Abstract
Provocative emerging evidence suggests that the N-methyl-d-aspartate (NMDA) receptor can signal in the absence of ion flux through the receptor. This non-ionotropic signaling is thought to be due to agonist-induced conformational changes in the receptor, independently of channel opening. Non-ionotropic NMDA receptor signaling has been proposed to be sufficient to induce synaptic long-term depression (LTD), directly challenging the decades-old model that prolonged low-level calcium influx is required to induce LTD. Here, we briefly review these recent findings, focusing primarily on the potential role of non-ionotropic signaling in NMDA receptor-mediated LTD. Further reports concerning additional roles of non-ionotropic NMDA receptor signaling are also discussed. If validated, this new view of NMDA receptor-mediated signaling will usher in an exciting new era of exploring synapse function and dysfunction.
Keywords: NMDA, Non-ionotropic, signaling, LTD
Introduction
N-methyl- d-aspartate receptors (NMDARs) are glutamate-gated cation channels that play crucial roles in neurodevelopment and bidirectional synaptic plasticity. Most of the functions of the NMDAR have been attributed to the influx of calcium ions during channel opening 1– 10. However, a flurry of recent studies 11– 18 have provided more systematic support for earlier studies 19– 22 suggesting that agonist binding to NMDARs can transmit information to signaling molecules independently of Ca 2+ influx through the channel. If confirmed, these findings may lead to new pharmacological approaches to target specific synaptic signaling cascades in the numerous disorders attributed to synaptic dysfunction, including autism, schizophrenia, post-traumatic stress disorder, epilepsy, addiction, and Alzheimer’s disease.
Synaptic plasticity is a well-established cellular model for learning and memory and involves the persistent increase (long-term potentiation, or LTP) and weakening (long-term depression, or LTD) of synaptic strength in response to various patterns of activity. At most excitatory synapses in the brain, NMDAR activation is important for the induction of both LTP and LTD 23– 26. The widely accepted model for how activation of a single receptor can produce opposite changes in synaptic strength involves the amount and duration of Ca 2+ influx 27– 30. This model posits that brief periods of high-frequency synaptic activity lead to a large, rapid increase in intracellular Ca 2+ through the NMDAR that activates a series of biochemical steps, leading to LTP 31. Conversely, prolonged periods of low-frequency synaptic activity drive a modest increase in Ca 2+ through NMDARs that activates a different series of biochemical steps, leading to LTD 24, 25, 32.
For NMDAR-dependent LTD, the model that a modest increase in intracellular Ca 2+ is necessary for LTD induction has recently been challenged. Using systematic pharmacological approaches that block ion flux but spare glutamate binding to the NMDAR, Nabavi et al. 15 found that LTD could still be induced in an NMDAR-dependent manner. Here, we will review this provocative finding and follow-up studies, highlighting a direct refutation 33 and confirmation 16 of this result. Furthermore, we will discuss possible explanations for the disparate findings and additional reports of NMDAR-mediated signaling independent of channel opening. Given the importance of NMDARs in synaptic development and plasticity, these findings have the potential to be transformative but need further detailed and rigorous follow-up.
Does NMDAR-dependent LTD involve non-ionotropic mechanisms?
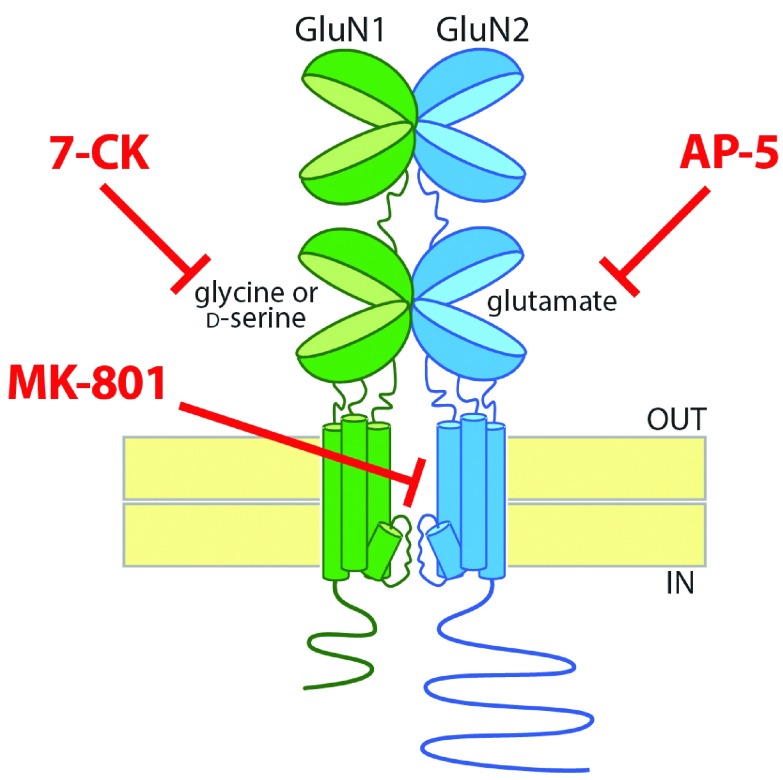
NMDARs have a rich and complex pharmacology. Most NMDARs are heterotetramers containing two GluN1 subunits and two GluN2 subunits. One of the notable properties of NMDARs is that they are blocked by magnesium ions at resting membrane potentials 35; opening of NMDARs requires simultaneous activation by glutamate and depolarization to relieve the Mg 2+ block. In addition, NMDARs are unique among neurotransmitter receptors in having an absolute requirement for the binding of a co-agonist in addition to glutamate in order to open the channel. Glutamate binds to the extracellular ligand-binding domain on the GluN2 subunits, whereas the co-agonist, which is either glycine or d-serine, binds to the homologous ligand-binding domain on the GluN1 subunits ( Figure 1).
Figure 1. Pharmacology of the N-methyl- d-aspartate receptor (NMDAR).
Most NMDARs are tetrameric proteins containing two GluN1 and two GluN2 subunits (for clarity, only one of each is pictured). For the NMDAR channel to open, both glutamate and a co-agonist, which can be glycine or d-serine, need to bind to clamshell-like ligand-binding domains on the GluN2 and GluN1 subunits, respectively. There are multiple approaches to block ion flow through the NMDAR channel: a competitive antagonist for the glutamate-binding site on the GluN2 subunits (e.g. AP-5), a competitive antagonist for the glycine/ d-serine binding site on the GluN1 subunits (e.g. 7-CK), or an uncompetitive blocker of the channel itself (e.g. MK-801). 7-CK, 7-chlorokynurenic acid; AP-5, (2R)-amino-5-phosphonovaleric acid; MK-801, dizocilpine.
There are multiple approaches to blocking ion flow through the NMDAR: (1) a competitive antagonist of the glutamate-binding site on GluN2 (e.g. AP-5), (2) a competitive antagonist of the co-agonist site on GluN1 (e.g. 7-chlorokynurenate, or 7-CK), or (3) an uncompetitive blocker of the channel pore (e.g. MK-801) ( Figure 1). Using each of these pharmacologic strategies to block ion flow through the NMDAR, Nabavi et al. 15 surprisingly found that only the glutamate site antagonist AP-5 blocked LTD, suggesting that glutamate binding is required for LTD but not co-agonist binding or ion flow through the NMDAR. Although metabotropic glutamate receptor (mGluR)-mediated forms of LTD can also occur at these synapses 36– 38, application of an mGluR5 inhibitor and an inhibitor of L-type Ca 2+ channels (which are required for mGluR1-mediated LTD 39) did not block the LTD observed in the presence of 7-CK or MK-801, supporting the notion that signaling through mGluRs did not provide an alternate source for a rise in intracellular Ca 2+ leading to LTD.
So, what about the two decades of evidence that support a fundamental role for NMDAR-mediated Ca 2+ influx in LTD 32? Indeed, introducing Ca 2+ chelators intracellularly to the post-synaptic neuron prevents LTD 24, 40– 43. In addition, increases in intracellular Ca 2+ levels through activation of voltage-gated calcium channels 44 or photolysis of caged Ca 2+ 30, 45 induces synaptic depression that occludes additional LTD. Furthermore, LTD requires signaling mechanisms which are dependent on Ca 2+, including the activation of calcineurin 46 and hippocalcin 47. Nabavi et al. 15 also saw that Ca 2+ chelation inhibited LTD, but they proposed that the reduction in basal intracellular Ca 2+ concentration by strong chelation is responsible for the loss of LTD. To test this idea, they buffered the intracellular Ca 2+ concentration with the strong chelator BAPTA (1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid) along with additional free Ca 2+ in order to prevent acute rises in Ca 2+ while maintaining baseline Ca 2+ near physiological levels. This clamping of Ca 2+ at baseline levels did not block LTD, suggesting that low levels of basal Ca 2+, but not acute elevations of Ca 2+ through the NMDAR, are required for LTD. Remarkably, when Ca 2+ influx was blocked through the NMDAR with MK-801, a high-frequency stimulus that normally induces LTP actually resulted in LTD. Together, these results support that NMDAR-dependent LTD requires glutamate binding to the NMDAR but not Ca 2+ influx.
The surprising and provocative work by Nabavi et al. 15 was soon directly challenged. Specifically, Babiec et al. 33 found that, in contrast to Nabavi et al., MK-801 effectively blocked LTD induction in slices from both young and adult animals. Furthermore, they showed that MK-801 blocked a chemical form of LTD induced by bath application of the glutamate site agonist NMDA. In addition, lowering extracellular Ca 2+ concentration also blocked LTD, supporting previous studies defining the role of Ca 2+ in LTD 32. These results stand in direct conflict with those of Nabavi et al., and the reasons for these inconsistent findings remain unclear (see below for additional discussion).
Others have begun to weigh in. Recently, Stein et al. 16 examined the role of non-ionotropic NMDAR signaling in activity-induced dendritic spine shrinkage, a structural correlate of LTD. Using two-photon glutamate uncaging and time-lapse imaging, they found that low-frequency uncaging led to spine shrinkage even when the NMDAR glycine/ d-serine site was blocked with 7-CK. Furthermore, the presence of 7-CK or MK-801 converted high-frequency uncaging-induced spine enlargement to spine shrinkage, similar to the findings by Nabavi et al. for LTD. This spine shrinkage evoked in the presence of 7-CK was not inhibited by co-application of antagonists of mGluR1 and mGluR5. Importantly, since two-photon glutamate uncaging was used to bypass presynaptic neurotransmitter release, the potential effects of the pharmacological agents on pre-synaptic NMDARs were avoided. These results further support the idea that non-ionotropic NMDAR-mediated signaling mechanisms can drive synaptic depression.
Why the inconsistent results among laboratories 48? It is not yet clear. One possibility is that ionotropic and non-ionotropic mechanisms coexist. Alternatively, there may be unrecognized experimental differences (for example, in slice preparation, solutions, timing of drug application and removal, perfusion, and temperature). Indeed, a major limitation of these studies is the reliance on pharmacology. In most previous studies, AP-5 or other competitive glutamate site antagonists are used to block NMDAR activity, and we are unaware of any published examples in which AP-5 did not block NMDAR-mediated LTD. The examination of glycine co-agonist site antagonists in LTD, however, is a newer development, and it would have been enlightening if Babiec et al. had included one in their analysis. 7-CK is an early derivative of the naturally occurring kynurenic acid, which is a non-selective antagonist of all ionotropic glutamate receptors as well as the α7-nicotinic receptor. Although 7-CK is more potent and selective than kynurenic acid, it still exhibits a significant blockade of other receptors 49. Follow-up studies using higher-potency glycine site antagonists, and ones of different chemical classes, such as MDL 105,519 50, will be of key importance.
Most of the conflicting results described above involve MK-801, an uncompetitive open-channel blocker of NMDARs 51. Oddly enough, it appears that this controversy is not new. Previous studies have shown an inhibition of LTD by MK-801 in the CA1 region of the hippocampus 52, 53 and the mouse visual cortex 54. However, an older report showed that although MK-801 blocked LTP, it did not block low-frequency stimulation-induced LTD in the hippocampus 19. Perhaps others have observed a lack of LTD inhibition by MK-801 but attributed this to experimental error or incomplete NMDAR blockade and therefore never reported it. Indeed, because of the use-dependent nature of MK-801, incomplete blockade of synaptic NMDARs may allow small local increases in Ca 2+ during repetitive stimulation right at the channel mouth that is not effectively buffered. Although this explanation is unlikely, it is difficult to rule out. Indeed, in an attempt to control for this use dependence of MK-801, slices are often incubated for a few hours in order to allow spontaneous activity to block all NMDARs prior to the onset of stimulation 15, 33, although others find that LTD is blocked when MK-801 is applied just minutes before induction 55– 57. Another issue is that the current tools for measuring changes in intracellular Ca 2+ are not sensitive enough to detect trace amounts of influx through the NMDAR, especially if that Ca 2+ is immediately bound to proteins within the NMDAR signaling complex. This could possibly be examined with a Ca 2+-impermeable or channel “dead” NMDAR or by attaching a genetically encoded Ca 2+ sensor directly to the NMDAR intracellular domains or associated proteins.
Agonist-induced conformational changes in the NMDAR intracellular domains
Of course, the possibility of non-ionotropic signaling by NMDARs requires evidence of conformational changes upon agonist binding. While perhaps surprising for ligand-gated ion channels, non-ionotropic signaling is extremely common. G-protein-coupled receptors (GPCRs) comprise the largest protein superfamily in mammalian genomes and act solely through conformational changes upon extracellular agonist binding 58, 59. Indeed, the β2-adrenergic receptor, a prototypical GPCR, has only 168 intracellularly located amino acids, whereas NMDARs with their tetrameric structure and long complex C-terminal tails can have upwards of 1700 intracellular residues. In addition, at the post-synaptic density, NMDARs are a central member of a large macromolecular complex comprising signaling molecules, scaffolding and adaptor proteins, and cytoskeletal proteins 60, 61. Through these complex interactions, NMDARs are in a key position to engage and regulate intracellular signaling machinery. Indeed, while the long C-terminal tails of NMDARs have been presumed to be intrinsically unstructured, the complex scaffolding and interactions at the post-synaptic density may impart the secondary and tertiary structure 62 required to transmit information via agonist-induced conformational changes 63.
Recently, Dore et al. 13 demonstrated that NMDA binding to the glutamate site of the GluN2 subunits drives conformational changes in the NMDAR intracellular domains. Specifically, either green fluorescent protein (GFP) or mCherry was fused to the C-terminal tails of GluN1 subunits, and primary hippocampal neurons were co-transfected with both GFP- and mCherry-containing GluN1 subunits. Importantly, although the GluN2 subunits contain the glutamate-binding domain, GluN1 was chosen because tagging GluN2 subunits affects their trafficking and synaptic targeting 64. They then used fluorescence lifetime imaging microscopy (FLIM) to measure the lifetime of GFP fluorescence, which is reduced when in close proximity to mCherry because of Förster resonance energy transfer (FRET) 65. They found that NMDA caused rapid changes in GFP fluorescence lifetime in the presence of 7-CK or MK-801, but not in the presence of AP-5, providing evidence for agonist-induced, but ion flow-independent, conformational changes in the NMDAR C-terminal tails. In an accompanying study using similar techniques, Aow et al. 11 showed that NMDA binding, even in the presence of 7-CK (but not AP-5), leads to changes in the interactions between GluN1-GFP and various signaling proteins known to associate with the NMDAR which were tagged with the FRET acceptor mCherry. Specifically, they measured a rapid transient change in the interaction between GluN1 and protein phosphatase 1 (PP1) and a delayed but persistent change in the interaction of GluN1 with calcium/calmodulin-dependent protein kinase II (CaMKII). Together, these findings provide support that agonist binding to the NMDAR can produce intracellular conformational changes independently of ion flow through the receptor channel. These conformational changes are consistent with non-ionotropic signaling following glutamate binding to NMDARs.
Other non-ionotropic NMDAR signaling
In addition to LTD, other studies have suggested additional roles for non-ionotropic NMDAR signaling. In Alzheimer’s disease, for example, impaired hippocampal synapse dysfunction is an early event 66, 67 that is associated with increased levels of diffusible oligomeric assemblies of the amyloid-beta (Aβ) protein 68– 70. These Aβ oligomers cause a rapid synaptic depression that is dependent on NMDAR activity 71– 75. Recently, it has been shown that this NMDAR-dependent, Aβ-induced synaptic depression does not require ion flux though the channel 12, 14, 17. Kessels et al. 14 found that increasing Aβ levels in organotypic hippocampal slice cultures through the viral expression of the β-secretase product of the amyloid precursor protein leads to a baseline synaptic depression that can be blocked by AP-5, but not 7-CK or MK-801. Similarly, Tamburri et al. 17 found that this synaptic depression can occur in acute hippocampal slices within 15 minutes of perfusion of oligomeric Aβ and that this rapid depression was dependent on NMDAR activity and was blocked by AP-5, but not MK-801, again suggesting that it did not require ion flux through the receptor 17. In addition to Aβ-induced synaptic depression, Aβ oligomer-induced synapse loss was recently demonstrated to be blocked by AP-5, but not MK-801 12. Together, these results suggest that non-ionotropic NMDAR signaling contributes to the Aβ-induced synaptic dysfunction in Alzheimer’s disease and may suggest a common mechanism between Aβ-induced synaptic depression and non-ionotropic NMDAR-dependent LTD.
Other studies have described non-ionotropic NMDAR signaling, providing additional support for its physiological importance. In the earliest of these studies, glutamate binding was shown to induce dephosphorylation of the GluN2A subunit, resulting in the endocytosis of the receptor in the absence of ion flux 21. In another, co-activation of NMDARs and mGluR5 led to extracellular signal-regulated kinase (ERK) activation and increased c-Fos expression independent of ion flux but dependent on the interaction of the GluN2 C-terminal tail with scaffolding proteins in the post-synaptic density 22. NMDAR activation is also central to pathological processes that lead to neuronal death and non-ionotropic NMDAR-mediated signaling through Src kinase, and pannexin-1 was recently reported to occur during excitotoxicity 18. In addition to glutamate, co-agonist binding to the glycine site on the GluN1 subunits may also be involved in non-ionotropic signaling. For example, glycine or D-serine binding has been found to prime the NMDAR for subsequent clathrin-mediated endocytosis in the presence of AP-5 but not glycine site antagonists 20.
A new horizon for NMDAR biology?
Here we have reviewed the current literature suggesting that activation of NMDARs can activate intracellular signaling independent of ion flux through the receptor. These results have been quite provocative, though from our perspective not entirely unexpected as NMDAR are part of a large multi-protein complex at the post-synaptic density and thus are in an ideal position to have conformation-based signaling. Although the physiological significance of potential parallel ionotropic and non-ionotropic NMDAR signaling processes remains controversial, their coexistence predicts the possibility for divergent signaling events based on agonist and co-agonist availability, channel opening, and receptor subunit composition. Ultimately, further exploration of this model may open a new frontier of NMDAR biology and lead to the development of novel approaches for targeting NMDAR signaling for the treatment of multiple neuropsychiatric disorders.
Abbreviations
7-CK, 7-chlorokynurenic acid; Aβ, amyloid-beta; AP-5, (2R)-amino-5-phosphonovaleric acid; FRET, Förster resonance energy transfer; GFP, green fluorescent protein; GPCR, G-protein-coupled receptor; LTD, long-term depression; LTP, long-term potentiation; mGluR, metabotropic glutamate receptor; MK-801, dizocilpine; NMDA, N-methyl D-aspartate; NMDAR, N-methyl D-aspartate receptor.
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
Yasunori Hayashi, RIKEN Brain Science Institute, Wako, Japan
Isabel Pérez-Otaño, University of Navarra, Navarra, Spain
Funding Statement
John A. Gray is supported by a NARSAD Young Investigator Award from the Brain and Behavior Research Foundation and by the National Institutes of Health (NIH) (K08 MH100562 and a pilot through P30 AG010129). Karen Zito is supported by the NIH (R01 NS062736). Johannes W. Hell is supported by the NIH (R01 NS078792, R01 AG017502, and R01 MH097887).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; referees: 2 approved]
References
- 1. Cole AJ, Saffen DW, Baraban JM, et al. : Rapid increase of an immediate early gene messenger RNA in hippocampal neurons by synaptic NMDA receptor activation. Nature. 1989;340(6233):474–6. 10.1038/340474a0 [DOI] [PubMed] [Google Scholar]
- 2. Hardingham GE, Arnold FJ, Bading H: A calcium microdomain near NMDA receptors: on switch for ERK-dependent synapse-to-nucleus communication. Nat Neurosci. 2001;4(6):565–6. 10.1038/88380 [DOI] [PubMed] [Google Scholar]
- 3. Hayashi Y, Shi SH, Esteban JA, et al. : Driving AMPA receptors into synapses by LTP and CaMKII: requirement for GluR1 and PDZ domain interaction. Science. 2000;287(5461):2262–7. 10.1126/science.287.5461.2262 [DOI] [PubMed] [Google Scholar]
- 4. Mayer ML, Westbrook GL, Guthrie PB: Voltage-dependent block by Mg 2+ of NMDA responses in spinal cord neurones. Nature. 1984;309(5965):261–3. 10.1038/309261a0 [DOI] [PubMed] [Google Scholar]
- 5. Nägerl UV, Eberhorn N, Cambridge SB, et al. : Bidirectional activity-dependent morphological plasticity in hippocampal neurons. Neuron. 2004;44(5):759–67. 10.1016/j.neuron.2004.11.016 [DOI] [PubMed] [Google Scholar]
- 6. Shi SH, Hayashi Y, Petralia RS, et al. : Rapid spine delivery and redistribution of AMPA receptors after synaptic NMDA receptor activation. Science. 1999;284(5421):1811–6. 10.1126/science.284.5421.1811 [DOI] [PubMed] [Google Scholar]
- 7. Zhou Q, Homma KJ, Poo MM: Shrinkage of dendritic spines associated with long-term depression of hippocampal synapses. Neuron. 2004;44(5):749–57. 10.1016/j.neuron.2004.11.011 [DOI] [PubMed] [Google Scholar]
- 8. Van Dongen AM, editor: Biology of the NMDA Receptor.Boca Raton, FL.2009. [PubMed] [Google Scholar]
- 9. Matsuzaki M, Honkura N, Ellis-Davies GC, et al. : Structural basis of long-term potentiation in single dendritic spines. Nature. 2004;429(6993):761–6. 10.1038/nature02617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zito K, Scheuss V: NMDA Receptor Function and Physiological Modulation. In Encyclopedia of Neuroscience.Squire LR, Editor, Oxford: Academic Press.2009;1157–1164. 10.1016/B978-008045046-9.01225-0 [DOI] [Google Scholar]
- 11. Aow J, Dore K, Malinow R: Conformational signaling required for synaptic plasticity by the NMDA receptor complex. Proc Natl Acad Sci U S A. 2015;112(47):14711–6. 10.1073/pnas.1520029112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Birnbaum JH, Bali J, Rajendran L, et al. : Calcium flux-independent NMDA receptor activity is required for A β oligomer-induced synaptic loss. Cell Death Dis. 2015;6(6):e1791. 10.1038/cddis.2015.160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dore K, Aow J, Malinow R: Agonist binding to the NMDA receptor drives movement of its cytoplasmic domain without ion flow. Proc Natl Acad Sci U S A. 2015;112(47):14705–10. 10.1073/pnas.1520023112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kessels HW, Nabavi S, Malinow R: Metabotropic NMDA receptor function is required for β-amyloid-induced synaptic depression. Proc Natl Acad Sci U S A. 2013;110(10):4033–8. 10.1073/pnas.1219605110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nabavi S, Kessels HW, Alfonso S, et al. : Metabotropic NMDA receptor function is required for NMDA receptor-dependent long-term depression. Proc Natl Acad Sci U S A. 2013;110(10):4027–32. 10.1073/pnas.1219454110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Stein IS, Gray JA, Zito K: Non-Ionotropic NMDA Receptor Signaling Drives Activity-Induced Dendritic Spine Shrinkage. J Neurosci. 2015;35(35):12303–8. 10.1523/JNEUROSCI.4289-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tamburri A, Dudilot A, Licea S, et al. : NMDA-receptor activation but not ion flux is required for amyloid-beta induced synaptic depression. PLoS One. 2013;8(6):e65350. 10.1371/journal.pone.0065350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Weilinger NL, Lohman AW, Rakai BD, et al. : Metabotropic NMDA receptor signaling couples Src family kinases to pannexin-1 during excitotoxicity. Nat Neurosci. 2016;19(3):432–42. 10.1038/nn.4236 [DOI] [PubMed] [Google Scholar]
- 19. Mayford M, Wang J, Kandel ER, et al. : CaMKII regulates the frequency-response function of hippocampal synapses for the production of both LTD and LTP. Cell. 1995;81(6):891–904. 10.1016/0092-8674(95)90009-8 [DOI] [PubMed] [Google Scholar]
- 20. Nong Y, Huang YQ, Ju W, et al. : Glycine binding primes NMDA receptor internalization. Nature. 2003;422(6929):302–7. 10.1038/nature01497 [DOI] [PubMed] [Google Scholar]
- 21. Vissel B, Krupp JJ, Heinemann SF, et al. : A use-dependent tyrosine dephosphorylation of NMDA receptors is independent of ion flux. Nat Neurosci. 2001;4(6):587–96. 10.1038/88404 [DOI] [PubMed] [Google Scholar]
- 22. Yang L, Mao L, Tang Q, et al. : A novel Ca 2+-independent signaling pathway to extracellular signal-regulated protein kinase by coactivation of NMDA receptors and metabotropic glutamate receptor 5 in neurons. J Neurosci. 2004;24(48):10846–57. 10.1523/JNEUROSCI.2496-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bliss TV, Collingridge GL: A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361(6407):31–9. 10.1038/361031a0 [DOI] [PubMed] [Google Scholar]
- 24. Dudek SM, Bear MF: Homosynaptic long-term depression in area CA1 of hippocampus and effects of N-methyl-D-aspartate receptor blockade. Proc Natl Acad Sci U S A. 1992;89(10):4363–7. 10.1073/pnas.89.10.4363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Malenka RC, Bear MF: LTP and LTD: an embarrassment of riches. Neuron. 2004;44(1):5–21. 10.1016/j.neuron.2004.09.012 [DOI] [PubMed] [Google Scholar]
- 26. Morris RG: Long-term potentiation and memory. Philos Trans R Soc Lond B Biol Sci. 2003;358(1432):643–7. 10.1098/rstb.2002.1230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kessels HW, Malinow R: Synaptic AMPA receptor plasticity and behavior. Neuron. 2009;61(3):340–50. 10.1016/j.neuron.2009.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lisman J: A mechanism for the Hebb and the anti-Hebb processes underlying learning and memory. Proc Natl Acad Sci U S A. 1989;86(23):9574–8. 10.1073/pnas.86.23.9574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Malenka RC: Synaptic plasticity in the hippocampus: LTP and LTD. Cell. 1994;78(4):535–8. 10.1016/0092-8674(94)90517-7 [DOI] [PubMed] [Google Scholar]
- 30. Neveu D, Zucker RS: Postsynaptic levels of [Ca 2+] i needed to trigger LTD and LTP. Neuron. 1996;16(3):619–29. 10.1016/S0896-6273(00)80081-1 [DOI] [PubMed] [Google Scholar]
- 31. Lüscher C, Malenka RC: NMDA receptor-dependent long-term potentiation and long-term depression (LTP/LTD). Cold Spring Harb Perspect Biol. 2012;4(6): pii: a005710. 10.1101/cshperspect.a005710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Collingridge GL, Peineau S, Howland JG, et al. : Long-term depression in the CNS. Nat Rev Neurosci. 2010;11(7):459–73. 10.1038/nrn2867 [DOI] [PubMed] [Google Scholar]
- 33. Babiec WE, Guglietta R, Jami SA, et al. : Ionotropic NMDA receptor signaling is required for the induction of long-term depression in the mouse hippocampal CA1 region. J Neurosci. 2014;34(15):5285–90. 10.1523/JNEUROSCI.5419-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gray JA, Shi Y, Usui H, et al. : Distinct modes of AMPA receptor suppression at developing synapses by GluN2A and GluN2B: single-cell NMDA receptor subunit deletion in vivo. Neuron. 2011;71(6):1085–101. 10.1016/j.neuron.2011.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nowak L, Bregestovski P, Ascher P, et al. : Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984;307(5950):462–5. 10.1038/307462a0 [DOI] [PubMed] [Google Scholar]
- 36. Bolshakov VY, Siegelbaum SA: Postsynaptic induction and presynaptic expression of hippocampal long-term depression. Science. 1994;264(5162):1148–52. 10.1126/science.7909958 [DOI] [PubMed] [Google Scholar]
- 37. Oliet SH, Malenka RC, Nicoll RA: Two distinct forms of long-term depression coexist in CA1 hippocampal pyramidal cells. Neuron. 1997;18(6):969–82. 10.1016/S0896-6273(00)80336-0 [DOI] [PubMed] [Google Scholar]
- 38. Volk LJ, Daly CA, Huber KM: Differential roles for group 1 mGluR subtypes in induction and expression of chemically induced hippocampal long-term depression. J Neurophysiol. 2006;95(4):2427–38. 10.1152/jn.00383.2005 [DOI] [PubMed] [Google Scholar]
- 39. Bernard PB, Castano AM, Bayer KU, et al. : Necessary, but not sufficient: insights into the mechanisms of mGluR mediated long-term depression from a rat model of early life seizures. Neuropharmacology. 2014;84:1–12. 10.1016/j.neuropharm.2014.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bröcher S, Artola A, Singer W: Intracellular injection of Ca2+ chelators blocks induction of long-term depression in rat visual cortex. Proc Natl Acad Sci U S A. 1992;89(1):123–7. 10.1073/pnas.89.1.123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cho K, Aggleton JP, Brown MW, et al. : An experimental test of the role of postsynaptic calcium levels in determining synaptic strength using perirhinal cortex of rat. J Physiol. 2001;532(Pt 2):459–66. 10.1111/j.1469-7793.2001.0459f.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Debanne D, Gähwiler BH, Thompson SM: Asynchronous pre- and postsynaptic activity induces associative long-term depression in area CA1 of the rat hippocampus in vitro. Proc Natl Acad Sci U S A. 1994;91(3):1148–52. 10.1073/pnas.91.3.1148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mulkey RM, Malenka RC: Mechanisms underlying induction of homosynaptic long-term depression in area CA1 of the hippocampus. Neuron. 1992;9(5):967–75. 10.1016/0896-6273(92)90248-C [DOI] [PubMed] [Google Scholar]
- 44. Cummings JA, Mulkey RM, Nicoll RA, et al. : Ca 2+ signaling requirements for long-term depression in the hippocampus. Neuron. 1996;16(4):825–33. 10.1016/S0896-6273(00)80102-6 [DOI] [PubMed] [Google Scholar]
- 45. Yang SN, Tang YG, Zucker RS: Selective induction of LTP and LTD by postsynaptic [Ca2 +] i elevation. J Neurophysiol. 1999;81(2):781–7. [DOI] [PubMed] [Google Scholar]
- 46. Mulkey RM, Endo S, Shenolikar S, et al. : Involvement of a calcineurin/inhibitor-1 phosphatase cascade in hippocampal long-term depression. Nature. 1994;369(6480):486–8. 10.1038/369486a0 [DOI] [PubMed] [Google Scholar]
- 47. Palmer CL, Lim W, Hastie PG, et al. : Hippocalcin functions as a calcium sensor in hippocampal LTD. Neuron. 2005;47(4):487–94. 10.1016/j.neuron.2005.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Nabavi S, Fox R, Alfonso S, et al. : GluA1 trafficking and metabotropic NMDA: addressing results from other laboratories inconsistent with ours. Philos Trans R Soc Lond B Biol Sci. 2014;369(1633): 20130145. 10.1098/rstb.2013.0145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Foster AC, Kemp JA, Leeson PD, et al. : Kynurenic acid analogues with improved affinity and selectivity for the glycine site on the N-methyl-D-aspartate receptor from rat brain. Mol Pharmacol. 1992;41(5):914–22. [PubMed] [Google Scholar]
- 50. Baron BM, Harrison BL, Kehne JH, et al. : Pharmacological characterization of MDL 105,519, an NMDA receptor glycine site antagonist. Eur J Pharmacol. 1997;323(2–3):181–92. 10.1016/S0014-2999(97)00045-9 [DOI] [PubMed] [Google Scholar]
- 51. Huettner JE, Bean BP: Block of N-methyl-D-aspartate-activated current by the anticonvulsant MK-801: selective binding to open channels. Proc Natl Acad Sci U S A. 1988;85(4):1307–11. 10.1073/pnas.85.4.1307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kollen M, Dutar P, Jouvenceau A: The magnitude of hippocampal long term depression depends on the synaptic location of activated NR2-containing N-methyl-D-aspartate receptors. Neuroscience. 2008;154(4):1308–17. 10.1016/j.neuroscience.2008.04.045 [DOI] [PubMed] [Google Scholar]
- 53. Raymond CR, Ireland DR, Abraham WC: NMDA receptor regulation by amyloid-beta does not account for its inhibition of LTP in rat hippocampus. Brain Res. 2003;968(2):263–72. 10.1016/S0006-8993(03)02269-8 [DOI] [PubMed] [Google Scholar]
- 54. Crozier RA, Wang Y, Liu CH, et al. : Deprivation-induced synaptic depression by distinct mechanisms in different layers of mouse visual cortex. Proc Natl Acad Sci U S A. 2007;104(4):1383–8. 10.1073/pnas.0609596104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Coultrap SJ, Freund RK, O'Leary H, et al. : Autonomous CaMKII mediates both LTP and LTD using a mechanism for differential substrate site selection. Cell Rep. 2014;6(3):431–7. 10.1016/j.celrep.2014.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sanderson JL, Gorski JA, Dell'Acqua ML: NMDA Receptor-Dependent LTD Requires Transient Synaptic Incorporation of Ca 2+-Permeable AMPARs Mediated by AKAP150-Anchored PKA and Calcineurin. Neuron. 2016;89(5):1000–15. 10.1016/j.neuron.2016.01.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sanderson JL, Gorski JA, Gibson ES, et al. : AKAP150-anchored calcineurin regulates synaptic plasticity by limiting synaptic incorporation of Ca 2+-permeable AMPA receptors. J Neurosci. 2012;32(43):15036–52. 10.1523/JNEUROSCI.3326-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ghanouni P, Steenhuis JJ, Farrens DL, et al. : Agonist-induced conformational changes in the G-protein-coupling domain of the beta 2 adrenergic receptor. Proc Natl Acad Sci U S A. 2001;98(11):5997–6002. 10.1073/pnas.101126198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Oldham WM, Hamm HE: Heterotrimeric G protein activation by G-protein-coupled receptors. Nat Rev Mol Cell Biol. 2008;9(1):60–71. 10.1038/nrm2299 [DOI] [PubMed] [Google Scholar]
- 60. Husi H, Ward MA, Choudhary JS, et al. : Proteomic analysis of NMDA receptor-adhesion protein signaling complexes. Nat Neurosci. 2000;3(7):661–9. 10.1038/76615 [DOI] [PubMed] [Google Scholar]
- 61. Paoletti P, Bellone C, Zhou Q: NMDA receptor subunit diversity: impact on receptor properties, synaptic plasticity and disease. Nat Rev Neurosci. 2013;14(6):383–400. 10.1038/nrn3504 [DOI] [PubMed] [Google Scholar]
- 62. Ryan TJ, Emes RD, Grant SG, et al. : Evolution of NMDA receptor cytoplasmic interaction domains: implications for organisation of synaptic signalling complexes. BMC Neurosci. 2008;9:6. 10.1186/1471-2202-9-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Choi UB, McCann JJ, Weninger KR, et al. : Beyond the random coil: stochastic conformational switching in intrinsically disordered proteins. Structure. 2011;19(4):566–76. 10.1016/j.str.2011.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Barria A, Malinow R: Subunit-specific NMDA receptor trafficking to synapses. Neuron. 2002;35(2):345–53. 10.1016/S0896-6273(02)00776-6 [DOI] [PubMed] [Google Scholar]
- 65. Yasuda R: Imaging spatiotemporal dynamics of neuronal signaling using fluorescence resonance energy transfer and fluorescence lifetime imaging microscopy. Curr Opin Neurobiol. 2006;16(5):551–61. 10.1016/j.conb.2006.08.012 [DOI] [PubMed] [Google Scholar]
- 66. Selkoe DJ: Alzheimer's disease is a synaptic failure. Science. 2002;298(5594):789–91. 10.1126/science.1074069 [DOI] [PubMed] [Google Scholar]
- 67. Terry RD, Masliah E, Salmon DP, et al. : Physical basis of cognitive alterations in Alzheimer's disease: synapse loss is the major correlate of cognitive impairment. Ann Neurol. 1991;30(4):572–80. 10.1002/ana.410300410 [DOI] [PubMed] [Google Scholar]
- 68. Haass C, Selkoe DJ: Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer's amyloid beta-peptide. Nat Rev Mol Cell Biol. 2007;8(2):101–12. 10.1038/nrm2101 [DOI] [PubMed] [Google Scholar]
- 69. Klein WL, Stine WB, Jr, Teplow DB: Small assemblies of unmodified amyloid beta-protein are the proximate neurotoxin in Alzheimer's disease. Neurobiol Aging. 2004;25(5):569–80. 10.1016/j.neurobiolaging.2004.02.010 [DOI] [PubMed] [Google Scholar]
- 70. Selkoe DJ, Schenk D: Alzheimer's disease: molecular understanding predicts amyloid-based therapeutics. Annu Rev Pharmacol Toxicol. 2003;43:545–84. 10.1146/annurev.pharmtox.43.100901.140248 [DOI] [PubMed] [Google Scholar]
- 71. Hsieh H, Boehm J, Sato C, et al. : AMPAR removal underlies Abeta-induced synaptic depression and dendritic spine loss. Neuron. 2006;52(5):831–43. 10.1016/j.neuron.2006.10.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Roselli F, Hutzler P, Wegerich Y, et al. : Disassembly of shank and homer synaptic clusters is driven by soluble beta-amyloid 1-40 through divergent NMDAR-dependent signalling pathways. PLoS One. 2009;4(6):e6011. 10.1371/journal.pone.0006011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Shankar GM, Bloodgood BL, Townsend M, et al. : Natural oligomers of the Alzheimer amyloid-beta protein induce reversible synapse loss by modulating an NMDA-type glutamate receptor-dependent signaling pathway. J Neurosci. 2007;27(11):2866–75. 10.1523/JNEUROSCI.4970-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Shankar GM, Li S, Mehta TH, et al. : Amyloid-beta protein dimers isolated directly from Alzheimer's brains impair synaptic plasticity and memory. Nat Med. 2008;14(8):837–42. 10.1038/nm1782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Wei W, Nguyen LN, Kessels HW, et al. : Amyloid beta from axons and dendrites reduces local spine number and plasticity. Nat Neurosci. 2010;13(2):190–6. 10.1038/nn.2476 [DOI] [PMC free article] [PubMed] [Google Scholar]