Abstract
While highly active anti-retroviral therapy has greatly improved the lives of HIV-infected individuals, current treatments are unable to completely eradicate the virus. This is due to the presence of HIV latently infected cells which harbor transcriptionally silent HIV. Latent HIV does not replicate or produce viral proteins, thereby preventing efficient targeting by anti-retroviral drugs. Strategies to target the HIV latent reservoir include viral reactivation, enhancing host defense mechanisms, keeping latent HIV silent, and using gene therapy techniques to knock out or reactivate latent HIV. While research into each of these areas has yielded promising results, currently no one mechanism eradicates latent HIV. Instead, combinations of these approaches should be considered for a potential HIV functional cure.
Keywords: HIV, latent HIV, targeting latent HIV, viral reactivation, host defense mechanisms, gene therapy
Introduction
In the twenty years since the implementation of highly active anti-retroviral therapy (HAART), the overall face of HIV as a global health issue has changed 1. HAART—composed of a cocktail of anti-retroviral drugs which target proteins expressed at different steps in the HIV replication cycle—can affect only cells that harbor actively replicating virus. HIV+ individuals are able to live fairly normal lives on maintenance HAART, with minimal side effects. Nevertheless, the effects of HIV infection continue to be evident in these suppressed individuals, who continue to suffer from a number of metabolic, immunologic, and neurologic co-morbidities 2. Thus, despite reducing plasma viremia below detection limits, the virus is not eliminated. There is evidence that low levels of replication occur in suppressed individuals, primarily in tissue reservoirs; however, this is not reflected in systemic plasma viremia in these individuals 3, 4. HAART requires life-long administration. Following even brief treatment interruption, HIV rebounds rapidly from its reservoirs 5– 7. Goals of the present research are to eliminate, suppress permanently, or render cells inhospitable to the hidden HIV in infected individuals.
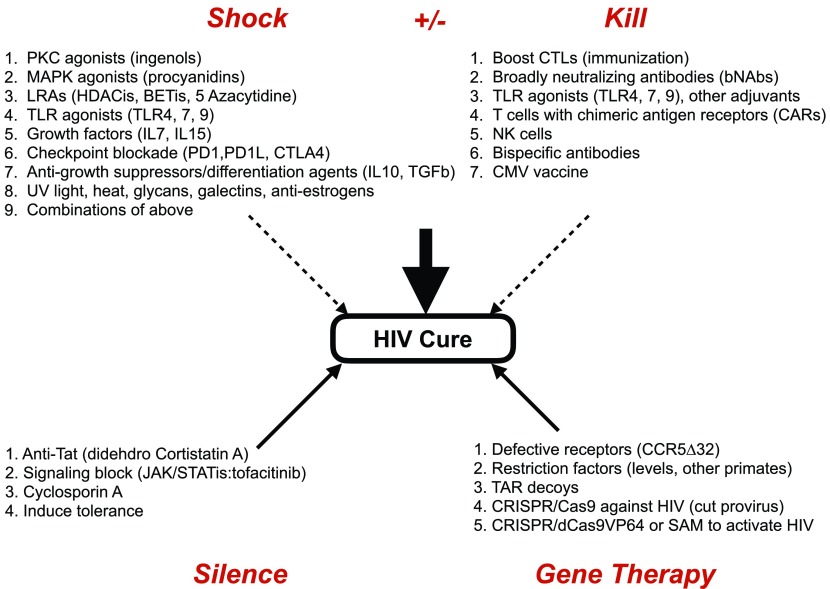
Research efforts to understand and target HIV reservoirs have focused on four main categories outlined in this review ( Figure 1): first, reactivation of latent HIV by capitalizing on the ability of host cellular activation signals and transcription factors (TFs) to ‘shock’ the virus out of hiding; second, killing of reactivated HIV by strengthening the immune system, which has been crippled by the infection; third, keeping latent reservoirs permanently suppressed; and, finally, targeting HIV and CD4+ T cells, which are the primary host cells for the virus, via new gene therapy approaches.
Figure 1. Four main approaches that target the latent reservoir of HIV.
Four research areas, which reactivate HIV (1. shock), eliminate HIV (2. kill), silence HIV (3. silence), or alter the immune system to resist HIV (4. gene therapy) should contribute to the functional or complete cure of HIV in infected individuals. Within each area are individual components of that therapy. They can be applied individually or in combinations, which should decrease their doses and deleterious effects. Most likely, there will be additional approaches in the future.
Shock
Chronic infection by HIV is characterized by severe depletion of CD4+ T cells and continuing inflammation, which contribute to HIV-associated co-morbidities 2. Continued exposure to inflammatory cytokines exhausts the immune system. It also elevates the expression of the receptors programmed death 1 (PD-1) 8 and cytotoxic T-lymphocyte–associated antigen 4 (CTLA-4) 9 on T cells. Blockade of these molecules is used as a treatment for solid tumors 10 and could reinvigorate exhausted T cells in HIV+ patients 11. These individuals also produce elevated levels of inhibitory cytokines interleukin (IL)-10 and transforming growth factor–beta (TGF-β) 12, 13. Indeed, blocking IL-10 results in increased T cell activity in a hepatitis C infection model 14, 15.
Growth factor therapy, including treatment with IL-2, -7, or -15, is being explored as a means to stimulate T cell recovery. IL-2 and IL-7 are important T cell growth and proliferation factors. Infusion with IL-2 and IL-7 results in enhanced T cell production and memory T cell proliferation 16– 18. IL-15 enhances cytotoxic CD8+ T lymphocyte (CTL) and natural killer (NK) cell activity in vitro. Indeed, the IL-15 super-agonist ALT-803 is currently in preclinical trials 19.
Latent HIV is primarily found in resting CD4+ T cells in the periphery. Resting cells have low levels of cellular TFs, which are also required for HIV replication, including NF-κB, P-TEFb, and CDK11 20, 21. Among the first examined latency reversing agents (LRAs) were histone deacetylase inhibitors (HDACis) and BET bromodomain inhibitors (BETis), which induce chromatin stress and induce the release of positive transcription elongation factor b (P-TEFb) from its repressive complex 22. HDACis—such as panobinostat 23, romidepsin 24, SAHA 25, and valproic acid 26—and BETis—such as JQ1 27—all reactivate HIV in cell line models of latency. However, they do not work in human primary resting infected T cells 28, 29 because they contain very low levels of necessary TFs 20, 21. Thus, clinical trials with SAHA resulted in only a modest and transient reactivation of HIV 30, making it an impractical mono-therapy for HIV reactivation.
Since HDACis and BETis do not increase levels of required TFs, some activation of CD4+ T cells is required. Indeed, protein kinase C (PKC) agonists, such as prostratin 31 and bryostatin 32, and the MAPK agonist procyanidin 33, 34 can reactivate HIV in cell line models and primary CD4+ T cells. However, prostratin is toxic at therapeutic levels, leading to muscle pain, respiratory distress, and hypertension. Bryostatin, derived from a marine animal, Bugula neritina, not only has similar side effects but is also cost prohibitive to manufacture. Because of these limitations, a number of synthetic analogues of prostratin and bryostatin with reduced toxicity in vitro are being developed 35– 37. Ingenols, which are purified from Euphorbia plants, represent additional PKC agonists of interest. Native and chemically modified ingenols reactivate HIV in cell lines and primary T cells 38– 40. These PKC agonists also increase cellular levels of necessary TFs 38. Thus, select MAPK and PKC agonists represent attractive candidates to reactivate latent HIV.
Combining several of these approaches has the greatest potential to purge the viral reservoir. Indeed, lower doses of a T cell activator and an LRA (HDACi or BETi) can be administered for increased potency and reduced pro-inflammatory responses 41– 43. Further understanding of HIV integration, transcription, and reactivation, as well as host cell behaviors, will inform optimal combinations of activators and LRAs.
Kill
Strategies to remove HIV by enhancing the killing by CTL and NK cells 44 or via broadly neutralizing antibodies (bNAbs) represent the second major field of research in HIV eradication. It is also important to investigate kill strategies in the context of the aforementioned shock therapies because many of the treatments proposed to reactivate latent HIV also dampen CTL function 45, which is already impaired in HIV+ individuals 11.
Using modified cytomegalovirus (CMV), a live vaccine expressing several simian immunodeficiency virus (SIV) antigens, was found to protect rhesus macaques against viral challenge 46– 48. Vaccinated animals initially appeared to be infected; however, they gained protection against SIV and showed enhanced effector T cell function against viral antigens.
Another approach involves bNAbs 49. Following infection, anti-HIV antibodies are abundant in HIV+ patients; however, owing to the ability of the virus to mutate, the majority of them fail to eliminate the virus. bNAbs are the exception, in that they recognize many clades of HIV as well as escape mutants of the virus. In several studies, they not only neutralized virions released from activated CD4+ T cells from patients 50 but also reduced the viral rebound following HIV reactivation in a humanized mouse model 51. However, even the most potent bNAbs are each only effective against a narrow subset of HIV clinical isolates, suggesting that effective bNAb approaches may require a combination of several bNAbs 52. A second antibody approach utilizes bispecific antibodies, wherein one arm of the Fab portion of the antibody recognizes HIV envelope and the second arm recognizes CD3, making the cell vulnerable to CTL-mediated killing.
Finally, in an effort to achieve more effective killing, chimeric antigen receptors (CARs), which increase T cell receptor avidity and activation, are being explored. They can be engineered to recognize specific viral proteins; CARs against CD19, which is a B cell receptor, led to an astounding 90% remission rate in acute leukemia 53, 54. However, one caveat to CARs is that these cells are long-lived and can have substantial off-target effects.
Silence
The success of HAART has demonstrated that keeping the virus suppressed results in markedly healthier individuals. Resting infected cells do not produce HIV. Thus, these strategies rely on reducing T cell activation, which should also reduce the HIV-associated inflammation found in chronically infected individuals 2. JAK and STAT molecules are important signaling molecules associated with many cytokine receptors. Ruxolitinib and tofacitinib, two JAK inhibitors that are approved for the treatment of rheumatoid arthritis and myelofibrosis, were tested against HIV, HIV2, and simian HIV (SHIV). They inhibited HIV reactivation 55, and, furthermore, ruxolitinib attenuated encephalitis symptoms in infected humanized mice 56. Cyclosporine A, an immunosuppressant used primarily to prevent transplant rejection 57, inhibits T cell proliferation by blocking IL-2 signaling in T cells 58. Infected patients treated with cyclosporine A had some T cell recovery 59 but limited suppression of HIV replication 60, 61.
The inhibitor didehydro-cortistatin A (dCA) acts via a suppressive mechanism that primarily targets HIV transcription. dCA binds to the basic domain in the HIV regulatory protein Tat, inhibits its interactions with the RNA response element TAR, and prevents its activation of HIV transcription 62. dCA inhibits HIV reactivation in cell lines, primary cells, and peripheral blood mononuclear cells (PBMCs) from HAART-suppressed patients 62. Furthermore, dCA may also contribute to continued HIV suppression by inhibiting inflammatory cytokine expression 63.
Gene therapy
Recently, a number of groups have taken advantage of cutting edge gene therapy approaches to HIV cure. However, as with any gene therapy approach, the barriers include delivery, specificity, off-target effects, costs, and ethical concerns.
The single case of successful HIV cure was achieved by the reconstitution of the patient’s immune system with donor bone marrow containing a natural mutation in the CCR5 HIV co-receptor 64. This patient was treated for acute leukemia with several courses of total lymphoid irradiation followed by two separate bone marrow transplantations. Attempts to replicate this therapy used the Zn++ finger nuclease 65 and more recently CRISPR/Cas9 targeting of CCR5 to induce the delta 32 mutation in patients’ own hematopoietic cells 66, 67, which were then returned to the host. Since only mature cells were used, the effects of these manipulated cells were not permanent 65. Recent work using CRISPR/Cas9 to target the second HIV co-receptor, CXCR4, has also yielded promising results 68, 69.
While HIV and SIV are highly related viruses, HIV cannot infect non-human primates, as their restriction factors block HIV infection more effectively than their human counterparts 70. Therefore, altering human restriction factors to behave like their simian counterparts represents an attractive strategy. One such factor is TRIM5. Of special interest is TRIM5 from owl monkeys, which is linked in frame to cyclophilin A, and this fusion protein blocks HIV 71. Using lentiviral vectors to deliver Trim-Cyp has blocked HIV effectively in cell lines and primary T cells 72. Additionally, it has been used successfully in a triple combination anti-HIV lentiviral vector approach in an infected humanized mouse model 73.
Recently, CRISPR/Cas9 technology has emerged as the most versatile and effective gene therapy approach. Using a DNA targeting strategy utilized by bacterial CRISPR, any number of specific guide RNAs can be loaded into the Cas9 protein to target specific areas of DNA for knock out or knock in of genes 74. Similarly, this technology has been used to knock out and reactivate latent HIV. Targeting various regions of the HIV LTR inactivated the virus in infected cell lines 75 and prevented their reinfection 76. However, viral target sequences can mutate, and HIV LTR-specific guide RNA can fail to recognize and target the mutant sequences, preventing long-term eradication by this method 77. To reactivate HIV, a defective Cas9 protein (dCas9) is used, which is fused to four copies of the herpes simplex VP16 activation domain (VP64) or a synergistic activation mediator (SAM) complex. Again, guide RNAs bring these dCas9 activators to the initiated transcription machinery. This targeting results in potent reactivation in latently infected cell lines 78– 80.
Summary
Although HIV infection in the era of HAART has become a manageable chronic infection, problems with adherence to drug regiment, co-morbidities, and the emergence of drug resistance emphasize the need for continued research into HIV cure. Since the barrier to cure is the HIV reservoir, targeting this persistent virus is critical. The approaches detailed in this review represent a spectrum of the current research: however, eliminating the remaining 10 6 to 10 8 latently infected cells 81 will require a combination of approaches. Mechanisms, such as HIV reactivation, will reveal hidden virus. However, the severely crippled immune system and further decreased CTL function indicate that it must be paired with the boosting of anti-viral host defenses. Likewise, keeping latent HIV in a suppressed state could keep HIV+ patients relatively healthy but less able to resist other infections and/or cancer. Using gene therapy to create a parallel immune system, where cells resist HIV infection, could complement all other approaches but is not scalable or affordable in resource-poor countries. While none of these approaches represent the eradication of HIV, combining several treatment modalities could bring us closer to a functional cure, where prolonged HAART-free and disease-free intervals would be achieved in infected patients.
Acknowledgements
We thank Koh Fujinaga and Wei Shao for helpful discussions.
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
Thomas Hope, Department of Cell and Molecular Biology, Northwestern University, Feinberg School of Medicine, Chicago, IL, 60611, USA
Nathaniel Landau, Department of Microbiology, NYU Langone Medical Center, New York, NY, 10016, USA
Greg Towers, Division of Infection and Immunity, University College London, London, WC1E 6BT, UK
Funding Statement
This work was supported by National Institutes of Health grants U19 AI096113 (Martin Delaney Collaboratory of AIDS Researchers for Eradication [CARE] Center Grant, David Margolis, PI), P50 GM082250 (HARC Center Grant, Alan Frankel and Nevan Krogan, coPIs) and AI1049104 (to B.M.P.).
[version 1; referees: 3 approved]
References
- 1. UNAIDS: AIDS by the numbers 2015. Joint United Nations Programme on HIV/AIDS (UNAIDS).2015. Reference Source [Google Scholar]
- 2. Marin B, Thiébaut R, Bucher HC, et al. : Non-AIDS-defining deaths and immunodeficiency in the era of combination antiretroviral therapy. AIDS. 2009;23(13):1743–53. 10.1097/QAD.0b013e32832e9b78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Buzón MJ, Massanella M, Llibre JM, et al. : HIV-1 replication and immune dynamics are affected by raltegravir intensification of HAART-suppressed subjects. Nat Med. 2010;16(4):460–5. 10.1038/nm.2111 [DOI] [PubMed] [Google Scholar]
- 4. Lorenzo-Redondo R, Fryer HR, Bedford T, et al. : Persistent HIV-1 replication maintains the tissue reservoir during therapy. Nature. 2016;530(7588):51–6. 10.1038/nature16933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chun TW, Stuyver L, Mizell SB, et al. : Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. Proc Natl Acad Sci U S A. 1997;94(24):13193–7. 10.1073/pnas.94.24.13193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Finzi D, Hermankova M, Pierson T, et al. : Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science. 1997;278(5341):1295–300. 10.1126/science.278.5341.1295 [DOI] [PubMed] [Google Scholar]
- 7. Wong JK, Hezareh M, Günthard HF, et al. : Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science. 1997;278(5341):1291–5. 10.1126/science.278.5341.1291 [DOI] [PubMed] [Google Scholar]
- 8. Day CL, Kaufmann DE, Kiepiela P, et al. : PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443(7109):350–4. 10.1038/nature05115 [DOI] [PubMed] [Google Scholar]
- 9. Kaufmann DE, Kavanagh DG, Pereyra F, et al. : Upregulation of CTLA-4 by HIV-specific CD4 + T cells correlates with disease progression and defines a reversible immune dysfunction. Nat Immunol. 2007;8(11):1246–54. 10.1038/ni1515 [DOI] [PubMed] [Google Scholar]
- 10. Callahan MK, Wolchok JD: At the bedside: CTLA-4- and PD-1-blocking antibodies in cancer immunotherapy. J Leukoc Biol. 2013;94(1):41–53. 10.1189/jlb.1212631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jones RB, Walker BD: HIV-specific CD8 + T cells and HIV eradication. J Clin Invest. 2016;126(2):455–63. 10.1172/JCI80566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Renga B, Francisci D, D'Amore C, et al. : HIV-1 infection is associated with changes in nuclear receptor transcriptome, pro-inflammatory and lipid profile of monocytes. BMC Infect Dis. 2012;12:274. 10.1186/1471-2334-12-274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yadav A, Collman RG: CNS inflammation and macrophage/microglial biology associated with HIV-1 infection. J Neuroimmune Pharmacol. 2009;4(4):430–47. 10.1007/s11481-009-9174-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Brooks DG, Ha SJ, Elsaesser H, et al. : IL-10 and PD-L1 operate through distinct pathways to suppress T-cell activity during persistent viral infection. Proc Natl Acad Sci U S A. 2008;105(51):20428–33. 10.1073/pnas.0811139106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rigopoulou EI, Abbott WG, Haigh P, et al. : Blocking of interleukin-10 receptor--a novel approach to stimulate T-helper cell type 1 responses to hepatitis C virus. Clin Immunol. 2005;117(1):57–64. 10.1016/j.clim.2005.06.003 [DOI] [PubMed] [Google Scholar]
- 16. Levy Y, Lacabaratz C, Weiss L, et al. : Enhanced T cell recovery in HIV-1-infected adults through IL-7 treatment. J Clin Invest. 2009;119(4):997–1007. 10.1172/JCI38052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Scripture-Adams DD, Brooks DG, Korin YD, et al. : Interleukin-7 induces expression of latent human immunodeficiency virus type 1 with minimal effects on T-cell phenotype. J Virol. 2002;76(24):13077–82. 10.1128/JVI.76.24.13077-13082.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lévy Y, Sereti I, Tambussi G, et al. : Effects of recombinant human interleukin 7 on T-cell recovery and thymic output in HIV-infected patients receiving antiretroviral therapy: results of a phase I/IIa randomized, placebo-controlled, multicenter study. Clin Infect Dis. 2012;55(2):291–300. 10.1093/cid/cis383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Seay K, Church C, Zheng JH, et al. : In Vivo Activation of Human NK Cells by Treatment with an Interleukin-15 Superagonist Potently Inhibits Acute In Vivo HIV-1 Infection in Humanized Mice. J Virol. 2015;89(12):6264–74. 10.1128/JVI.00563-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bartholomeeusen K, Xiang Y, Fujinaga K, et al. : Bromodomain and extra-terminal (BET) bromodomain inhibition activate transcription via transient release of positive transcription elongation factor b (P-TEFb) from 7SK small nuclear ribonucleoprotein. J Biol Chem. 2012;287(43):36609–16. 10.1074/jbc.M112.410746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yu W, Ramakrishnan R, Wang Y, et al. : Cyclin T1-dependent genes in activated CD4 + T and macrophage cell lines appear enriched in HIV-1 co-factors. PLoS One. 2008;3(9):e3146. 10.1371/journal.pone.0003146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bartholomeeusen K, Fujinaga K, Xiang Y, et al. : Histone deacetylase inhibitors (HDACis) that release the positive transcription elongation factor b (P-TEFb) from its inhibitory complex also activate HIV transcription. J Biol Chem. 2013;288(20):14400–7. 10.1074/jbc.M113.464834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rasmussen TA, Schmeltz Søgaard O, Brinkmann C, et al. : Comparison of HDAC inhibitors in clinical development: effect on HIV production in latently infected cells and T-cell activation. Hum Vaccin Immunother. 2013;9(5):993–1001. 10.4161/hv.23800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wei DG, Chiang V, Fyne E, et al. : Histone deacetylase inhibitor romidepsin induces HIV expression in CD4 T cells from patients on suppressive antiretroviral therapy at concentrations achieved by clinical dosing. PLoS Pathog. 2014;10(4):e1004071. 10.1371/journal.ppat.1004071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Contreras X, Schweneker M, Chen CS, et al. : Suberoylanilide hydroxamic acid reactivates HIV from latently infected cells. J Biol Chem. 2009;284(11):6782–9. 10.1074/jbc.M807898200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Routy JP, Tremblay CL, Angel JB, et al. : Valproic acid in association with highly active antiretroviral therapy for reducing systemic HIV-1 reservoirs: results from a multicentre randomized clinical study. HIV Med. 2012;13(5):291–6. 10.1111/j.1468-1293.2011.00975.x [DOI] [PubMed] [Google Scholar]
- 27. Boehm D, Calvanese V, Dar RD, et al. : BET bromodomain-targeting compounds reactivate HIV from latency via a Tat-independent mechanism. Cell Cycle. 2013;12(3):452–62. 10.4161/cc.23309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Spina CA, Anderson J, Archin NM, et al. : An in-depth comparison of latent HIV-1 reactivation in multiple cell model systems and resting CD4+ T cells from aviremic patients. PLoS Pathog. 2013;9(12):e1003834. 10.1371/journal.ppat.1003834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Blazkova J, Chun TW, Belay BW, et al. : Effect of histone deacetylase inhibitors on HIV production in latently infected, resting CD4 + T cells from infected individuals receiving effective antiretroviral therapy. J Infect Dis. 2012;206(5):765–9. 10.1093/infdis/jis412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Archin NM, Liberty AL, Kashuba AD, et al. : Administration of vorinostat disrupts HIV-1 latency in patients on antiretroviral therapy. Nature. 2012;487(7408):482–5. 10.1038/nature11286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Korin YD, Brooks DG, Brown S, et al. : Effects of prostratin on T-cell activation and human immunodeficiency virus latency. J Virol. 2002;76(16):8118–23. 10.1128/JVI.76.16.8118-8123.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pérez M, de Vinuesa AG, Sanchez-Duffhues G, et al. : Bryostatin-1 synergizes with histone deacetylase inhibitors to reactivate HIV-1 from latency. Curr HIV Res. 2010;8(6):418–29. 10.2174/157016210793499312 [DOI] [PubMed] [Google Scholar]
- 33. Hori T, Barnor J, Huu TN, et al. : Procyanidin trimer C1 derived from Theobroma cacao reactivates latent human immunodeficiency virus type 1 provirus. Biochem Biophys Res Commun. 2015;459(2):288–93. 10.1016/j.bbrc.2015.02.102 [DOI] [PubMed] [Google Scholar]
- 34. Wang C, Yang S, Lu H, et al. : A Natural Product from Polygonum cuspidatum Sieb. Et Zucc. Promotes Tat-Dependent HIV Latency Reversal through Triggering P-TEFb's Release from 7SK snRNP. PLoS One. 2015;10(11):e0142739. 10.1371/journal.pone.0142739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Beans EJ, Fournogerakis D, Gauntlett C, et al. : Highly potent, synthetically accessible prostratin analogs induce latent HIV expression in vitro and ex vivo. Proc Natl Acad Sci U S A. 2013;110(29):11698–703. 10.1073/pnas.1302634110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. DeChristopher BA, Loy BA, Marsden MD, et al. : Designed, synthetically accessible bryostatin analogues potently induce activation of latent HIV reservoirs in vitro. Nat Chem. 2012;4(9):705–10. 10.1038/nchem.1395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wender PA, Nakagawa Y, Near KE, et al. : Computer-guided design, synthesis, and protein kinase C affinity of a new salicylate-based class of bryostatin analogs. Org Lett. 2014;16(19):5136–9. 10.1021/ol502491f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pandeló José D, Bartholomeeusen K, da Cunha RD, et al. : Reactivation of latent HIV-1 by new semi-synthetic ingenol esters. Virology. 2014;462-463:328–39. 10.1016/j.virol.2014.05.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jiang G, Mendes EA, Kaiser P, et al. : Reactivation of HIV latency by a newly modified Ingenol derivative via protein kinase Cδ-NF-κB signaling. AIDS. 2014;28(11):1555–66. 10.1097/QAD.0000000000000289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Abreu CM, Price SL, Shirk EN, et al. : Dual role of novel ingenol derivatives from Euphorbia tirucalli in HIV replication: inhibition of de novo infection and activation of viral LTR. PLoS One. 2014;9(5):e97257. 10.1371/journal.pone.0097257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jiang G, Mendes EA, Kaiser P, et al. : Synergistic Reactivation of Latent HIV Expression by Ingenol-3-Angelate, PEP005, Targeted NF-kB Signaling in Combination with JQ1 Induced p-TEFb Activation. PLoS Pathog. 2015;11(7):e1005066. 10.1371/journal.ppat.1005066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Darcis G, Kula A, Bouchat S, et al. : An In-Depth Comparison of Latency-Reversing Agent Combinations in Various In Vitro and Ex Vivo HIV-1 Latency Models Identified Bryostatin-1+JQ1 and Ingenol-B+JQ1 to Potently Reactivate Viral Gene Expression. PLoS Pathog. 2015;11(7):e1005063. 10.1371/journal.ppat.1005063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Laird GM, Bullen CK, Rosenbloom DI, et al. : Ex vivo analysis identifies effective HIV-1 latency-reversing drug combinations. J Clin Invest. 2015;125(5):1901–12. 10.1172/JCI80142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Scully E, Alter G: NK Cells in HIV Disease. Curr HIV/AIDS Rep. 2016;13(2):85–94. 10.1007/s11904-016-0310-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jones RB, O'Connor R, Mueller S, et al. : Histone deacetylase inhibitors impair the elimination of HIV-infected cells by cytotoxic T-lymphocytes. PLoS Pathog. 2014;10(8):e1004287. 10.1371/journal.ppat.1004287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cicin-Sain L, Sylwester AW, Hagen SI, et al. : Cytomegalovirus-specific T cell immunity is maintained in immunosenescent rhesus macaques. J Immunol. 2011;187(4):1722–32. 10.4049/jimmunol.1100560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hansen SG, Ford JC, Lewis MS, et al. : Profound early control of highly pathogenic SIV by an effector memory T-cell vaccine. Nature. 2011;473(7348):523–7. 10.1038/nature10003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hansen SG, Sacha JB, Hughes CM, et al. : Cytomegalovirus vectors violate CD8 + T cell epitope recognition paradigms. Science. 2013;340(6135): 1237874. 10.1126/science.1237874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Halper-Stromberg A, Nussenzweig MC: Towards HIV-1 remission: potential roles for broadly neutralizing antibodies. J Clin Invest. 2016;126(2):415–23. 10.1172/JCI80561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Chun TW, Murray D, Justement JS, et al. : Broadly neutralizing antibodies suppress HIV in the persistent viral reservoir. Proc Natl Acad Sci U S A. 2014;111(36):13151–6. 10.1073/pnas.1414148111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Halper-Stromberg A, Lu CL, Klein F, et al. : Broadly neutralizing antibodies and viral inducers decrease rebound from HIV-1 latent reservoirs in humanized mice. Cell. 2014;158(5):989–99. 10.1016/j.cell.2014.07.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bruel T, Guivel-Benhassine F, Amraoui S, et al. : Elimination of HIV-1-infected cells by broadly neutralizing antibodies. Nat Commun. 2016;7: 10844. 10.1038/ncomms10844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Grupp SA, Kalos M, Barrett D, et al. : Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med. 2013;368(16):1509–18. 10.1056/NEJMoa1215134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Maude SL, Frey N, Shaw PA, et al. : Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371(16):1507–17. 10.1056/NEJMoa1407222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Gavegnano C, Detorio M, Montero C, et al. : Ruxolitinib and tofacitinib are potent and selective inhibitors of HIV-1 replication and virus reactivation in vitro. Antimicrob Agents Chemother. 2014;58(4):1977–86. 10.1128/AAC.02496-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Haile WB, Gavegnano C, Tao S, et al. : The Janus kinase inhibitor ruxolitinib reduces HIV replication in human macrophages and ameliorates HIV encephalitis in a murine model. Neurobiol Dis. 2016; pii: S0969-9961(16)30028-6. 10.1016/j.nbd.2016.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Starzl TE, Weil R, 3rd, Iwatsuki S, et al. : The use of cyclosporin A and prednisone in cadaver kidney transplantation. Surg Gynecol Obstet. 1980;151(1):17–26. [PMC free article] [PubMed] [Google Scholar]
- 58. Bunjes D, Hardt C, Röllinghoff M, et al. : Cyclosporin A mediates immunosuppression of primary cytotoxic T cell responses by impairing the release of interleukin 1 and interleukin 2. Eur J Immunol. 1981;11(8):657–61. 10.1002/eji.1830110812 [DOI] [PubMed] [Google Scholar]
- 59. Andrieu JM, Even P, Venet A, et al. : Effects of cyclosporin on T-cell subsets in human immunodeficiency virus disease. Clin Immunol Immunopathol. 1988;47(2):181–98. 10.1016/0090-1229(88)90071-2 [DOI] [PubMed] [Google Scholar]
- 60. Markowitz M, Vaida F, Hare CB, et al. : The virologic and immunologic effects of cyclosporine as an adjunct to antiretroviral therapy in patients treated during acute and early HIV-1 infection. J Infect Dis. 2010;201(9):1298–302. 10.1086/651664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Rizzardi GP, Harari A, Capiluppi B, et al. : Treatment of primary HIV-1 infection with cyclosporin A coupled with highly active antiretroviral therapy. J Clin Invest. 2002;109(5):681–8. 10.1172/JCI14522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Mousseau G, Kessing CF, Fromentin R, et al. : The Tat Inhibitor Didehydro-Cortistatin A Prevents HIV-1 Reactivation from Latency. MBio. 2015;6(4):e00465. 10.1128/mBio.00465-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Mediouni S, Jablonski J, Paris JJ, et al. : Didehydro-cortistatin A inhibits HIV-1 Tat mediated neuroinflammation and prevents potentiation of cocaine reward in Tat transgenic mice. Curr HIV Res. 2015;13(1):64–79. 10.2174/1570162X13666150121111548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hütter G, Nowak D, Mossner M, et al. : Long-term control of HIV by CCR5 Delta32/Delta32 stem-cell transplantation. N Engl J Med. 2009;360(7):692–8. 10.1056/NEJMoa0802905 [DOI] [PubMed] [Google Scholar]
- 65. Tebas P, Stein D, Tang WW, et al. : Gene editing of CCR5 in autologous CD4 T cells of persons infected with HIV. N Engl J Med. 2014;370(10):901–10. 10.1056/NEJMoa1300662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ye L, Wang J, Beyer AI, et al. : Seamless modification of wild-type induced pluripotent stem cells to the natural CCR5Δ32 mutation confers resistance to HIV infection. Proc Natl Acad Sci U S A. 2014;111(26):9591–6. 10.1073/pnas.1407473111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Wang W, Ye C, Liu J, et al. : CCR5 gene disruption via lentiviral vectors expressing Cas9 and single guided RNA renders cells resistant to HIV-1 infection. PLoS One. 2014;9(12):e115987. 10.1371/journal.pone.0115987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Schumann K, Lin S, Boyer E, et al. : Generation of knock-in primary human T cells using Cas9 ribonucleoproteins. Proc Natl Acad Sci U S A. 2015;112(33):10437–42. 10.1073/pnas.1512503112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Hou P, Chen S, Wang S, et al. : Genome editing of CXCR4 by CRISPR/cas9 confers cells resistant to HIV-1 infection. Sci Rep. 2015;5: 15577. 10.1038/srep15577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Chan E, Towers GJ, Qasim W: Gene therapy strategies to exploit TRIM derived restriction factors against HIV-1. Viruses. 2014;6(1):243–63. 10.3390/v6010243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Carthagena L, Parise MC, Ringeard M, et al. : Implication of TRIM alpha and TRIMCyp in interferon-induced anti-retroviral restriction activities. Retrovirology. 2008;5:59. 10.1186/1742-4690-5-59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Neagu MR, Ziegler P, Pertel T, et al. : Potent inhibition of HIV-1 by TRIM5-cyclophilin fusion proteins engineered from human components. J Clin Invest. 2009;119(10):3035–47. 10.1172/JCI39354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Walker JE, Chen RX, McGee J, et al. : Generation of an HIV-1-resistant immune system with CD34 + hematopoietic stem cells transduced with a triple-combination anti-HIV lentiviral vector. J Virol. 2012;86(10):5719–29. 10.1128/JVI.06300-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Wright AV, Nuñez JK, Doudna JA: Biology and Applications of CRISPR Systems: Harnessing Nature's Toolbox for Genome Engineering. Cell. 2016;164(1–2):29–44. 10.1016/j.cell.2015.12.035 [DOI] [PubMed] [Google Scholar]
- 75. Ebina H, Misawa N, Kanemura Y, et al. : Harnessing the CRISPR/Cas9 system to disrupt latent HIV-1 provirus. Sci Rep. 2013;3: 2510. 10.1038/srep02510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Hu W, Kaminski R, Yang F, et al. : RNA-directed gene editing specifically eradicates latent and prevents new HIV-1 infection. Proc Natl Acad Sci U S A. 2014;111(31):11461–6. 10.1073/pnas.1405186111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Wang G, Zhao N, Berkhout B, et al. : CRISPR-Cas9 Can Inhibit HIV-1 Replication but NHEJ Repair Facilitates Virus Escape. Mol Ther. 2016;24(3):522–6. 10.1038/mt.2016.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Zhang Y, Yin C, Zhang T, et al. : CRISPR/gRNA-directed synergistic activation mediator (SAM) induces specific, persistent and robust reactivation of the HIV-1 latent reservoirs. Sci Rep. 2015;5: 16277. 10.1038/srep16277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Saayman SM, Lazar DC, Scott TA, et al. : Potent and Targeted Activation of Latent HIV-1 Using the CRISPR/dCas9 Activator Complex. Mol Ther. 2016;24(3):488–98. 10.1038/mt.2015.202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Limsirichai P, Gaj T, Schaffer DV: CRISPR-mediated Activation of Latent HIV-1 Expression. Mol Ther. 2016;24(3):499–507. 10.1038/mt.2015.213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Massanella M, Richman DD: Measuring the latent reservoir in vivo. J Clin Invest. 2016;126(2):464–72. 10.1172/JCI80567 [DOI] [PMC free article] [PubMed] [Google Scholar]