The trouble began for Toledo, Ohio, in the spring of 2014, when extreme storms washed an unusually high pulse of nutrients into Lake Erie. By late summer, a massive bloom of blue-green algae, otherwise known as cyanobacteria, had shut down the city’s drinking water system.
Powered by nutrients from agricultural runoff and the warm, calm summer waters, the algae spread across the lake and slowly began releasing toxins into the water. The most common of these, microcystin, showed up at levels of 2.5 μg/L, exceeding the safe threshold of 1 μg/L set by the World Health Organization (WHO). The city has been spending $3 million to $4 million per year treating the water supply.
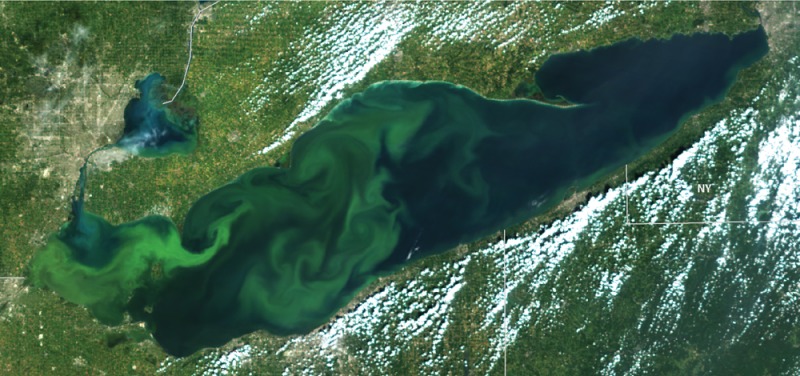
Cyanobacteria clog Lake Erie, which is about 92 km wide, in this satellite image. Credit: Richard Stumpf.
“Harmful algal blooms are one of the worst water quality issues that we need to deal with across the globe,” says Diane Orihel, an aquatic ecologist at the University of Ottawa. The U.S. economy loses $2 billion per year from damages that require expensive water treatment and result in property devaluation and declines in fish stocks. “Because cyanobacteria produce brain and liver toxins, there are cases of livestock, pets, and humans getting sick and even dying from these blooms,” she says (see appendix, “Meet the Cyanotoxins”).
Cyanobacteria have evolved over 3 billion years to become wily competitors for light and nutrients in lakes and rivers. Conditions today—a warmer climate combined with nutrient-polluted waters—allow them to dominate as they never have before, says Hans W. Paerl, an aquatic ecologist at the University of North Carolina, Chapel Hill. A recent study found lakes in every province of Canada with microcystin levels above the WHO guideline. And the U.S. Geological Survey reported this year that scientists found microcystin in 39% of streams in the southeastern U.S. at levels up to 3.2 μg/L.
Scientists and regulators have been tackling the issue, calling for cuts to pollution of phosphorus, a nutrient of choice for toxic algae. But researchers have been noticing that in some polluted lakes, such as China’s Lake Taihu, cyanobacteria have become so sated with phosphorus that their growth is held back by the relative scarcity of another favorite nutrient, nitrogen. So some scientists are now controversially suggesting that nitrogen should be controlled along with phosphorus to tame algal blooms.

China’s Lake Taihu has become so overenriched with phosphorus that its algal blooms are nitrogen-limited. Credit: Hans Paerl.
Algae require 10 to 40 times as much nitrogen as phosphorus to thrive and grow. In general, when the ratio of nitrogen to phosphorus is low, the microorganisms’ growth is limited by nitrogen, and when the ratio is high, phosphorus controls the growth rate. But these simple rules get more complicated and difficult to apply when water quality managers attempt to control blooms on an ecosystem scale.
The Phosphorus Paradigm
When growing numbers of lakes turned pea green in the 1960s, researchers couldn’t agree on what was driving the overgrowth of algae, says David W. Schindler, an aquatic ecologist with the University of Alberta. A leading theory promoted by the detergent industry held that carbon, not phosphorus, caused blooms. Industry based the claim on short-term experiments in which scientists scooped up lake water in a bottle, added carbon, and waited to see how much algal production ensued.
“But a bottle is not a lake, and a few days are not a summer,” says Stephen R. Carpenter, an aquatic ecologist at the University of Wisconsin-Madison. He explains that although short-term assays can tell you which nutrient is limiting growth during a brief moment in time, they don’t capture the complicated long-term dynamics of a whole ecosystem.
Schindler put an end to the carbon theory in the mid-1970s when he and his colleagues added phosphorus and nitrogen to an entire lake at Ontario’s Experimental Lakes Area. The lake turned green with algae, even though carbon levels were very low. In a second lake with an hourglass shape, the researchers constructed a barrier at the neck to split the lake in two. They then added carbon and nitrogen to both basins, but phosphorus only to one. The iconic photo of the green scummy phosphorus side against the clear blue nonphosphorus side convinced regulators worldwide to ban phosphates in detergent and cut phosphorus discharges from sewage plants.
When scientists add phosphorus to lakes, Schindler explains, algae respond in the springtime with a big bloom dominated by diatoms and other species that aquatic crustaceans like to eat. However, as the bloom grows, the algae consume all the dissolved inorganic nitrogen in the lake and become what is called “nitrogen-limited.”
So in midsummer to early fall, cyanobacteria that can extract gaseous nitrogen from the atmosphere and convert it into more useful molecular forms take over the lake. In fact, scientists arguing against the idea that nitrogen should be controlled to tame algal blooms point to cyanobacteria’s ability to “fix” nitrogen to bolster their case. They say that the nitrogen fixers never actually become nitrogen-limited.
In any case, when the algal crop dies as cold weather descends, its remnants sink to the bottom of the lake where they enrich the sediment. In spring, that sediment releases stored phosphorus and, along with external inputs such as agricultural runoff, drives another year’s bloom. Over years and years, a lake accrues a vast store of phosphorus, nitrogen, and carbon that can cycle from sediment to algal bloom and back again.
With excess phosphorus added to lakes these days from pollution, these bodies of water might seem nitrogen-limited. Still, phosphorus-control advocates point to case studies of the Great Lakes and many European lakes showing that, when phosphorus additions cease, a lake can regain its clarity, demonstrating that the lake is actually phosphorus-limited.
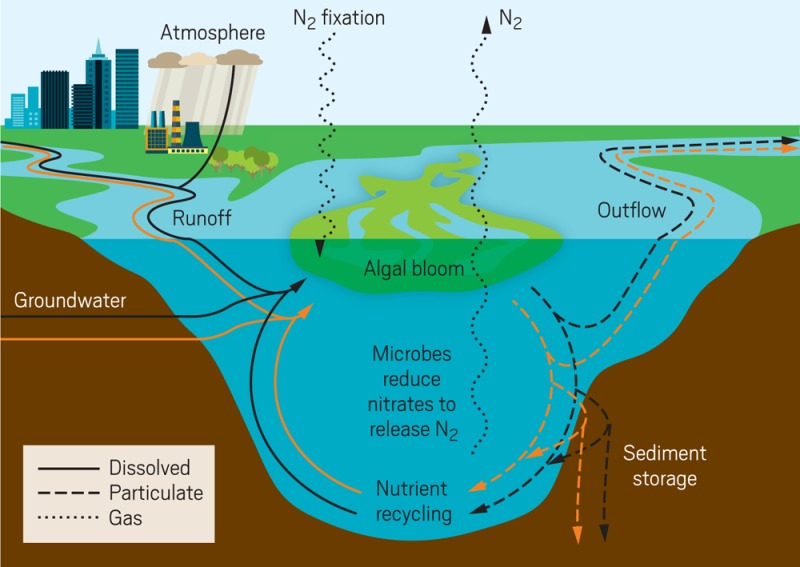
When lakes receive excess nitrogen (black) and phosphorus (orange), the nutrients accumulate in the lake as they cycle between algae, the water, the air, and the sediment. Credit: Adapted from Environ. Microbiol. 2016, DOI: 10.1111/1462–2920.13035. Copyright 2016 John Wiley & Sons.
Dual Nutrient Control
Still, one group of scientists doesn’t think phosphorus is the end of the story.
“We need to worry about controlling both nitrogen and phosphorus in lakes because freshwater ecosystems are linked to coastal waters that are typically nitrogen-limited,” says Wayne A. Wurtsbaugh, an aquatic ecologist at Utah State University. Saltwater chemistry makes phosphorus much more abundant than nitrogen in marine waters. In freshwater systems rich in iron, phosphorus binds to the iron, forming ferrous phosphate minerals that are not available for algae to consume. But in saline systems, iron has lots of salt-based sulfide to react with, leaving phosphorus much more available to algae compared with nitrogen in saltwater.
Add nitrogen to these coastal systems—either from agricultural runoff or from the air—and blooms take off, Paerl says. His research on North Carolina’s Neuse River estuary has demonstrated that although phosphate detergent bans and phosphorus controls on sewage plants improved the upper freshwater reaches of the watershed, nitrogen levels increased downstream in the coastal waters, generating nuisance algal blooms. Scientists have documented nitrogen-driven blooms along coasts worldwide, including those from the Gulf of Mexico to China’s Yangtze River Delta, Paerl says.
The European Union’s Water Framework Directive sets nutrient criteria for both phosphorus and nitrogen to protect coastal waters. But some experts are saying that dual nutrient control could benefit freshwater lakes as well. Wurtsbaugh has pored over past studies in which scientists manipulated nutrient loads in 20 freshwater lakes. He found that lakes fertilized with phosphorus alone or nitrogen alone experienced a two- to threefold increase in algal biomass. But when researchers enriched lakes with both phosphorus and nitrogen, the algal crop ballooned by a factor of 10. The findings hint that phosphorus and nitrogen act in a synergistic way on algal production, he says.
“Lake Taihu is dominated by cyanobacteria that don’t fix nitrogen, so they require external nitrogen inputs,” Paerl says. The lake receives urban and agricultural waste from a watershed that supports about 11% of the Chinese economy. When Paerl and his team added varying levels of nutrients to mesocosms—fiberglass closed-bottom cylinders—suspended in the lake, they triggered the most growth when they added phosphorus and nitrogen together. Paerl and his Chinese colleagues have convinced officials to reduce both phosphorus and nitrogen inputs to the lake until the nutrients stored in the sediment are depleted.
“But it’s not so easy to remove nitrogen because nitrogen compounds are very soluble, and they can’t be precipitated out like phosphorus is at the wastewater treatment plant,” Paerl says. Removing nitrogen from wastewater will require running the water over special bacteria that sequester the element and convert it into other forms.
Time and Space Matter
While the different scientific camps—those that advocate for only phosphorus control versus those that advocate for dual phosphorus and nitrogen control—continue to argue, others take a middle ground. “The arguments over limiting nutrients are compatible if you think deeply enough about the importance of time and spatial scales,” says Robert W. Sterner, an aquatic ecologist and director of the Large Lakes Observatory at the University of Minnesota, Duluth.
The researchers who advocate for phosphorus as the limiting nutrient are looking at lake processes over annual and decadal time scales for which phosphorus is important. The scientists championing nitrogen as the controlling factor are looking at short-term events such as a sudden late-summer bloom so immense it has consumed most of the available nitrogen and is now limited by that nutrient.
A growing body of research shows that elevated nitrogen levels influence the mix of cyanobacteria species in blooms and make them more toxic, Sterner says. Other studies indicate that the amount of nitrogen in the system has a secondary role in controlling the biomass of a bloom. But municipalities may want to pause before removing phosphorus and nitrogen from sewage because it can cost four to eight times as much as removing phosphorus alone. “In addition, the science right now isn’t strong enough to predict the benefits of a given amount of nitrogen reduction,” Sterner says.
“Because of all the remarkable research that has been done on nutrients, it is shocking to find ourselves in the year 2016 still arguing about what’s limiting algal growth,” Sterner says. The argument will continue, though, until there is a long-term whole-lake experiment like the one conducted by Schindler in the 1970s on adding nitrogen alone rather than phosphorus, he says.
New Strategies To Cope
As with many of Canada’s prairie lakes and reservoirs, Alberta’s Lake Nakamun is high in phosphorus but low in iron. So the University of Ottawa’s Orihel, a phosphorus-control advocate, wondered whether adding iron could lock up the phosphorus in the water by forming ferrous phosphate.
When Orihel and her team fertilized open-bottom mesocosms in Nakamun with iron, the treatment cut phosphorus concentrations by 56–72% in open water, suppressed cyanobacteria blooms, and even kept microcystin concentrations below water quality guidelines. “But this is not a silver bullet strategy; it must be used in conjunction with efforts to cut nutrient pollution,” Orihel says.
Adding iron to tie up phosphorus may work for small anemic lakes but wouldn’t be practical or affordable for a large body such as Lake Erie. To protect human health around Lake Erie, researchers have developed a new forecasting and tracking system for blooms. The seasonal forecast models rely primarily on measurements of nutrient runoff from farms. “The model shows that while nitrogen may explain some of the bloom size, phosphorus is the best predictor of the bloom,” says Daniel R. Obenour, an environmental engineer at North Carolina State University. The weekly tracking system employs sample data, models of cyanobacteria growth, and satellite data to predict the size and locations of blooms on Lake Erie.
The U.S. and Canada recently agreed to cut phosphorus loading into Lake Erie by 40%. Even if the management goals for Lake Erie emphasize phosphorus at the expense of nitrogen, the best management practices to control phosphorus runoff from farms, such as improving fertilizer application and planting vegetative buffer zones that sop up nutrients, will have a positive impact on cutting both phosphorus and nitrogen, Obenour says.
But a specific focus on nitrogen would be expensive and difficult to achieve because it might entail changing or cutting back crop production. “We know we can manage nitrogen,” Obenour says, “but we haven’t decided if the costs are worth the improvement in water quality.”
APPENDIX
Meet the Cyanotoxins
More deadly than cobra venom and potent enough to be weaponized, cyanotoxins come in a wide variety of forms that scientists are working hard to analyze. “We need good tools to identify toxins so we can see whether our management interventions are improving water quality,” says Keith Loftin, a research chemist with the U.S. Geological Survey.
When Loftin’s team descends on a bloom, the researchers take water samples and run them through a quick screening assay to identify which general classes of toxins are present. “Assays such as enzyme-linked immunosorbent assays (ELISAs) are relatively quick, easy to use, and inexpensive,” he says.
Once the screening process pinpoints what class of toxin is present in a sample, the scientists turn to triple-quadrupole mass spectrometry to precisely determine the specific identity of the toxin they are dealing with. “We have good tools for identifying toxins, but one thing we lack are certified reference toxins to use with the mass spectrometry because the toxins are expensive and difficult to synthesize,” Loftin says. Currently, he has to make his own reference standards for the toxins.
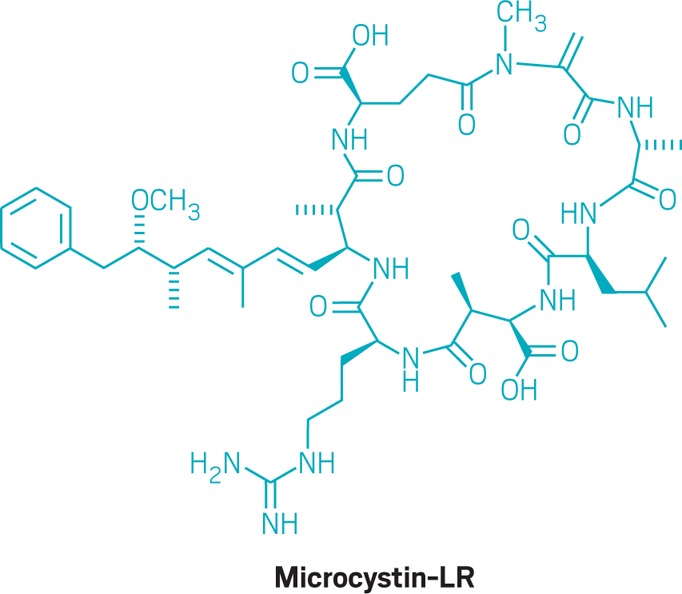
• When researchers in North America probe an algal bloom, the most common cyanotoxin they encounter is microcystin, a large cyclic peptide ring sporting seven amino acids that can vary (one version containing lysine and arginine is shown here). Some researchers have compared the structure with that of the peptide antibiotic gramicidin. Microcystin is produced by the cyanobacteria Microcystis and many other species. If ingested by an animal, microcystin causes bleeding into liver tissue that rapidly leads to death. Chronic exposure to the toxin can promote tumors in the liver and colon.
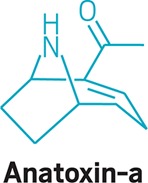
• Much smaller than microcystin, anatoxin-a is a bicyclic amine alkaloid with a cocainelike architecture. It is a neurotoxin that leads to respiratory failure so swiftly it has its own handle, very fast death factor.
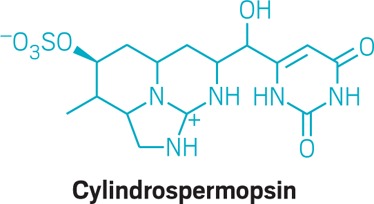
• Many types of cyanobacteria produce cylindrospermopsin, an alkaloid toxin with a complex heterocyclic multiring system. The toxin targets the liver and kidneys. Symptoms can include vomiting, bloody diarrhea, and liver and kidney damage. Cylindrospermopsin, like all the cyanotoxins, bioaccumulates in freshwater food webs.
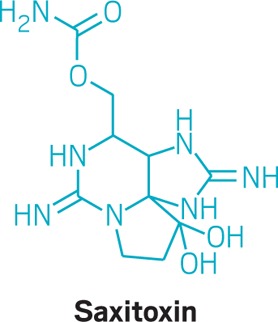
• Saxitoxin is a sodium channel blocker so lethal that the U.S. once considered developing it as a chemical weapon. The toxin paralyzes the respiratory system, killing victims in a matter of minutes. Francis Gary Powers, the U-2 spy plane pilot who was shot down over the U.S.S.R. in 1960, refrained from using the small dose of saxitoxin he carried with him to commit suicide in case he was captured.
Janet Pelley is a freelance contributor toChemical & Engineering News, the weekly newsmagazine of the American Chemical Society. A version of this story first appeared in C&EN.
In collaboration with C&EN.


