Abstract
Since initial reports, cross-coupling technologies employing photoredox catalysts to access novel reactivity have developed with increasing pace. In this Outlook, prominent examples from the recent literature are organized on the basis of the elementary transformation enabled by photoredox catalysis and are discussed in the context of relevant historical precedent in stoichiometric organometallic chemistry. This treatment allows mechanistic similarities inherent to odd-electron transition metal reactivity to be generalized to a set of lessons for future reaction development.
Short abstract
The influence of photoredox catalysis on transition metal catalyzed cross-coupling is reviewed, with single-electron analogues of each elementary step discussed from a mechanistic perspective.
Introduction
The ability for transition metal catalysts to forge bonds between ligated fragments has become a cornerstone of modern synthetic chemistry. Cross-coupling methodologies allow access to a variety of carbon–carbon and carbon–heteroatom coupled products via straightforward retrosynthetic disconnections, and the systematic exploration of this paradigm has expanded the canon of accessible reactivity to cover a wide swath of chemical space.1
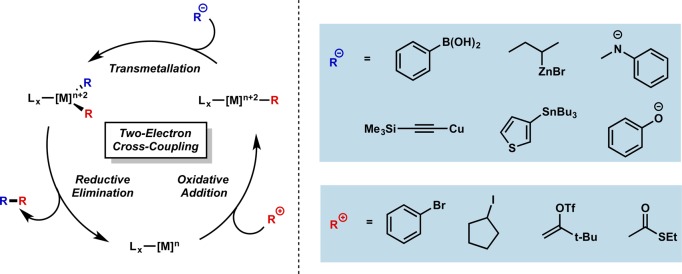
The vast majority of these methodologies rely on the same three two-electron elementary steps as a means to accomplish their target transformation: oxidative addition, transmetalation, and reductive elimination (Figure 1). The centrality of these reactions is underscored by the numerous mechanistic studies conducted that outline the influence of the metal catalyst and its ligand environment. Despite the attention given to these polar mechanisms, many worthwhile challenges remain in this field.2
Figure 1.
Generalized mechanism for cross-coupling and representative nucleophiles and electrophiles. Tf = trifluoromethanesulfonyl.
Meanwhile, it has long been known that the chemistry of 17- and 19-electron transition metal complexes is marked by dramatically faster rates compared to their even-electron congeners for virtually all elementary transformations.3 Despite the wealth of stoichiometric precedent in this area, translation of odd-electron organometallic reactivity into mild catalytic reactions remained, until recently, far rarer than the two-electron analogues.
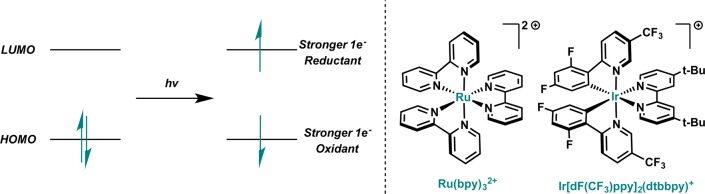
The key development in recent reports has been the implementation of visible-light photocatalysts as a means to induce the desired redox processes in a mild and selective manner. Examination of a generalized photoabsorption scheme reveals that such an activation mode is a natural means of accessing odd-electron intermediates (Figure 2). As noted by early studies on these photoredox catalysts, excited states become both stronger single-electron oxidants and stronger single-electron reductants.4−6 By employing catalysts with absorption bands in the visible region of the spectrum (rather than in the ultraviolet), the source of open shell intermediates can be effectively controlled.7,8
Figure 2.
Simplified photoabsorption scheme and commonly employed photoredox catalysts.
The rapid pace of recent developments combining photocatalysis with transition metal catalysis has prompted a survey of the field.9 We have endeavored here, for instructive purposes, to organize these landmark examples by virtue of the elementary step in which the photocatalyst is engaged, as the guiding principles and mechanistic homologies of this new field are clearer when the catalyzed elementary steps are delineated. In each case, we aim to tie these novel catalytic methodologies back to their prior stoichiometric analogues as a means to both rationalize the observed reactivity and inspire the next generation of technologies.
It is prudent to note that, in many cases, the photocatalysts may serve as chain initiators, with chain lengths measured for many photoredox processes exceeding one.10 As such, we have chosen to abstract the involvement of these catalysts to the level of a photoinduced electron transfer (signified by an electron), rather than imply the specific species involved in any given reduction or oxidation event.11 This Outlook is not meant as a comprehensive review12−15 but rather as a tutorial, and the lessons involved do not hinge on the presence or absence of chain processes.16
Photocatalysis of Oxidative Addition
Oxidative addition involves the formation of bonds between a metal and an electrophilic substrate concomitant with the formal two-electron oxidation of the metal center.2 In cross-coupling this step typically involves concerted oxidative addition of a carbon–halogen or carbon–pseudohalogen bond to generate the oxidized organometallic species. However, many metals and electrophiles undergo prohibitively slow reactions by this mechanism.
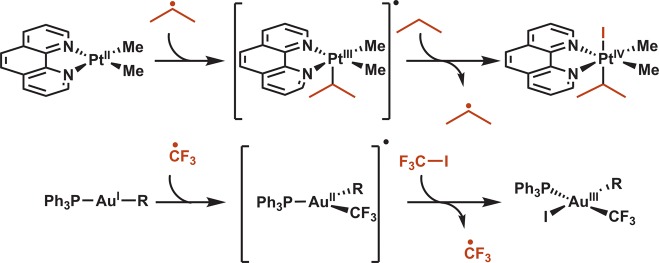
On the other hand, the facile addition of alkyl radicals to transition metals in both chain and nonchain processes has been studied in detail for a variety of complexes (Figure 3).17 For example, photochemical initiation to generate isopropyl radical was shown to accelerate the oxidative addition of isopropyl iodide to Pt(II) via a radical chain process.18 Fluoroalkyl iodides similarly undergo radical chain oxidative additions to Au(I).19,20 These precedents highlight the ability of otherwise sluggish oxidative addition processes to be facilitated by injection into a radical manifold.
Figure 3.
Stoichiometric precedent: radical chain oxidative addition to Pt(II) and Au(I).
With a photoredox catalyst, the involvement of a photochemical process allows for the generation of the intermediate radical species in milder fashion and with transition metal catalysts kinetically incompetent for such an initiation on their own. In the most general scheme, an electrophilic reagent is reduced by a photoexcited species, leading to its radical congener. Subsequent addition to the metal species followed by oxidation yields the net oxidative addition product. In this way, the photocatalyst catalyzes the oxidative addition (Figure 4).
Figure 4.
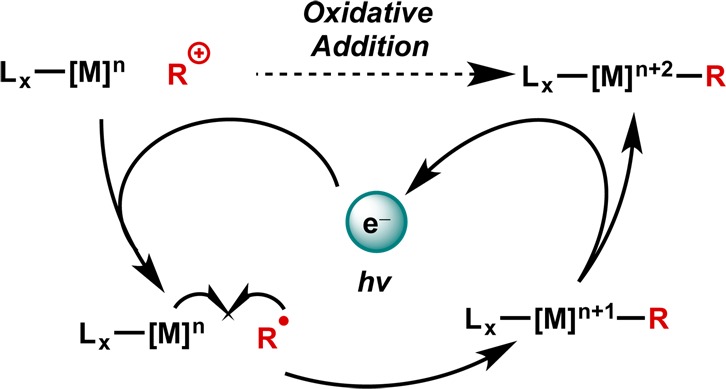
Generalized scheme for photoredox catalysis of oxidative addition.
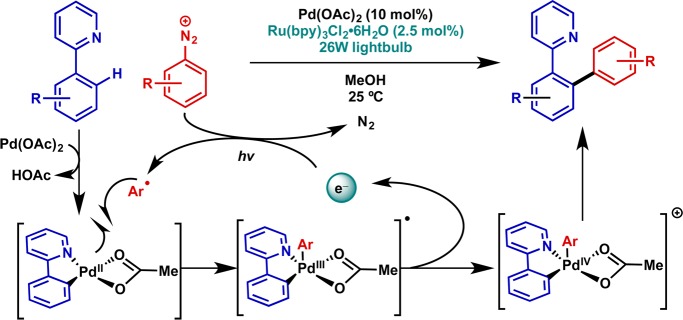
One of the earliest examples of this activation mode in the context of catalysis is in Pd-catalyzed directed C–H functionalization (Figure 5).21 Sanford and co-workers showed that, in contrast to much harsher methods for accessing Pd(IV), which require elevated temperature, the use of aryldiazonium cations in combination with a photoredox catalyst allowed for the generation of the high-valent intermediate at room temperature. This was found to be broadly applicable with respect to the directing group employed and to the diazonium structure. Subsequent studies expanded this manifold to diaryliodonium electrophiles.22
Figure 5.
Catalytic application: palladium-catalyzed C–H functionalization at room temperature via Pd(IV) intermediates generated by photoredox catalysis.
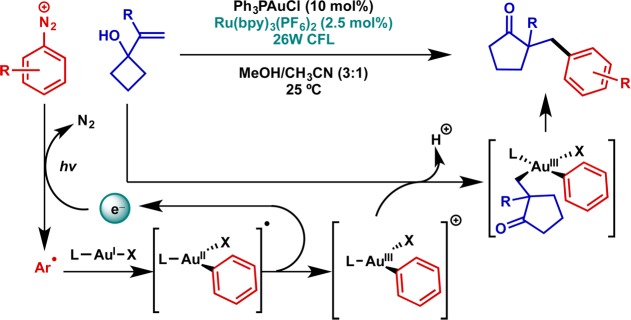
This same strategy employing diazonium electrophiles was subsequently applied in the context of gold catalysis by the Glorius and Toste groups, providing access to highly reactive Au(III) intermediates in a straightforward fashion.23,24 Initial studies focused on the activation of alkenes to provide oxy- and aminoarylated as well as ring expanded products (Figure 6).25 Mechanistic investigations including time-resolved FT-IR, labeling studies, and DFT support a mechanism in which photocatalysis generates the Au(III)–aryl intermediate prior to intervention of the unsaturated substrate.23,26 Subsequent studies have expanded the scope to a wide variety of Au-catalyzed reactions of alkynes,27−30 allenes,31 and heteroatom nucleophiles32 allowing arylation to terminate catalytic cycles typically closed via protodeauration.
Figure 6.
Catalytic application: gold-catalyzed arylative ring expansion of vinylcyclobutanols via aryl–Au(III) intermediates generated by photoredox catalysis.
Finally, a variant on this activation mode was demonstrated by Fu and Peters in which a copper catalyst serves as both the photocatalyst and ultimate bond-forming agent, albeit with UV rather than visible light in most cases. These processes vary in their nucleophiles and electrophiles, encompassing both aryl and alkyl halide substrates in C–S,33 C–N,34−37 C–C,38,39 and C–O40 forming reactions. Recent mechanistic studies have shown that the copper-mediated pathway involves photoinduced electron transfer from the nucleophile–cuprate complex followed by in-cage radical recombination to afford the coupled product, though oxidative addition and outer-sphere recombination could not be distinguished (Figure 7).41 As such, it remains unclear the extent to which these precedents are analogous to other photoredox-catalyzed oxidative additions. Nonetheless, a recent study demonstrated an enantioselective variant of this process for C–N bond formation.42
Figure 7.
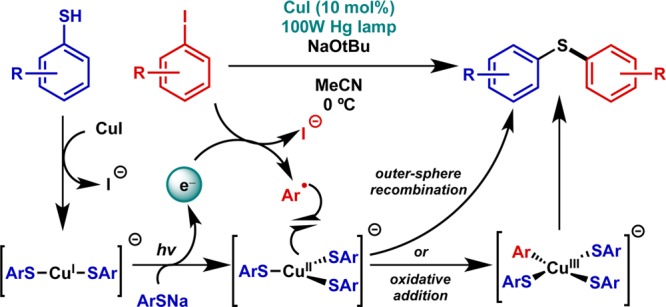
Catalytic application: copper-catalyzed C–S bond formation via photoinduced electron transfer. Mechanistic experiments have not distinguished the two potential pathways.
In addition to these illustrative examples, several other reports have emerged utilizing photoredox catalysis to enable oxidative addition. Notably, the combination of photoredox catalysts with copper has enabled mild C-fluoroalkyl and C–N coupling reactions to occur.43,44
Photocatalysis of Reductive Elimination
Reductive elimination involves the formation of a new bond between ligands bound to a metal with concomitant 2-electron reduction of the metal center.2 As the key bond-forming step in cross-coupling reactions, this process has been widely explored for diverse combinations of metals and ligands, and virtually all classes of reductive elimination are precedented in the literature. Nonetheless, there remain examples for which bond formation is prohibitively slow.
Again, the literature surrounding single-electron processes provides a potential solution;45,46 seminal studies by Kochi and Hillhouse showed that complexes that are inert to reductive elimination or undergo unselective decomposition can be coaxed to perform the desired bond-forming process by single-electron oxidation with a diverse array of oxidants (Figure 8).47,48
Figure 8.
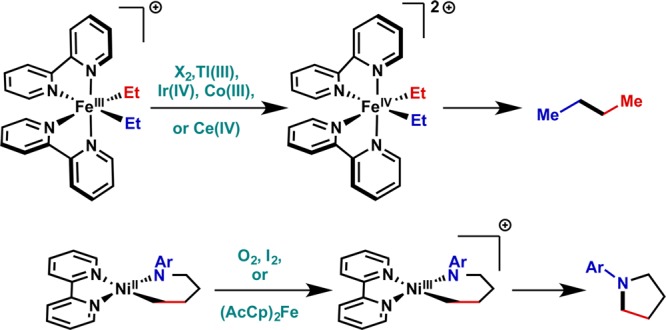
Stoichiometric precedent: oxidatively induced reductive elimination from Fe(IV) and Ni(III). Cp = cyclopentadienyl.
On the basis of these precedents, it is conceivable that a photoredox catalyst can be applied to accomplish such a transformation in a dual catalytic process. “Temporary” oxidation of the metal catalyst allows photocatalysis of the desired reductive elimination, which is followed by rereduction of the catalyst, as outlined in Figure 9.
Figure 9.
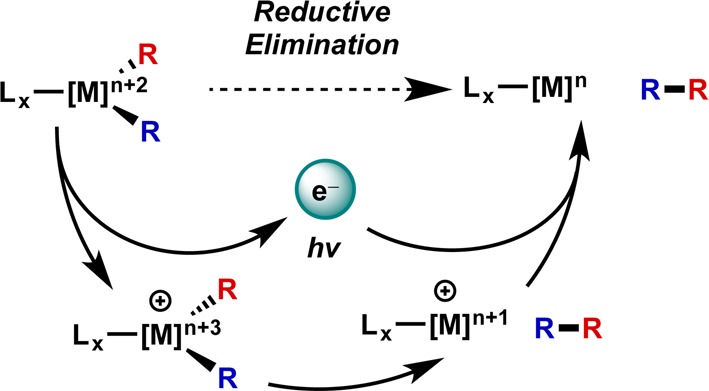
Generalized scheme for photoredox catalysis of reductive elimination.
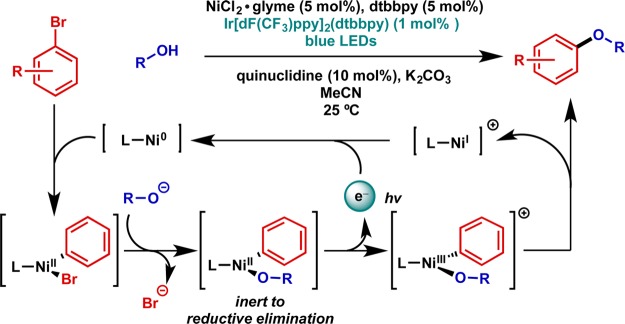
A powerful realization of this activation strategy was employed by the Macmillan group in a nickel-catalyzed C–O coupling aided by an iridium photocatalyst (Figure 10).49 In addition to demonstrating the scope of this process for a range of coupling partners, stoichiometric experiments on an isolated Ni(II)–aryl alkoxide complex clearly demonstrate the crucial influence of the photoredox catalyst on the desired reductive elimination process.
Figure 10.
Catalytic application: nickel-catalyzed etherification with C–O reductive elimination enabled via photoredox catalysis.
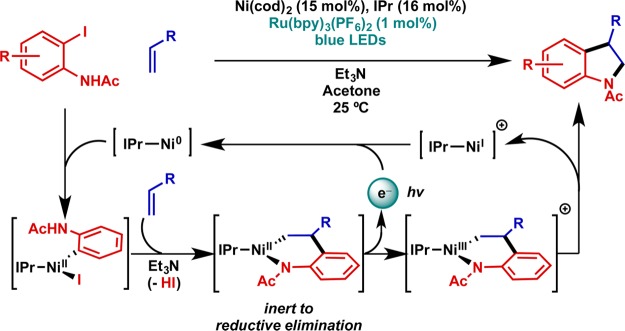
A second example from Jamison and co-workers concerns the synthesis of indolines via the coupling of ortho-iodoaniline derivatives with alkenes (Figure 11).50 Having observed that small amounts of the desired indoline product were formed upon exposure of the reaction mixture to air, they employed a photoredox catalyst to serve as a reversible means to access the requisite Ni(III) intermediate. The proposed mechanism bears many similarities to the example in Figure 10.
Figure 11.
Catalytic application: nickel-catalyzed Larock-type indoline synthesis with C–N reductive elimination enabled via photoredox catalysis. IPr = 1,3-bis(2,6-diisopropylphenyl)imidazol-2-ylidene.
Photocatalysis of Transmetalation
Transmetalation involves the formation of a bond between a nucleophilic substrate and a metal, with no change in formal oxidation state of the metal.2 These reactions typically involve displacement of a metal halide, and are mechanistically the most complex of the steps discussed herein. Despite several in-depth studies into the underlying elementary steps, many transmetalation processes remain poorly understood.51−53 Worse yet, transmetalation of sp3 alkyl fragments is typically challenging, requiring the use of sensitive reagents or harsh conditions.
In this arena, limited stoichiometric precedent employing open-shell intermediates has been reported; as such, the simultaneous discovery by the Macmillan, Doyle, and Molander groups that photoredox catalysis can be used to enable mild transmetalation from otherwise weakly nucleophilic coupling partners is perhaps even more impressive.54,55
Though many mechanisms have been proposed, the general scheme for such a photocatalyzed transmetalation can be conceived as follows: single-electron oxidation of a nucleophile and subsequent reduction of a transition metal complex generates complementary odd-electron species that can then efficiently undergo recombination (Figure 12). The order of these two steps can likely be inverted, and other elementary steps can intervene, but the sense of redox is likely maintained regardless of the conditions employed.
Figure 12.
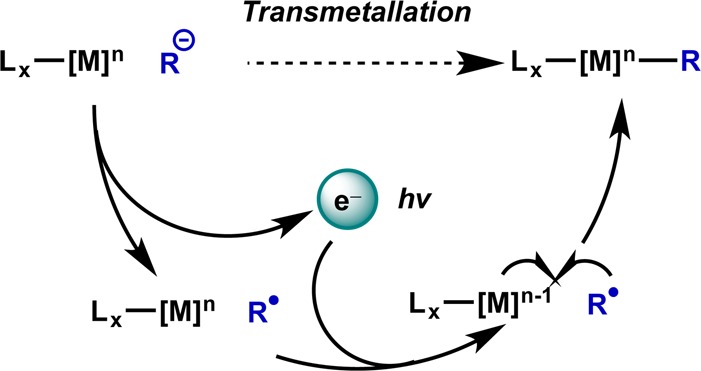
Generalized scheme for photoredox catalysis of transmetalation.
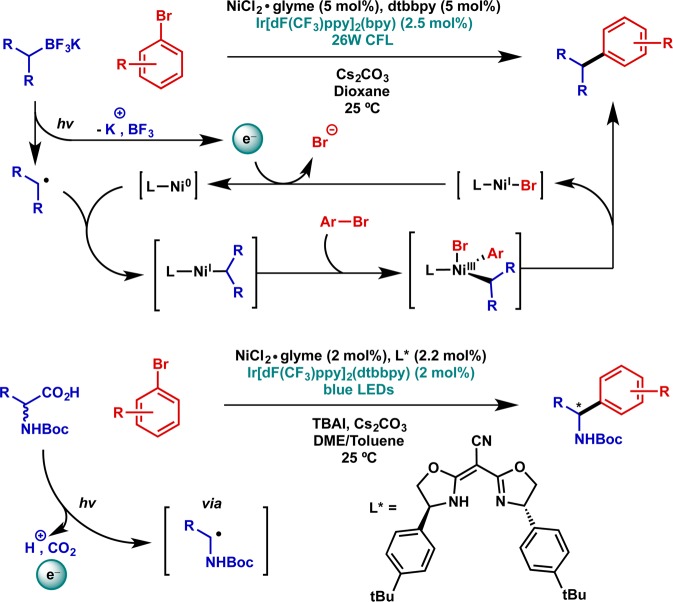
Beyond the mechanistic novelty in these processes, a striking feature of all systems developed to date is that the nucleophiles employed are all air-stable, easily prepared, and yet still capable of effectively delivering sp3 nucleophiles to transition metal centers. Four main classes of photoredox-activated alkyl nucleophiles have been developed: carboxylates,54,56−59 alkyl trifluoroborates,55,60−65 alkyl silicates,66−70 and α-heteroatom C–H bonds.54,71,72 In each, oxidation generates an alkyl radical via a subsequent fragmentation process, generating CO2, BF3, bis(catecholato)silane, or acid, respectively.
The most comprehensive mechanistic experiments and DFT calculations have been carried out for the nickel-catalyzed variants of these processes, and the studies support a process in which a Ni(0) intermediate combines with the nascent radical species to generate the corresponding Ni(I) alkyl.73,74 The overall cycle is then closed via oxidative addition of the sp2 halide coupling partner, reductive elimination from Ni(III), and single-electron reduction to regenerate the Ni(0) complex. Two representative examples are shown in Figure 13, including a recently reported enantioselective method leveraging this protocol to generated enantioenriched benzylic amines in a stereoconvergent fashion.75
Figure 13.
Catalytic applications: nickel-catalyzed Csp3–Csp2 coupling reactions of alkyltrifluoroborates (racemic) and amino acids (enantioselective). TBAI = tetrabutylammonium iodide, DME = 1,2-dimethoxyethane, Boc = tertbutoxycarbamoyl.
An interesting application of the carboxylate nucleophiles has been reported in which the carboxylate moiety is generated by oxidative addition to palladium forming a π-allyl intermediate.76,77 Other photoredox-facilitated transmetalations in cross-coupling have also been reported.78−80
Conclusions and Outlook
The leveraging of single-electron chemistry via the enabling technology of photoredox catalysis has clearly opened new doors in the realm of transition metal catalyzed cross-coupling. The motifs outlined herein are only some of the powerful new strategies accessed by this fruitful marriage of catalytic modes.81−85 For example, several reports have employed photoredox catalysts to access oxidative cross-couplings with a variety of transition metals.86−93
Moreover, there still exist stoichiometric precedents in single-electron acceleration of reactivity that have yet to be realized in a catalytic sense. One potentially instructive example of an accelerated migratory insertion is the documented catalysis of alkyl carbonylation at iron via single-electron oxidation (Figure 14); a dual catalytic coupling reaction making use of this behavior is likely possible by some combination of photoredox and transition metal catalysts.94
Figure 14.
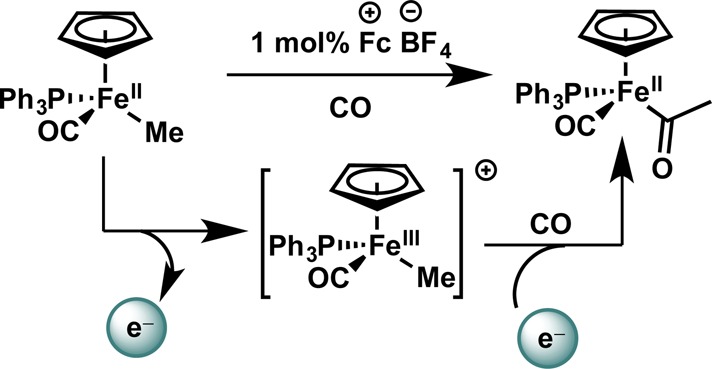
Redox catalysis of migratory insertion. Fc+ = ferrocenium.
Though the power of open-shell intermediates to provide rapid turnover has evidently been demonstrated, there are a number of challenges that lie ahead. For one, while the stereoconvergent nature of radical addition to transition metals has allowed the development of enantioselective processes,75 it also imposes limitations in the diastereoselectivity for radical transmetalation,62 making the synthesis of mismatched stereoisomers a more complex problem (Figure 15).95
Figure 15.
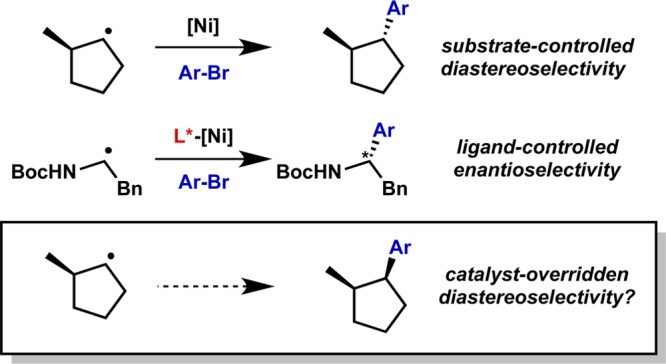
Stereoconvergence and diastereoselectivity in nickel-catalyzed radical cross-coupling.
These challenges should be taken as a call to action, as the power demonstrated in the reports thus far suggests a wealth of untapped reactivity. Most tantalizingly, the involvement of a photoprocess suggests that endergonic transformations may be possible in these catalytic reactions.96
Additionally, there are a wide variety of photoredox catalysts and metals yet to be engaged in dual catalytic reactions. Recent reports of photoinduced electron transfer from supramolecular host complexes97 are particularly intriguing given the synergy displayed between transition metal catalysts and supramolecular catalysts.98
Furthermore, the combination of more than one of the activation modes presented herein, though challenging, promises to enable progressively more complex transformations to be developed. To date, no sp3–sp3 C–C coupling protocols have taken advantage of the newly discovered photoredox manifolds, but such an advance, not to mention many others, is undoubtedly on the horizon.
Acknowledgments
We gratefully acknowledge NIHGMS (RO1 GM073932) for financial support. M.D.L thanks the NSF GRFP and ARCS foundation for graduate research fellowships. We thank Richard Thornbury and Roman Sarott for helpful discussions.
The authors declare no competing financial interest.
References
- Johansson Seechurn C. C. C.; Kitching M. O.; Colacot T. J.; Snieckus V. Palladium-Catalyzed Cross-Coupling: A Historical Contextual Perspective to the 2010 Nobel Prize. Angew. Chem., Int. Ed. 2012, 51, 5062–5085. 10.1002/anie.201107017. [DOI] [PubMed] [Google Scholar]
- Hartwig J. F.Organotransition Metal Chemistry: From Bonding to Catalysis; University Science Books: Herndon, VA, 2010. [Google Scholar]
- Stiegman A. E.; Tyler D. R. Reactivity of Seventeen- and Nineteen-Valence Electron Complexes in Organometallic Chemistry. Comments Inorg. Chem. 1986, 5, 215–245. 10.1080/02603598608079840. [DOI] [Google Scholar]
- Gafney H. D.; Adamson A. W. Excited State Ru(bipyr)32+ as an Electron-Transfer Reductant. J. Am. Chem. Soc. 1972, 94, 8238–8239. 10.1021/ja00778a054. [DOI] [Google Scholar]
- Lowry M. S.; Goldsmith J. I.; Slinker J. D.; Rohl R.; Pascal R. A. Jr.; Malliaras G. G.; Bernhard S. Single-Layer Electroluminescent Devices and Photoinduced Hydrogen Production from an Ionic Iridium(III) Complex. Chem. Mater. 2005, 17, 5712–5719. 10.1021/cm051312+. [DOI] [Google Scholar]
- Slinker J. D.; Gorodetsky A. A.; Lowry M. S.; Wang J.; Parker S.; Rohl R.; Bernhard S.; Malliaras G. G. Efficient Yellow Electroluminescence from a Single Layer of a Cyclometalated Iridium Complex. J. Am. Chem. Soc. 2004, 126, 2763–2767. 10.1021/ja0345221. [DOI] [PubMed] [Google Scholar]
- Yoon T. P.; Ischay M. A.; Du J. Visible Light Photocatalysis as a Greener Approach to Photochemical Synthesis. Nat. Chem. 2010, 2, 527–532. 10.1038/nchem.687. [DOI] [PubMed] [Google Scholar]
- Prier C. K.; Rankic D. A.; MacMillan D. W. C. Visible Light Photoredox Catalysis with Transition Metal Complexes: Applications in Organic Synthesis. Chem. Rev. 2013, 113, 5322–5363. 10.1021/cr300503r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkinson M. N.; Sahoo B.; Li J.; Glorius F. Dual Catalysis Sees the Light: Combining Photoredox with Organo-, Acid, and Transition-Metal Catalysis. Chem. - Eur. J. 2014, 20, 3874–3886. 10.1002/chem.201304823. [DOI] [PubMed] [Google Scholar]
- Cismesia M. A.; Yoon T. P. Characterizing Chain Processes in Visible Light Photoredox Catalysis. Chem. Sci. 2015, 6, 5426–5434. 10.1039/C5SC02185E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studer A.; Curran D. P. The Electron Is a Catalyst. Nat. Chem. 2014, 6, 765–773. 10.1038/nchem.2031. [DOI] [PubMed] [Google Scholar]
- We have elected not to discuss examples of photoredox and transition metal dual catalysis (refs (13, 14)) and sequential, photoredox- and metal-catalyzed steps (refs (15, 16)).
- Huo H.; Shen X.; Wang C.; Zhang L.; Rose P.; Chen L.; Harms K.; Marsch M.; Hilt G.; Meggers E. Asymmetric Photoredox Transition-Metal Catalysis Activated by Visible Light. Nature 2014, 515, 100–103. 10.1038/nature13892. [DOI] [PubMed] [Google Scholar]
- Du J.; Skubi K. L.; Schultz D. M.; Yoon T. P. A Dual-Catalysis Approach to Enantioselective [2 + 2] Photocycloadditions Using Visible Light. Science 2014, 344, 392–396. 10.1126/science.1251511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueping M.; Koenigs R. M.; Poscharny K.; Fabry D. C.; Leonori D.; Vila C. Dual Catalysis: Combination of Photocatalytic Aerobic Oxidation and Metal Catalyzed Alkynylation Reactions—C–C Bond Formation Using Visible Light. Chem. - Eur. J. 2012, 18, 5170–5174. 10.1002/chem.201200050. [DOI] [PubMed] [Google Scholar]
- Two mechanistic possibilities not discussed herein include photoinduced energy transfer and outer-sphere free radical coupling. Where possible we have relied on studies where mechanistic experiments discount these alternatives.
- Halpern J. Oxidative-Addition Reactions of Transition Metal Complexes. Acc. Chem. Res. 1970, 3, 386–392. 10.1021/ar50035a004. [DOI] [Google Scholar]
- Hill R. H.; Puddephatt R. J. A Mechanistic Study of the Photochemically Initiated Oxidative Addition of Isopropyl Iodide to dimethyl(1,10-phenanthroline)platinum(II). J. Am. Chem. Soc. 1985, 107, 1218–1225. 10.1021/ja00291a022. [DOI] [Google Scholar]
- Johnson A.; Puddephatt R. J. Reactions of Trifluoromethyl Iodide with methylgold(I) Complexes. Preparation of Trifluoromethyl-gold(I) and -gold(III) Complexes. J. Chem. Soc., Dalton Trans. 1976, 1360–1363. 10.1039/dt9760001360. [DOI] [Google Scholar]
- Winston M. S.; Wolf W. J.; Toste F. D. Photoinitiated Oxidative Addition of CF3I to Gold(I) and Facile Aryl-CF3 Reductive Elimination. J. Am. Chem. Soc. 2014, 136, 7777–7782. 10.1021/ja503974x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalyani D.; McMurtrey K. B.; Neufeldt S. R.; Sanford M. S. Room-Temperature C–H Arylation: Merger of Pd-Catalyzed C–H Functionalization and Visible-Light Photocatalysis. J. Am. Chem. Soc. 2011, 133, 18566–18569. 10.1021/ja208068w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeldt S. R.; Sanford M. S. Combining Transition Metal Catalysis with Radical Chemistry: Dramatic Acceleration of Palladium-Catalyzed C–H Arylation with Diaryliodonium Salts. Adv. Synth. Catal. 2012, 354, 3517–3522. 10.1002/adsc.201200738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu X.; Zhang M.; He Y.; Frei H.; Toste F. D. Dual Visible Light Photoredox and Gold-Catalyzed Arylative Ring Expansion. J. Am. Chem. Soc. 2014, 136, 5844–5847. 10.1021/ja500716j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahoo B.; Hopkinson M. N.; Glorius F. Combining Gold and Photoredox Catalysis: Visible Light-Mediated Oxy- and Aminoarylation of Alkenes. J. Am. Chem. Soc. 2013, 135, 5505–5508. 10.1021/ja400311h. [DOI] [PubMed] [Google Scholar]
- Hopkinson M. N.; Sahoo B.; Glorius F. Dual Photoredox and Gold Catalysis: Intermolecular Multicomponent Oxyarylation of Alkenes. Adv. Synth. Catal. 2014, 356, 2794–2800. 10.1002/adsc.201400580. [DOI] [Google Scholar]
- Zhang Q.; Zhang Z.; Fu Y.; Yu H. Mechanism of the Visible Light-Mediated Gold-Catalyzed Oxyarylation Reaction of Alkenes. ACS Catal. 2016, 6, 798–808. 10.1021/acscatal.5b01971. [DOI] [Google Scholar]
- Tlahuext-Aca A.; Hopkinson M. N.; Garza-Sanchez R. A.; Glorius F. Alkyne Difunctionalization by Dual Gold/Photoredox Catalysis. Chem. - Eur. J. 2016, 22, 5909. 10.1002/chem.201600710. [DOI] [PubMed] [Google Scholar]
- Tlahuext-Aca A.; Hopkinson M. N.; Sahoo B.; Glorius F. Dual Gold/photoredox-Catalyzed C(sp)–H Arylation of Terminal Alkynes with Diazonium Salts. Chem. Sci. 2016, 7, 89–93. 10.1039/C5SC02583D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Um J.; Yun H.; Shin S. Cross-Coupling of Meyer–Schuster Intermediates under Dual Gold–Photoredox Catalysis. Org. Lett. 2016, 18, 484–487. 10.1021/acs.orglett.5b03531. [DOI] [PubMed] [Google Scholar]
- Kim S.; Rojas-Martin J.; Toste F. D. Visible Light-Mediated Gold-Catalysed carbon(sp2)-Carbon(sp) Cross-Coupling. Chem. Sci. 2016, 7, 85–88. 10.1039/C5SC03025K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil D. V.; Yun H.; Shin S. Catalytic Cross-Coupling of Vinyl Golds with Diazonium Salts under Photoredox and Thermal Conditions. Adv. Synth. Catal. 2015, 357, 2622–2628. 10.1002/adsc.201500525. [DOI] [Google Scholar]
- He Y.; Wu H.; Toste F. D. A Dual Catalytic Strategy for Carbon-Phosphorus Cross-Coupling via Gold and Photoredox Catalysis. Chem. Sci. 2015, 6, 1194–1198. 10.1039/C4SC03092C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uyeda C.; Tan Y.; Fu G. C.; Peters J. C. A New Family of Nucleophiles for Photoinduced, Copper-Catalyzed Cross-Couplings via Single-Electron Transfer: Reactions of Thiols with Aryl Halides Under Mild Conditions (0 °C). J. Am. Chem. Soc. 2013, 135, 9548–9552. 10.1021/ja404050f. [DOI] [PubMed] [Google Scholar]
- Bissember A. C.; Lundgren R. J.; Creutz S. E.; Peters J. C.; Fu G. C. Transition-Metal-Catalyzed Alkylations of Amines with Alkyl Halides: Photoinduced, Copper-Catalyzed Couplings of Carbazoles. Angew. Chem., Int. Ed. 2013, 52, 5129–5133. 10.1002/anie.201301202. [DOI] [PubMed] [Google Scholar]
- Ziegler D. T.; Choi J.; Muñoz-Molina J. M.; Bissember A. C.; Peters J. C.; Fu G. C. A Versatile Approach to Ullmann C–N Couplings at Room Temperature: New Families of Nucleophiles and Electrophiles for Photoinduced, Copper-Catalyzed Processes. J. Am. Chem. Soc. 2013, 135, 13107–13112. 10.1021/ja4060806. [DOI] [PubMed] [Google Scholar]
- Do H.; Bachman S.; Bissember A. C.; Peters J. C.; Fu G. C. Photoinduced, Copper-Catalyzed Alkylation of Amides with Unactivated Secondary Alkyl Halides at Room Temperature. J. Am. Chem. Soc. 2014, 136, 2162–2167. 10.1021/ja4126609. [DOI] [PubMed] [Google Scholar]
- Creutz S. E.; Lotito K. J.; Fu G. C.; Peters J. C. Photoinduced Ullmann C–N Coupling: Demonstrating the Viability of a Radical Pathway. Science 2012, 338, 647–651. 10.1126/science.1226458. [DOI] [PubMed] [Google Scholar]
- Yang F.; Koeller J.; Ackermann L. Photoinduced Copper-Catalyzed C–H Arylation at Room Temperature. Angew. Chem., Int. Ed. 2016, 55, 4759. 10.1002/anie.201512027. [DOI] [PubMed] [Google Scholar]
- Ratani T. S.; Bachman S.; Fu G. C.; Peters J. C. Photoinduced, Copper-Catalyzed Carbon–Carbon Bond Formation with Alkyl Electrophiles: Cyanation of Unactivated Secondary Alkyl Chlorides at Room Temperature. J. Am. Chem. Soc. 2015, 137, 13902–13907. 10.1021/jacs.5b08452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan Y.; Munoz-Molina J. M.; Fu G. C.; Peters J. C. Oxygen Nucleophiles as Reaction Partners in Photoinduced, Copper-Catalyzed Cross-Couplings: O-Arylations of Phenols at Room Temperature. Chem. Sci. 2014, 5, 2831–2835. 10.1039/c4sc00368c. [DOI] [Google Scholar]
- Johnson M. W.; Hannoun K. I.; Tan Y.; Fu G. C.; Peters J. C. A Mechanistic Investigation of the Photoinduced, Copper-Mediated Cross-Coupling of an Aryl Thiol with an Aryl Halide. Chem. Sci. 2016, 10.1039/C5SC04709A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kainz Q. M.; Matier C. D.; Bartoszewicz A.; Zultanski S. L.; Peters J. C.; Fu G. C. Asymmetric Copper-Catalyzed C–N Cross-Couplings Induced by Visible Light. Science 2016, 351, 681–684. 10.1126/science.aad8313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Y.; Sanford M. S. Merging Visible-Light Photocatalysis and Transition-Metal Catalysis in the Copper-Catalyzed Trifluoromethylation of Boronic Acids with CF3I. J. Am. Chem. Soc. 2012, 134, 9034–9037. 10.1021/ja301553c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo W.; Tsukamoto T.; Kobayashi S. Visible Light-Mediated Ullmann-Type C–N Coupling Reactions of Carbazole Derivatives and Aryl Iodides. Org. Lett. 2015, 17, 3640–3642. 10.1021/acs.orglett.5b01645. [DOI] [PubMed] [Google Scholar]
- Komiya S.; Albright T. A.; Hoffmann R.; Kochi J. K. The Stability of Organogold Compounds. Hydrolytic, Thermal, and Oxidative Cleavages of dimethylaurate(I) and tetramethylaurate(III). J. Am. Chem. Soc. 1977, 99, 8440–8447. 10.1021/ja00468a010. [DOI] [Google Scholar]
- Han R.; Hillhouse G. L. Carbon–Oxygen Reductive-Elimination from Nickel(II) Oxametallacycles and Factors That Control Formation of Ether, Aldehyde, Alcohol, or Ester Products. J. Am. Chem. Soc. 1997, 119, 8135–8136. 10.1021/ja9714999. [DOI] [Google Scholar]
- Lau W.; Huffman J. C.; Kochi J. K. Electrochemical Oxidation-Reduction of Organometallic Complexes. Effect of the Oxidation State on the Pathways for Reductive Elimination of Dialkyliron Complexes. Organometallics 1982, 1, 155–169. 10.1021/om00061a027. [DOI] [Google Scholar]
- Koo K.; Hillhouse G. L. Carbon-Nitrogen Bond Formation by Reductive Elimination from Nickel(II) Amido Alkyl Complexes. Organometallics 1995, 14, 4421–4423. 10.1021/om00009a054. [DOI] [Google Scholar]
- Terrett J. A.; Cuthbertson J. D.; Shurtleff V. W.; MacMillan D. W. C. Switching on Elusive Organometallic Mechanisms with Photoredox Catalysis. Nature 2015, 524, 330–334. 10.1038/nature14875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasker S. Z.; Jamison T. F. Highly Regioselective Indoline Synthesis under Nickel/Photoredox Dual Catalysis. J. Am. Chem. Soc. 2015, 137, 9531–9534. 10.1021/jacs.5b05597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrow B. P.; Hartwig J. F. Distinguishing Between Pathways for Transmetalation in Suzuki–Miyaura Reactions. J. Am. Chem. Soc. 2011, 133, 2116–2119. 10.1021/ja1108326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amatore C.; Grimaud L.; Le Duc G.; Jutand A. Three Roles for the Fluoride Ion in Palladium-Catalyzed Hiyama Reactions: Transmetalation of [ArPdFL2] by Ar′Si(OR)3. Angew. Chem., Int. Ed. 2014, 53, 6982–6985. 10.1002/anie.201400956. [DOI] [PubMed] [Google Scholar]
- Casado A. L.; Espinet P. Mechanism of the Stille Reaction. 1. The Transmetalation Step. Coupling of R1I and R2SnBu3 Catalyzed by Trans-[PdR1IL2] (R1 = C6Cl2F3; R2 = Vinyl, 4-Methoxyphenyl; L = AsPh3). J. Am. Chem. Soc. 1998, 120, 8978–8985. 10.1021/ja9742388. [DOI] [Google Scholar]
- Zuo Z.; Ahneman D. T.; Chu L.; Terrett J. A.; Doyle A. G.; MacMillan D. W. C. Merging Photoredox with Nickel Catalysis: Coupling of α-Carboxyl sp3-Carbons with Aryl Halides. Science 2014, 345, 437–440. 10.1126/science.1255525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tellis J. C.; Primer D. N.; Molander G. A. Single-Electron Transmetalation in Organoboron Cross-Coupling by Photoredox/nickel Dual Catalysis. Science 2014, 345, 433–436. 10.1126/science.1253647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu L.; Lipshultz J. M.; MacMillan D. W. C. Merging Photoredox and Nickel Catalysis: The Direct Synthesis of Ketones by the Decarboxylative Arylation of α-Oxo Acids. Angew. Chem., Int. Ed. 2015, 54, 7929–7933. 10.1002/anie.201501908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le C. C.; MacMillan D. W. C. Fragment Couplings via CO2 Extrusion–Recombination: Expansion of a Classic Bond-Forming Strategy via Metallaphotoredox. J. Am. Chem. Soc. 2015, 137, 11938–11941. 10.1021/jacs.5b08304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble A.; McCarver S. J.; MacMillan D. W. C. Merging Photoredox and Nickel Catalysis: Decarboxylative Cross-Coupling of Carboxylic Acids with Vinyl Halides. J. Am. Chem. Soc. 2015, 137, 624–627. 10.1021/ja511913h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J.; Zhang J. Donor–Acceptor Fluorophores for Visible-Light-Promoted Organic Synthesis: Photoredox/Ni Dual Catalytic C(sp3)–C(sp2) Cross-Coupling. ACS Catal. 2016, 6, 873–877. 10.1021/acscatal.5b02204. [DOI] [Google Scholar]
- El Khatib M.; Serafim R. A. M.; Molander G. A. α-Arylation/Heteroarylation of Chiral α-Aminomethyltrifluoroborates by Synergistic Iridium Photoredox/Nickel Cross-Coupling Catalysis. Angew. Chem., Int. Ed. 2016, 55, 254–258. 10.1002/anie.201506147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu D.; Primer D. N.; Tellis J. C.; Molander G. A. Single-Electron Transmetalation: Synthesis of 1,1-Diaryl-2,2,2-Trifluoroethanes by Photoredox/Nickel Dual Catalytic Cross-Coupling. Chem. - Eur. J. 2016, 22, 120–123. 10.1002/chem.201504079. [DOI] [PubMed] [Google Scholar]
- Primer D. N.; Karakaya I.; Tellis J. C.; Molander G. A. Single-Electron Transmetalation: An Enabling Technology for Secondary Alkylboron Cross-Coupling. J. Am. Chem. Soc. 2015, 137, 2195–2198. 10.1021/ja512946e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karakaya I.; Primer D. N.; Molander G. A. Photoredox Cross-Coupling: Ir/Ni Dual Catalysis for the Synthesis of Benzylic Ethers. Org. Lett. 2015, 17, 3294–3297. 10.1021/acs.orglett.5b01463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amani J.; Sodagar E.; Molander G. A. Visible Light Photoredox Cross-Coupling of Acyl Chlorides with Potassium Alkoxymethyltrifluoroborates: Synthesis of α-Alkoxyketones. Org. Lett. 2016, 18, 732–735. 10.1021/acs.orglett.5b03705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita Y.; Tellis J. C.; Molander G. A. Protecting Group-Free, Selective Cross-Coupling of Alkyltrifluoroborates with Borylated Aryl Bromides via Photoredox/nickel Dual Catalysis. Proc. Natl. Acad. Sci. U. S. A. 2015, 112, 12026–12029. 10.1073/pnas.1509715112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouffroy M.; Primer D. N.; Molander G. A. Base-Free Photoredox/Nickel Dual-Catalytic Cross-Coupling of Ammonium Alkylsilicates. J. Am. Chem. Soc. 2016, 138, 475–478. 10.1021/jacs.5b10963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouffroy M.; Kelly C. B.; Molander G. A. Thioetherification via Photoredox/Nickel Dual Catalysis. Org. Lett. 2016, 18, 876–879. 10.1021/acs.orglett.6b00208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel N. R.; Kelly C. B.; Jouffroy M.; Molander G. A. Engaging Alkenyl Halides with Alkylsilicates via Photoredox Dual Catalysis. Org. Lett. 2016, 18, 764–767. 10.1021/acs.orglett.6b00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouffroy M.; Davies G. H. M.; Molander G. A. Accessing Elaborated 2,1-Borazaronaphthalene Cores Using Photoredox/Nickel Dual-Catalytic Functionalization. Org. Lett. 2016, 18, 1606. 10.1021/acs.orglett.6b00466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leveque C.; Chenneberg L.; Corce V.; Goddard J.; Ollivier C.; Fensterbank L. Primary Alkyl Bis-Catecholato Silicates in Dual Photoredox/nickel Catalysis: Aryl- and Heteroaryl-Alkyl Cross Coupling Reactions. Org. Chem. Front. 2016, 3, 462–465. 10.1039/C6QO00014B. [DOI] [Google Scholar]
- Joe C. L.; Doyle A. G. Direct Acylation of C(sp3)–H Bonds Enabled by Nickel and Photoredox Catalysis. Angew. Chem., Int. Ed. 2016, 55, 4040–4043. 10.1002/anie.201511438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xuan J.; Zeng T.; Feng Z.; Deng Q.; Chen J.; Lu L.; Xiao W.; Alper H. Redox-Neutral α-Allylation of Amines by Combining Palladium Catalysis and Visible-Light Photoredox Catalysis. Angew. Chem., Int. Ed. 2015, 54, 1625–1628. 10.1002/anie.201409999. [DOI] [PubMed] [Google Scholar]
- Gutierrez O.; Tellis J. C.; Primer D. N.; Molander G. A.; Kozlowski M. C. Nickel-Catalyzed Cross-Coupling of Photoredox-Generated Radicals: Uncovering a General Manifold for Stereoconvergence in Nickel-Catalyzed Cross-Couplings. J. Am. Chem. Soc. 2015, 137, 4896–4899. 10.1021/ja513079r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oderinde M. S.; Varela-Alvarez A.; Aquila B.; Robbins D. W.; Johannes J. W. Effects of Molecular Oxygen, Solvent, and Light on Iridium-Photoredox/Nickel Dual-Catalyzed Cross-Coupling Reactions. J. Org. Chem. 2015, 80, 7642–7651. 10.1021/acs.joc.5b01193. [DOI] [PubMed] [Google Scholar]
- Zuo Z.; Cong H.; Li W.; Choi J.; Fu G. C.; MacMillan D. W. C. Enantioselective Decarboxylative Arylation of α-Amino Acids via the Merger of Photoredox and Nickel Catalysis. J. Am. Chem. Soc. 2016, 138, 1832–1835. 10.1021/jacs.5b13211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang S. B.; O’Nele K. M.; Tunge J. A. Decarboxylative Allylation of Amino Alkanoic Acids and Esters via Dual Catalysis. J. Am. Chem. Soc. 2014, 136, 13606–13609. 10.1021/ja508317j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang S. B.; O’Nele K. M.; Douglas J. T.; Tunge J. A. Dual Catalytic Decarboxylative Allylations of α-Amino Acids and Their Divergent Mechanisms. Chem. - Eur. J. 2015, 21, 18589–18593. 10.1002/chem.201503644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng W.; Shang R.; Yu H.; Fu Y. Room-Temperature Decarboxylative Couplings of α-Oxocarboxylates with Aryl Halides by Merging Photoredox with Palladium Catalysis. Chem. - Eur. J. 2015, 21, 13191–13195. 10.1002/chem.201502286. [DOI] [PubMed] [Google Scholar]
- Xuan J.; Zeng T.; Chen J.; Lu L.; Xiao W. Room Temperature C–P Bond Formation Enabled by Merging Nickel Catalysis and Visible-Light-Induced Photoredox Catalysis. Chem. - Eur. J. 2015, 21, 4962–4965. 10.1002/chem.201500227. [DOI] [PubMed] [Google Scholar]
- Oderinde M. S.; Frenette M.; Robbins D. W.; Aquila B.; Johannes J. W. Photoredox Mediated Nickel Catalyzed Cross-Coupling of Thiols With Aryl and Heteroaryl Iodides via Thiyl Radicals. J. Am. Chem. Soc. 2016, 138, 1760–1763. 10.1021/jacs.5b11244. [DOI] [PubMed] [Google Scholar]
- This includes cases where the role of the photoredox catalyst is unclear (refs (83, 84)).
- Zhang G.; Liu C.; Yi H.; Meng Q.; Bian C.; Chen H.; Jian J.; Wu L.; Lei A. External Oxidant-Free Oxidative Cross-Coupling: A Photoredox Cobalt-Catalyzed Aromatic C–H Thiolation for Constructing C–S Bonds. J. Am. Chem. Soc. 2015, 137, 9273–9280. 10.1021/jacs.5b05665. [DOI] [PubMed] [Google Scholar]
- Sagadevan A.; Ragupathi A.; Lin C.; Hwu J. R.; Hwang K. C. Visible-Light Initiated Copper(I)-Catalysed Oxidative C–N Coupling of Anilines with Terminal Alkynes: One-Step Synthesis of α-Ketoamides. Green Chem. 2015, 17, 1113–1119. 10.1039/C4GC01623H. [DOI] [Google Scholar]
- Osawa M.; Nagai H.; Akita M. Photo-Activation of Pd-Catalyzed Sonogashira Coupling Using a Ru/bipyridine Complex as Energy Transfer Agent. Dalton Trans. 2007, 827–829. 10.1039/b618007h. [DOI] [PubMed] [Google Scholar]
- Inagaki A.; Edure S.; Yatsuda S.; Akita M. Highly Selective Photo-Catalytic Dimerization of α-Methylstyrene by a Novel Palladium Complex with Photosensitizing Ruthenium(II) Polypyridyl Moiety. Chem. Commun. 2005, 5468–5470. 10.1039/b508013d. [DOI] [PubMed] [Google Scholar]
- Choi S.; Chatterjee T.; Choi W. J.; You Y.; Cho E. J. Synthesis of Carbazoles by a Merged Visible Light Photoredox and Palladium-Catalyzed Process. ACS Catal. 2015, 5, 4796–4802. 10.1021/acscatal.5b00817. [DOI] [Google Scholar]
- Yoo W.; Tsukamoto T.; Kobayashi S. Visible-Light-Mediated Chan–Lam Coupling Reactions of Aryl Boronic Acids and Aniline Derivatives. Angew. Chem., Int. Ed. 2015, 54, 6587–6590. 10.1002/anie.201500074. [DOI] [PubMed] [Google Scholar]
- Fabry D. C.; Zoller J.; Raja S.; Rueping M. Combining Rhodium and Photoredox Catalysis for C–H Functionalizations of Arenes: Oxidative Heck Reactions with Visible Light. Angew. Chem., Int. Ed. 2014, 53, 10228–10231. 10.1002/anie.201400560. [DOI] [PubMed] [Google Scholar]
- Fabry D. C.; Ronge M. A.; Zoller J.; Rueping M. C–H Functionalization of Phenols Using Combined Ruthenium and Photoredox Catalysis: In Situ Generation of the Oxidant. Angew. Chem., Int. Ed. 2015, 54, 2801–2805. 10.1002/anie.201408891. [DOI] [PubMed] [Google Scholar]
- Zoller J.; Fabry D. C.; Ronge M. A.; Rueping M. Synthesis of Indoles Using Visible Light: Photoredox Catalysis for Palladium-Catalyzed C–H Activation. Angew. Chem., Int. Ed. 2014, 53, 13264–13268. 10.1002/anie.201405478. [DOI] [PubMed] [Google Scholar]
- Xu N.; Li P.; Xie Z.; Wang L. Merging Visible-Light Photocatalysis and Palladium Catalysis for C–H Acylation of Azo- and Azoxybenzenes with α-Keto Acids. Chem. - Eur. J. 2016, 22, 2236–2242. 10.1002/chem.201504530. [DOI] [PubMed] [Google Scholar]
- Zhou C.; Li P.; Zhu X.; Wang L. Merging Photoredox with Palladium Catalysis: Decarboxylative Ortho-Acylation of Acetanilides with α-Oxocarboxylic Acids under Mild Reaction Conditions. Org. Lett. 2015, 17, 6198–6201. 10.1021/acs.orglett.5b03192. [DOI] [PubMed] [Google Scholar]
- Zhong J.; Wu C.; Meng Q.; Gao X.; Lei T.; Tung C.; Wu L. A Cascade Cross-Coupling and in Situ Hydrogenation Reaction by Visible Light Catalysis. Adv. Synth. Catal. 2014, 356, 2846–2852. 10.1002/adsc.201400588. [DOI] [Google Scholar]
- Magnuson R. H.; Meirowitz R.; Zulu S. J.; Giering W. P. Redox-Catalyzed Carbonylation of an Iron Methyl Complex. Organometallics 1983, 2, 460–462. 10.1021/om00075a021. [DOI] [Google Scholar]
- This is in contrast to 2-electron transmetalations and oxidative additions which have been shown to occur with both retentive and invertive stereospecificity depending on the conditions employed.
- Many reported transformations occur with concomitant release of a gaseous byproduct, complicating analysis of the energetics due to entropic factors.
- Dalton D. M.; Ellis S. R.; Nichols E. M.; Mathies R. A.; Toste F. D.; Bergman R. G.; Raymond K. N. Supramolecular Ga4L612– Cage Photosensitizes 1,3-Rearrangement of Encapsulated Guest via Photoinduced Electron Transfer. J. Am. Chem. Soc. 2015, 137, 10128–10131. 10.1021/jacs.5b06317. [DOI] [PubMed] [Google Scholar]
- Kaphan D. M.; Levin M. D.; Bergman R. G.; Raymond K. N.; Toste F. D. A Supramolecular Microenvironment Strategy for Transition Metal Catalysis. Science 2015, 350, 1235–1238. 10.1126/science.aad3087. [DOI] [PubMed] [Google Scholar]