Abstract
Human milk is the ideal nutrition source for healthy infants during the first six months of life and a detailed characterisation of the composition of milk from mothers that deliver prematurely (<37 weeks gestation), and of how human milk changes during lactation, would benefit our understanding of the nutritional requirements of premature infants. Individual milk samples from mothers delivering prematurely and at term were collected. The human milk metabolome, established by nuclear magnetic resonance (NMR) spectroscopy, was influenced by gestational and lactation age. Metabolite profiling identified that levels of valine, leucine, betaine, and creatinine were increased in colostrum from term mothers compared with mature milk, while those of glutamate, caprylate, and caprate were increased in mature term milk compared with colostrum. Levels of oligosaccharides, citrate, and creatinine were increased in pre-term colostrum, while those of caprylate, caprate, valine, leucine, glutamate, and pantothenate increased with time postpartum. There were differences between pre-term and full-term milk in the levels of carnitine, caprylate, caprate, pantothenate, urea, lactose, oligosaccharides, citrate, phosphocholine, choline, and formate. These findings suggest that the metabolome of pre-term milk changes within 5–7 weeks postpartum to resemble that of term milk, independent of time of gestation at pre-mature delivery.
Keywords: pre-term, infant, nutrition, human milk, metabolites, NMR, metabolomics
1. Introduction
Human milk (HM) is the recognised gold standard for feeding new-born full-term healthy infants. HM is a unique food source that contains all the exacting amounts of required nutrients to support the growth and development of term infants during the first six months of life. The health benefits of HM have been well documented [1]. Current data suggest that the quantity of HM consumed by healthy term infants is on average 0.778 kg human milk/day, with males consuming 0.056 kg/day more than females. Human milk consumption rises rapidly during the first month postpartum to ~0.6 kg/day and increases to ~0.82 kg/day in 3–4 month old infants [2].
Human milk is also regarded as particularly important for feeding pre-mature infants. There are many health benefits related to providing a pre-term infant with HM, including improvements in digestion, nutrient absorption, gastrointestinal function, and neurodevelopment of the infant [3,4]. In addition, an exclusive HM diet has been associated with lower rates of necrotizing enterocolitis, a potentially fatal gastrointestinal complication, in premature infants [5]. Human milk oligosaccharides (HMO) are known to affect the gut microbiota and it has been speculated that HMOs could account for the lower observed incidences of necrotizing enterocolitis [6].
Mother’s milk is inadequate for rapidly growing pre-term neonates, which can be ascribed to a higher protein and energy requirement of pre-term neonates, particularly those of low birth weight and <28 weeks gestation, leading to insufficient weight gain and nutrition deficits [7,8]. For extremely pre-term and very low birth weight infants, the fortification of HM using commercial fortifiers is often recommended in order to supply the neonate with the nutrients required to support its rapid rate of growth. Studies have shown that the addition of HM fortifier can improve weight, length, and head circumference growth, in addition to bone mineralization and neurological outcomes [9,10,11,12,13].
It is known that the composition of pre-term milk differs from that of milk of full-term mothers, with higher reported levels of total protein, fat, carbohydrate and energy in pre-term milk [14]. Differences in the proteome of the two types of milk have also been reported [15]. Generally, studies have focused on the macronutrient content of pre-term and term HM without consideration of micronutrients such as biologically significant metabolites [14].
Previous work on milk metabolomes has predominantly considered bovine milk [16,17,18,19,20] and human milk [21,22,23,24,25,26,27,28]. Recently, a multi-analytical platform study identified 710 metabolites in human milk by using a combination of MS- and NMR-based analytical techniques [21]. Moreover, a recent study identified subtle differences between some milk metabolites during the first month of lactation [27]. However, the changes in pre-term and full-term human milk metabolomes over a full lactation time course have not yet been reported in detail.
In this study, we report how gestational age affects the low-molecular-weight metabolome of HM from mothers of pre-term and term infants over range of stages of lactation. The milk metabolomes were compared using proton nuclear magnetic resonance spectroscopy (1H NMR)-based metabolomics from milk samples from mothers at different gestational ages. Longitudinal samples were also examined to analyse how pre-term milk develops with time postpartum compared with term milk.
2. Materials and Methods
2.1. Ethical Approval
Ethical approval for this study was granted by The Clinical Research Ethics Committee of the Cork Teaching Hospitals, Cork, Ireland (clinical number reference ECM 4(s) 06/08/13).
2.2. Samples and Sample Collection
2.2.1. Pre-term Samples
Multiple frozen (−20 °C) longitudinal pre-term HM samples were collected from 15 individual mothers of pre-term infants (Table 1). The HM samples were collected from the freezers of the neonatal intensive care unit of Cork University Maternity Hospital (Wilton, Co. Cork, Ireland). Each bottle of HM was dated allowing for an accurate calculation of the gestational age of the infant when the milk was expressed. The HM samples from each individual mother were pooled according to ‘day postpartum’ up to 14 days and according to ‘week postpartum’ after this, creating n = 62 pre-term HM samples. For one donor (Pre-1), information on number of days postpartum was unavailable and accordingly samples from this donor were removed from multivariate models using gestational age or days postpartum for modelling or visualization purposes.
Table 1.
Sample information from mothers of pre-term infants.
| Sample Id | Secretor Status | Gestation | Infant Weight, Grams | Age of Mother, Years | Gravida 1 | Para 2 | Number of Samples | Postpartum Span 3 |
|---|---|---|---|---|---|---|---|---|
| Pre 01 | Se+ | – | – | – | – | – | 4 | – |
| Pre 02 | Se+ | 38 weeks | 3210 | 35 | 2 | 2 | 4 | 7–11 weeks |
| Pre 03 | Se+ | 28 weeks | 1190 | 33 | 1 | 1 | 9 | 4–12 days |
| Pre 04 | Se+ | 31 weeks | 1830 | 36 | 3 | 3 | 5 | 1–5 weeks |
| Pre 05 | Se+ | 30 weeks | 1340 | 29 | 1 | 1 | 7 | 4 days–7 weeks |
| Pre 06 | Se+ | 32 weeks + 2 days | 2340 | 42 | 2 | 2 | 1 | 8 days |
| Pre 07 | Se− | 24 weeks + 4 days | 540 | 33 | 1 | 1 | 4 | 1–4 days |
| Pre 08 | Se− | 31 weeks | 1680 | 35 | 2 | 2 | 2 | 5–14 weeks |
| Pre 09 | Se+ | 35 weeks + 6 days | 2410 | 33 | 1 | 1 | 3 | 2–5 weeks |
| Pre 10 | Se+ | 35 weeks + 2 days | 3100 | 26 | 1 | 1 | 2 | 4–5 days |
| Pre 11 | Se+ | 33 weeks + 4 days | 1800 | 29 | 6 | 5 | 2 | 4–6 days |
| Pre 12 | Se+ | 35 weeks + 4 days | 3260 | 45 | 4 | 3 + 2 4 | 2 | 9–10 days |
| Pre 13 | Se+ | 26 weeks | 650 | 40 | – | – | 15 | 6 days–8 weeks |
| Pre 14 | Se− | 28 weeks + 3 days | – | – | – | – | 1 | 13 weeks |
| Pre 15 | Se+ | 32 weeks | – | – | – | – | 1 | 9 weeks |
1 Number of pregnancies, including the current pregnancy; 2 number of times the mother has given birth; 3 time range indicates days postpartum of first and last samples from that donor; 4 indicates twin pregnancy.
2.2.2. Full-term Samples
Frozen (−20 °C) HM samples (n = 30) were obtained from The Western Trust Milk Bank, Irvinestown, Co. Fermanagh, Ireland (Table 2). The HM samples were from 30 individual mothers of healthy full-term infants who donated milk with consent for use for research purposes. The mothers expressed milk in a domestic setting and stored milk at −20 °C before shipping to The Western Trust Milk Bank, Ireland. HM was pasteurised and microbiologically screened before being frozen and shipped to University College Cork where it was stored at −20 °C. Milk samples were categorized according to the length of time postpartum; colostrum (<5 days postpartum; n = 5), transitional (6 days–2 weeks postpartum; n = 4), mature (>2 weeks, n = 21).
Table 2.
Sample information from mothers of term infants.
| Sample ID | Secretor status | Time postpartum | Group |
|---|---|---|---|
| Term 01 | Se+ | <5 days | Colostrum |
| Term 02 | Se+ | <5 days | Colostrum |
| Term 03 | Se+ | <5 days | Colostrum |
| Term 04 | Se+ | <5 days | Colostrum |
| Term 05 | Se+ | <5 days | Colostrum |
| Term 06 | Se+ | 2 weeks | Transitional |
| Term 07 | Se+ | 2 weeks | Transitional |
| Term 08 | Se− | 6 days | Transitional |
| Term 09 | Se− | 2 weeks | Transitional |
| Term 10 | Se− | 15 weeks | Mature |
| Term 11 | Se− | 9 weeks | Mature |
| Term 12 | Se+ | 15 weeks | Mature |
| Term 13 | Se− | 11 weeks | Mature |
| Term 14 | Se− | 15 weeks | Mature |
| Term 15 | Se− | 11 weeks | Mature |
| Term 16 | Se+ | 16 weeks | Mature |
| Term 17 | Se− | 9 weeks | Mature |
| Term 18 | Se+ | 19 weeks | Mature |
| Term 19 | Se+ | 24 weeks | Mature |
| Term 20 | Se+ | 11 weeks | Mature |
| Term 21 | Se+ | 7–10 weeks | Mature |
| Term 22 | Se+ | 13 weeks | Mature |
| Term 23 | Se+ | 13 weeks | Mature |
| Term 24 | Se+ | 14 weeks | Mature |
| Term 25 | Se+ | 5 weeks | Mature |
| Term 26 | Se+ | 27 weeks | Mature |
| Term 27 | Se− | 33 weeks | Mature |
| Term 28 | Se− | 27 weeks | Mature |
| Term 29 | Se+ | 29 weeks | Mature |
| Term 30 | Se+ | 33 weeks | Mature |
2.3. NMR Spectroscopy
NMR spectroscopy was essentially performed as described earlier [29]. Briefly, the samples skimmed by centrifugation at 4000 g for 15 min and removal of the top fat layer before filtering to remove residual lipids and protein using Amicon Ultra 0.5 mL 10 kDa (Millipore, Billerica, MA, USA) spin filters at 10,000 g for 30 min at 4 °C. A filtered sample (500 µL) was mixed with 100 µL D2O containing 0.025% 3-(trimethylsilyl) propionic-2,2,3,3-d4 acid, sodium salt (TSP; Sigma-Aldrich, St. Louis, MO, USA) as an internal chemical shift reference. 1H NMR spectroscopy was performed at 298 K on a Bruker Avance III 600 spectrometer, operating at a 1H frequency of 600.13 MHz, and equipped with a 5-mm 1H TXI probe (Bruker BioSpin, Rheinstetten, Germany). The sample sequence was randomized prior to acquisition and standard one-dimensional spectra were acquired using a single 90° pulse experiment with a relaxation delay of 5 s. Water suppression was achieved by irradiating the water peak during the relaxation delay, and a total of 64 scans were collected into 32,768 data points spanning a spectral width of 12.15 ppm. All 1H spectra were initially referenced to the TSP signal at 0 ppm. Prior to Fourier transformation, the data were multiplied by a 0.3 Hz line-broadening function. The proton NMR spectra were phase and baseline corrected manually using Topspin 3.2 (Bruker Biospin, Rheinstetten, Germany). NMR signals were assigned in accordance with existing literature [20,21,25,29], 2D NMR spectroscopy, Chenomx NMR Suite 8.1.2 (Chenomx Inc, Edmonton, AB, Canada) and the Human Metabolome Database [30].
2.4. Secretor Status
Maternal secretor status was determined as previously described [25]. In brief the absence or presence of 2-FL was used to determine secretor status quantified by NMR spectroscopy (2-FL corresponds to NMR signal at δ 5.32 ppm). Milk from mothers classified as non-secretors did not have any detectable levels of 2-FL, whereas milk from mothers classified as secretors did contain 2-FL.
2.5. Multivariate Data and Statistical Analyses
NMR spectra of milk samples were aligned using Icoshift by co-shifting of the whole spectra according to the anomeric lactose proton at 5.23 ppm [31]. The proton NMR spectra were subdivided into 0.01 ppm bins, reducing each spectrum into 957 separate variables in the regions 10.00–5.00 and 4.72–0.5 ppm. Principal component analysis (PCA) and orthogonal partial least squares discriminant analysis (OPLS-DA) were performed in order to identify differences in the metabolite profiles. The data was mean-centred and Pareto-scaled prior to analysis. The OPLS-DA model was cross-validated using segmentation with seven splits. Covariance was investigated by analysis of OPLS-DA regression coefficients back-transformed to original data and colour coded by the loading weights [32]. The multivariate data analysis was performed using SIMCA-P + 13 (Umetrics AB, Umeå, Sweden). Alignment by Icoshift, binning, and analysis of OPLS-DA plots were performed in MATLAB 7.13 using in-house developed scripts (MathWorks Inc., Natick, MA, USA). Univariate statistical significance was evaluated by Student’s t-test using the Statistics Toolbox in MATLAB 7.13 (MathWorks Inc., Natick, MA, USA).
3. Results
HM samples (n = 92) were collected from 45 individual mothers with different gestational ages at delivery, ranging from 24.6–35.8 weeks in the case of pre-term samples and more than 37 weeks for full-term samples (Table 1 and Table 2). For pre-mature deliveries, infant birth weight ranged from 540 g to 3260 g. Age of the mothers of pre-term infants ranged from 26–45 years, and all had at least one previous pregnancy longer than 24 weeks gestation (Table 1).
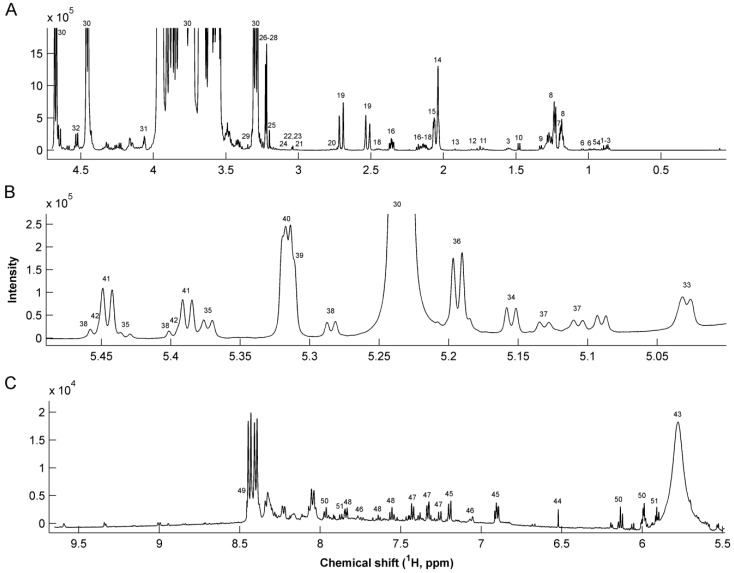
The median spectrum of all 92 milk samples is shown in Figure 1. Each signal corresponds to proton resonances in milk metabolites according to assignments in Table 3. Differences in fucosylated human milk oligosaccharides (HMO) have previously been described to depend on maternal secretor status [33]. Accordingly, secretor status of the mothers was determined by examining the specific patterns of fucosylated oligosaccharides (Figure 1B) [25]. Thirteen mothers were found to be non-secretors, accounting for 28.9% of the mothers included in the study (Table 1 and Table 2).
Figure 1.
Median 1H NMR spectrum of 92 human milk samples. (A) Aliphatic region 4.6–0 ppm (B) Human milk oligosaccharides in 5.45–5.00 ppm region; and (C) aromatic region 9.7–5.5 ppm region. For peak assignments refer to Table 3.
Table 3.
List of metabolites in human milk with chemical shifts in ppm from internal TSP standard and assignment of resonances.
| # | Metabolite | 1H Chemical shift (ppm) | Assignment | # | Metabolite | 1H Chemical shift (ppm) | Assignment |
|---|---|---|---|---|---|---|---|
| 1 | Caprylate | 0.85 | CH3 | 29 | Methanol | 3.37 | CH3 |
| 1.53 | CH2 | 30 | Lactose | 3.23, 3.5–4.0 | Multiple | ||
| 2 | Caprate | 0.85 | CH3 | 31 | Gluconate | 4.05 | CH |
| 1.53 | CH2 | 32 | Galactose | 4.57 | CH | ||
| 3 | Butyrate | 0.88 | CH3 | 33 | Fuc α1,4 GlcNAc | 5.03 | CH-1 |
| 1.55 | β-CH2 | 34 | Fuc α1,3 GlcNAc | 5.19 | CH-1 | ||
| 2.16 | α-CH2 | 35 | LNDFH II | 5.03 | Fuc (α1-4) CH-1 | ||
| 4 | Pantothenate | 0.91 | CH3 | 5.38 | Fuc (α1-3) αGlc CH-1 | ||
| 5 | Leucine | 0.94 | CH3 | 5.43 | Fuc (α1-3) βGlc CH-1 | ||
| 6 | Valine | 0.98 | γ-CH3 | 36 | LNDFH I | 5.16 | Fuc (α1-2) CH-1 |
| 1.03 | γ’-CH3 | 5.03 | Fuc (α1-4) CH-1 | ||||
| 7 | 3-BHBA | 1.18 | CH3 | 37 | LNFP III | 5.11 | Fuc (α1-3) GlcNAc CH-1 |
| 2.39 | CH2 | 5.13 | Fuc (α1-3) GlcNAc CH-1 | ||||
| 8 | Fucosyl moieties | 1.19 | CH3-6 | 38 | LDFT | 5.29 | Fuc (α1-2) CH-1 |
| 1.23–1.29 | CH3-6 | 5.4 | Fuc (α1-3) αGlc CH-1 | ||||
| 9 | Lactate | 1.32 | CH3 | 5.46 | Fuc (α1-3) βGlc CH-1 | ||
| 10 | Alanine | 1.47 | CH3 | 39 | LNFP I | 5.32 | Fuc (α1-2) CH-1 |
| 11 | 6’-SL | 1.73 | CH3 | 40 | 2’FL | 5.32 | Fuc (α1-2) CH-1 |
| 12 | 3’-SL | 1.78 | CH3 | 41 | 3’FL | 5.39 | Fuc (α1-3) αGlc CH-1 |
| 13 | Acetate | 1.91 | CH3 | 5.44 | Fuc (α1-3) βGlc CH-1 | ||
| 14 | GlcNAc | 2.04 | CH3 | 42 | LNFP V | 5.39 | Fuc(α1-3)αGlc CH-1 |
| 15 | Sialic acid | 2.06 | CH3 | 5.44 | Fuc (α1-3) βGlc CH-1 | ||
| 16 | Glutamate | 2.12 | β-CH2 | 43 | Urea | 5.76 | NH2 |
| 2.33 | γ-CH2 | 44 | Fumarate | 6.51 | CH = CH | ||
| 17 | Methionine | 2.13 | CH3 | 45 | Tyrosine | 6.9 | CH-3,5 |
| 18 | Glutamine | 2.11 | β-CH2 | 7.2 | CH-2,6 | ||
| 2.46 | γ-CH2 | 46 | Methylhistidine | 7.06 | CH-4 | ||
| 19 | Citrate | 2.51 | α-CH2 | 7.8 | CH-2 | ||
| 2.69 | α’-CH2 | 47 | Phenylalanine | 7.32 | CH-2,6 | ||
| 20 | Dimethylamine | 2.72 | 2 CH3 | 7.37 | CH-4 | ||
| 21 | 2-oxogluturate | 2.99 | CH2 | 7.42 | CH-3,5 | ||
| 22 | Creatinine | 3.02 | CH3 | 48 | Hippurate | 7.54 | CH-2,6 |
| 23 | Creatine | 3.03 | CH3 | 7.63 | CH-4 | ||
| 24 | Malonate | 3.12 | CH2 | 7.82 | CH-3,5 | ||
| 25 | Choline | 3.19 | 3 CH3 | 49 | Formate | 8.44 | CH |
| 26 | O-Phosphocholine | 3.21 | 3 CH3 | 50 | Cytidine triphosphate | 5.99 | CH-2 |
| 4.16 | CH2 | 6.13 | CH-10 | ||||
| 27 | Carnitine | 2.42 | CH2 | 7.97 | CH-11 | ||
| 3.21 | 3 CH3 | 51 | Uridine | 5.89 | CH-2 | ||
| 28 | Betaine | 3.24 | 3 CH3 | 5.91 | CH-10 | ||
| 7.87 | CH-11 |
Abbreviations: 3-BHBA, 3-betahydroxybutyrate; FL, fucosyllactose; Fuc, Fucose; GlcNAc, N-acetylglucosamine; LDFT, lactodifucotetraose; LNDFH, lacto-N-difucohexaose, LNFP, lacto-N-fucopentaose; SL, sialyllactose.
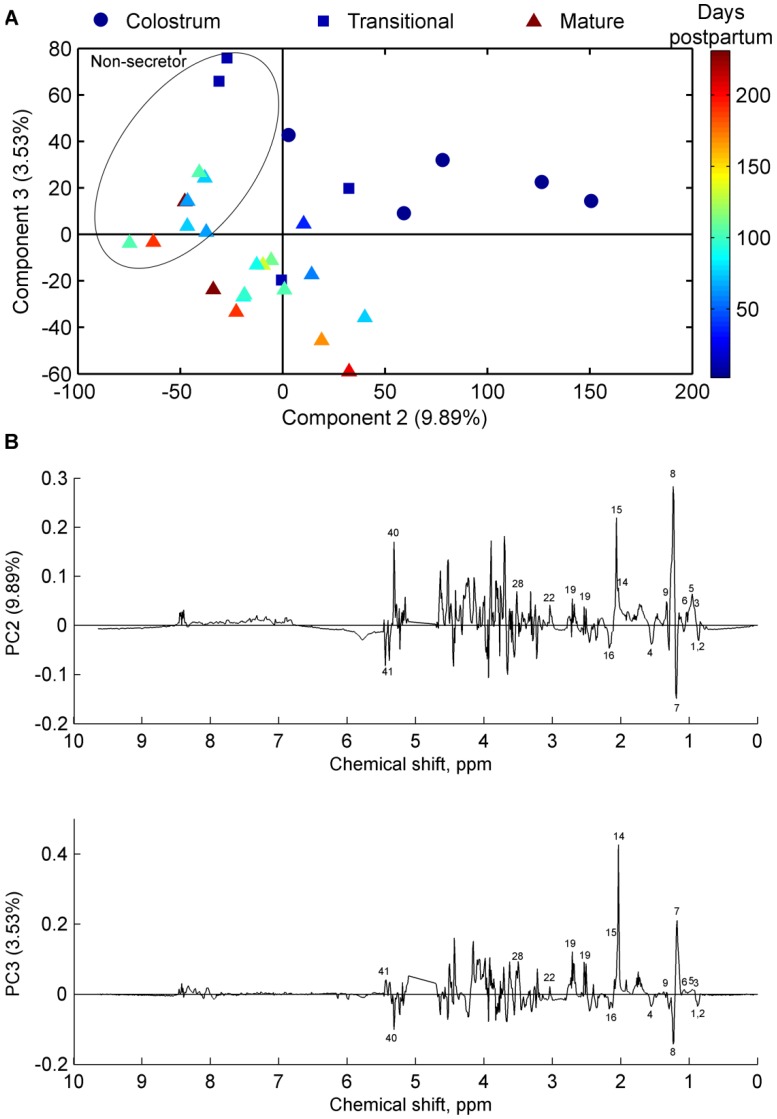
The collected milk samples were analysed for changes during lactation following both full-term (Figure 2) and pre-term (Figure 3) delivery. Figure 2 shows a scores plot of principal component (PC) 2 and PC3 from a principal component analysis (PCA) model of full-term milk samples. The samples are grouped into colostrum, transitional, and mature groups and coloured according to days postpartum. The scores plot shows the distinct groupings of the milk samples, and indicates a gradual change in the milk metabolome as the milk develops from colostrum towards mature milk (Figure 2). In addition, no detectable differences were observed between mature milk samples in the intervals before 26 weeks and after 26 weeks (Figure 2). There was an apparent difference in milk metabolites depending on secretor status in term milk samples, as the milk samples were separated into secretor or non-secretor groups along PC2 (Figure 2).
Figure 2.
(A) Principal component analysis scores plot of full-term milk samples from 30 mothers (n = 30)); samples are coloured according to days postpartum; Colostrum (dots), transitional (squares), and mature (triangles); the circle denotes samples from non-secretor mothers, while remaining samples are from secretor mothers; and (B) corresponding loading line plots. For peak assignments refer to Table 3.
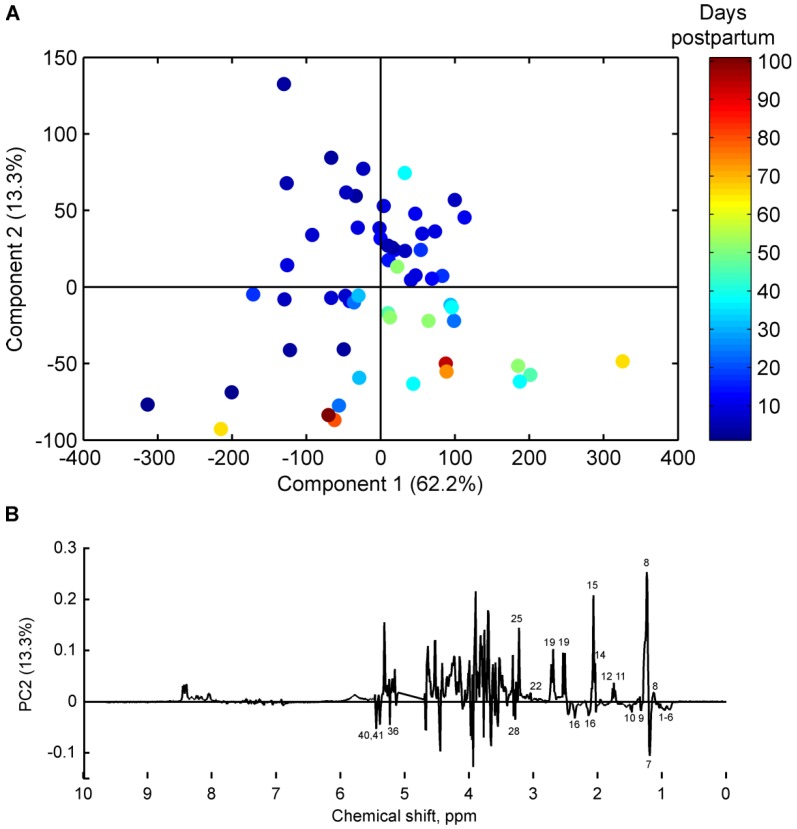
Figure 3.
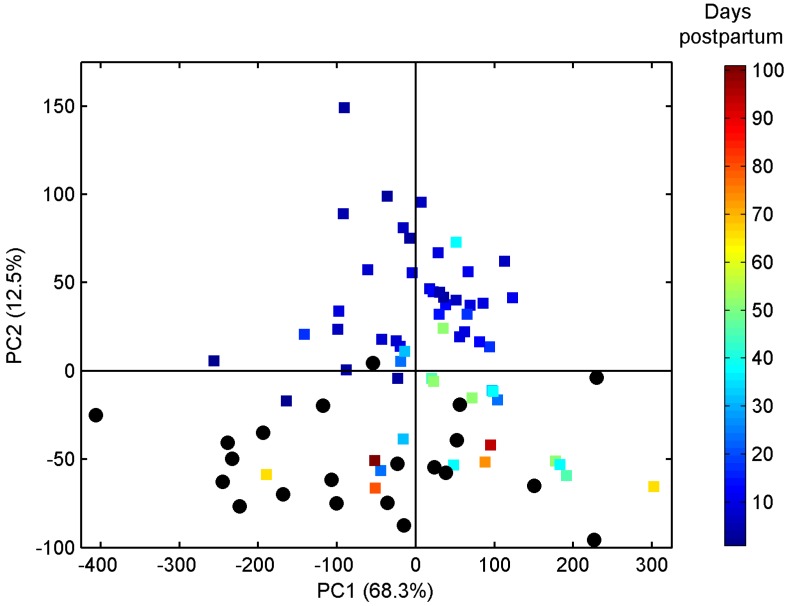
(A) Principal component analysis scores plot of pre-term milk samples from 15 mothers (n = 58), coloured according to number of days postpartum; and (B) corresponding loading line plot. For peak assignments refer to Table 3.
Several milk metabolites were found to be present in significantly different concentration in colostrum, transitional, and mature milk. Fucosylated oligosaccharides, and also components of oligosaccharides (Fucose, N-acetylneuraminic acid, N-acetylglucosamine), were found at the highest levels in colostrum, and levels decreased in mature milk samples (Figure 2B). Moreover, levels of valine, leucine, pantothenate, citric acid, lactic acid, betaine, and creatinine were higher in colostrum and transitional milk compared with mature HM, and levels of glutamate, butyrate, caprylate, and caprate were higher in mature HM compared with colostrum and transitional milk. The level of β-hydroxybutyrate was found to be independent of milk maturity in full-term HM (Figure 2B). Milk from non-secretor mothers did not contain oligosaccharides with α1-2 fucosylated structures (Figure 2B); however, the level of 3’ fucosyllactose (3’-FL) was increased in non-secretor mothers compared with secretor mothers.
Similarly, the pre-term milk samples were also examined for an effect of stage of lactation on the milk metabolome. The scores plot of a two component PCA model of milk samples from pre-term mothers is shown in Figure 3, in which samples are coloured according to number of days postpartum. The scores and loadings plots clearly show the changes in milk metabolome occurring over time in pre-term milk. Levels of fucosyl moieties, N-acetylneuraminic acid, N-acetylglucosamine, 3’-sialyllactose, 6’-sialyllactose, 2’-fucosyllactose, citric acid, choline, and creatinine decreased with time postpartum (Figure 3B), while levels of 3-FL, lacto-N-difucohexaose I (LNDFH I), butyrate, caprylate, caprate, lactic acid, valine, leucine, alanine, glutamate, and pantothenate increased with time postpartum (Figure 3B). Only two mothers, who donated seven milk samples in total, were identified as non-secretors (Table 1). Thus, it was not possible to identify differences in the PCA model for pre-term samples on the basis of secretor status.
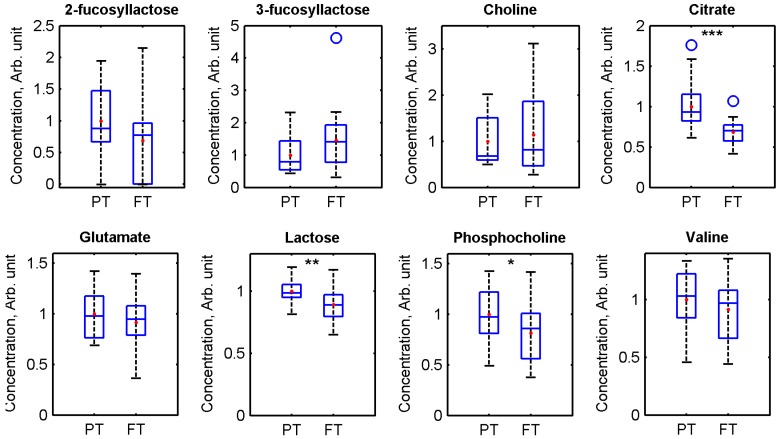
As some of the same time-related changes were apparent in both pre-term and full-term milk samples, the difference between the pre-term and full-term milk metabolomes of similar days postpartum was investigated. Citrate (P = 0.00057), lactose (P = 0.0039), and phosphocholine (P = 0.049 were found to be present in significantly higher levels in pre-term milk samples compared with full-term milk samples (Figure 4). Citrate level is known to decrease with time postpartum [28], while lactose is the major milk metabolite identified by NMR spectroscopy [20].
Figure 4.
Box plots showing the distributions of 2-fucosyllactose, 3-fucosyllactose, choline, citrate, glutamate, lactose, phosphocholine, and valine concentrations in pre-term (PT) and full-term (FT) milk. Pre-term milk samples <14 days postpartum and full-term colostrum, transitional, and mature >26 weeks were excluded from the analysis in order to compare milk with a similar range of days postpartum. Horizontal lines indicate medians; coloured boxes specify interquartile ranges and dashed lines the ranges without outliers. The open circles indicate outliers (falls in-between 1.5× and 3× the interquartile range). *** = P < 0.001, ** = P < 0.01, * = P < 0.05; the comparisons were made using two-tailed Student’s t-test.
Thus, in the following analysis, NMR resonances originating from lactose were removed in order to increase the weight of other metabolites in the multivariate models. A PCA of pre-term and full-term milk samples (n = 88) was generated using two principal components which, together, explained 80.8% of the total variance described by the PCA model (Figure 5). From the PCA model, it is apparent that the milk metabolite profile from mothers of pre-term infants older than 5–7 weeks resembles that of full-term milk, as they are positioned in close proximity to the full-term milk samples, independent of time of gestation at delivery (Figure 5).
Figure 5.
Principal component analysis scores plot of mature milk samples from pre-term (squares, n = 58) and full-term (circles, n = 30) mothers. Pre-term milk samples are coloured according to days postpartum.
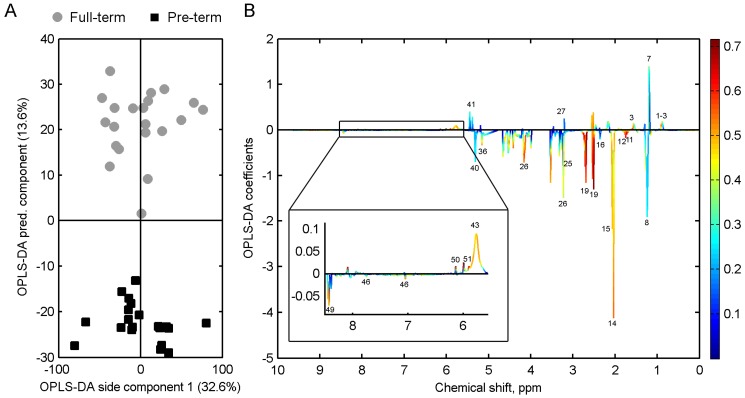
OPLS-DA was also performed on the pre-term and full-term milk samples (Figure 6). Full-term and pre-term colostrum and transitional milk were excluded from the analysis, due to there being too few samples of these, and to be able to compare milk samples with similar days postpartum. The OPLS-DA model shows that significant differences exist between the metabolite profiles of pre-term and full-term milk (Figure 6B). In full-term milk, carnitine, caprylate, caprate, pantothenate, beta-hydroxybutyrate, and urea were found to be present in higher levels compared with pre-term milk, while lactose (Figure 4), Fucosyl moieties, N-acetylneuraminic acid, N-acetylglucosamine, 3’-sialyllactose, 6’-sialyllactose, lacto-N-difucohexaose I (LNDFH I), glutamate, citric acid, phosphocholine, choline, and formic acid were found in higher levels in pre-term milk (Figure 6).
Figure 6.
(A) Orthogonal partial least squares discriminant analysis of pre-term (n = 20, days postpartum range 3–14 weeks) and full-term (n = 21, days postpartum range 3–26 weeks) milk. Full-term colostrum, transitional, and pre-term milk <2 weeks postpartum have been excluded. Cross-validation, Q2: 0.70; (B) corresponding OPLS-DA coefficients plot. Each variable has been coloured according to the OPLS-DA loadings (correlation between NMR variables and pre-term/full-term classes); for peak assignments refer to Table 3.
4. Discussion
Pre-mature birth is considered to have long-lasting adverse effects on health, and it has been proposed that the introduction of metabolomics technologies may facilitate better understanding of these effects [34]. The exact causes of preterm delivery are not clear, but often associated with inflammation [35]. In the present study, causes of the preterm delivery were not known. In this study, differences in metabolomic profiles of HM were associated with gestational age. Previous studies have shown many health benefits of breast-feeding pre-term infants. However, mother’s milk may not always be adequate for pre-term infants with high nutrition density requirements, which can lead to insufficient weight gain and nutrition deficits. To date, only a few metabolomics studies have been performed on pre-term human milk in the first few weeks of lactation [27]. To our knowledge, this is the first study to comprehensively characterize and compare the pre-term and full-term human milk metabolome over a lactation time-course (up to 14 weeks postpartum). Milk metabolomics may be used in a clinical setting [23], in order to establish the milk metabolome for infant nutrition in cases where the optimal nutrition is a key advantage as in pre-term infants. Milk metabolomics studies in cows’ milk have previously shown an ability to yield information about the health status of the cow [29,36]. It can likewise be hypothesized that the milk metabolome of lactating mothers can give information that can be used in a clinical setting.
Previous studies have shown that several factors influence the variability in the milk metabolome. The epithelial cells in the mammary gland are accountable for the milk production, and these cells are ultimately responsible for converting most precursors into milk components, but many other cell types are also involved in milk production [37]. Development of the mammary epithelial cells during pregnancy has been shown to influence transport pathways in the mammary gland responsible for different protein profiles of milk [38]. Moreover, insulin has been shown to be actively transported into milk and maternal diabetes affect milk insulin levels [39]. Maternal factors such as diet, lifestyle and phenotype have been shown to influence the milk composition [26]. Moreover, secretor status is also important for the biosynthesis of milk oligosaccharides [26]. The presence of α1,2 fucosyltransferase gene (FUT2) is important for the milk oligosaccharides since non-secretors, which lack the FUT2 gene, are unable to biosynthesize HMOs with a fucose bound to a N-acetylglucosamine via a glycosidic α1,2 linkage. Non-secretor status occurs in approximately 20% of most human populations [40,41]. In the present study, non-secretor status, identified by increased level of 3-FL, was found in 28.9 % of milk donors. Gestational age and the stage of lactation are also known to influence the milk proteins [14], lipids [42], and lactose [43]. Preliminary HM metabolomic studies have shown a difference between the metabolite profiles of pre-term and full-term HM [22,24]. Additionally, the metabolite extraction procedure in sample preparation prior to NMR spectroscopy has shown to influence the milk metabolite levels. In a recent paper, a sample preparation method using ultrafiltration as employed in the present study, was found to be superior compared with methanol/chloroform extraction in terms of separating small molecules from proteins and lipids [28]. Levels of HMO and its components are known to decrease with time [44]. A specific HMO, lacto-N-tetraose, has also been shown to be elevated in pre-term milk [45]. Interestingly, specific HMOs, LNDFH I, 3’-sialyllactose, and 6’-sialyllactose, and common components of HMO, including fucose, N-acetylglucosamine, and N-acetylneuraminic acid were identified to be present in higher concentrations in pre-term milk. HMOs have previously been shown to prevent necrotizing enterocolitis in neonatal rats [6], which may be linked to alterations in the microbiota of the infant’s gut [46]. Thus, the elevated amounts of HMOs in pre-term milk may be a factor in the observed association of lower rates of necrotizing enterocolitis in premature infants fed HM [5].
Most of the free amino acids in HM steadily decrease in level as the milk matures [42]. However, the pre-term milk amino acid composition has not been well characterized throughout the course of lactation [47,48,49]. Glutamate is known to increase with lactation time [47] and this was also found in both pre-term and full-term milk in the present study. Valine and leucine, two essential amino acids, were found at the highest concentration in full-term colostrum and decreased with time postpartum, in agreement with other human milk metabolomics studies [27]. In contrast, increases in valine and leucine levels were observed in pre-term milk with time post-partum, which is in contrast to the pattern observed in full-term milk and that previously reported for pre-term milk [48,49].
Short- and medium-chain fatty acids (SMCFA; butyrate, caprylate, and caprate) were found in higher concentrations in full-term milk compared with pre-term milk. Moreover, the levels of SMCFA increased with time postpartum, as previously reported [50]. Choline is of high nutritional value, as it is abundant in cell membranes and acts as a precursor for the important messenger acetylcholine, and infants require high amounts of choline in order to sustain their rapid growth. Phosphocholine was found in higher levels in pre-term milk than in full-term milk, which is in agreement with previous reports on phosphocholine [51]. In contrast, pre-term milk has previously been shown to contain lesser amounts of choline [51], which is contradictory to findings in the present study.
In this study, it was shown that, after 5–7 weeks the metabolite profiles of pre-term milk resembled that of full-term milk, independently of gestational age. For extremely early pre-term infants born at a gestational age of, e.g., 24 weeks, these findings suggest that mother’s milk becomes equivalent to mature milk for term infants by the time the infant reaches ~29 weeks post-menstrual age. This is a significant finding concerning a period in which the infant still requires advanced nutritional support to maintain its growth and long-term developmental requirements. Clinical metabolomics could potentially be advantageous to determine when milk metabolites levels become inadequate due to maturation of milk, if causal relationships between milk metabolites and infant growth parameters can be established. Previous studies have shown that protein concentration remains at higher levels in pre-term human milk even after 8 weeks of lactation [13]; however, we believe this to be the first study to document the changes in the metabolomics profile of pre-term human milk as it evolves to resemble that of full-term milk, and to show that this occurs independent of gestational age at delivery.
In conclusion, specific differences in milk metabolites exist between milk from mothers delivering pre-maturely and at term. Moreover, milk metabolite composition is associated with gestational age and the metabolome of pre-term milk changes within 5–7 weeks postpartum to resemble that of full-term milk, independent of time of gestation at delivery. However, as this study did not systematically collect samples from a cohort of mothers in a longitudinal design, further studies of this kind are recommended to more fully understand the significance of the changes reported herein.
Acknowledgments
The authors would like to acknowledge the Western Trust Milk Bank, Irvinestown, Co. Fermanagh, Ireland, the staff of the neonatal intensive care unit of Cork University Maternity Hospital and all of the mother and infants that kindly donated their milk. The authors are grateful for financial support from the Danish Research Council FTP through the project “Advances in Food quality and Nutrition Research through implementation of metabolomic strategies”.
Abbreviations
The following abbreviations are used in this manuscript:
- HM
Human milk
- HMO
Human milk oligosaccharide
- NMR
Nuclear magnetic resonance
Author Contributions
All authors participated in the conception, design and writing of the manuscript. All authors read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Wu T.C., Chen P.H. Health consequences of nutrition in childhood and early infancy. Pediatr. Neonatol. 2009;50:135–142. doi: 10.1016/S1875-9572(09)60051-6. [DOI] [PubMed] [Google Scholar]
- 2.Da Costa T.H.M., Haisma H., Wells J.C.K., Mander A.P., Whitehead R.G., Bluck L.J.C. How much human milk do infants consume? Data from 12 countries using a standardized stable isotope methodology. J. Nutr. 2010;140:2227–2232. doi: 10.3945/jn.110.123489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Isaacs E.B., Morley R., Lucas A. Early diet and general cognitive outcome at adolescence in children born at or below 30 weeks gestation. J. Pediatr. 2009;155:229–234. doi: 10.1016/j.jpeds.2009.02.030. [DOI] [PubMed] [Google Scholar]
- 4.Lawrence R.M. Breastfeeding, Host-resistance factors and immunologic significance of human milk. In: Lawrence R.A., Lawrence R.M., editors. Breastfeeding. A guide for the medical profession. Elsevier; Philadelphia, PA, USA: 2006. pp. 171–214. [Google Scholar]
- 5.Sullivan S., Schanler R.J., Kim J.H., Patel A.L., Trawöger R., Kiechl-Kohlendorfer U., Chan G.M., Blanco C.L., Abrams S., Cotten C.M., et al. An exclusively human milk-based diet is associated with a lower rate of necrotizing enterocolitis than a diet of human milk and bovine milk-based products. J. Pediatr. 2010;156:562–567. doi: 10.1016/j.jpeds.2009.10.040. [DOI] [PubMed] [Google Scholar]
- 6.Jantscher-Krenn E., Zherebtsov M., Nissan C., Goth K., Guner Y.S., Naidu N., Choudhury B., Grishin A.V., Ford H.R., Bode L. The human milk oligosaccharide disialyllacto-N-tetraose prevents necrotising enterocolitis in neonatal rats. Gut. 2012;61:1417–1425. doi: 10.1136/gutjnl-2011-301404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mikkola K., Ritari N., Tommiska V., Salokorpi T., Lehtonen L., Tammela O., Pääkkönen L., Olsen P., Korkman M., Fellman V. Neurodevelopmental outcome at 5 years of age of a national cohort of extremely low birth weight infants who were born in 1996–1997. Pediatrics. 2005;116:1391–1400. doi: 10.1542/peds.2005-0171. [DOI] [PubMed] [Google Scholar]
- 8.Sauer P.J. Can extrauterine growth approximate intrauterine growth? Should it? Am. J. Clin. Nutr. 2007;85:608S–613S. doi: 10.1093/ajcn/85.2.608S. [DOI] [PubMed] [Google Scholar]
- 9.Faerk J., Petersen S., Peitersen B., Michaelsen K.F. Diet and bone mineral content at term in premature infants. Pediatr. Res. 2000;47:148–156. doi: 10.1203/00006450-200001000-00025. [DOI] [PubMed] [Google Scholar]
- 10.Gross S.J. Bone mineralization in preterm infants fed human milk with and without mineral supplementation. J. Pediatr. 1987;111:450–458. doi: 10.1016/S0022-3476(87)80478-X. [DOI] [PubMed] [Google Scholar]
- 11.Martins E.C., Krebs V.L.J. Effects of the use of fortified raw maternal milk on very low birth weight infants. J. Pediatr. 2009;85:157–162. doi: 10.2223/JPED.1878. [DOI] [PubMed] [Google Scholar]
- 12.Nicholl R.M., Gamsu H.R. Changes in growth and metabolism in very low birthweight infants fed with fortified breast milk. Acta. Paediatr. 1999;88:1056–1061. doi: 10.1111/j.1651-2227.1999.tb00987.x. [DOI] [PubMed] [Google Scholar]
- 13.Pettifor J.M., Rajah R., Venter A., Moodley G.P., Opperman L., Cavaleros M., Ross F.P. Bone mineralization and mineral homeostasis in very low-birth-weight infants fed either human milk or fortified human milk. J. Pediatr. Gastroenterol. Nutr. 1989;8:217–224. doi: 10.1097/00005176-198902000-00016. [DOI] [PubMed] [Google Scholar]
- 14.Bauer J., Gerss J. Longitudinal analysis of macronutrients and minerals in human milk produced by mothers of preterm infants. Clin. Nutr. 2011;30:215–220. doi: 10.1016/j.clnu.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 15.Armaforte E., Curran E., Huppertz T., Ryan C.A., Caboni M.F., O’Connor P.M., Ross R.P., Hirtz C., Sommerer N., Chevalier F., et al. Proteins and proteolysis in pre-term and term human milk and possible implications for infant formulae. Int. Dairy J. 2010;20:715–723. doi: 10.1016/j.idairyj.2010.03.008. [DOI] [Google Scholar]
- 16.Boudonck K.J., Mitchell M.W., Wulff J., Ryals J.A. Characterization of the biochemical variability of bovine milk using metabolomics. Metabolomics. 2009;5:375–386. doi: 10.1007/s11306-009-0160-8. [DOI] [Google Scholar]
- 17.Buitenhuis A.J., Sundekilde U.K., Poulsen N.A., Bertram H.C., Larsen L.B., Sørensen P. Estimation of genetic parameters and detection of quantitative trait loci for metabolites in Danish Holstein milk. J. Dairy Sci. 2013;96:3285–3295. doi: 10.3168/jds.2012-5914. [DOI] [PubMed] [Google Scholar]
- 18.Klein M.S., Almstetter M.F., Schlamberger G., Nurnberger N., Dettmer K., Oefner P.J., Meyer H.H.D., Wiedemann S., Gronwald W. Nuclear magnetic resonance and mass spectrometry-based milk metabolomics in dairy cows during early and late lactation. J. Dairy Sci. 2010;93:1539–1550. doi: 10.3168/jds.2009-2563. [DOI] [PubMed] [Google Scholar]
- 19.Maher A.D., Hayes B., Cocks B., Marett L., Wales W.J., Rochfort S. Latent biochemical relationships in the blood-milk metabolic axis of dairy cows revealed by statistical integration of 1H NMR spectroscopic data. J. Proteome Res. 2013;12:1428–1435. doi: 10.1021/pr301056q. [DOI] [PubMed] [Google Scholar]
- 20.Sundekilde U.K., Larsen L.B., Bertram H.C. NMR-Based Milk Metabolomics. Metabolites. 2013;3:204–222. doi: 10.3390/metabo3020204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Andreas N.J., Hyde M.J., Gomez-Romero M., Lopez-Gonzalvez M.A., Villaseñor A., Wijeyesekera A., Barbas C., Modi N., Holmes E., Garcia-Perez I. Multiplatform characterization of dynamic changes in breast milk during lactation. Electrophoresis. 2015;36:2269–2285. doi: 10.1002/elps.201500011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Longini M., Tataranno M.L., Proietti F., Tortoriello M., Belvisi E., Vivi A., Tassini M., Perrone S., Buonocore G. A metabolomic study of preterm and term human and formula milk by proton MRS analysis: Preliminary results. J. Matern. Neonatal Med. 2014;27:27–33. doi: 10.3109/14767058.2014.955958. [DOI] [PubMed] [Google Scholar]
- 23.Marincola F.C., Dessì A., Corbu S., Reali A., Fanos V. Clinical impact of human breast milk metabolomics. Clin. Chim. Acta. 2015;451:103–106. doi: 10.1016/j.cca.2015.02.021. [DOI] [PubMed] [Google Scholar]
- 24.Marincola F.C., Noto A., Caboni P., Reali A., Barberini L., Lussu M., Murgia F., Santoru M.L., Atzori L., Fanos V. A metabolomic study of preterm human and formula milk by high resolution NMR and GC/MS analysis: Preliminary results. J. Matern. Neonatal Med. 2012;25:62–67. doi: 10.3109/14767058.2012.715436. [DOI] [PubMed] [Google Scholar]
- 25.Praticò G., Capuani G., Tomassini A., Baldassarre M.E., Delfini M., Miccheli A. Exploring human breast milk composition by NMR-based metabolomics. Nat. Prod. Res. 2014;28:95–101. doi: 10.1080/14786419.2013.843180. [DOI] [PubMed] [Google Scholar]
- 26.Smilowitz J.T., O’Sullivan A., Barile D., German J.B., Lönnerdal B., Slupsky C.M. The human milk metabolome reveals diverse oligosaccharide profiles. J. Nutr. 2013;143:1709–1718. doi: 10.3945/jn.113.178772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spevacek A.R., Smilowitz J.T., Chin E.L., Underwood M.A., German J.B., Slupsky C.M. Infant maturity at birth reveals minor differences in the maternal milk metabolome in the first month of lactation. J. Nutr. 2015;145:1698–1708. doi: 10.3945/jn.115.210252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu J., Domellöf M., Zivkovic A.M., Larsson G., Öhman A., Nording M.L. NMR-based metabolite profiling of human milk: A pilot study of methods for investigating compositional changes during lactation. Biochem. Biophys. Res. Commun. 2015;469:626–632. doi: 10.1016/j.bbrc.2015.11.114. [DOI] [PubMed] [Google Scholar]
- 29.Sundekilde U.K., Poulsen N.A., Larsen L.B., Bertram H.C. Nuclear magnetic resonance metabonomics reveals strong association between milk metabolites and somatic cell count in bovine milk. J. Dairy Sci. 2013;96:290–299. doi: 10.3168/jds.2012-5819. [DOI] [PubMed] [Google Scholar]
- 30.Wishart D.S., Knox C., Guo A.C., Eisner R., Young N., Gautam B., Hau D.D., Psychogios N., Dong E., Bouatra S., et al. HMDB: A knowledgebase for the human metabolome. Nucleic Acids Res. 2009;37:D603–D610. doi: 10.1093/nar/gkn810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Savorani F., Tomasi G., Engelsen S.B. icoshift: A versatile tool for the rapid alignment of 1D NMR spectra. J. Magn. Reson. 2010;202:190–202. doi: 10.1016/j.jmr.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 32.Cloarec O., Dumas M.E., Trygg J., Craig A., Barton R.H., Lindon J.C., Nicholson J.K., Holmes E. Evaluation of the orthogonal projection on latent structure model limitations caused by chemical shift variability and improved visualization of biomarker changes in 1H NMR spectroscopic metabonomic studies. Anal. Chem. 2005;77:517–526. doi: 10.1021/ac048803i. [DOI] [PubMed] [Google Scholar]
- 33.Thurl S., Munzert M., Henker J., Boehm G., Müller-Werner B., Jelinek J., Stahl B. Variation of human milk oligosaccharides in relation to milk groups and lactational periods. Br. J. Nutr. 2010;104:1261–1271. doi: 10.1017/S0007114510002072. [DOI] [PubMed] [Google Scholar]
- 34.Dessì A., Ottonello G., Fanos V. Physiopathology of intrauterine growth retardation: From classic data to metabolomics. J. Matern. Neonatal Med. 2012;25(Suppl. 5):13–18. doi: 10.3109/14767058.2012.714639. [DOI] [PubMed] [Google Scholar]
- 35.Palmas F., Fattuoni C., Noto A., Barberini L., Dessì A., Fanos V. The choice of amniotic fluid in metabolomics for the monitoring of fetus health. Expert Rev. Mol. Diagn. 2016;7159:1–14. doi: 10.1586/14737159.2016.1139456. [DOI] [PubMed] [Google Scholar]
- 36.Klein M.S., Buttchereit N., Miemczyk S.P., Immervoll A.K., Louis C., Wiedemann S., Junge W., Thaller G., Oefner P.J., Gronwald W. NMR metabolomic analysis of dairy cows reveals milk glycerophosphocholine to phosphocholine ratio as prognostic biomarker for risk of ketosis. J. Proteome Res. 2012;11:1373–1381. doi: 10.1021/pr201017n. [DOI] [PubMed] [Google Scholar]
- 37.McManaman J.L., Neville M.C. Mammary physiology and milk secretion. Adv. Drug Deliv. Rev. 2003;55:629–641. doi: 10.1016/S0169-409X(03)00033-4. [DOI] [PubMed] [Google Scholar]
- 38.Molinari C.E., Casadio Y.S., Hartmann B.T., Livk A., Bringans S., Arthur P.G., Hartmann P.E. Proteome mapping of human skim milk proteins in term and preterm milk. J. Proteome Res. 2012;11:1696–1714. doi: 10.1021/pr2008797. [DOI] [PubMed] [Google Scholar]
- 39.Whitmore T.J., Trengove N.J., Graham D.F., Hartmann P.E. Analysis of insulin in human breast milk in mothers with type 1 and type 2 diabetes mellitus. Int. J. Endocrinol. 2012;2012:296368. doi: 10.1155/2012/296368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kelly R.J., Rouquier S., Giorgi D., Lennon G.G., Lowe J.B. Sequence and Expression of a Candidate for the Human Secretor Blood Group (1,2) Fucosyltransferase Gene (FUT2): Homozygosity for an enzyme-inactivating nonsense mutation commonly correlates with the non-secretor phenotype. J. Biol. Chem. 1995;270:4640–4649. doi: 10.1074/jbc.270.9.4640. [DOI] [PubMed] [Google Scholar]
- 41.Totten S.M., Zivkovic A.M., Wu S., Ngyuen U., Freeman S.L., Ruhaak L.R., Darboe M.K., German J.B., Prentice A.M., Lebrilla C.B. Comprehensive profiles of human milk oligosaccharides yield highly sensitive and specific markers for determining secretor status in lactating mothers. J. Proteome Res. 2012;11:6124–6133. doi: 10.1021/pr300769g. [DOI] [PubMed] [Google Scholar]
- 42.Beijers R.J.W., Schaafsma A. Long-chain polyunsaturated fatty acid content in Dutch preterm breast milk; differences in the concentrations of docosahexaenoic acid and arachidonic acid due to length of gestation. Early Hum. Dev. 1996;44:215–223. doi: 10.1016/0378-3782(95)01710-0. [DOI] [PubMed] [Google Scholar]
- 43.Coppa G.V., Gabrielli O., Pierani P., Catassi C., Carlucci A., Giorgi P.L. Changes in carbohydrate composition in human milk over 4 months of lactation. Pediatrics. 1993;91:637–641. [PubMed] [Google Scholar]
- 44.Villaseñor A., Garcia-Perez I., Garcia A., Posma J.M., Fernández-López M., Nicholas A.J., Modi N., Holmes E., Barbas C. Breast milk metabolome characterization in a single-phase extraction, multiplatform analytical approach. Anal. Chem. 2014;86:8245–8252. doi: 10.1021/ac501853d. [DOI] [PubMed] [Google Scholar]
- 45.De Leoz M.L.A., Gaerlan S.C., Strum J.S., Dimapasoc L.M., Mirmiran M., Tancredi D.J., Smilowitz J.T., Kalanetra K.M., Mills D.A., German J.B., et al. Lacto-N-tetraose, fucosylation, and secretor status are highly variable in human milk oligosaccharides from women delivering preterm. J. Proteome Res. 2012;11:4662–4672. doi: 10.1021/pr3004979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Torrazza R.M., Neu J. The altered gut microbiome and necrotizing enterocolitis. Clin. Perinatol. 2013;40:93–108. doi: 10.1016/j.clp.2012.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang Z., Adelman A.S., Rai D., Boettcher J., Lőnnerdal B. Amino acid profiles in term and preterm human milk through lactation: A systematic review. Nutr. 2013;5:4800–4821. doi: 10.3390/nu5124800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Atkinson S., Anderson G., Bryan M. Human milk: Comparison of the nitrogen composition in milk from mothers of premature and full-term infants. Am. J. Clin. Nutr. 1980;33:811–815. doi: 10.1093/ajcn/33.4.811. [DOI] [PubMed] [Google Scholar]
- 49.Chuang C.K., Lin S.P., Lee H.C., Wang T.J., Shih Y.S., Huang F.Y., Yeung C.Y. Free amino acids in full-term and pre-term human milk and infant formula. J. Pediatr. Gastroenterol. Nutr. 2005;40:496–500. doi: 10.1097/01.MPG.0000150407.30058.47. [DOI] [PubMed] [Google Scholar]
- 50.Moltó-Puigmartí C., Castellote A.I., Carbonell-Estrany X., López-Sabater M.C. Differences in fat content and fatty acid proportions among colostrum, transitional, and mature milk from women delivering very preterm, preterm, and term infants. Clin. Nutr. 2011;30:116–123. doi: 10.1016/j.clnu.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 51.Holmes-McNary M.Q., Cheng W.L., Mar M.H., Fussell S., Zeisel S.H. Choline and choline esters in human and rat milk and in infant formulas. Am. J. Clin. Nutr. 1996;64:572–576. doi: 10.1093/ajcn/64.4.572. [DOI] [PubMed] [Google Scholar]