantify the amplitudes of the CEST signals with excellent precision. The CEST signal at 9.5 ppm was assigned to the salicylic acid moiety, which was consistent with previous reports (11). The CEST signal at 5.2 ppm was assigned to the aryl amide group, which was consistent with the CEST signals of aryl amides reported for other diaCEST agents (13). The CEST signal at 2.2 ppm was assigned to the guanidinium functional group from the amino acid side chain of arginine. A small CEST signal was also detected at 3.5 ppm, which was assigned to the amide of the Arg residue. This CEST signal of the Arg amide was not evaluated during subsequent analyses due to its small amplitude, and because evaluation of the Arg amide’s CEST signal was not relevant for this specific study.
Figure 3.
Experimental catalyCEST MRI. A) The CEST spectrum and B) Lorentzian line shape spectrum of compound 1 (black) and product (grey) shows that the CEST signal from the aryl amide at 5.2 ppm disappears after four hours of catalysis. The CEST signal from the salicylic acid at 9.5 ppm also decreases. C) the CEST spectrum and D) Lorentzian line shape spectrum of separate samples of amino-salicylic acid (grey) and the dipeptide (black) show CEST signals at 9.6 ppm and 2.5 ppm, respectively, which matched the CEST signals observed for the product.
A solution of 1 underwent cleavage by the protease cathepsin B under physiological conditions (37°C, pH = 7) in the presence of a thiol source that generates a reducing environment needed for enzyme function. As expected, the addition of cathepsin B to 1 caused the CEST signal at 5.2 ppm to rapidly disappear. To confirm this result, the CEST signals of the two expected products, 4-amino salicylic acid and the dipeptide, were found to also be missing a CEST signal at 5.2 ppm (Fig. 3C,D). The addition of cathepsin B also caused the CEST signal at 9.5 ppm to decrease, indicating that the enzyme catalyzed the degradation of the salicylic acid moiety. Fortunately, this degradation occurred slowly, so that this CEST signal could still be detected during a catalyCEST MRI study. The ratio of these two CEST signals (CEST @ 5.2 ppm / CEST @ 9.5 ppm) was 88.4% before enzyme catalysis, and dropped to 0% after adding the enzyme. This result demonstrated that the comparison of two CEST signals from the same agent can improve the detection of enzyme activity. Also, the CEST signal at 2.2 ppm remained the same after the addition of cathepsin B, so that the ratio of the CEST signals from the aryl amide and guanidinium group dropped from 26.8% to 0% after adding the enzyme.
2.2. Chemical exchange rates
The chemical exchange rates were estimated for the salicylic acid moiety, aryl amide, and guanidinium group of 1 using the HW-QUESP analysis method (Table 1; Fig. 4) (14). The same procedure was used to measure the chemical exchange rates of the product, 4-amino salicylic acid, and a Phe-Arg dipeptide. The salicylic acid moiety of the reactant had a chemical exchange rate of 391 Hz, and had a small 4.3% decrease after enzyme catalysis. Similarly, the guanidinium group had a chemical exchange rate of 307 Hz and decreased by only 5.2% after enzyme catalysis. These results validated that the CEST signals of salicylic acid and guanidinium moieties can be used as enzyme-unresponsive CEST signals for comparison to the responsive CEST signal from the aryl amide moiety.
Table 1.
Chemical exchange rates
| Sample | Saturation Frequency |
||
|---|---|---|---|
| 9.5 ppm | 5.25 ppm | 2.25 ppm | |
| 1 | 391 Hz | 279 Hz | 307 Hz |
| Product | 374 Hz | --- | 291 Hz |
| 4-aminosalicylic acid | 384 Hz | --- | --- |
| Phe-Arg dipeptide | --- | --- | 119 Hz |
Figure 4.
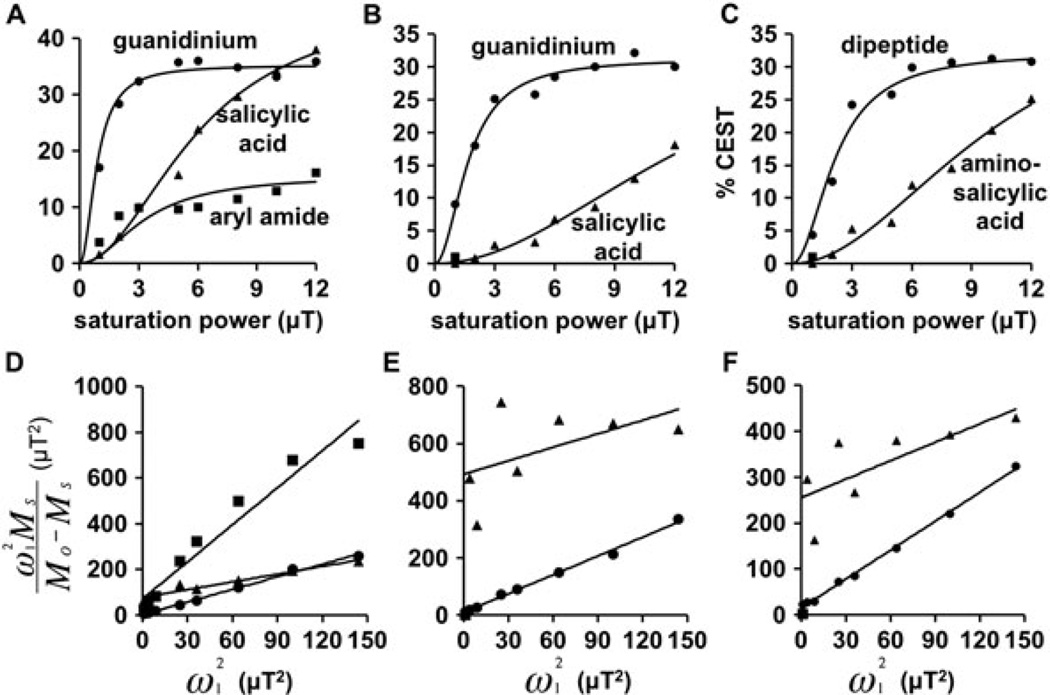
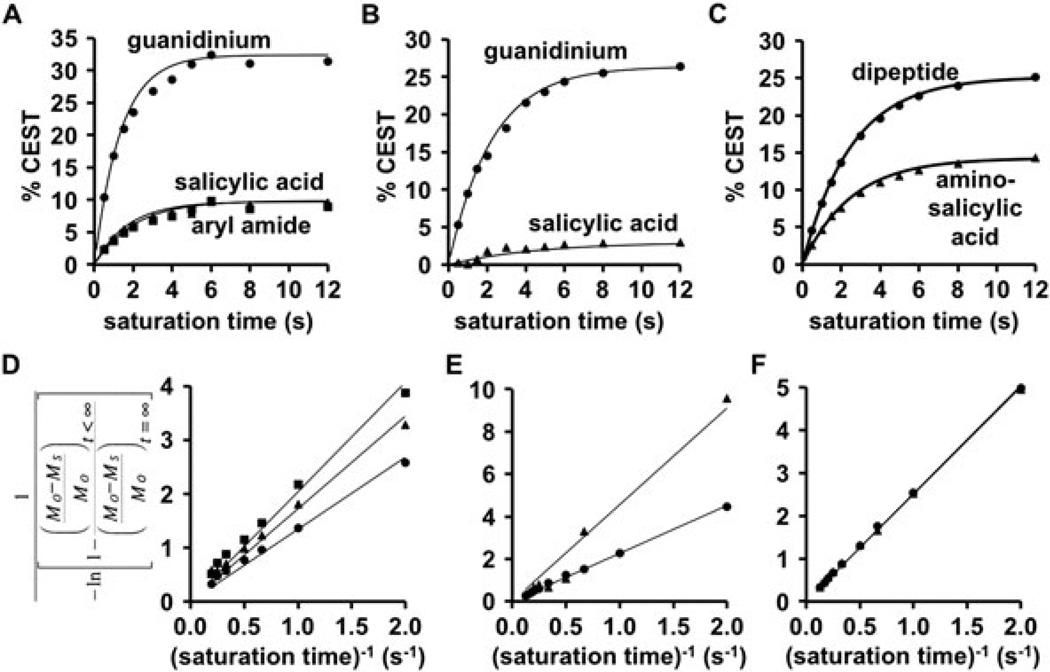
Optimization of saturation power. A) The CEST signals from the salicylic acid (triangles), aryl amide (squares), and guanidinium group (circles) of 1, B) the product’s CEST signals from the salicylic acid (triangles) and guanidinium group (circles), and C) separate samples of aminosalicylic acid (triangles) and the dipeptide (circles) showed an expected dependence on saturation power. D–F) The HW-QUESP fitting of experimental results was used to determine the lines in graphs A, B, and C, respectively.
2.3. CEST MRI conditions
The signals of saturation power were evaluated and analyzed with the HW-QUESP method (Fig. 4) (14). A saturation power of 3 µT generated greater than 90% of the maximum CEST signal amplitude from the guanidinium group, but only 26% and 43% of the maximum observed CEST signal amplitude from the salicylic acid moiety and the aryl amide. Yet a 3 µT saturation power was used for subsequent studies, because this lower power created narrower peaks in the CEST spectrum that facilitated Lorentzian line shape fitting, which improved the accuracy of measuring CEST signal amplitudes (5). Overall, these results showed that one saturation power could be used to detect both CEST signals for the ratiometric analysis.
The saturation conditions for catalyCEST MRI with this agent were optimized by iterating the saturation time and analyzing the results with the RL-QUEST method (Fig. 5) (13). A saturation time of 6 seconds was sufficient to generate greater than 90% of the maximum CEST amplitude from the salicylic acid moiety and the aryl amide, both from compound 1 and the product. A 6 second saturation time generated greater than 78% of the maximum CEST amplitude from the guanidinium group, which was still sufficient to generate a large CEST amplitude from the five equivalent protons of this group. These results showed that one saturation time could be used to detect both CEST signals with good sensitivity. Subsequent studies were performed with a 6 second saturation time.
Figure 5.
Optimization of saturation time. A) The CEST signals from the salicylic acid (triangles), aryl amide (squares), and guanidinium group (circles) of 1, B) the product’s CEST signals from the salicylic acid (triangles) and guanidinium group (circles), and C) separate samples of aminosalicylic acid (triangles) and the dipeptide (circles) reached > 90% of the maximum value after 6 seconds of saturation time. D–F) The RL-QU EST fitting of experimental results was used to determine the lines in graphs A, B, and C, respectively.
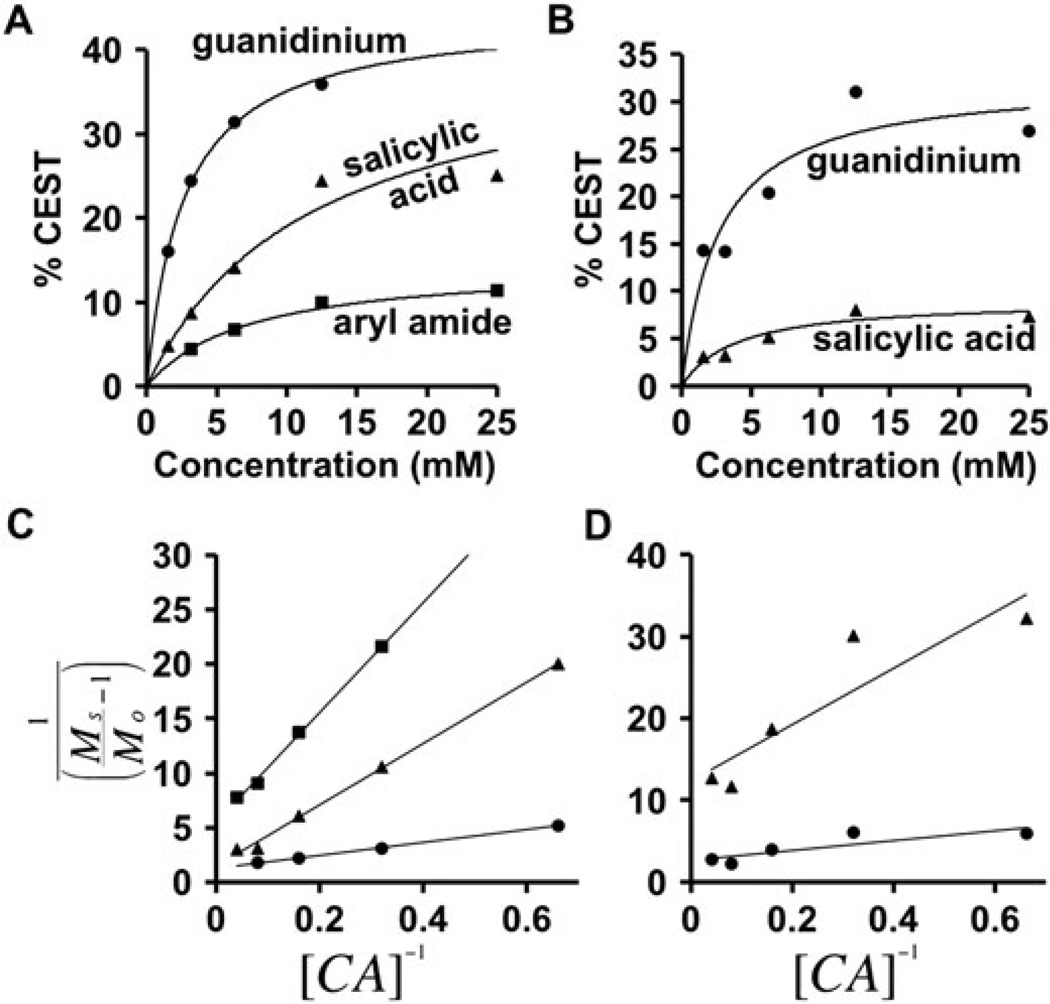
The CEST signals of the salicylic acid and aryl amide could be detected at concentrations in the single-millimolar concentration range as assessed by the HW-Conc method (Fig. 6) (16). Even at a low concentration of 3.125 mM, the salicylic acid generated a 8.6% CEST signal and the aryl amide generated a 4.4% CEST signal. These strong CEST signal amplitudes matched similar published results with other salicylic acid derivatives (11), and also agreed with a published theoretical analysis of CEST signal magnitudes for agents with high chemical shifts (17). The ability to detect CEST at lower concentrations of contrast agent has advantages for in vivo imaging applications.
Figure 6.
The dependence of CEST on concentration. A) The CEST signals from the salicylic acid (triangles), aryl amide (squares), and guanidinium group (circles) of 1, B) and the product’s CEST signals from the salicylic acid (triangles) and guanidinium group (circles) could be detected at low millimolar concentrations. C,D) The HW-QUESP fitting of experimental results was used to determine the lines in graphs A and B, respectively.
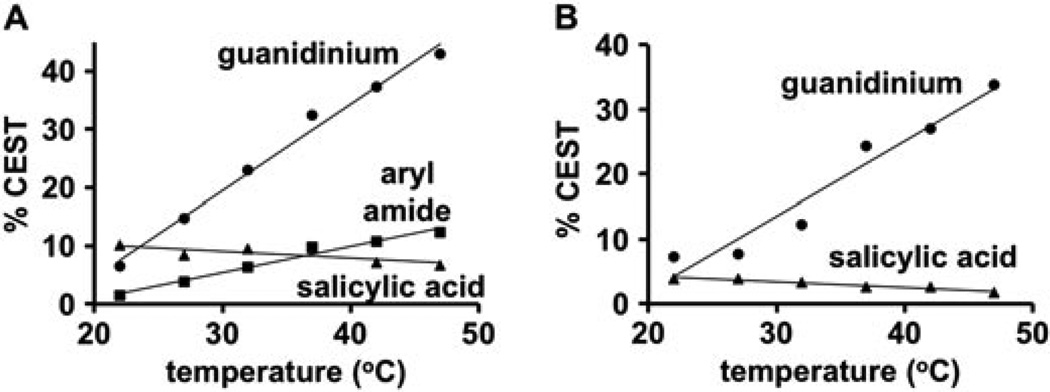
As expected, an increase in temperature caused the CEST amplitudes from the aryl amide and guanidinium group to increase (Fig. 7). Conversely, an increase in temperature caused a decrease in the CEST amplitude from the salicylic acid moiety for both compound 1 and the product as well as from the commercially available 4-amino salicylic acid. This unexpected behaviour indicated that the chemical exchange of salicylic acid follows a non-Arrhenius process. This unusual condition can be explained by a two-step kinetic model (18). The first step involves the formation of a hydrogen-bonded complex between the salicylic acid moiety and water (or a hydroxide ion) with highly entropic conformational flexibility. The second step involves extraction of the hydrogen bonded proton of salicylic acid by water (or a hydroxide ion), which requires an entropically constrained configuration. Therefore, an increase in temperature favours the more entropic first step, which reduces the chemical exchange rate. This explanation also justifies the relatively poor fitting of the CEST dependence on saturation power for the salicylic acid moiety, which is based on a single-step kinetic model (Fig. 4A–C).
Figure 7.
The effects of temperature on CEST. A) The CEST signals from the salicylic acid (triangles), aryl amide (squares), and guanidinium group (circles) of 1, B) and the product’s CEST signals from the salicylic acid (triangles) and guanidinium group (circles) each showed a dependence on temperature. This temperature dependence for the CEST signals of aryl amide and guanidinium group matched the Arrhenius equation, but the temperature dependence of the CEST signal of salicylic acid was non-Arrhenius.
3. DISCUSSION
Our results demonstrated that we have developed a single catalyCEST MRI contrast agent with two CEST signals with chemical shifts greater than 5 ppm, and which can be detected with good sensitivity with a single saturation power and saturation time. This agent can detect the enzyme activity of cathepsin B by comparing the ratio of the CEST signal amplitudes of the aryl amide at 5.2 ppm and the salicylic acid moiety at 9.5 ppm. The CEST signal from the salicylic acid moiety slowly decreased, demonstrating that this moiety is not truly enzyme-unresponsive. However, the slow decrease in signal still provided the opportunity to apply the ratiometric method for improved detection of enzyme activity. This difference in the rates of decreasing CEST signals further demonstrates the advantages of detecting two CEST signals, because future studies could temporally monitor each CEST signal to improve the analysis.
Although in vivo investigations are beyond the scope of our initial studies, our studies show characteristics that facilitate translation to in vivo imaging. Our studies in this report used a saturation time of 6 seconds, a saturation power of 3 µT, and a Lorentzian line shape fitting method that can detect CEST signal amplitudes of 4.4%. These conditions are similar to our previously reported in vivo CEST MRI studies with diaCEST agents that used a satuation time of 3.8 to 5 seconds, 2.8 µT saturation power, and Lorentzian line shape fitting analyses to detect CEST signal amplitudes of approximately 2% (13,19–21). Therefore, translation of our agent to in vivo studies appears feasible.
The in vivo detection of cathepsin B enzyme activity via catalyCEST MRI potentially provides new opportunities for molecular imaging. Extracellular cathepsin B activity is a useful biomarker of tumour initiation, proliferation, invasion and metastasis (22). Notably, this enzyme can be expressed as an inactive proenzyme, and therefore detection of the enzyme’s activity may be more informative than monitoring the enzyme’s expression (23). Contrast agents for other imaging modalities can also detect cathepsin B activity, including optical imaging agents and radionuclide agents that typically require concentrations in the nanomolar concentration range (24,25). However, as shown in these studies, the detection of the activity of a nanomolar concentration of cathepsin B enzyme is feasible if enzyme catalysis can rapidly convert a high concentration of the agent to product. We refer to the detection of enzyme activity with catalyCEST MRI as ‘agent limited’, whereby the concentration for adequate detection is dictated by the agent’s concentration, rather than a ‘targeted limited’ case where the concentration of the molecular target dictates the level of detection. For these reasons, catalyCEST MRI can exploit the merits of MRI to detect enzyme activity (e.g., avoids the use of ionizing radiation that confounds radionuclide imaging, and can image deeper tissues that confounds optical imaging), which further justifies the translation of this agent to future in vivo studies.
This diaCEST agent represents a platform technology that may be exploited to create many new types of responsive MRI contrast agents for molecular imaging. Other peptide sequences can be incorporated into the synthesis scheme to create catalyCEST MRI contrast agents that sense other proteases (26). Other classes of enzymes that modify an aryl functional group may also be detected using this platform technology, such as esterases (7), phosphatases (27), and sulfatases (28). The salicylic acid moiety may be replaced by other molecules that have intramolecular hydrogen bonding that causes highly shifted CEST signals and are less likely to undergo slow degradation (11,17,29). These additional chemical structures can provide more variety for developing this platform technology and expand catalyCEST MRI for molecular imaging.
4. CONCLUSIONS
To summarize, (Phe-Arg)-4-amino-2-hydroxybenzoic acid is a diaCEST MRI contrast agent that can be employed in a catalyCEST MRI protocol to detect the enzyme activity of cathepsin B. This derivative of salicylic acid generated two CEST signals above 5 ppm, and generated both signals from a single agent, which improves on the design of other catalyCEST MRI agents that require two agents or have smaller chemical shifts. This seminal example of a responsive diaCEST agent with two highly shifted CEST signals represents a platform technology that may be easily modified to detect other enzyme activities, and may facilitate clinical translation of catalyCEST MRI for molecular imaging.
5. EXPERIMENTAL
5.1. Synthesis
All reactions were performed under argon unless otherwise indicated. Dry solvents were purchased and used without further purification or drying treatment. Protected L-amino acids were used for this synthesis. ACS grade solvents for chromatography, NaHCO3, Na2SO4, and PBS buffer (pH 7) were purchased from Fisher Scientific (part of Thermo Fisher Scientific, Waltham, MA, USA). Dithiotreitol (DTT), 4-amino-2-hydroxybenzoic acid, KOtBu, conc. HCl, anhydrous DMF, absolute ethanol (EtOH), Pd/C and Pd (OH)2/C were purchased from Sigma-Aldrich (St. Louis, MO, USA). Z-Phe-OH, benzotriazol-1-yloxytris(dimethylamino)-phosphonium hexafluorophosphate (BOP) were purchased from Advanced Chem Tech (Louisville, KY, USA). BnBr was acquired from Sigma-Aldrich (St. Louis, MO, USA). Hydroxybenzotriazole (HOBt) was purchased from Anaspec, Inc. (Fremont, CA, USA). Anhydrous THF was purchased from Acros Organics (part of Thermo Fisher Scientific, Waltham, MA, USA) and HPLC grade THF was purchased from EMD Millipore, Inc. (Billerica, MA, USA). Di-tert-butyl dicarbonate (Boc2O) and Boc-Arg(Z)2-OH were purchased from Chem Impex Intl., Inc. (Wood Dale, IL, USA). NMR spectroscopy was performed with a 400 MHz NMR spectrometer (Bruker Biospin, Corp., Billerica, MA, USA). An XBridge™ Prep C18 10 µm OBD™ 19*250mm column was used for HPLC.
5.1.1. Benzyl 4-amino-2-(benzyloxy)benzoate (BnSal)
Compound 2 was synthesized using a previously reported method with minor modifications (30). 1.5 g (9.8 mmol, 1 equi) of 4-amino-2-hydroxybenzoic acid and 1.21 g (10.8 mmol, 1.1 equi) KOtBu was dissolved in 70 mL of dry DMF and cooled to 0°C with vigorous stirring. 1.285 mL (10.8 mmol, 1.1 equi) BnBr was dissolved in 10 mL of dry DMF and added to the solution dropwise over 30 min. After 4 hours of stirring at 0 °C, 1.21 g of KOtBu was added and after 15 min a solution of 1.285 mL of BnBr in 10 mL dry DMF was added to the flask. The reaction was allowed to warm to room temperature overnight. The reaction was quenched with an equal volume of water and then extracted with EtOAc three times. The organic layer was then washed with distilled water twice, then washed with brine, and then dried over Na2SO4. The solvent was removed using rotary evaporation. The product was purified using column chromatography using 40:60 EtOAc:hexanes to obtain an yellow powder (yield: 1.4 g, 42.9%). 1H NMR (CDCl3): 7.81 (d, J = 8 Hz, 1H), 7.28–7.48 (m, 10H), 6.25 (dd, J = 8 Hz, 2 Hz, 1H), 6.24 (d, J = 2 Hz, 1H), 5.31 (s, 2H), 5.12 (s, 2H), 4.01 (bs, 2H). 13C NMR (CDCl3): 165.7, 160.8, 151.9, 136.7, 136.6, 134.5, 128.6, 128.5, 128.4, 128.1, 128.0, 127.8, 127.6, 126.9, 109.6, 106.8, 99.4, 70.4, 66.0. ESI MS: Exact mass (C21H19NO3): 333.14, MH+ observed: 334.1.
5.1.2. BnSal-Arg(Z)2-NHBoc
Compound 3 was synthesized using a previously reported method with minor modifications (31). A solution of 2.44 g (4.5 mmol, 1.5 equi) Boc-Arg(Z)2-OH was prepared in 10 mL dry THF. To this solution 0.484 mL (6 mmol, 2 equi) of anhydrous pyridine was added dropwise followed by 1.31 g (6 mmol, 2 equi) Boc2O. After 1 hour of constant stirring, a solution of 1 g (3 mmol, 1 equi) benzyl 4-amino-2-(benzyloxy)benzoate in 5 mL of dry THF was added. The solution was stirred overnight at room temperature. The reaction mixture was diluted with EtOAc and washed with sat. NaHCO3 solution twice, then washed with 1 N HCl solution twice, then washed with brine, and then dried over Na2SO4. The solvent was removed using rotary evaporation. The product was purified using column chromatography using 95:5 DCM: MeOH to obtain an off-white powder (yield: 2.35 g, 89%). 1H NMR (CDCl3): 7.64 (d, J = 8 Hz, 1H), 7.33–7.36 (m, 2H), 7.12–7.30 (m, 24H), 5.22 (s, 2H), 5.14 (s, 2H), 1.65 (m, 4H), 1.36 (s, 9H). 13C NMR (CDCl3): 171.2, 165.6, 163.5, 160.9, 159.4, 156.0, 155.7, 142.7, 136.7, 136.5, 136.3, 134.5, 133.0, 129.0, 128.8, 128.5, 128.5, 128.4, 128.4, 128.2, 128.1, 128.0, 127.7, 127.2, 115.4, 111.2, 104.7, 83.5, 70.4, 69.1, 67.3, 66.5, 60.5, 44.0, 28.4, 24.8. ESI MS: Exact mass (C48H49N5O10): 857.3, MH+ observed: 858.1.
5.1.3. BnSal-Arg(Z)2-NH2. TFA salt
To synthesize compound 4, 0.5 g (0.058 mmol) of 3 was dissolved in 10 mL DCM and cooled in an ice bath. 10 mL of TFA was added dropwise and the reaction was allowed to stir for 30 min on ice and then 1 hour at room temperature. The completion of the reaction was followed using TLC (90:10 DCM:MeOH). The solvent was removed using rotary evaporation, and then re-dissolved and re-dried with toluene three times and ether twice to obtain a yellowish powder, which was used immediately for the next reaction without purification.
5.1.4. BnSal-Arg(Z)2-Phe-NH-Z
Compound 5 was synthesized using a previously reported method with minor modifications (32). 4mL of DMF was added to a flask containing 0.44 g (0.58 mmol, 1 equi) 4 and the solution was cooled to 0 °C. Then 0.192 g (0.64 mmol, 1.1 equi) Z-Phe-OH, 0.094 g (0.7 mmol, 1.2 equi) HOBt, 0.357 g (0.7 mmol, 1.2 equi) BOP were added sequentially. The reaction mixture was allowed to stir for 15 min on ice followed by addition of 128 µL of NMM. The reaction was allowed to warm to room temperature and stirred for six hours, followed by diluting the solution with EtOAc. The organic solution was washed with 5% NaHCO3 three times, with 5% citric acid three times, and with brine and water. Then the organic layer was dried over Na2SO4, the excess solution was removed by rotary evaporation, and the product was precipitated with ether. TLC (9.5 mL DCM, 0.4 mL MeOH, 0.1mL acetic acid) was used to follow the reaction and the extraction process. Column chromatography was performed using 95:5 DCM:MeOH (yield: 0.49 g, 80%). 1H NMR (CDCl3): 8.00 (d, 1H) 7.84 (d, 1H) 7.76 (d, 1H) 7.0–7.5 (m, 30H), 5.0–5.4 (m, 10H), 2.92 (d, J = 28 Hz, 2H), 1.41 (m, 4H). ESI MS: Exact mass (C60H56N6O11): 1038.42, MH+ observed: 1040.0
5.1.5. (Phe-Arg)-4-amino-2-hydroxybenzoate
To synthesize compound 1, 0.49 g of 5 was dissolved in 10 mL absolute ethanol with 0.4 mL 1 N HCl and 3mL EtOAc. A mixture of 15% w/w of Pd/C and 15% w/w of Pd(OH)2/C were added and the flask was allowed to shake vigorously on a hydrogenation apparatus (Parr Instrument Co., Moline, IL, USA) with H2 gas at room temperature and 50 psi for 24 hours. Then 20 mL of DCM was added to the flask and the solution was filtered over a celite plug. The filtrate was evaporated to give pale yellow oil that was purified using preparative HPLC (RP C-18, 90:10 0.1% TFA: MeCN to 50:50 0.1% TFA: MeCN in 20 min, Rt = 17 min) (yield: 63 mg, 30%). ESI MS: Exact mass (C22H30N6O5): 456.21, MH+ observed: 457.41
5.2. Enzyme reaction
A solution of 12.5mM of 1 with 4mM DTT was prepared in 0.2mL of a 50mM PBS buffer at pH7. Using a stock solution of enzyme, 1.25 µg of cathepsin B was added to the sample of 1 to achieve a concentration of 250 nM cathepsin B. The reaction was allowed to stand in a water bath at 37.0°C for 4 hours. 10 µL reaction solution was then dissolved in 0.99 mL water and analyzed with LC-MS to confirm that the enzyme reaction had produced the intended product. The exact mass matched the mass of 4-amino-2-hydroxybenzoate. ESI MS: Exact mass (C7H7NO3): 153.04, MH+ observed: 153.64
5.3. Acquisition and processing of CEST spectra
NMR samples were prepared by adding solution from 1 to a 3mm O.D. tube and inserting this tube into a larger 5mm O.D. tube that contained 100% D2O. CEST spectra were recorded using a 600 MHz Bruker NMR spectrometer. The temperature of the sample was set to 37.0 °C. The sample was locked and shimmed using the signal from D2O, and the transmitter offset was set to the Larmor frequency of the water. The saturation power output was calibrated with the attenuator values of the power amplifier, because the power amplifier of the Bruker NMR spectrometer had a linear power response. The power deposition into the sample was controlled by modifying the time of the 360° pulse at a preselected power. A continuous wave saturation pulse was applied for a specific duration at a specific saturation power followed by immediate acquisition of a 1D NMR spectrum. This procedure was repeated for each saturation radio frequency from −15 ppm to 15 ppm in 0.25 ppm increments. All saturation frequencies were assigned relative to the resonance frequency of water. The 1D NMR spectra were processed to determine the amplitude of the water signal in a magnitude spectrum with line broadening of 10 Hz. The CEST spectra were created by plotting the water signal amplitude for each saturation frequency.
5.4. Evaluation of saturation conditions and measurement of chemical exchange rates
Chemical exchange rates are typically measured by fitting experimental CEST spectra with the Bloch-McConnell equations (33). However, this non-linear fitting method requires technical expertise to obtain acceptable fitting results, especially for a CEST spectrum with multiple CEST signals. In addition, recent evidence suggests that fitting Bloch-McConnell equations to CEST spectra may systematically overestimate the chemical exchange rates of systems with multiple CEST signals (34). Therefore, a more simplified analysis can be made, based on the assumption that CEST agent immediately reaches steady state saturation when radio frequency saturation is initially applied. The QUEST and QUESP methods can estimate chemical exchange rates by fitting non-linear equations to CEST amplitudes that are acquired using a series of saturation times or saturation powers (35). These two non-linear fitting methods can be further simplified to create linear equations, which accelerates the fitting process and compensates for systematic experimental errors. The HW-QUESP method generates accurate estimates of chemical exchange rates of fast exchanging protons and in a concentration-independent manner (15), while the RL-QUEST method provides the most accurate estimates for slow exchanging protons if the agent’s concentration is known (14). Both linear fitting methods can also be used to evaluate the saturation power and time for optimizing CEST MRI signals.
5.4.1. CEST vs. Saturation Power
CEST spectra of 1 (Fig. 4A), product (Fig. 4B), and control samples of 4-aminosalicylic acid and the Phe-Arg dipeptide (Fig. 4C) were acquired using a range of saturation powers from 1 to 12 µT. These studies used a 6 s saturation time, samples with 25mM concentration, and a temperature of 37.0°C. The HW-QUESP method was used to analyse these results (Fig. 4D–F) (14). These results were used to determine that a saturation power of 3 µT was adequate to produce sufficient CEST amplitudes for 1 and the product. This fitting method was used to estimate chemical exchange rates because this method does not require a high concentration of agent and does not require that the concentration of the agent be known. In addition, this method compensates for B1 inhomogeneity that may alter the absolute value of the saturation power applied to a specific sample.
5.4.2. CEST vs. Saturation Time
CEST spectra of compound 1 (Fig. 5A), product (Fig. 5B), and control samples of 4-aminosalicylic acid and the Phe-Arg dipeptide (Fig. 5C) were acquired using a range of saturation times from 0.1 to 12.0s. These studies used a 3 µT saturation power, samples with 25mM concentration, and a temperature of 37.0 °C. The RL-QUEST method was used to analyse these results (Fig. 5D–F) (15). These results were used to determine that a saturation time of 6s was sufficient to produce optimal CEST amplitudes for 1 and the product. This fitting method was not used to estimate chemical exchange rates because this method requires a high concentration of agent to accurately estimate chemical exchange rates, and our analysis used samples at 25mM concentration.
5.4.3. CEST vs. Concentration
CEST spectra of compound 1 (Fig. 6A) and the product (Fig. 6B) were acquired using a range of concentrations from 1 to 25 mM. These studies used a 6s saturation time, 3 µT saturation power, and a temperature of 37.0 °C. The HW-Conc method was used to analyse these results (Fig. 6C,D) (16). These results were used to determine that CEST signals can be measured at low single-mM concentrations. This fitting method was not used to estimate chemical exchange rates because this method has a lower dynamic range than the RL-QUEST and HW-QUESP methods.
5.5. Temperature studies
CEST spectra were acquired between 23.0°C and 47.0 °C in steps of 5 °C (Fig. 7). Care was taken to allow the sample to equilibrate for more than five minutes after the NMR spectrometer had reached the desired temperature. The temperature of the NMR spectrometer was calibrated using neat methanol (36).
Acknowledgments
This research is supported by the Phoenix Friends of the Arizona Cancer Center, the Community Foundation of Southern Arizona, the Better Than Ever Program, the Anne Rita Monahan Foundation, R01CA167183-01 and P50 CA95060.
REFERENCES
- 1.Yoo B, Sheth VR, Howison CM, Douglas MJK, Pineda CT, Maine EA, Baker AF, Pagel MD. Detection of in vivo enzyme activity with catalyCEST MRI. Magn Reson Med. 2013;71:1221–1230. doi: 10.1002/mrm.24763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yoo B, Pagel MD. A PARACEST MRI contrast agent to detect enzyme activity. J Am Chem Soc. 2006;128:14032–14033. doi: 10.1021/ja063874f. [DOI] [PubMed] [Google Scholar]
- 3.Yoo B, Sheth VR, Pagel MD. An amine-derivatized, DOTA-loaded polymeric support for Fmoc solid phase peptide synthesis. Tet Lett. 2009;50:4459–4462. doi: 10.1016/j.tetlet.2009.05.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suchy M, Ta R, Li A, Wojciechowski F, Pasternak SH, Bartha R, Hudson RHE. A paramagnetic chemical exchange-based MRI probe metabolized by cathepsin D: design, synthesis and cellular uptake studies. Org Biomolec Chem. 2010;8:2560–2566. doi: 10.1039/b926639a. [DOI] [PubMed] [Google Scholar]
- 5.Hingorani DV, Randtke EA, Pagel MD. A catalyCEST MRI contrast agent that detects the enzyme- catalyzed creation of a covalent bond. J Am Chem Soc. 2013;135:6396–6398. doi: 10.1021/ja400254e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chauvin T, Durand P, Bernier M, Meudal H, Doan BT, Noury F, Badet B, Beloeil JC, Tóth E. Detection of enzymatic activity by PARACEST MRI: a general approach to target a large variety of enzymes. Angew Chemie Int Ed. 2008;47:4370–4372. doi: 10.1002/anie.200800809. [DOI] [PubMed] [Google Scholar]
- 7.Li Y, Sheth VR, Liu G, Pagel MD. A self-calibrating PARACEST MRI contrast agent that detects esterase enzyme activity. Contrast Media Molec Imaging. 2011;6:219–228. doi: 10.1002/cmmi.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Airan RD, Bar-Shir A, Liu G, Pelled G, McMahon MT, van Zijl PCM, Bulte JWM, Gilad AA. MRI biosensor for protein kinase A encoded by a single synthetic gene. Magn Reson Med. 2012;68:1919–1923. doi: 10.1002/mrm.24483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu G, Liang Y, Bar-Shir AA, Chan KWYC, Galpoththawela CS, Bernard SM, Tse T. Monitoring enzyme activity using a diamagnetic chemical exchange saturation transfer magnetic resonance imaging contrast agent. J Am Chem Soc. 2011;133:16326–16329. doi: 10.1021/ja204701x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuo PH, Kanal E, Abu-Alfa AK, Cowper SE. Gadolinium-based MR contrast agents and nephrogenic systemic fibrosis. Radiology. 2007;242:647–649. doi: 10.1148/radiol.2423061640. [DOI] [PubMed] [Google Scholar]
- 11.Yang X, Song X, Li Y, Liu G, Ray Banerjee S, Pomper MG, McMahon MT. Salicylic acid and analogues as diaCEST MRI contrast agents with highly shifted exchangeable proton frequencies. Angew Chem Int Ed Engl. 2013;52:8116–8119. doi: 10.1002/anie.201302764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choe Y, Leonetti F, Greenbaum DC, Lecaille F, Bogyo M, Bromme D, Ellman JA, Craik CS. Substrate profiling of cysteine proteases using a combinatorial peptide library identifies functionally unique specificities. J Bio Chem. 2006;281:12824–12832. doi: 10.1074/jbc.M513331200. [DOI] [PubMed] [Google Scholar]
- 13.Chen LQ, Howison CM, Jeffery JJ, Robey IF, Kuo PF, Pagel MD. Evaluations of extracellular pH within in vivo tumors using acidoCEST MRI. Magn Reson Med. 2014;72:1408–1417. doi: 10.1002/mrm.25053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Randtke EA, Chen LQ, Corrales LR, Pagel MD. The Hanes-Woolf Linear QUESP method improves the measurements of fast chemical exchange rates with CEST MRI. Magn Reson Med. 2013;74:1063–1612. doi: 10.1002/mrm.24792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Randtke EA, Chen LQ, Pagel MD. The reciprocal linear QUEST analysis method facilitates the measurements of chemical exchange rates with CEST MRI. Contrast Media Molec Imaging. 2013;9:252–258. doi: 10.1002/cmmi.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ali MM, Liu G, Shah T, Flask CA, Pagel MD. Using two chemical exchange saturation transfer magnetic resonance imaging contrast agents for molecular imaging studies. Acc Chem Res. 2009;42:915–924. doi: 10.1021/ar8002738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang X, Yadav NN, Song X, Ray Banerjee S, Edelman H, Minn I, van Zijl PCM, Pomper MG, McMahon MT. Salicylic acid and analogs: diamagnetic chemical exchange saturation transfer (diaCEST) magnetic resonance imaging (MRI) contrast agents with highly shifted exchangeable protons. Chemistry. 2014;20:15824–15832. [Google Scholar]
- 18.Revell LE, Williamson BE. Why are some reactions slower at higher temperatures? J Chem Educ. 2013;90:1024–1027. [Google Scholar]
- 19.Jones KM, Randtke EA, Howison CM, Cárdenas-Rodríguez J, Sime PJ, Kottmann RM, Pagel MD. Measuring Extracellular pH in a Lung Fibro-sis Model with acidoCEST MRI. Molec Imaging Biol. 2015;17:177–184. doi: 10.1007/s11307-014-0784-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen LQ, Randtke EA, Jones KM, Moon BF, Howison CM, Pagel MD. Evaluations of tumor acidosis within in vivo tumor models using parametric maps generated with acidoCEST MRI. Mol Imaging Biol. 2015;17:488–496. doi: 10.1007/s11307-014-0816-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moon BF, Jones KM, Chen LQ, Liu P, Randtke EA, Howison CM, Pagel MD. A comparison of iopromide and iopamidol, two acidoCEST MRI contrast media that measure tumor extracellular pH. Contrast Media Molec Imaging. 2015 doi: 10.1002/cmmi.1647. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aggarwal N, Sloane BF, Cathepsin B. Multiple roles in cancer. Proteo-mics: Clin App. 2014;8:427–437. doi: 10.1002/prca.201300105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roshy S, Sloane BF, Moin K. Pericellular cathepsin B and malignant progression. Cancer Metast Rev. 2003;22:271–286. doi: 10.1023/a:1023007717757. [DOI] [PubMed] [Google Scholar]
- 24.Mahmood U, Weissleder R. Near-infrared optical imaging of proteases in cancer. Mol Cancer Ther. 2003;2:489–496. [PubMed] [Google Scholar]
- 25.Edem PE, Czorny S, Valliant JF. Synthesis and evaluation of radioiodinated acyloxymethyl ketones as activity-based probes for cathepsin B. J Med Chem. 2014;57:9564–9577. doi: 10.1021/jm501357r. [DOI] [PubMed] [Google Scholar]
- 26.Yoo B, Raam M, Rosenblum R, Pagel MD. Enzyme-responsive PARACEST MRI contrast agents: A new biomedical imaging approach for studies of the proteasome. Contrast Media Molec Imaging. 2007;2:189–198. doi: 10.1002/cmmi.145. [DOI] [PubMed] [Google Scholar]
- 27.Levine MN, Raines RT. Sensitive fluorogenic substrate for alkaline phosphatase. Anal Biochem. 2011;418:247–252. doi: 10.1016/j.ab.2011.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beatty KE, Williams M, Carlson BL, Swarts BM, Warren RM, van Helden PD, Bertozzi CR. Sulfatase-activated fluorophores for rapid discrimination of mycobacterial species and strains. Proc. Nat. Acad. Sci. USA. 2013;110:12911–12916. doi: 10.1073/pnas.1222041110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Song X, Yang X, Ray Banerjee S, Pomper MG, McMahon MT. Anthranilic acid analogs as diamagnetic CEST MRI contrast agents that feature an intramolecular-bond shifted hydrogen. Contrast Media Molec Imaging. 2015;10:74–80. doi: 10.1002/cmmi.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Page BDG, Fletcher S, Yue P, Li Z, Zhang X, Sharmeen S, Datti A, Wrana JL, Trudel S, Schimmer AD, Turkson J, Gunning PT. Identification of a non-phosphorylated, cell permeable, small molecule ligand for the Stat3 SH2 domain. Bioorg Med Chem Lett. 2011;21:5605–5609. doi: 10.1016/j.bmcl.2011.06.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Furlong ST, Mauger RC, Strimpler AM, Liu YP, Morris FX, Edwards PD. Synthesis and physical characterization of a P1 arginine combinatorial library, and its application to the determination of the substrate specificity of serine peptidases. Bioorg Med Chem. 2002;10:3637–3647. doi: 10.1016/s0968-0896(02)00174-8. [DOI] [PubMed] [Google Scholar]
- 32.Lee YS, Agnes RS, Badghisi H, Davis P, Ma S, Lai J, Porreca F, Hruby VJ. Design and synthesis of novel hydrazide-linked bifunctional pep-tides as delta/mu opioid receptor agonists and CCK-1/CCK-2 receptor antagonists. J Med Chem. 2006;49:1773–1780. doi: 10.1021/jm05085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Woessner DE, Zhang S, Merritt ME, Sherry AD. Numerial solution of the Bloch equations provides insights into the optimum design of PARACEST agents for MRI. Magn Reson Med. 2005;53:790–799. doi: 10.1002/mrm.20408. [DOI] [PubMed] [Google Scholar]
- 34.Evbuomwan OM, Lee J, Woods M, Sherry AD. The presence of fast-exchanging proton species in aqueous solutions of paraCEST agents can impact rate constants measured for slower exchanging species when fitting CEST spectra to the Bloch equations. Inorg Chem. 2014;53:10012–10014. doi: 10.1021/ic501290q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McMahon MT, Gilad AA, Zhou J, Sun PZ, Bulte JW, van Zijl PCM. Quantifying exchange rates in chemical exchange saturation transfer agents using the saturation time and saturation power dependencies of the magnetization transfer effect on the magnetic resonance imaging signal (QUEST and QUESP): Ph calibration for poly-L-lysine and a starburst dendrimer. Magn Reson Med. 2006;55:836–847. doi: 10.1002/mrm.20818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Amman C, Meier P, Merbach AE. A simple multinuclear thermometer. J Magn Reson. 1982;46:319–321. [Google Scholar]