Abstract
Incorporation of all-trans retinoic acid (ATRA) and arsenic trioxide (ATO) into the management paradigms of acute promyelocytic leukemia (APL) has markedly improved outcomes. Significant progress occurred in understanding the molecular pathogenesis of APL. ATO, in contrast with ATRA, is capable of eradicating the APL-initiating cells and can result in cure. Preclinical and clinical data confirmed the synergy of ATO and ATRA, and the ATRA–ATO combination was proved noninferior to a standard ATRA–chemotherapy regimen in patients with non–high-risk APL. Oral formulations of arsenic exhibited excellent activity in advanced clinical testing and their combinations with ATRA offer an opportunity for a completely oral, chemotherapy-free regimen for curing APL. Nonetheless, significant challenges remain. Reducing early death due to bleeding complications is an important area of unmet need. Data suggest that delays in initiation of ATRA upon suspecting APL continue to occur in the community and contribute to early mortality. Questions remain about the optimal place and schedule of arsenic in the therapeutic sequence and the role of the oral formulations. Refining the role of minimal residual disease in directing treatment decisions is important. Development of novel targeted agents to treat relapsed disease requires deeper understanding of the secondary resistance mechanisms to ATRA and ATO.
Background
At one time a highly lethal malignancy, significant progress has transformed acute promyelocytic leukemia (APL) into the most curable form of acute myelogenous leukemia (1). APL was one of the first leukemias linked to a recurrent mechanistically important genetic translocation, and remains the only type of leukemia in which oncoprotein-targeting therapy cures patients (2, 3). Approximately 98% of patients have a reciprocal translocation, involving the retinoic acid receptor-alpha gene (RARA) on chromosome 17 with the promyelocytic leukemia gene (PML) on chromosome 15 resulting in creation of the chimeric oncogene t (15;17)(q24.1;q21.1) PML–RARA (2, 4). A few patients have translocations involving RARA with other partner genes, the most common of which are t(11;17)(q23; q21.1)PLZF/RARA, t(5;17)(q35;q21.1)NPM/RARA, and t(11;17)(q13;q21.1)NuMA/RARA (5–7).
RARA is a nuclear hormone receptor that heterodimerizes with retinoid X receptor to recruit transcription corepressor complexes (CoC) and induces gene transcription repression, except when bound to ligand (4, 8). In APL, PML–RARA proteins recruit CoCs, leading to sustained transcriptional inhibition, blocking myeloid differentiation in a negative dominant fashion, and increasing self-renewal of leukemic progenitor cells (Fig. 1; refs. 8, 9). PML–RARA also exerts negative dominant effects on PML, which also contribute to the pathogenesis of APL (10). At pharmacologic doses, all-trans retinoic acid (ATRA) binds to the RARA moiety of PML–RARA, leading to dissociation of the CoCs and recruitment of transcription coactivators (8, 9). This results in epigenetic reprogramming with transcriptional derepression and eventually into terminal myeloid differentiation (11).
Figure 1.
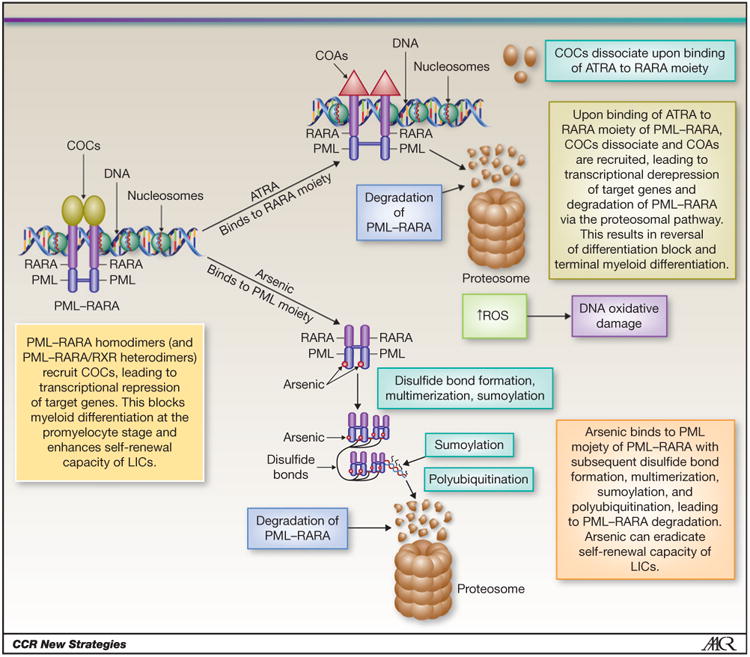
Mechanisms of action of ATRA and arsenic in APL. COA, coactivator complex; LIC, leukemia-initiating cells; ROS, reactive oxygen species. Modified from Tomita et al. (4). This figure is reused by the courtesy of the International Journal of Hematology.
A high index of suspicion for APL is essentially based on morphologic (promyelocytic blasts in the peripheral blood smear) and clinical features (e.g., coagulopathy and pancytopenia). Suspected APL is a medical emergency, and prompt initiation of ATRA and aggressive transfusion support should be implemented without waiting for pathologic and/or genetic conformation (12). The microgranular variant of APL presents a particular challenge for early diagnosis and treatment (13). These monoblast-like cells often have few or no granules visible by Wright–Giemsa staining; a high index of suspicion is required when evaluating patients with apparent monoblastic leukemia and coagulopathy (14). Unlike most monoblastic leukemias, the micro-granular variant often has bilobed nuclei (“wasp-waisted”; ref. 14). Flow cytometric analysis can support the diagnosis of APL; definitive diagnosis requires documentation of a pathognomic translocation using reverse transcriptase PCR (RT-PCR), FISH, or karyotyping(12). A sensitive and highly specific immunocytochemical labeling assay uses an anti-PML monoclonal antibody to establish the diagnosis within hours (15). Administration of granulocyte growth factors in cases of unexplained neutropenia before excluding APL is contraindicated because administration can lead to clinical deterioration (16).
Pre-arsenic treatment paradigms for APL included an induction phase using ATRA, an anthracycline ± cytarabine with a complete remission (CR) occurring in 86% to 95% of patients (17–21). Once CR is achieved, consolidation therapy usually combines ATRA with anthracyclines aiming at inducing a molecular CR (mCR; refs. 22, 23). The need for maintenance therapy with 1 to 2 years of ATRA 6-mercaptopurine (6-MP) and methotrexate (MTX) remains controversial (24). The long-term survival rate with this treatment paradigm has been estimated at 69% to 84% (18, 22–25).
Early mortality in APL (mostly due to bleeding complications) has been particularly difficult to improve (20, 26). Registry and retrospective studies suggest an even higher rate of early mortality than observed in clinical trials (15%–25%), because many deaths occur before the diagnosis is made premortem, enrollment in clinical trials, or even receiving ATRA therapy (27–29). Despite the recommendations to initiate ATRA as soon as the diagnosis is suspected, a retrospective study showed that ATRA was started on the day APL was suspected in only 31% of patients (28). A SEER analysis that spanned the era from 1974 to 2008 did not show a significant reduction in early death despite a significant prolongation in overall survival (OS), suggesting that the survival advantage was mostly due to reduced relapse rather than reduction in early death (30).
Arsenic trioxide (ATO) has been shown to be the most active single agent in APL (31–33). Two prospective studies of ATO monotherapy for newly diagnosed APL reported 5-year event-free survival (EFS), disease-free survival (DFS), and OS rates of 69%, 80% and 67%, and 74% and 64%, respectively (31, 34). ATO-associated toxicities, mainly cytopenias, liver enzyme abnormalities, QTc prolongation, and differentiation syndrome, were generally manageable and reversible (31, 34, 35). Arsenic directly binds to the PML moiety and exhibits dose-dependent dual effects with preferential a proapoptotic effect at higher concentrations and partial differentiation at lower concentrations (Fig. 1; refs. 36, 37). In contrast with ATO, which is associated with high rates of mCR and potential for cure, mCR with ATRA monotherapy is rare and all patients relapse within months if not treated with additional agents, presumably due to the persistence of ATRA-resistant cells (38, 39). Arsenic abolishes the aberrant stem cell capacity of PML–RARA-positive leukemia-initiating cells (LIC), which explains the cure potential of the drug (40). The highly synergistic non–cross-resistant pathways of degradation of the PML–RARA oncoproteins activated by ATO and ATRA probably explain the greatly enhanced LIC clearance with the combination (41, 42).
On the Horizon
A completely oral, chemotherapy-free combination regimen for newly diagnosed APL?
Clinical trials that used both arsenic and ATRA in the upfront management of APL
Given the high cure rates in APL, many patients, especially those with low-risk disease, are potentially overtreated. Therefore, an expanding focus of research relates to reducing chemotherapy exposure without comprising outcomes by incorporating non-chemotherapeutic agents into risk-adapted management approaches. The distinct but complementary mechanisms of action of ATO and ATRA provided a biologic rationale for combining the two agents to achieve synergistic efficacy with low toxicity (43). Although ATRA functions mainly through transcriptional modulation, the main effects of arsenic occur at the proteomic level, therefore, explaining the lack of cross-resistance between the two agents (37, 44). ATRA also causes upregulation of the gene encoding for the transmembrane protein aquaglyceroporin 9, which leads to increased arsenic uptake into the APL cells (45).
Trials that used combination ATRA-ATO in upfront therapy for APL are shown in Table 1. The first trial that evaluated ATO–ATRA used this combination for induction but used a chemotherapy-based consolidation (46). Patients randomized to ATRA–ATO combination instead of single-agent ATRA or ATO had a shorter time to CR, a greater reduction in PML–RARA transcript levels, and a lower probability of relapse (46); 5-year EFS and OS in the combination arm were 89% and 92%, respectively (38). Researchers from the University of Texas MD Anderson Cancer Center (Houston, TX) reported 3-year OS and DFS of 85% and 81%, respectively in patients treated with a chemotherapy-free combination approach using ATO and ATRA for induction and consolidation [with the addition of gemtuzumab ozogamicin (GO) for high-risk patients] (47, 48).
Table 1. A summary of prospective trials that used arsenic along with other agents (not as single agent) in the upfront management of patients with newly diagnosed APL.
| Risk groupa (# patients) | ||||||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| Study, type of study, # of patients (ref.) | Follow-up, median (range) in months | Age, median (range) in years | High | Non-high | Induction (CR%) | Consolidation | Maintenance | Survival |
| Induction consolidation maintenance in combination with other agents | ||||||||
|
| ||||||||
| Shen et al., randomized, 61, (46) | 18 (8–30) | 30.5–39.5 (14–74) | 14 | 47 | Randomized to:
|
Three courses of sequential DA-cytarabine “pulse” regimen-HA | Five cycles of either:
|
Median DFS (mo):
|
|
| ||||||||
| Estey et al., single-arm, 44, (47) | 16 (N/A) | 45 | 19 | 25 | ATRA+ATO (+GO for high-risk; 89%) | Four courses of ATRA–ATO (28 weeks) | No additional therapy | 2-year OS: 86% 2-year DFS: 86% |
|
| ||||||||
| Ravandi et al., single-arm, 82, (48) | 23 (0.47–65.6) | 47 (14–81) | 26 | 56 | ATRA+ATO (+GO for high-risk; 92%) | Four courses of ATRA–ATO (28 weeks) | No additional therapy | 3-year OS: 85% 3-year DFS: 81 % |
|
| ||||||||
| Hu et al., single-arm, 85, (38) | 70 (21–88) | N/A(<15–>55) | 19 | 66 | ATRA+ATO (+hydroxyurea for high-risk; 94.1%) | Three courses of sequential DA-cytarabine “pulse” regimen-HA | Five cycles of either:
|
5-year OS: 91.7% 5-year EFS: 89.2% 5-year DFS: 94.8% |
|
| ||||||||
| Dai et al., nonrandomized, two-arm, 162, (69) | 30–32 (1–59) | 32–34 (14–69) | 39 | 123 | Group A (72 patients): ATRA (90.3%) Group B (90 patients): ATRA+ATO (93.3%, P = 0.57) |
Group A: A2: Five cycles of ATRA+DA+HA; A1: Four cycles of ATRA+DA+ HA+ one cycle of ATO Group B: Four cycles of ATRA+DA+HA+ one cycle of ATO |
Group A: A2: 2 years of ATRA+DA+HA; A1: 2 years of ATRA+ATO+DA Group B: 2 years of ATRA+ATO+DA |
3-year DFS:
|
|
| ||||||||
| Iland et al., single-arm, 124, (51) | 24 (2–61) | 44 (3–78) | 24 | 99 | ATRA+ ATO+lda (95%) | Two courses of ATO+ATRA | 2 years (eight cycles) of ATRA, 6-MP, and MTX | 2-year OS: 93.2% 2-year EFS: 88.1% 2-year DFS: 98% |
|
| ||||||||
| Lo-Coco et al., randomized noninferiority, 156, (43) | 34.4 (0.5–55.8) | 44.6–46.6 (18.7–70.2) | 0 | 156 | Randomized (1:1) to:
|
For ATRA–ATO group: four courses of ATRA–ATO (28 weeks) For ATRA-lda group: three sequential cycles of ATRA-lda, ATRA-Mitroxantrone, ATRA-lda. |
For ATRA-ATO: No additional therapy For ATRA-lda group: 2 years of ATRA, 6-MP, and MTX |
2-year EFS:
|
|
| ||||||||
| Zhu et al., randomized noninferiority, 242, (56) | 39 (21–64) | 36 (15–60) | 46 | 185 | Randomized (1:1) to: Oral RIF (an AS4S4-containing formula) + ATRA(99.1%) ntravenous ATO+ATRA (97.4%, P = 0.62) | Both groups received three sequential cycles of HA, MA, and DA. | For RIF+ATRA group: 2 years of RIF-ATRA For ATO+ATRA group: 2 years of ATO-ATRA |
2-year DFS:
|
|
| ||||||||
| Consolidation | ||||||||
|
| ||||||||
| Gore et al., single-arm, 45, (49) | 32.4 (18–66) | 50 (19–70) | 14 | 31 | ATRA+daunorubicin | One course of cytarabine + daunorubicin+ATO | High-risk: 2 years of ATRA, 6-MP, and MTX Low-risk: 2-year (ATRA) | 3-year OS: 88% 3-year DFS: 88.7% 3-year EFS: 76% |
|
| ||||||||
| Powell et al., randomized, 481, (21) | 28.8 (N/A) | N/A (15–79) | 113 | 368 | ATRA+ cytarabine+ D aunorubicin (90%) | Randomized (1:1) to: 2 courses of ATRA+daunorubicin Two courses of ATO followed by two courses of ATRA+ Daunorubicin | Randomized to one year of:
|
3-year EFS:
|
|
| ||||||||
| Maintenance therapy | ||||||||
|
| ||||||||
| Au et al., single-arm, 76, (54) | 24 (1–115) | 44 (16–83) | 10 | 66 | For patients <65 years old: ATRA+ 7 and three regimen (daunorubicin and cytarabine)b For older patients: ATRA-ATOb |
For patients <65 years old: two cycles of daunorubicin and cytarabineb For older patients: no consolidation, went directly to maintenanceb |
One of 3 regimens (evolved over course of study):
|
3-year OS: 90.6% 3-year DFS: 87.7% 3-year EFS: 83.7% (type of maintenance did not affect survival rates) |
Abbreviations: DA, daunorubicin + cytarabine; HA, homoharringtonine + cytarabine; Ida, Idarubicin; N/A, not available; MA, mitoxantrone + cytarabine; RIF, Realgar-Indigo naturalis (an AS4S4-containing formulation).
Risk grouping according to Sanz et al. (70).
All patients were required to be in CR after induction and consolidation at registration for the trial.
A randomized noninferiority trial conducted by Lo-Coco and colleagues (43) compared ATRA-ATO combination with ATRA–idarubicin for induction and consolidation therapy for newly diagnosed patients with non-high-risk APL (white blood cell count at presentation ≤ 10 × 109/L). The ATO–ATRA combination was associated with significantly better 2-year EFS and OS rates (97% and 99%) than those for the ATRA-chemotherapy arm (86% and 91%, respectively; ref. 43). Follow-up was relatively short compared with the long experience with chemotherapy–ATRA regimens. Importantly, this approach was not assessed in patients with high-risk APL in this study. It is hoped that the anticipated results of another randomized trial of ATRA + ATO versus ATRA + chemotherapy from the U.K. National Cancer Research Institute will address this question, as high-risk patients are included in this study (32). ATO-based consolidation after ATRA–chemotherapy induction have not been compared with ATO-ATRA induction and consolidation; therefore, the differential benefit of using ATO in induction, consolidation, or both phases remains unclear. Finally, the consolidation phase with ATO–ATRA combination in this trial lasted significantly longer (28 weeks of intensive therapy) than the chemotherapy-based consolidation, and the value of maintenance therapy was not assessed in the ATO–ATRA arm.
The addition of ATO to ATRA–anthracycline consolidation platforms has also been studied. The large randomized trial C9710 added two cycles of ATO to ATRA + daunorubicin consolidation and reported a 3-year EFS of 80% compared with 63% among those who received only ATRA + daunorubicin consolidation (P < 0.0001; ref. 21). A phase II trial used a single cycle of ATO-based consolidation chemotherapy for upfront management and showed 2.7-year DFS and OS rates of 90% and 88%, respectively (49). An updated analysis of this trial of 63 patients that also included consecutive patients treated on-protocol but off-study showed 5-year EFS, OS, and DFS rates of 89%, 93%, and 92%, respectively (50). Another trial also reduced anthracycline exposure by incorporating ATO into induction with ATRA–idarubicin followed by two cycles of chemotherapy-free ATRA–ATO consolidation and ATRA-based maintenance (51).
Combinations of oral arsenic formulations with ATRA in upfront management of APL
The intravenous route of administration of ATO is inconvenient, involves frequent patient visits for administration and possibly hospitalization, and requires maintenance of vascular access. These limitations pose important obstacles that might prevent patients from receiving the most active drug for APL, especially for prolonged consolidation therapy. An oral formulation of ATO has been found to be active in relapsed APL (52, 53), and has subsequently been evaluated in upfront management. One study of 76 patients used 2 years of maintenance therapy with oral ATO–ATRA after ATRA–chemotherapy induction and consolidation (54). The 3-year DFS, EFS, and OS rates were 88%, 84%, and 91%, respectively, despite the omission of 6-MP and MTX from maintenance. In contrast with intravenous ATO, QTc interval prolongation and ventricular arrhythmias were not observed with the oral formulation, probably due to the lower peak plasma arsenic concentrations achieved with oral ATO therapy (54, 55).
Another formulation of arsenic in advanced clinical investigation is tetra-arsenic tetra-sulfide [AS4S4]. A total of 242 newly diagnosed non–high-risk patients were randomized to an AS4S4-containing oral formulation or to intravenous ATO for induction and maintenance (56). All patients received ATRA during induction, three cycles of consolidation chemotherapy, and maintenance with sequential ATRA followed by either AS4S4 or ATO for 2 years. The 2-year DFS (98.1% vs. 95.5%) and the 3-year OS rates (99.1% vs. 96.6%) were not inferior for the AS4S4 group compared with the ATO group (56). No severe long-term toxicity or increased secondary malignancy rates were observed in either trial. These two trials establish the feasibility and safety of prolonged oral arsenic administration but long-term safety data are still required. Oral arsenic formulations are not currently available in the United States or Europe.
Importance of minimal residual disease in APL
In APL, early detection of minimal residual disease (MRD) has been shown to predict relapse effectively (57–59). Rigorous serial monitoring of MRD is usually achieved with serial RT-PCR or quantitative RT-PCR assays to detect leukemia-specific transcripts in peripheral blood or bone marrow samples, a surveillance approach that has become a recommended standard of care (24, 58, 60). In one large analysis of 406 patients who received ATRA– chemotherapy regimens, the majority of relapsed patients were successfully identified with serial MRD monitoring, which was the strongest predictor of relapse-free survival (RFS) in multivariable analysis (HR, 17.87; 95% confidence interval, 6.88–46.41; P < 0.0001; ref. 58). In another analysis of 151 patients with APL treated with single-agent ATO, serial MRD monitoring successfully predicted relapse in 60% of cases with an overall sensitivity and specificity of 60% and 93.2%, respectively (59). It should be noted that a positive RT-PCR at the end of induction is a common finding when ATRA–chemotherapy regimens are used and does not predict relapse, whereas a positive RT-PCR at the end of the first consolidation course in these patients is a predictor of subsequent relapse (57–59). In contrast, a positive RT-PCR at the end of initial induction therapy in patients treated with upfront single-agent ATO seemed to correlate best with subsequent relapse (59).
More importantly, aggressive preemptive therapy at the time of molecular relapse has been associated with significantly better survival outcomes when compared with treatment at time of florid hematologic relapse (58, 59, 61, 62). Grimwade and colleagues (58) found that early intervention with ATO salvage therapy at the time of molecular relapse prevented progression to florid relapse in most patients, with a 1-year RFS rate of 73% among those with molecular relapse. In another study that compared 16 patients who were salvaged at the time of molecular relapse with 36 patients salvaged at the time of hematologic relapse, the patients re-treated at the time of molecular relapse had higher 5-year OS rates (64% vs. 24%, P = 0.01) and lower 5-year relapse rates (30% vs. 64%, P = 0.044), respectively (62). The National Comprehensive Cancer Network guidelines currently recommend a bone marrow sample at the end of consolidation therapy to document negativity for MRD (24). If there is persistence of MRD or subsequent reappearance of MRD during serial monitoring, then the patient should be treated for relapsed disease (after confirmation of the PCR positivity within 2–4 weeks; ref. 24). The utility of MRD monitoring in ATO-treated patients is unclear because so few patients have relapses (63).
Elucidation of the mechanisms of resistance to ATRA and ATO and their management
Primary resistance to ATRA or ATO is very rare except for some forms of APL in which the RARA gene partners with other genes aside from PML [e.g., t(11;17) PLZF–RARA; refs. 5, 8]. Secondary resistance to ATRA, on the other hand, is more common. Genetic mutations in the ligand-binding domain of the RARA moiety that interfere with ATRA binding are believed to be the most common cause of resistance to ATRA in APL (64). In addition, aberrant CoCs that cannot be dissociated with ATRA therapy can contribute to resistance (11).
Secondary resistance to ATO is rare and poorly understood. Resistance-inducing genetic mutations in the PML moiety of the PML–RARA oncogene have been described, and some subclones carry these mutations in addition to ATRA resistance-inducing mutations (11, 65). In addition, posttreatment acquisition of secondary chromosomal aberrations and FLT3 mutations has been implicated in the resistance and relapse of APL (66). Most patients who have relapses after chemotherapy and/or ATRA respond to salvage therapy with ATO. Histone deacetylase and histone demethylase (e.g., PHF8) inhibitors can potentially overcome resistance in APL by inducing derepression of transcription (11). GO, an anti-CD33–calicheamicin conjugate, has also been evaluated in this setting (67, 68). A better understanding of the mechanisms underlying resistance to ATRA and ATO will be important for the development of newer targeted agents to salvage relapsed patients.
Final Remarks
Although evidence strongly supports the incorporation of arsenic in the management of APL, questions remain with regard to its appropriate place in the therapeutic sequence, the optimal schedule, and the role of the oral formulations. Longer follow-up is required to assess this treatment approach for long-term toxicity and risk of relapse. Nonetheless, the prospect of a completely oral, chemotherapy-free combination of arsenic and ATRA for some patients with newly diagnosed APL, potentially allowing outpatient management for at least part of the course, is possible in the near future. Oral arsenic compounds are still not available commercially in the United States despite the positive randomized data, and this lack of access potentially limits the utility of the most active drug for APL to those patients willing to receive several months of daily intravenous therapy.
Many other questions remain unanswered in APL such as the role of maintenance therapy, its duration, and the specific drugs to be used. As outcomes of patients with APL continue to improve, the issue of central nervous system relapse is becoming more problematic, and, therefore, more data about intrathecal prophylaxis are required (12). Refining the role of monitoring of MRD in directing treatment decisions is also important. The development of novel targeted agents to treat relapsed disease will likely require a deeper understanding of the secondary resistance mechanisms to ATRA and ATO. Despite all of the exciting therapeutic advances, early death due to coagulopathy and bleeding complications remains the major challenge to further improvement of outcomes in patients with APL and constitutes a top research priority.
Acknowledgments
Financial or Other Support: This activity does not receive commercial support.
Grant Support: A.M. Zeidan is supported by a Young Investigator Award (YIA) from the American Society of Clinical Oncology and an “Evans Fellow” award from the MDS Clinical Research Consortium of the Aplastic Anemia and Myelo-dysplastic Syndromes International Foundation and the Ed Evans Foundation. S.D. Gore was supported by the NCI of the NIH under award number K24CA111717.
Footnotes
Disclosure of Potential Conflicts of Interest: No potential conflicts of interest were disclosed.
CME Staff Planners' Disclosures: The members of the planning committee have no real or apparent conflicts of interest to disclose.
Learning Objectives: Upon completion of this activity, the reader should have a better understanding of the biologic rationale and clinical evidence of combining arsenic with all-trans retinoic acid (ATRA) in the treatment of acute promyelocytic leukemia and how would the ongoing research of oral formulations of arsenic lead to an entirely oral, chemotherapy-free management approaches for the disease.
Authors' Contributions:
Conception and design: A.M. Zeidan, S.D. Gore
Development of methodology: A.M. Zeidan
Analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis): A.M. Zeidan
Writing, review, and/or revision of the manuscript: A.M. Zeidan, S.D. Gore
References
- 1.Wang ZY, Chen Z. Acute promyelocytic leukemia: from highly fatal to highly curable. Blood. 2008;111:2505–15. doi: 10.1182/blood-2007-07-102798. [DOI] [PubMed] [Google Scholar]
- 2.de The H, Chomienne C, Lanotte M, Degos L, Dejean A. The t(15;17) translocation of acute promyelocytic leukaemia fuses the retinoic acid receptor alpha gene to a novel transcribed locus. Nature. 1990;347:558–61. doi: 10.1038/347558a0. [DOI] [PubMed] [Google Scholar]
- 3.Dos Santos GA, Kats L, Pandolfi PP. Synergy against PML-RARa: targeting transcription, proteolysis, differentiation, and self-renewal in acute promyelocytic leukemia. J Exp Med. 2013;210:2793–802. doi: 10.1084/jem.20131121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tomita A, Kiyoi H, Naoe T. Mechanisms of action and resistance to all-trans retinoic acid (ATRA) and arsenic trioxide (As2O3) in acute promyelocytic leukemia. Int J Hematol. 2013;97:717–25. doi: 10.1007/s12185-013-1354-4. [DOI] [PubMed] [Google Scholar]
- 5.Licht JD, Chomienne C, Goy A, Chen A, Scott AA, Head DR, et al. Clinical and molecular characterization of a rare syndrome of acute promyelocytic leukemia associated with translocation (11;17) Blood. 1995;85:1083–94. [PubMed] [Google Scholar]
- 6.Redner RL, Rush EA, Faas S, Rudert WA, Corey SJ. The t(5;17) variant of acute promyelocytic leukemia expresses a nucleophosmin-retinoic acid receptor fusion. Blood. 1996;87:882–6. [PubMed] [Google Scholar]
- 7.Wells RA, Hummel JL, De Koven A, Zipursky A, Kirby M, Dube I, et al. A new variant translocation in acute promyelocytic leukaemia: molecular characterization and clinical correlation. Leukemia. 1996;10:735–40. [PubMed] [Google Scholar]
- 8.Tomita A, Buchholz DR, Obata K, Shi YB. Fusion protein of retinoic acid receptor alpha with promyelocytic leukemia protein or promyelocytic leukemia zinc finger protein recruits N-CoR-TBLR1 corepressor complex to repress transcription in vivo. J Biol Chem. 2003;278:30788–95. doi: 10.1074/jbc.M303309200. [DOI] [PubMed] [Google Scholar]
- 9.de The H, Chen Z. Acute promyelocytic leukaemia: novel insights into the mechanisms of cure. Nat Rev Cancer. 2010;10:775–83. doi: 10.1038/nrc2943. [DOI] [PubMed] [Google Scholar]
- 10.Zhu J, Lallemand-Breitenbach V, de The H. Pathways of retinoic acid- or arsenic trioxide-induced PML/RARalpha catabolism, role of oncogene degradation in disease remission. Oncogene. 2001;20:7257–65. doi: 10.1038/sj.onc.1204852. [DOI] [PubMed] [Google Scholar]
- 11.Fung TK, So CW. Overcoming treatment resistance in acute promyelocytic leukemia and beyond. Oncotarget. 2013;4:1128–9. doi: 10.18632/oncotarget.1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mi JQ, Li JM, Shen ZX, Chen SJ, Chen Z. How to manage acute promyelocytic leukemia. Leukemia. 2012;26:1743–51. doi: 10.1038/leu.2012.57. [DOI] [PubMed] [Google Scholar]
- 13.Tallman MS, Kim HT, Montesinos P, Appelbaum FR, de la Serna J, Bennett JM, et al. Does microgranular variant morphology of acute promyelocytic leukemia independently predict a less favorable outcome compared with classical M3 APL? A joint study of the North American Intergroup and the PETHEMA Group. Blood. 2010;116:5650–9. doi: 10.1182/blood-2010-06-288613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neame PB, Soamboonsrup P, Leber B, Carter RF, Sunisloe L, Patterson W, et al. Morphology of acute promyelocytic leukemia with cytogenetic or molecular evidence for the diagnosis: characterization of additional microgranular variants. Am J Hematol. 1997;56:131–42. doi: 10.1002/(sici)1096-8652(199711)56:3<131::aid-ajh1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 15.Falini B, Flenghi L, Fagioli M, Lo Coco F, Cordone I, Diverio D, et al. Immunocytochemical diagnosis of acute promyelocytic leukemia (M3) with the monoclonal antibody PG-M3 (anti-PML) Blood. 1997;90:4046–53. [PubMed] [Google Scholar]
- 16.Tsimberidou AM, Estey E, Kantarjian H, Keating MJ, Pierce S, Garcia-Manero G. Granulocyte colony stimulating factor administration associated with cerebral hemorrhage in acute promyelocytic leukemia. Leukemia. 2006;20:1452–3. doi: 10.1038/sj.leu.2404272. [DOI] [PubMed] [Google Scholar]
- 17.Tallman MS, Andersen JW, Schiffer CA, Appelbaum FR, Feusner JH, Ogden A, et al. All-trans-retinoic acid in acute promyelocytic leukemia. N Engl J Med. 1997;337:1021–8. doi: 10.1056/NEJM199710093371501. [DOI] [PubMed] [Google Scholar]
- 18.Ades L, Guerci A, Raffoux E, Sanz M, Chevallier P, Lapusan S, et al. Very long-term outcome of acute promyelocytic leukemia after treatment with all-trans retinoic acid and chemotherapy: the European APL Group experience. Blood. 2010;115:1690–6. doi: 10.1182/blood-2009-07-233387. [DOI] [PubMed] [Google Scholar]
- 19.Sanz MA, Vellenga E, Rayon C, Diaz-Mediavilla J, Rivas C, Amutio E, et al. All-trans retinoic acid and anthracycline monochemotherapy for the treatment of elderly patients with acute promyelocytic leukemia. Blood. 2004;104:3490–3. doi: 10.1182/blood-2004-04-1642. [DOI] [PubMed] [Google Scholar]
- 20.de la Serna J, Montesinos P, Vellenga E, Rayon C, Parody R, Leon A, et al. Causes and prognostic factors of remission induction failure in patients with acute promyelocytic leukemia treated with all-trans retinoic acid and idarubicin. Blood. 2008;111:3395–402. doi: 10.1182/blood-2007-07-100669. [DOI] [PubMed] [Google Scholar]
- 21.Powell BL, Moser B, Stock W, Gallagher RE, Willman CL, Stone RM, et al. Arsenic trioxide improves event-free and overall survival for adults with acute promyelocytic leukemia: North American Leukemia Inter-group Study C9710. Blood. 2010;116:3751–7. doi: 10.1182/blood-2010-02-269621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lo-Coco F, Avvisati G, Vignetti M, Breccia M, Gallo E, Rambaldi A, et al. Front-line treatment of acute promyelocytic leukemia with AIDA induction followed by risk-adapted consolidation for adults younger than 61 years: results of the AIDA-2000 trial of the GIMEMA group. Blood. 2010;116:3171–9. doi: 10.1182/blood-2010-03-276196. [DOI] [PubMed] [Google Scholar]
- 23.Avvisati G, Lo-Coco F, Paoloni FP, Petti MC, Diverio D, Vignetti M, et al. AIDA 0493 protocol for newly diagnosed acute promyelocytic leukemia: very long-term results and role of maintenance. Blood. 2011;117:4716–25. doi: 10.1182/blood-2010-08-302950. [DOI] [PubMed] [Google Scholar]
- 24.O'Donnell MR, Tallman MS, Abboud CN, Altman JK, Appelbaum FR, Arber DA, et al. Acute myeloid leukemia, version 2.2013. J Natl Compr Canc Netw. 2013;11:1047–55. doi: 10.6004/jnccn.2013.0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sanz MA, Montesinos P, Vellenga E, Rayon C, de la Serna J, Parody R, et al. Risk-adapted treatment of acute promyelocytic leukemia with all-trans retinoic acid and anthracycline monochemotherapy: long-term outcome of the LPA 99 multicenter study by the PETHEMA Group. Blood. 2008;112:3130–4. doi: 10.1182/blood-2008-05-159632. [DOI] [PubMed] [Google Scholar]
- 26.Kwaan HC, Cull EH. The coagulopathy in acute promyelocytic leukaemia - what have we learned in the past twenty years. Best Pract Res Clin Haematol. 2014;27:11–8. doi: 10.1016/j.beha.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 27.Lehmann S, Ravn A, Carlsson L, Antunovic P, Deneberg S, Mollgard L, et al. Continuing high early death rate in acute promyelocytic leukemia: a population-based report from the Swedish Adult Acute Leukemia Registry. Leukemia. 2011;25:1128–34. doi: 10.1038/leu.2011.78. [DOI] [PubMed] [Google Scholar]
- 28.Altman JK, Rademaker A, Cull E, Weitner BB, Ofran Y, Rosenblat TL, et al. Administration of ATRA to newly diagnosed patients with acute promyelocytic leukemia is delayed contributing to early hemorrhagic death. Leuk Res. 2013;37:1004–9. doi: 10.1016/j.leukres.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 29.Park JH, Qiao B, Panageas KS, Schymura MJ, Jurcic JG, Rosenblat TL, et al. Early death rate in acute promyelocytic leukemia remains high despite all-trans retinoic acid. Blood. 2011;118:1248–54. doi: 10.1182/blood-2011-04-346437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen Y, Kantarjian H, Wang H, Cortes J, Ravandi F. Acute promyelocytic leukemia: a population-based study on incidence and survival in the United States, 1975–2008. Cancer. 2012;118:5811–8. doi: 10.1002/cncr.27623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ghavamzadeh A, Alimoghaddam K, Rostami S, Ghaffari SH, Jahani M, Iravani M, et al. Phase II study of single-agent arsenic trioxide for the front-line therapy of acute promyelocytic leukemia. J Clin Oncol. 2011;29:2753–7. doi: 10.1200/JCO.2010.32.2107. [DOI] [PubMed] [Google Scholar]
- 32.Breccia M, Cicconi L, Lo-Coco F. ATRA + ATO: has a new standard of care been established in low-risk acute promyelocytic leukaemia? Curr Opin Hematol. 2014;21:95–101. doi: 10.1097/MOH.0000000000000023. [DOI] [PubMed] [Google Scholar]
- 33.Powell BL. Acute promyelocytic leukemia: progress far and wide. Blood. 2013;121:1925–6. doi: 10.1182/blood-2012-12-470757. [DOI] [PubMed] [Google Scholar]
- 34.Mathews V, George B, Chendamarai E, Lakshmi KM, Desire S, Balasubramanian P, et al. Single-agent arsenic trioxide in the treatment of newly diagnosed acute promyelocytic leukemia: long-term follow-up data. J Clin Oncol. 2010;28:3866–71. doi: 10.1200/JCO.2010.28.5031. [DOI] [PubMed] [Google Scholar]
- 35.Zhang Y, Zhang Z, Li J, Li L, Han X, Han L, et al. Long-term efficacy and safety of arsenic trioxide for first-line treatment of elderly patients with newly diagnosed acute promyelocytic leukemia. Cancer. 2013;119:115–25. doi: 10.1002/cncr.27650. [DOI] [PubMed] [Google Scholar]
- 36.Chen GQ, Shi XG, Tang W, Xiong SM, Zhu J, Cai X, et al. Use of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia (APL): I. As2O3 exerts dose-dependent dual effects on APL cells. Blood. 1997;89:3345–53. [PubMed] [Google Scholar]
- 37.Zhu J, Koken MH, Quignon F, Chelbi-Alix MK, Degos L, Wang ZY, et al. Arsenic-induced PML targeting onto nuclear bodies: implications for the treatment of acute promyelocytic leukemia. Proc Natl Acad Sci U S A. 1997;94:3978–83. doi: 10.1073/pnas.94.8.3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hu J, Liu YF, Wu CF, Xu F, Shen ZX, Zhu YM, et al. Long-term efficacy and safety of all-trans retinoic acid/arsenic trioxide-based therapy in newly diagnosed acute promyelocytic leukemia. Proc Natl Acad Sci U S A. 2009;106:3342–7. doi: 10.1073/pnas.0813280106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lallemand-Breitenbach V, de The H. Retinoic acid plus arsenic trioxide, the ultimate panacea for acute promyelocytic leukemia? Blood. 2013;122:2008–10. doi: 10.1182/blood-2013-06-505115. [DOI] [PubMed] [Google Scholar]
- 40.Zheng X, Seshire A, Ruster B, Bug G, Beissert T, Puccetti E, et al. Arsenic but not all-trans retinoic acid overcomes the aberrant stem cell capacity of PML/RARalpha-positive leukemic stem cells. Haematologica. 2007;92:323–31. doi: 10.3324/haematol.10541. [DOI] [PubMed] [Google Scholar]
- 41.Nasr R, Guillemin MC, Ferhi O, Soilihi H, Peres L, Berthier C, et al. Eradication of acute promyelocytic leukemia-initiating cells through PML-RARA degradation. Nat Med. 2008;14:1333–42. doi: 10.1038/nm.1891. [DOI] [PubMed] [Google Scholar]
- 42.Lallemand-Breitenbach V, Zhu J, Chen Z, de The H. Curing APL through PML/RARA degradation by As2O3. Trends Mol Med. 2012;18:36–42. doi: 10.1016/j.molmed.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 43.Lo-Coco F, Avvisati G, Vignetti M, Thiede C, Orlando SM, Iacobelli S, et al. Retinoic acid and arsenic trioxide for acute promyelocytic leukemia. N Engl J Med. 2013;369:111–21. doi: 10.1056/NEJMoa1300874. [DOI] [PubMed] [Google Scholar]
- 44.Breccia M, Lo-Coco F. Arsenic trioxide for management of acute promyelocytic leukemia: current evidence on its role in front-line therapy and recurrent disease. Expert Opin Pharmacother. 2012;13:1031–43. doi: 10.1517/14656566.2012.677436. [DOI] [PubMed] [Google Scholar]
- 45.Leung J, Pang A, Yuen WH, Kwong YL, Tse EW. Relationship of expression of aquaglyceroporin 9 with arsenic uptake and sensitivity in leukemia cells. Blood. 2007;109:740–6. doi: 10.1182/blood-2006-04-019588. [DOI] [PubMed] [Google Scholar]
- 46.Shen ZX, Shi ZZ, Fang J, Gu BW, Li JM, Zhu YM, et al. All-trans retinoic acid/As2O3 combination yields a high quality remission and survival in newly diagnosed acute promyelocytic leukemia. Proc Natl Acad Sci U S A. 2004;101:5328–35. doi: 10.1073/pnas.0400053101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Estey E, Garcia-Manero G, Ferrajoli A, Faderl S, Verstovsek S, Jones D, et al. Use of all-trans retinoic acid plus arsenic trioxide as an alternative to chemotherapy in untreated acute promyelocytic leukemia. Blood. 2006;107:3469–73. doi: 10.1182/blood-2005-10-4006. [DOI] [PubMed] [Google Scholar]
- 48.Ravandi F, Estey E, Jones D, Faderl S, O'Brien S, Fiorentino J, et al. Effective treatment of acute promyelocytic leukemia with all-trans-retinoic acid, arsenic trioxide, and gemtuzumab ozogamicin. J Clin Oncol. 2009;27:504–10. doi: 10.1200/JCO.2008.18.6130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gore SD, Gojo I, Sekeres MA, Morris L, Devetten M, Jamieson K, et al. Single cycle of arsenic trioxide-based consolidation chemotherapy spares anthracycline exposure in the primary management of acute promyelocytic leukemia. J Clin Oncol. 2010;28:1047–53. doi: 10.1200/JCO.2009.25.5158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Leech M, Stewart M, Zhang X, Bashey A, Holland HK, Solomon SR, et al. Validation of a brief arsenic trioxide (ATO)-based consolidation chemotherapy in the upfront management of acute promyelocytic leukemia (APL): less anthracycline exposure and faster completion of consolidation therapy with equivalent survival [abstract] Blood. 2013;122:3963. [Google Scholar]
- 51.Iland HJ, Bradstock K, Supple SG, Catalano A, Collins M, Hertzberg M, et al. All-trans-retinoic acid, idarubicin, and IV arsenic trioxide as initial therapy in acute promyelocytic leukemia (APML4) Blood. 2012;120:1570–80. doi: 10.1182/blood-2012-02-410746. [DOI] [PubMed] [Google Scholar]
- 52.Au WY, Kumana CR, Kou M, Mak R, Chan GC, Lam CW, et al. Oral arsenic trioxide in the treatment of relapsed acute promyelocytic leukemia. Blood. 2003;102:407–8. doi: 10.1182/blood-2003-01-0298. [DOI] [PubMed] [Google Scholar]
- 53.Au WY, Lie AK, Chim CS, Liang R, Ma SK, Chan CH, et al. Arsenic trioxide in comparison with chemotherapy and bone marrow transplantation for the treatment of relapsed acute promyelocytic leukaemia. Ann Oncol. 2003;14:752–7. doi: 10.1093/annonc/mdg208. [DOI] [PubMed] [Google Scholar]
- 54.Au WY, Kumana CR, Lee HK, Lin SY, Liu H, Yeung DY, et al. Oral arsenic trioxide-based maintenance regimens for first complete remission of acute promyelocytic leukemia: a 10-year follow-up study. Blood. 2011;118:6535–43. doi: 10.1182/blood-2011-05-354530. [DOI] [PubMed] [Google Scholar]
- 55.Siu CW, Au WY, Yung C, Kumana CR, Lau CP, Kwong YL, et al. Effects of oral arsenic trioxide therapy on QT intervals in patients with acute promyelocytic leukemia: implications for long-term cardiac safety. Blood. 2006;108:103–6. doi: 10.1182/blood-2006-01-0054. [DOI] [PubMed] [Google Scholar]
- 56.Zhu HH, Wu DP, Jin J, Li JY, Ma J, Wang JX, et al. Oral tetra-arsenic tetra-sulfide formula versus intravenous arsenic trioxide as first-line treatment of acute promyelocytic leukemia: a multicenter randomized controlled trial. J Clin Oncol. 2013;31:4215–21. doi: 10.1200/JCO.2013.48.8312. [DOI] [PubMed] [Google Scholar]
- 57.Diverio D, Rossi V, Avvisati G, De Santis S, Pistilli A, Pane F, et al. Early detection of relapse by prospective reverse transcriptase-polymerase chain reaction analysis of the PML/RARalpha fusion gene in patients with acute promyelocytic leukemia enrolled in the GIMEMA-AIEOP multicenter “AIDA” trial. GIMEMA-AIEOP multicenter “AIDA” Trial. Blood. 1998;92:784–9. [PubMed] [Google Scholar]
- 58.Grimwade D, Jovanovic JV, Hills RK, Nugent EA, Patel Y, Flora R, et al. Prospective minimal residual disease monitoring to predict relapse of acute promyelocytic leukemia and to direct pre-emptive arsenic trioxide therapy. J Clin Oncol. 2009;27:3650–8. doi: 10.1200/JCO.2008.20.1533. [DOI] [PubMed] [Google Scholar]
- 59.Chendamarai E, Balasubramanian P, George B, Viswabandya A, Abraham A, Ahmed R, et al. Role of minimal residual disease monitoring in acute promyelocytic leukemia treated with arsenic trioxide in frontline therapy. Blood. 2012;119:3413–9. doi: 10.1182/blood-2011-11-393264. [DOI] [PubMed] [Google Scholar]
- 60.Sanz MA, Grimwade D, Tallman MS, Lowenberg B, Fenaux P, Estey EH, et al. Management of acute promyelocytic leukemia: recommendations from an expert panel on behalf of the European LeukemiaNet. Blood. 2009;113:1875–91. doi: 10.1182/blood-2008-04-150250. [DOI] [PubMed] [Google Scholar]
- 61.Lo Coco F, Diverio D, Avvisati G, Petti MC, Meloni G, Pogliani EM, et al. Therapy of molecular relapse in acute promyelocytic leukemia. Blood. 1999;94:2225–9. [PubMed] [Google Scholar]
- 62.Esteve J, Escoda L, Martin G, Rubio V, Diaz-Mediavilla J, Gonzalez M, et al. Outcome of patients with acute promyelocytic leukemia failing to front-line treatment with all-trans retinoic acid and anthracycline-based chemotherapy (PETHEMA protocols LPA96 and LPA99): benefit of an early intervention. Leukemia. 2007;21:446–52. doi: 10.1038/sj.leu.2404501. [DOI] [PubMed] [Google Scholar]
- 63.Grimwade D, Jovanovic JV, Hills RK. Can we say farewell to monitoring minimal residual disease in acute promyelocytic leukaemia? Best Pract Res Clin Haematol. 2014;27:53–61. doi: 10.1016/j.beha.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 64.Cote S, Zhou D, Bianchini A, Nervi C, Gallagher RE, Miller WH., Jr Altered ligand binding and transcriptional regulation by mutations in the PML/RARalpha ligand-binding domain arising in retinoic acid-resistant patients with acute promyelocytic leukemia. Blood. 2000;96:3200–8. [PubMed] [Google Scholar]
- 65.Goto E, Tomita A, Hayakawa F, Atsumi A, Kiyoi H, Naoe T. Missense mutations in PML-RARA are critical for the lack of responsiveness to arsenic trioxide treatment. Blood. 2011;118:1600–9. doi: 10.1182/blood-2011-01-329433. [DOI] [PubMed] [Google Scholar]
- 66.Gallagher RE, Moser BK, Racevskis J, Poire X, Bloomfield CD, Carroll AJ, et al. Treatment-influenced associations of PML-RAR-alpha mutations, FLT3 mutations, and additional chromosome abnormalities in relapsed acute promyelocytic leukemia. Blood. 2012;120:2098–108. doi: 10.1182/blood-2012-01-407601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhou DC, Kim SH, Ding W, Schultz C, Warrell RP, Jr, Gallagher RE. Frequent mutations in the ligand-binding domain of PML-RARalpha after multiple relapses of acute promyelocytic leukemia: analysis for functional relationship to response to all-trans retinoic acid and histone deacetylase inhibitors in vitro and in vivo. Blood. 2002;99:1356–63. doi: 10.1182/blood.v99.4.1356. [DOI] [PubMed] [Google Scholar]
- 68.Ito Y, Wakita A, Takada S, Mihara M, Gotoh M, Ohyashiki K, et al. Phase 1 trial of gemtuzumab ozogamicin in combination with enocitabine and daunorubicin for elderly patients with relapsed or refractory acute myeloid leukemia: Japan Adult Leukemia Study Group (JALSG)-GML208 study. Int J Hematol. 2012;96:485–91. doi: 10.1007/s12185-012-1165-z. [DOI] [PubMed] [Google Scholar]
- 69.Dai CW, Zhang GS, Shen JK, Zheng WL, Pei MF, Xu YX, et al. Use of all-trans retinoic acid in combination with arsenic trioxide for remission induction in patients with newly diagnosed acute promyelocytic leukemia and for consolidation/maintenance in CR patients. Acta Haematol. 2009;121:1–8. doi: 10.1159/000204472. [DOI] [PubMed] [Google Scholar]
- 70.Sanz MA, Lo Coco F, Martin G, Avvisati G, Rayon C, Barbui T, et al. Definition of relapse risk and role of nonanthracycline drugs for consolidation in patients with acute promyelocytic leukemia: a joint study of the PETHEMA and GIMEMA cooperative groups. Blood. 2000;96:1247–53. [PubMed] [Google Scholar]


