Abstract
Compelling evidence indicates that α-synuclein (α-syn) aggregation plays a central role in the pathogenesis of Parkinson's disease (PD) and other synucleinopathies. Identification of compounds that inhibit or reverse the aggregation process may thus represent a viable therapeutic strategy against PD and related disorders. Ginseng is a well-known medicinal plant that has been used in East Asia for more than two thousand years to treat several conditions. It is now understood that the pharmacological properties of ginseng can be attributed to its biologically active components, the ginsenosides, which in turn have been shown to have neuroprotective properties. We therefore sought to determine for the first time, the potential of the most frequently used and studied ginsenosides, namely Rg1, Rg3 and Rb1, as anti-amyloidogenic agents. The effect of Rg1, Rg3 and Rb1 on α-syn aggregation and toxicity was determined by an array of biophysical, biochemical and cell-culture-based techniques. Among the screened ginsenosides, only Rb1 was shown to be a potent inhibitor of α-syn fibrillation and toxicity. Additionally, Rb1 exhibited a strong ability to disaggregate preformed fibrils and to inhibit the seeded polymerization of α-syn. Interestingly, Rb1 was found to stabilize soluble non-toxic oligomers with no β-sheet content, that were susceptible to proteinase K digestion, and the binding of Rb1 to those oligomers may represent a potential mechanism of action. Thus, Rb1 could represent the starting point for designing new molecules that could be utilized as drugs for the treatment of PD and related disorders.
Keywords: α-synuclein, Parkinson's disease, aggregation, amyloid fibrils, ginsenosides, drug discovery
Graphical Abstract
INTRODUCTION
Parkinson's disease (PD) is a neurodegenerative disorder caused by the gradual loss of dopaminergic neurons (Obeso et al., 2008). As a consequence, the neurotransmitter dopamine is depleted, resulting in severe debilitation in motor skills (Obeso et al., 2008). Neuropathological studies of PD have revealed the presence of cytoplasmic inclusions, which are abundantly found in the degenerating dopaminergic neurons of the substantia nigra and other cortical and subcortical neurons (Galvin et al., 1999). These inclusions are known as Lewy bodies (LBs) and Lewy neurites (LNs), formed due to α-syn deposition (Spillantini, Crowther, Jakes, Hasegawa, & Goedert, 1998). Intracellular α-syn inclusions are also a prominent feature of other neurodegenerative diseases, including dementia with Lewy bodies and multiple system atrophy (reviewed by Goedert, Spillantini, Del Tredici, & Braak, 2013). Genetic, biochemical and animal model studies also provide strong evidence in support of the central role of α-syn aggregation during the pathogenesis of PD and related disorders.
In its native form, α-syn, has little or no ordered structure, existing mostly as an unfolded protein (Weinreb, Zhen, Poon, Conway, & Lansbury, 1996). However, α-syn can undergo conformational changes that promote the self-assembly and aggregation of the protein. α-Syn aggregation proceeds through the formation of oligomers (early aggregates), which ultimately convert into well-ordered fibrils (late aggregates) (Uversky, Lee, Li, Fink, & Lee, 2001). Recent studies indicate that early aggregates of α-syn, the so-called soluble oligomers, form the pathogenic species that drive neurodegeneration and neuronal cell death rather than mature amyloid fibrils (Conway et al., 2000; El-Agnaf, Walsh, & Allsop, 2003; Winner et al., 2011).
The currently available drugs for the treatment of PD provide symptomatic relief but do not alter the course of the disease (He et al., 2013). However, the effectiveness of these drugs diminishes after several years of treatment. Together with the increasing incidence of PD due to population aging, these facts indicate the compelling need for more effective drugs and treatments for PD. Although modern therapeutic options that target disease modifications are on the rise, the multiple mechanisms involved in the pathogenesis of PD create considerable difficulty for producing effective treatments. Hence, inhibition of α-syn aggregation may represent a viable strategy for therapeutic intervention in PD and related disorders. It is therefore essential to identify compounds that can serve as potent inhibitors and interrupt the early stages of aggregation.
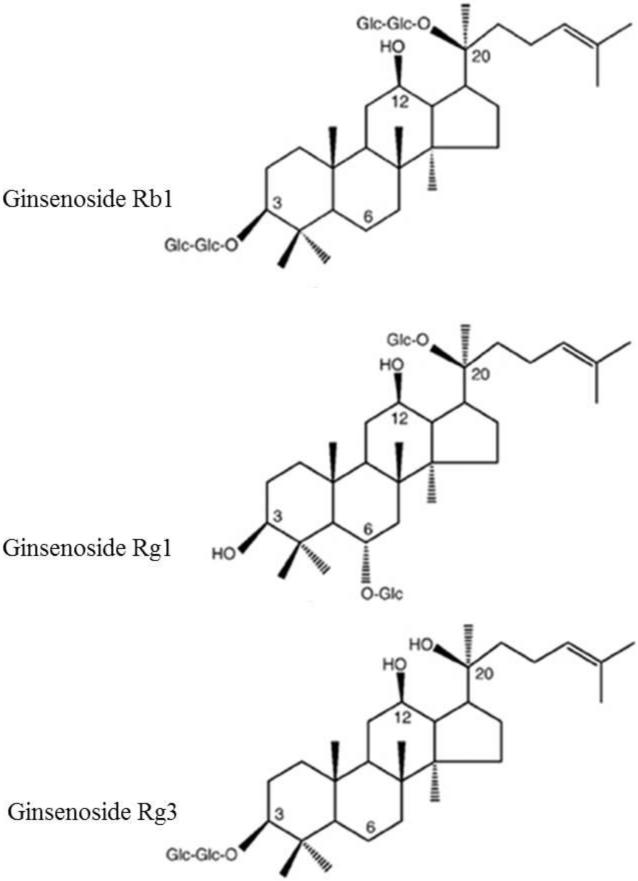
Ginseng is a well-known medicinal herb that has been used for more than two thousand years in China, Korea and Japan to promote well-being and alleviate fatigue. Although there are eleven different species of ginseng that belong to the genus Panax of the Aralliaceae family, the most commonly used species are Panax ginseng (Asian or Korean ginseng), P. quinquefolius (American ginseng), P. japonicus (Japanese ginseng) and P. notoginseng (Chinese notoginseng or Sanchi) (F. Chen, Eckman, & Eckman, 2006). Named after its ability to treat several conditions - Panax means panacea in Greek language- ginseng is now a well-documented anti-carcinogenic, anti-diabetic, anti-oxidant and vasorelaxing agent that exhibits immunomodulatory properties and improves the function of central nervous system (Lü, Yao, & Chen, 2009). The numerous pharmacological properties of ginseng are attributed to its biologically active ingredients, the ginsenosides (reviewed by Im & Nah, 2013), which can be extracted from many parts of the ginseng plant, including the root, the leaves and the ginseng berries (Attele, Wu, & Yuan, 1999). Ginsenosides, which are also referred to as ginseng saponins, are derivatives of triterpenoid dammarane with a four-ring, steroidal structure bearing sugar moieties and an aliphatic side chain (Wee JJ, 2011). The variations in the structure of ginsenosides, namely the type of aglycone (triterpene), the type of sugar moieties (glucose, maltose, fructose, saccharose etc), their number and their site of attachment (Wee JJ, 2011), give rise to three categories of ginseng saponins, the panaxadiol group, the panaxatriol group and the oleanolic acid group (H. J. Kim, Kim, & Shin, 2013). More than 100 ginsenosides have been identified so far (Nag et al., 2012), but the most frequently studied ones are Rb1, Rg1, Rg3, Rd, Re, Rh1 and Rh2. Ginsenosides have been shown to affect voltage-gated ion channels, such as the Ca2+, Na+, K+ channels, as well as the ligand-gated ion channels, such as the 5-HT3-, the α7 nicotinic acetylcholine and the N-methyl-d-aspartate (NMDA)-gated channels (reviewed by Nag et al., 2012; Radad, Moldzio, & Rausch, 2011)). This property of ginsenosides appears to underlie many pharmacological effects of ginseng, including neuroprotection (J. H. Kim et al., 2007), since it has a beneficial effect on many neurological conditions, including neurodegenerative diseases such as PD (reviewed by Cho, 2012; H. J. Kim et al., 2013). However, despite the numerous studies exploring the effect of various ginsenosides on the nervous system, there are no reports on the effect of ginsenosides on the aggregation propensity of amyloidogenic proteins such as α-syn. As a consequence, we sought to determine the potential of the most frequently used and studied ginsenosides, namely Rb1, Rg1 and (20S)-Rg3, i.e. the stereoisomer of Rg3 with its C-20 OH being spatially close to the C-12 OH group (Fig. 1).
Figure 1.
Chemical structure of ginsenoside Rb1, ginsenoside Rg1, ginsenoside Rg3.
MATERIALS AND METHODS
Expression and purification of recombinant human α-syn
A GST-α-syn fusion construct in the pGEX-4T1 vector (kindly provided by Dr. Hyangshuk Rhim of the Catholic University College of Medicine, Seoul, Korea). The expression and purification was carried out as described elsewhere (Ardah et al., 2014). Briefly, the construct was inserted into BL21 E.coli bacteria by heat shock. The transformed bacteria were grown in LB medium supplemented with 0.1 mg/ml ampicillin at 37°C in an orbital shaker to an OD600 of 0.5. Expression was then induced by adding 0.5 mM IPTG (Sigma-Aldrich Chemie GmbH, Germany), and the culture was incubated for 2 hours at 37°C. The cells were harvested by a 15 minute centrifugation at 9000 × g, and the resulting pellet was then resuspended in lysis buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 2 mM EDTA, 1% NP-40, 0.1% DTT) and shaken for 10 minutes at room temperature. To improve the efficiency of cell lysis, the resuspended pellet was subjected to 6 freeze-thaw cycles in liquid nitrogen and a 37°C water bath. The lysate was then centrifuged at 27,000 × g for 15 minutes, and the resulting supernatant was retained for purification by affinity chromatography using sepharose beads conjugated to glutathione, which has a high affinity for the GST tag. The cell lysate was mixed with glutathione sepharose beads and incubated for 1 hour at room temperature, followed by centrifugation at 500 × g at 4°C for 8 minutes. The beads were then washed twice with wash buffer (50 mM Tris-HCl, 150 mM NaCl, 10 mM EDTA, 1% Triton X-100, pH 8.0); twice with 50 mM Tris-HCl, pH 8.0; and once with 1X PBS. The washed beads were resuspended in 5 ml of 1X PBS, and the GST tag was cleaved by human plasma thrombin (1 unit/μl), (Sigma-Aldrich, USA). The thrombin-catalyzed cleavage reaction was incubated overnight at room temperature with continuous mixing followed by 5 minutes incubation at 37°C. The reaction mixture was then centrifuged for 8 minutes at 500 × g at 4°C, and benzamidine sepharose beads (Amersham, Sweden) were used to ‘fish out’ thrombin. Pure α-syn was collected by centrifugation at 500 × g for 8 minutes at 4°C. The α-syn concentration was estimated using a BCA assay (Pierce Biotechnology, Rockford, IL).
α-Syn purification and characterization
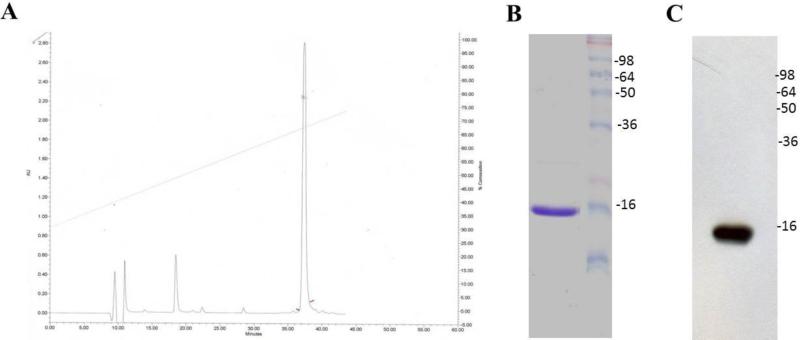
Crude α-syn protein after GST tag cleavage was purified using reversed phase HPLC. Columns used were an analytical Phenomenex Jupiter C4 (250 3 4.6 mm) and a preparative Phenomenex Jupiter C4 (250 3 10 mm). α-Syn was loaded into the column and eluted with a linear gradient (30-80%) of acetonitrile (70% acetonitrile, 0.1% trifluoroacetic acid,) versus water (0.1% trifluoracetic acid) in 38 minutes, at a flow rate of 0.5 ml/minute. (Fig. 2). Homogeneity of α-syn protein was ascertained by analytical HPLC and SDS-page (Fig. 2).
Figure 2.
HPLC analysis and characterization of crude α-syn. A. HPLC analysis was done using phenomenex Jupiter C4 (250 3 4.6 mm) column, with a gradient of 30%-80% solvent B in solvent A at 0.5 ml/minute over 38 minutes. B. Coomassie blue staining of 15% SDS-PAGE for HPLC purified recombinant α-syn. C. Immunoblotting for HPLC purified α-syn detected by mAb 211.
Aggregation of α-syn in vitro
The protein purity was estimated to be >95% using an SDS gel. The ginsenosides were purchased from the National institute for the control of pharmaceutical and biological products, China (NICPBP). Ginsenosides stock solutions (10 mM) were prepared in 100% DMSO such that the final amount of DMSO in the sample solutions was 1%. Samples of 25 μM α-syn in PBS were aged alone or with ginsenosides at various molar ratios (ginsenoside: μ-syn molar ratios of 4:1, 2:1 and 1:1). The samples were placed in 1.5 ml sterile polypropylene tubes, drops of mineral oil were added to prevent sample evaporation, and the tubes were then sealed with parafilm and incubated at 37°C for 5 days with continuous shaking at 800 rpm in a Thermomixer (Eppendorf). Samples were collected at the indicated time points, and a Thioflavin-T assay was performed immediately at each time point, while the rest of the samples were stored at −80°C until required for further analyses.
Thioflavin-T (Th-T) assay
α-Syn fibril formation was monitored by Th-T binding assay. Th-T is a fluorescent dye that interacts with fibrils containing a β-sheet structure. A total of 10 μl of each sample was diluted in 40 μl of Th-T in PBS. Fluorescence was then measured in a 384-well, untreated black micro-well plate (Nunc, Denmark) using a microplate reader (Victor X3 2030, Perkin Elmer) with the excitation and emission wavelengths set at 450 and 486 nm, respectively. To allow for background fluorescence, the fluorescence intensity of a blank PBS solution was subtracted from all readings.
Transmission electron microscopy (TEM)
Electron images were produced from α-syn aged alone or in the presence of ginsenosides. The samples (5 μL) were deposited on Formvar-coated 400-mesh copper grids (Agar Scientific, UK), fixed briefly with 0.5% glutaraldehyde (5 μl), negatively stained with 2% uranyl acetate (Sigma–Aldrich, USA) and examined with a Philips CM-10 TEM electron microscope.
Immunoblotting
The supernatant (20 ng) of samples of α-syn incubated alone or with the ginsenosides after sedimentation at 35,000 g was mixed with 2X non-denaturing sample loading buffer (250 mM Tris-HCl, pH 6.8, 30% glycerol, 0.02% bromophenol blue) to prevent the high molecular weight aggregates from denaturing, and then separated on 15% 1 mm SDS-PAGE gels. The separated proteins were transferred to 0.45 μm nitrocellulose membranes (Whatman Gmbh-Germany) at 90 V for 80 minutes. The membranes were boiled for 5 minutes in PBS and then blocked for 1 hour with 5% non-fat milk prepared in PBS-Tween-20 (0.05%; PBST) following, they were incubated overnight at 4°C with the primary mouse monoclonal anti-α-syn (211) antibody, which recognizes human α-syn (121-125) (Santa Cruz Biotechnology, USA), at a dilution of 1:1000. The membranes were then washed several times with PBST, followed by incubation with an HRP-conjugated goat anti-mouse antibody (Dako Ltd., Ely, UK) at a dilution of 1:70,000 for 60 minutes at room temperature with gentle agitation. Following extensive washes of the membrane for 25 minutes, immunoreactive bands were visualized with the Super Signal West Femto Chemiluminescent Substrate Kit (Pierce, Rockford, USA) according to the manufacturer's instructions. The amount of monomeric α-syn in the samples of α-syn aged alone or in the presence of the tested compounds was quantified against a fresh α-syn sample that contained only the monomeric species using the ImageJ software.
Congo red binding assay
Congo red (Sigma-Aldrich, USA) (20 μM) was prepared in PBS (pH 7.4) and filtered through a 0.45 μm filter. Samples of α-syn (5 μM), aged alone or with ginsenosides at different molar ratios, were mixed with Congo red (final concentration 5 μM), and the reaction samples were thoroughly mixed. The UV absorbance spectrum was recorded between 400 and 600 nm in a spectrophotometer (DU-800, Beckman-Coulter) using 10-mm quartz cuvettes (Hellma Analytics-Germany). Congo red alone was used as blank.
Proteinase K (PK) digestion
Samples of α-syn (25 μM), aged alone or with Rb1, Rg1 or Rg3 (α-syn: compound molar ratio of 1:1) were incubated for 15 minutes at 37°C with PK (Sigma-Aldrich, USA) to a 2.5 μg/ml. The reaction was stopped by the addition of 2x sample loading buffer (250 mM Tris-HCl, pH 6.8, 30% glycerol, 0.02% bromophenol blue, 8% SDS, 5% beta-mercaptoethanol), and then heated for 10 minutes at 95°C. The samples were loaded and run into 15% SDS-PAGE gels which were then silver stained.
Tissue culture of BE(2)-M17 human neuroblastoma cells
BE(2)-M17 human neuroblastoma cells were routinely cultured in Dulbecco's MEM/Nutrient Mix F-12 (1:1) (Gibco BRL, Rockville, MD) containing 15% fetal bovine serum and 1% penicillin-streptomycin (P/S; 100 U/ml penicillin, 100 mg/ml streptomycin). The cells were maintained at 37°C in a humidified incubator with 5% CO2/95% air.
Measurement of cell viability
BE(2)-M17 cells suspended in DMEM medium were plated at a density of 15,000 cells (100 μl /well) in a 96-well plate. After 24 hours, the medium was replaced with 200 μl of OPTI-MEM (Gibco-USA) serum-free medium containing aged α-syn solutions with or without ginsenosides. Aged α-syn and ginsenosides solutions were diluted in OPTI-MEM to obtain the desired concentration. Cells were then incubated at 37°C in 5% CO2 for 48 hours. A total of 20 μl of MTT (3-(4, 5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) (Sigma-Aldrich, USA) (6 mg/ml) in PBS was dispensed into each well, and the plate was incubated at 37°C for 4.5 hours. The MTT-containing medium was carefully removed, and 100 μl of lysis buffer (15% SDS, 50% N,N-dimethylformamide, pH 4.7) was added to each well. The lysis buffer was incubated overnight at 37°C before the absorbance values at 590 nm were determined by a microplate reader (Perkin Elmer).
Immunocytochemistry staining
M17 cells expressing were plated on 12-mm coverslips in 24-well plate for 24 hours. The cells were then washed twice with PBS and fixed with 4% paraformaldehyde for 15 minutes at room temperature. Following two washes (5 minutes each with PBS) the cells were permeabilized by incubating for 10 minutes at room temperature with PBS-1% triton and then incubated in blocking buffer [5% normal goat serum (NGS) in PBS + 1% Triton] for 1 hour at room temperature. Fixed cells were incubated with anti α-syn 211 Ab for 3 hours at room temperature and then washed three times with PBS. FITC conjugated Goat anti-mouse secondary Ab (Sigma) was added to the cells for 1 hour at room temperature, and after three washes with PBS the cells were counterstained with DAPI to reveal nuclei, and mounted in Shandon immu-mount (Thermo Scientific). Fluorescent images were taken with an inverted Axiovert 40 CFL fluorescence microscope (Carl Zeiss), equipped with AxioCam HRc (Carl Zeiss) using the 63x oil objective.
α-Syn disaggregation assay
α-Syn solution in PBS (pH 7.4) was aggregated at a concentration of 25 μM as indicated above. The resulting aggregated α-syn was incubated either alone or with Gn Rb1 at various molar ratios (Gn Rb1: α-syn molar ratios of 6:1, 4:1 and 2:1). It should be noted that for the purpose of the experiment, the concentration of α-syn taken into account was the concentration of fresh α-syn. The samples were incubated at 37°C for 48 hours on a thermomixer with continuous shaking at 800 rpm. Samples were collected at regular time points, and Th-T fluorescence was measured immediately.
Seeding polymerization assay
The aggregation of monomeric α-syn with or without seeding was performed as described elsewhere (Di Giovanni et al., 2010). The seeds were prepared by fragmenting the mature α-syn fibrils by sonication to obtain short fibrils, which were employed as ‘seeds’. Briefly, monomeric α-syn at a concentration of 100 μM was seeded with 2 μM of seeds and incubated in the presence or absence of Gn Rb1 (10 μM or 50 μM) at 37°C for 6 hours with continuous shaking. The fibrillization was monitored by Th-T binding assay as described above.
Size Exclusion Chromatography (SEC) for separating α-syn oligomers and monomers
SEC was carried out using an AKTA FPLC system (GE Healthcare-Sweden) and a superdex 200 column at 4 °C, in order to separate the oligomers generated from the aggregation of α-syn with Gn Rb1 (Gn Rb1: α-syn molar ratio of 4:1). Monomeric α-syn at a concentration of 100 μM was aggregated in the presence of Gn Rb1 for 5 days as described above. At the end of the aggregation process, the sample was centrifuged for 45 minutes at 14,000 × g at 4°C generating a supernatant free from insoluble material. Prior to injecting 80% of the generated supernatant, the column was thoroughly equilibrated with SEC running buffer (1x PBS, pH 7.4) and the flow rate was set to 0.1 ml/minute (0.5 ml/fraction). The elution of α-syn was monitored at absorbance wavelengths of 215 nm, 254 nm, and 280 nm. To determine the elution time of monomeric α-syn, molecular weight standards (ferritin 440 kDa, aldolase 171 kDa, abmumin 68 kDa and chymotrypsinogenA 25 kDa) and monomeric α-syn were co-injected into the column and eluted at the same conditions mentioned above. The fractions eluting between 2-4 ml CV were combined and labeled as oligomers (sample P1), and fractions eluting between 10-14 ml CV were combined and labeled as oligomers (sample P2), whereas the fractions eluting in the 14–16 ml CV were combined and labeled as monomers (sample P3). The P1, P2 and P3 fractions were further characterized by western blotting and TEM.
UV scanning
The P1, P2 and P3 samples, representing the oligomeric and monomeric fractions of SEC, were concentrated using a speed vac (CentriVap, Labconco). Their protein concentration was estimated by a BCA assay. The UV absorbance spectrum was recorded from 200- 600 nm in a spectrophotometer (DU-800, Beckman-Coulter) using 10 mm quartz cuvettes (Hellma Analytics-Germany) and employing equal concentrations of the P1, P2 and P3. Fresh monomeric α-syn was used as negative control.
NMR studies
For NMR studies, recombinant 15N-labeled α-syn was expressed and purified as previously described (Bussell & Eliezer, 2003; Eliezer, Kutluay, Bussell, & Browne, 2001), and resuspended in PBS buffer at pH 6.6. Two-dimensional 1H-15N HQSC spectra were acquired for α-syn at 100 μM concentration in the absence of Gn Rb1 and in the presence of increasing Gn Rb1: α-syn stoichiometries of 1:1, 4:1 and 6:1. Data were collected on a Bruker 900 MHz spectrometer equipped with a cold probe.
RESULTS
The effect of ginsenosides on α-syn fibrillation
A 25 μM solution of α-syn , which was purified by reverse-phase HPLC chromatography and its purity was estimated by SDS-PAGE and immunoblotting (Scheme 2 B, C) was incubated at 37°C with continuous shaking for 5 days, leading to fibril formation, which was monitored by Th-T fluorescence at regular time intervals. α-Syn was incubated with Gn Rg1, Gn (20S)-Rg3 and Gn Rb1 at molar ratios of 4:1, 2:1 and 1:1 (molar ratio ginsenosides: α-syn) with a constant α-syn concentration of 25 μM.
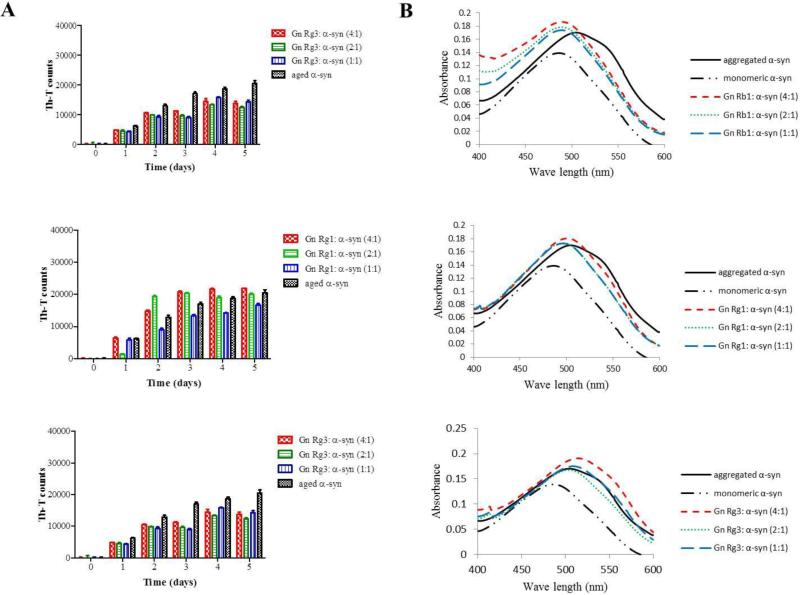
From the three tested compounds, only Gn Rb1 exhibited a significant inhibitory effect on α-syn fibrillation as indicated by the reduced Th-T fluorescence at all tested concentrations (Fig. 3 A). This effect was observed as early as 2 days of incubation, when the inhibition percentage was approximately 90%. After 5 days of incubation, the fibrillation of α-syn was reduced by approximately 80%. In contrast, Gn Rg3 showed a partial inhibitory effect, which was most prominent on the third day of incubation. After 5 days of incubation, Gn Rg3 inhibited the fibrillation of α-syn by approximately 25%, (Fig. 3 A). The extent of inhibition was comparable for all tested concentrations. The third tested compound, Gn Rg1, had no significant effect on α-syn fibrillation given the comparable Th-T measurements produced by the Gn Rg1 containing samples and the control, that is the α-syn solution aged alone (Fig. 3 A).
Figure 3. Gn Rb1 inhibits α-syn fibrillation.
A. Samples of α-syn (25 μM) were incubated for 5 days at 37°C with continuous shaking in the presence of various concentrations of the ginsenosides Rb1, Rg1 and (20S)-Rg3 (100 μM, 50 μM and 25 μM). Fibril formation was measured by Th-T binding assay. The assay was performed in triplicate, and the means ± standard deviations are shown. B. Congo red binding for Gn Rb1, Gn Rg1 and Gn (20S)-Rg3. α-Syn (5 μM) aged alone or in the presence of different concentrations of ginsenosides was mixed with Congo red (final concentration of 5 μM). The UV absorbance spectrum was recorded from 400 to 600 nm in a spectrophotometer. C. Silver staining for 15% SDS gel of α-syn monomers, α-syn aged alone or in the presence of Rb1, Rg1 and Rg3 at molar ratio 1:1 after 2.5 μg/ml PK digestion. D. Electron microscopy images of negatively stained samples of α-syn (25 μM) aged alone or in the presence of the ginsenosides (gincenoside: α-syn molar ratios of 4:1, 2:1, and 1:1) for 5 days with continuous shaking at 37°C. Scale bar, 500 nm.
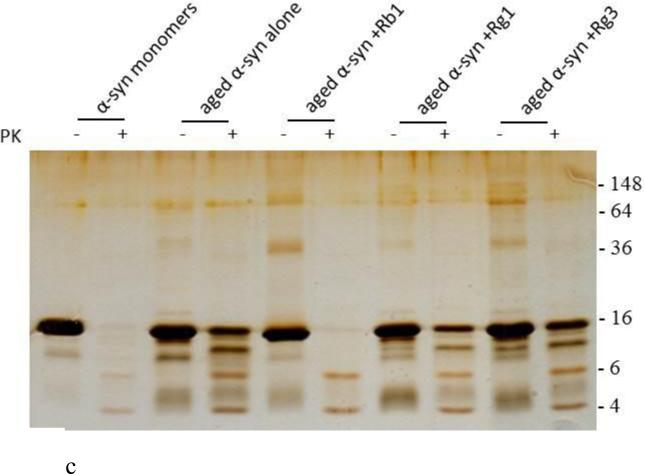
The ability of the three ginsenosides to block α-syn fibril formation was further assessed by the Congo red (CR) binding assay. CR is a dye with high affinity for amyloid fibrils (Groenning, 2010). Upon binding to α-syn fibrils, the absorption maximum of CR shifts from 490 to 508 nm. This shift was quite pronounced for the α-syn sample incubated in the absence of any of the three compounds (i.e. control) (Fig. 3 B). However, this characteristic shift was not observed for α-syn samples aged in the presence of Gn Rb1, indicating that this compound inhibited the formation of amyloid fibrils (Fig. 3 B). Indeed, the absorption maximum of CR bound to the α-syn samples containing Gn Rb1 (at all tested concentrations) only shifted a few nm and did not exceed the wavelength of 495 nm (Fig. 3 B). Interestingly, the CR shift was comparable to the shift observed for monomeric α-syn. In contrast, the samples incubated in the presence of Gn Rg1 and (20S)-Rg3 behaved similarly to the control sample, exhibiting the characteristic shift from 490 nm to approximately 508 nm. To test if the formed α-syn aggregates is resistant to PK digestion, samples of α-syn aggregated alone or in the presence of Gn Rb1, Rg1 and Rg3 at molar ratio of 1:1 were treated with 2.5 μg/ml PK; the digested samples were then run on 15% SDS-PAGE and the gel was silver stained as described in the materials and methods. The α-syn aged in the presence of Rb1 was easily digested by PK and behaved exactly as the monomeric α-syn (Fig. 3 C), while the samples of aged α-syn alone or in the presence of Rg1 and Rg3 were resistant to digestion (Fig. 3 C) possibly due to presence of β-sheets in these samples (Fig. 3 A, B).
These findings were further confirmed by electron microscopy. TEM images of α-syn aged in the presence of Gn Rb1 showed that α-syn formed thin, short, rod-like fibrils, with a fragmented appearance (Fig. 3 D), unlike the dense meshes of long fibrils formed by α-syn aged alone (Fig.3). For both Gn Rg1 and Gn (20S)-Rg3, TEM confirmed that there was little or no change in fibril morphology compared with the control, consistent with the Th-T fluorescence and CR binding findings (Fig. 3 D).
The effect of ginsenosides on α-syn oligomerization
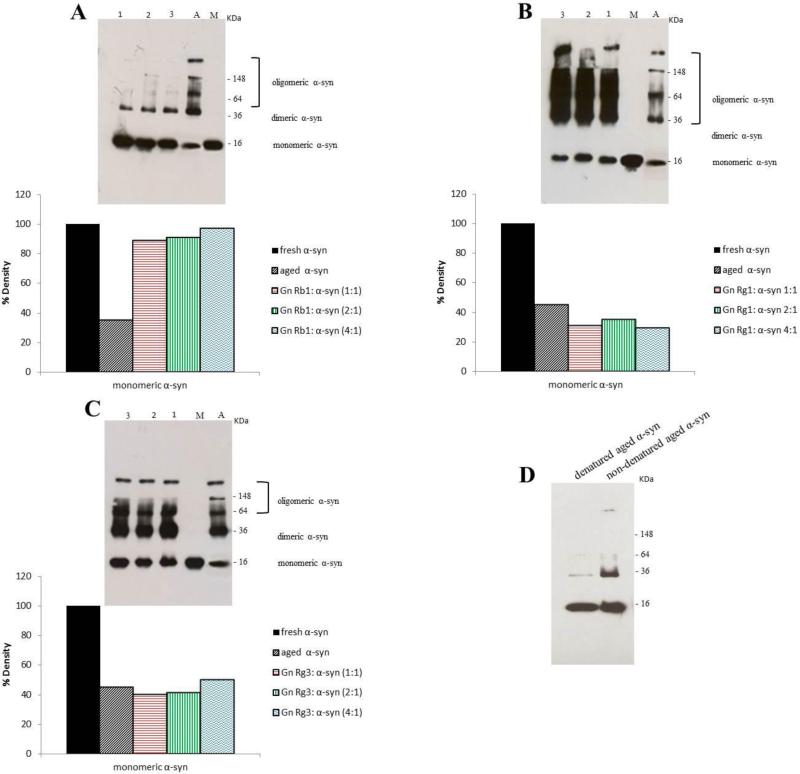
The effect of the Gn Rg1, Gn (20S)-Rg3 and Gn Rb1 over α-syn oligomerization was assessed by western blotting. The samples of α-syn aged alone or in the presence of the three ginsenosides were centrifuged (14,000 rpm, 15 minutes, 4°C) and the supernatants were separated on 15% SDS gels, transferred onto nitrocellulose membranes and probed with an antibody that recognizes the amino acid residues 121-125 of α-syn (anti-α-syn clone 211). Gn Rb1, which was shown to inhibit α-syn fibrillation, was also a potent inhibitor of α-syn oligomerization at all molar ratios (Fig. 4 A). More specifically, Gn Rb1 inhibited the formation of larger aggregates (MW >250 kDa) and high MW oligomers, generating a prominent monomeric band and a weak dimeric band (Fig. 4 A). Densitometric analysis of the monomeric bands revealed that Gn Rb1 hindered α-syn oligomerization to the same extent in all tested concentrations, (Fig. 4 A). It should be noted that even at the lowest concentration, Gn Rb1 was able to inhibit the formation of larger aggregates.
Figure 4. Immunoblot analysis showing the effect of ginsenosides on α-syn oligomerization.
α-Syn alone or in the presence of ginsenosides at ginsenoside: α-syn molar ratios of 1:1, 2:1 and 4:1, was incubated for 5 days. Lane 1, ginsenoside: α-syn 1:1, lane 2, ginsenoside: α-syn 2:1, lane 3, ginsenoside: α-syn 4:1, A: aged α-syn and M: fresh α-syn. A. Gn Rb1, B. Gn Rg1, C. Gn Rg3. The amount of the monomeric α-syn in the samples was quantified using ImageJ software. D. Immunoblotting for aged α-syn samples under denaturing and non-denaturing conditions.
The other two ginsenosides, Gn Rg1 and Gn (20S)-Rg3, failed to inhibit α-syn oligomerization, similarly to their effect on α-syn fibrillation. In fact, in the presence of Gn Rg1, α-syn oligomerization was enhanced compared with the control, as indicated by the detection of numerous strong bands corresponding to the oligomeric species (Fig. 4 B). Densitometric analysis of the monomeric bands showed that Gn Rg1 stimulated α-syn oligomerization to the same extent at all tested concentrations (Fig. 4 B). In the case of Gn (20S)- Rg3, at all tested concentrations of the compound, the separation of α-syn in the gel generated bands comparable with that of the control, indicating that Gn (20S)-Rg3 failed to inhibit α-syn oligomerization (Fig. 4 C).
The effect of ginsenosides on α-syn-induced cytotoxicity
BE(2)-M17 human neuroblastoma cells were treated with aged α-syn solutions at three different concentrations, 0.5 μM, 1 μM and 5 μM, either alone or in the presence of Gn Rb1, Gn Rg1 and Gn Rg3 and the viability of the treated cells was determined by MTT assay. Prior to the experiments with α-syn and ginsenosides, we assessed the effect of the ginsenosides alone on cell viability (Fig. 5 left panel), employing the same non-toxic ginsenoside concentrations that were later employed for the experiments with aged α-syn.
Figure 5. The effect of the ginsenosides on the toxicity induced by the aggregates of α-syn.
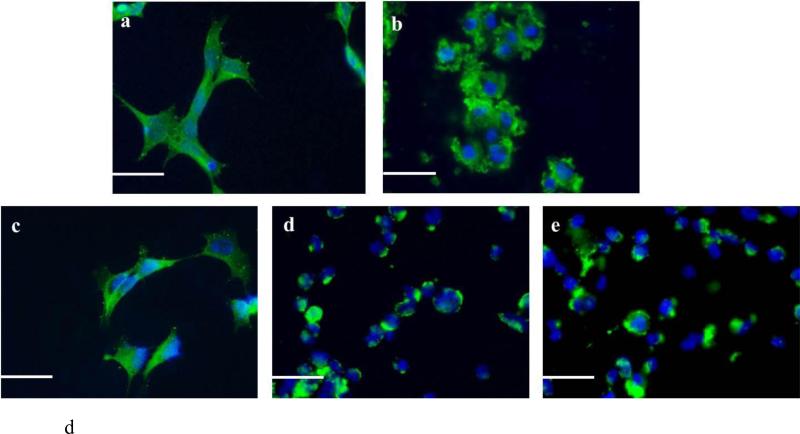
The viability of BE(2)-M17 human cells was evaluated by MTT assay. The results are expressed as percentages of the average of the control (i.e. untreated cells). The cells were treated with either α-syn aged with or without the A. Rb1, B. Rg1, and C. Rg3 for 48 hours prior to the addition of MTT. The graphs appearing on the left panel illustrate the toxicity of the compounds alone (average of 3 wells ± standard deviation). Statistical analysis was performed using a two-tailed unpaired t-test. ***, p< 0.001; **, p< 0.01; *, p< 0.05. D. Immunocytochemistry against α-syn of BE(2)-M17 cells. a.The cells were either non-treated or treated for 48 hours with 5 μM of b. aged α-syn alone, or with ginsenoside c. Rb1, d. Rg1, e. Rg3. at a molar ratio of aged α-syn: compound 1:4. Scale bar 30 μm.
As shown in Fig. 5 A-C, aged α-syn inhibited the reduction of MTT in a dose-dependent fashion. Given that MTT reduction is directly proportional to the number of surviving cells, it becomes apparent that fewer cells survived at higher concentrations of aged α-syn (Fig. 5 A-C). However, the α-syn aged in the presence of Gn Rb1 was less toxic to the cells, as indicated by the increase in MTT reduction (Figs. 5 A). Indeed, at 5 μM, the aged α-syn resulted in a decrease in the number of viable cells by almost 50%, whereas in the presence of all concentrations of Gn Rb1, the viability of the cells increased by approximately 30%, with approximately 80% of the cells surviving (Fig. 5 A). A similar trend was observed in the case of 1 μM aged α-syn; in all used concentrations, Gn Rb1 improved the viabilty of the cells treated with 1 μM aged α-syn by approximately 30 % (Fig. 5 A). Gn Rg1 and Gn (20S)-Rg3, however, did not show any protective effect on neuroblastoma cells against the toxicity of aged α-syn (Fig. 5 B, C), possibly due to their inability to inhibit α-syn fibrillation (Fig. 3) and oligomerization (Fig. 4). To confirm the toxicity effect observed in the MTT assay, BE(2)-M17 neuroblastoma cells were exposed to 5 μM α-syn aged in the presence or absence of the ginsenocide at a molar ratio of 1:4 (α-syn: Gn) and subjected to immunostaining for α-syn. Fluorescence microscopy revealed that, whereas untreated cells were healthy and displayed diffuse cytoplasmic α-syn staining (Fig. 5 D a), the cells treated with aged α-syn lost their neuronal shape, appearing rounded and unhealthy, with aggregated fibrils being accumulate at the cell membranes (Fig. 5 D b). This effect was dramatically reversed when the cells were treated with α-syn aged in the presence of Gn Rb1, with no apparent aggregates detected on the cell membranes (Fig. 5 D c). In contrast, the cells treated with the α-syn aged in the presence of Rg1 and Rg3, which were shown above not to inhibit α-syn aggregation, appeared rounded, unhealthy and bearing extracellular, membrane-bound aggregates (Fig. 5 D d, e).
The effect of Gn Rb1 on preformed α-syn amyloid fibrils
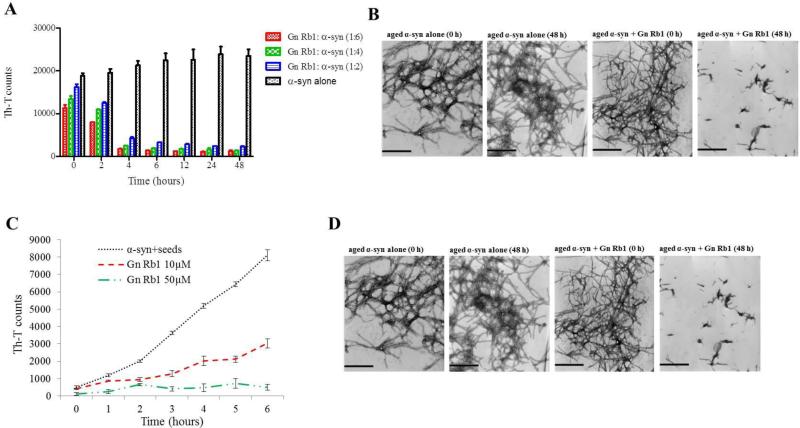
Due to its high efficiency in inhibiting α-syn fibrillation, Gn Rb1 was also assessed for its effectiveness in reversing fibrillation. Hence, 25 μM of preformed α-syn fibrils were incubated at 37°C in the presence of Gn Rb1 at molar ratios representing Gn Rb1: α-syn of 6:1, 4:1 and 2:1 for a period of 48 hours. By measuring the Th-T fluorescence counts, the fibril content was estimated at different time points. Even at time 0, the Th-T counts for α-syn incubated alone were much higher compared to the Th-T counts of the samples containing Gn Rb1, especially the one with the highest concentration of the particular ginsenoside (Fig. 6 A). During the course of the experiment, the α-syn fibrils that were incubated in the absence of Gn Rb1 continued to aggregate further, as indicated by the increase in Th-T counts (Fig. 6 A), whereas the fibrils incubating in the presence of the ginsenoside disaggregated in a dose-dependent fashion, given the decrease in Th-T counts (Fig. 6 A). This trend was apparent after 4 hours of incubation, with the Th-T counts for the Gn Rb1 containing samples being approximately 1/5 of the Th-T counts of the control.
Figure 6. The effect of Gn Rb1 on preformed α-syn fibrils and on the seeded polymerization of α-syn.
A. Samples of aggregated α-syn were incubated for 48 hours at 37°C in the absence or presence of various concentrations of Gn Rb1 (Gn Rb1: α-syn at 6:1, 4:1, and 2:1). The fibril content was then measured by the Th-T binding assay. The assay was performed in triplicate (average of triplicate measurements ± standard deviations). B. Electron microscopy images of negatively stained samples of the pre-aggregated α-syn incubated alone or in the presence of Gn Rb1 (1:4) for 0 and 48 hours with continuous shaking at 37°C. Scale bar, 500 nm. C. Samples of α-syn monomers (100 μM) were seeded with 2 μM sonicated α-syn fibrils, which were incubated in the presence or absence of Gn Rb1 at different concentrations (10 and 50 μM) for 6 hours with continuous shaking at 37°C. The extent of fibrillation was estimated by the Th-T binding assay. The assay was performed in triplicate (average of triplicate measurements ± standard deviations). D. Electron microscopy images of negatively stained samples of the α-syn seeds alone and of the seeded α-syn incubated in the absence or presence of Gn Rb1 (50 μM) for 6 hours with continuous shaking at 37°C. Scale bar, 1,000 nm.
The effect of Gn Rb1 on the seeding of α-syn monomers
It has been previously shown that the process of amyloid fibril formation follows a nucleation-dependent polymerization model (Jarrett & Lansbury, 1992). According to this model, soluble species generate via nucleation oligomeric species (nucleation or lag time phase), which in turn polymerize (polymerization or growth phase) to generate fibrils, thus reaching a final plateau known as the equilibrium phase (Harper, Wong, Lieber, & Lansbury, 1999). Small aggregates or seeds have been shown to accelerate the nucleation phase of amyloid formation in vitro and in vivo via a process known as seeding (Harper & Lansbury, 1997; Jarrett & Lansbury, 1993; Luk et al., 2012; Volpicelli-Daley et al., 2011). Given that Gn Rb1 inhibited α-syn fibrillation and disaggregated preformed α-syn fibrils, we sought to identify the effect of this ginsenoside on the seeding of α-syn aggregation. More specifically, mature α-syn fibrils were fragmented by sonication to obtain short fibrils, which were employed as ‘seeds’ (Fig. 6 C). These short fibrillar seeds were then added to monomeric α-syn, which was allowed to aggregate as described above. As expected, the addition of the short fibrillar seeds accelerated the fibrillation process of α-syn monomers, as indicated by the increase in Th-T counts.
To assess the effect of Gn Rb1 on the seeding of α-syn aggregation, the compound, employed at two different concentrations, 10 or 50 μM, was added to 100 μM monomeric α-syn containing seeds at a final concentration of 2 μM. The mixture was then incubated with continuous agitation at 37°C for 6 hours. The effect of Gn Rb1 on the seeded aggregation of α-syn was apparent after 2 hours of incubation, with the Th-T counts for the control being double of the counts for the Gn Rb1 containing samples. At this time point, both the Gn Rb1 containing samples, which represented two different concentrations of the ginsenoside, gave comparable measurements. However, after 6 hours of incubation the sample containing 50 μM of Gn Rb1 appeared much more efficient than the one containing 10 μM of the particular ginsenoside. Indeed, at a concentration of 50 μM, Gn Rb1 inhibited the seeding process by approximately 90%, whereas at a concentration of 10 μM, it only inhibited the seeding process by approximately 60% (Fig. 6 C). These findings were further confirmed by TEM (Fig. 6 D). After 6h of incubation, the seeded α-syn formed networks of fibrils, unlike the sample that contained Gn Rb1which consisted of mostly globular forms of α-syn (Fig. 6 D).
Gn Rb1 inhibition of α-syn fibrillation is mediated via binding to the intermediate species and formation of stable oligomers
The strong inhibitory effect that Gn Rb1 showed on fibrillation, prompted us to investigate further the interaction of Gn Rb1 with α-syn oligomers. For this purpose, monomeric α-syn (100 μM) was aggregated in the presence of Gn Rb1 (Gn Rb1: α-syn molar ratio 4:1). After 5 days of incubation, the samples were centrifuged and the supernatant was injected in a superdex 200 SE column. The elution volume for monomeric α-syn was determined by molecular weight standards, and was eluted in a peak corresponding to column volume of 14-16 ml, while oligomeric α-syn eluted in a peak corresponding to column volume of approximately 2-3 and 10-14 ml (Fig. 7 A). The fractions corresponding to the oligomeric and monomeric α-syn peaks were separately pooled together giving rise to P1, P2 and P3 samples (Fig. 7 A), which were concentrated using a speed vac. The α-syn species in the samples were characterized by western blotting and EM (Fig. 7 A, B). In agreement with the imunoblotting results (Fig. 7 A), electron microscopy of the samples indicated the presence of different species of oligomers in P1 and P2 (Fig. 7 B). In order to detect the incorporated Gn Rb1 in the P1, P2 and P3 samples, we exploited the property of Gn Rb1 to produce UV absorbance spectra with three notable peaks (Fig. 7 C).
Figure 7. Gn Rb1 binds to α-syn oligomers (Gn Rb1: α-syn molar ratio of 4:1).
A. Gel filtration profile of the 5-day aggregated α-syn in the presence of Gn Rb1 at a (Gn Rb1: α-syn) molar ratio 4:1 (α-syn concentration =100 μM) using a superdex 200 SE column. P1 and P2 samples contain the isolated fractions corresponding to the oligomeric peak and P3 the isolated fractions corresponding to the monomeric peak. The elution was monitored at the absorbance wavelength of 215 nm, immunoblot analysis of the samples P1 P2 and P3 separated by electrophoresis in a 15% SDS-PAGE gel. B. Electron microscopy images of negatively stained samples P1, P2 and P3 of α-syn in the presence of Gn Rb1 (molar ratio of Gn Rb1: α-syn 4:1) purified by SEC. Scale bar, 500 nm. C. UV absorbance spectra of Gn Rb1 alone. The UV absorbance was recorded between 200-600 nm employing a 10 mm quartz cuvette. D. UV absorbance spectra P1, P2 and P3. The UV absorbance was recorded between 200-600 nm employing a 10 mm quartz cuvette.
In the sample containing Gn Rb1: α-syn at 4:1 molar ratio, we detected Gn Rb1 only in the oligomeric P2 samples (Fig. 7 D). These findings strongly support the hypothesis that Gn Rb1 binds to the oligomeric intermediate species and stabilizes them.
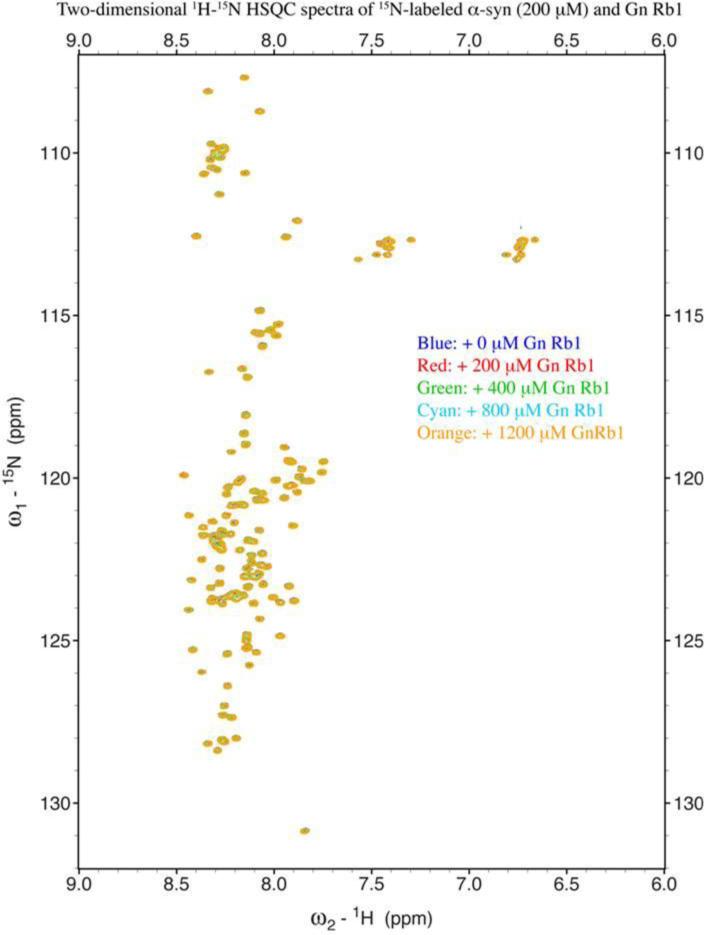
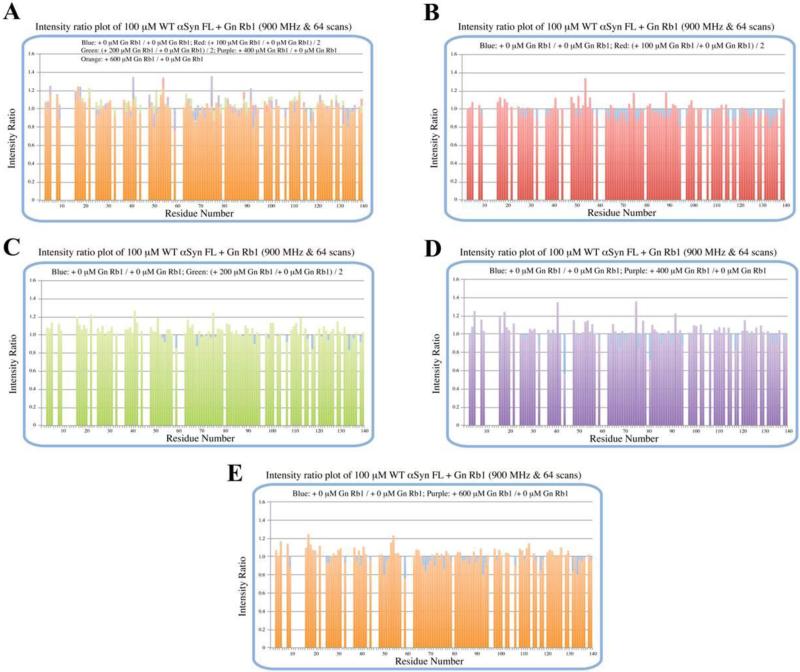
To further evaluate whether Gn Rb1 interacts with α-syn monomers, we monitored a titration of Gn Rb1 into a solution of monomeric α-syn using two-dimensional NMR spectroscopy, which provides signals covering the entire amino acid sequence of α-syn (Fig. 8). At stoichiometries of up to 6:1 Gn Rb1: α-syn, we observed no significant chemical shift or resonance intensity changes (Fig. 9 A-D), confirming that Gn Rb1, does not interact significantly with monomeric α-syn.
Figure 8. Analysis of Gn Rb1 binding to monomeric α-syn by NMR spectroscopy.
Proton-Nitrogen correlation (HSQC) spectra of monomeric α-syn in the presence of increasing ratios of Gn Rb1: α-syn demonstrating that there are no significant changes in the positions of the NMR resonances, indicating the lack of an interaction between Gn Rb1 and monomeric α-syn. Protein concentration: 200 μM.
Figure 9.
Peak intensity ratio plot of 200 μM 15N-labeled wt α-syn FL + Gn Rb1 (Gn Rb1: α-syn 1:1, 2:1, 4:1 and 6:1). From the HSQC spectra and the intensity plot, there might be no interaction between α-syn monomer and Gn Rb1. A. all; B. 1:1/0:1 and 0:1/0:1; C. 2:1/0:1 and 0:1/0:1; D. 4:1/0:1 and 0:1/0:1; E. 6:1/0:1 and 0:1/0:1.
DISCUSSION
PD is a progressive neurodegenerative disorder, the incidence of which is expected to rise sharply worldwide by 2030, with PD cases doubling by that time (Dorsey et al., 2007). Existing treatments for PD cannot cure the disease and can only offer moderate symptom relief, which is moreover accompanied by adverse side effects. Hence, there is a definite and imperative need for the development of new and more efficient treatments. Although PD pathology is considered to be induced by various etiological factors (reviewed by Di Monte, 2003), there is ample evidence indicating that α-syn misfolding and aggregation play a central role in the pathogenesis of PD and related disorders (Baba et al., 1998; Spillantini et al., 1998; Spillantini et al., 1997). As a result, there is an ongoing endeavor to identify or design molecules that can suppress or even reverse α-syn aggregation (reviewed by Findeis, 2000). Ginseng and its biologically active components, the ginsenosides, have been shown to have a beneficial effect on neurological conditions such as PD, due to their neuroprotective properties (Cho, 2012; H. J. Kim et al., 2013), but their potential as anti-amyloidogenic agents have never been assessed. This gap in knowledge prompted us to investigate the effect of the most frequently used ginsenosides, namely Rb1, Rg1 and Rg3, on the formation of the early aggregates (soluble oligomers) and late aggregates (fibrils) of α-syn. For this purpose we employed an array of biophysical, biochemical and cell-cultured based techniques.
Among the ginsenosides screened, ginsenoside Rb1 was shown to be the only potent inhibitor of α-syn fibrillation that adequately blocked α-syn-induced toxicity; it disaggregated preformed α-syn fibrils and inhibited the seeded aggregation of α-syn. More specifically, our results show for the first time that Gn Rb1 can disrupt the fibrillation of α-syn in vitro (Fig. 3 A, B) allowing only the formation of small, sheared, rod-like species that are easily digested by PK (Fig. 3 C) and fail to elongate even after an extended aggregation period (Fig. 3 D). The accumulation of α-syn monomers was first detected by immunoblotting and densitometric analysis of the monomeric bands (Fig. 4 A) and was further confirmed by SEC (Fig. 7 A). Furthermore, Gn Rb1 could satisfactorily curb the neurotoxic effect that was provoked by the aged α-syn, demonstrating a marked increase in cell survival and exhibiting a neuroprotective effect on neuroblastoma cells (Fig. 5 A, D). Gn Rb1 also disaggregated preformed α-syn fibrils (Fig. 6 A) and inhibited the seeded aggregation of α-syn in a concentration-dependent manner (Fig. 6 B, C).
These results are in accordance with previous reports that described Gn Rb1 as a neurotrophic and anti-inflammatory agent. Gn Rb1 has been previously shown to exert cell protective effects against the toxicity induced by Aβ42 (Qian, Han, Hu, & Shi, 2009) and Aβ25-35 (X. Xie et al., 2010). In animal models, Gn Rb1 was demonstrated to have anti-inflammatory activity (Wang et al., 2011) and to reduce soluble Aβ40 in a dose-dependent fashion (Shi et al., 2010). Interestingly, Gn Rb1 was shown to attenuate the Aβ25-35-induced hyperphosphorylation of tau protein in vitro (Y. H. Xie et al., 2007) and Aβ25-35-induced memory impairment, axonal atrophy and synaptic loss in vivo (Tohda, Matsumoto, Zou, Meselhy, & Komatsu, 2004).
In terms of structure, Gn Rb1is a triterpene saponin, containing a four-ring steroidal structure that bears an aliphatic side chain and two disaccharide moieties attached to C-3 and C-20 of the triterpene structure (Fig. 1). Since one of the disaccharides of Rb1 is attached to the 3-position of the dammarane-type triterpine, Gn Rb1 is a protopanaxadiol-type of gindenoside, like Gn (20S)-Rg3. By comparing the structure of Gn Rb1 with that of Gn (20S)-Rg3 that partially inhibited fibrillation but failed to inhibit α-syn oligomerisation and toxicity (Fig. 1), it becomes apparent that the difference between the two ginsenosides lies in the presence of two additional sugar rings in Gn Rb1 at C-20 of the triterpene. As a consequence, Gn Rb1 possesses overall four sugar rings that are clustered in the form of disaccharides in each side of the dammarane-type triterpene, thus conferring a more symmetrical structure to Gn Rb1 as compared to Gn (20S)-Rg3, which has only a disaccharide group attached to C-3 of the triterpene. Although structural symmetry is not a prerequisite for the inhibition of amyloid aggregation, there are many naturally occurring compounds whose structural symmetry renders them potent anti-aggregating agents in vitro (Caruana et al., 2011; Porat, Abramowitz, & Gazit, 2006).
Furthermore, it is well understood that the specific action of each ginsenoside may depend on the type of sugar components, their number and their position on the aglycone (Liu et al., 2003; Nag et al., 2012; Popovich & Kitts, 2002; Qi, Wang, & Yuan, 2010; Sun, Yang, & Ye, 2006). It is therefore likely that the number of sugar moieties may also play an important role in the anti-amyloidogenic properties of the ginsenosides. Gn Rb1 that was shown to be the most potent inhibitor possesses four sugar rings, while Gn (20S)-Rg3, which partially inhibited α-syn fibrillation but failed to exhibit any inhibitory effect in the rest of the assays, and possesses two sugar moieties. Although both Gn (20S)-Rg3 and Gn Rg1 (which failed to inhibit fibrillation and stimulated the oligomerisation of α-syn) contain two sugar rings, there is a major difference between the two ginsenosides. Gn (20)-Rg3 belongs to the protopanaxadiol group of gindenosides, whereas Gn Rg1 belongs to the protopanaxatriol group, which is characterized by the presence of an OH or a sugar group at C-6 of the triterpene. Gn Rg1 bears a sugar moiety at C-6 (Fig. 1). It has been reported before that the sugar linkage at C-6 is associated with a decreased ginsenoside anticancer activity compared to the linkages at C-3 or C-20 (R. J. Chen, Chung, Li, Lin, & Tzen, 2009). Also, the sugar linkage at C-6 has been shown to confer an antioxidant quality to the ginsenosides (Liu et al., 2003). There is hence the possibility that the C-6 linkage also renders the ginsenosides poor anti-amyloidogenic agents. Based on our findings using both NMR and SEC combined with UV spectroscopy, Gn Rb1 inhibits α-syn fibrillation not by interacting with the monomeric α-syn and preventing it from fibrillation, but rather by stabilizing the structure of soluble oligomeric α-syn without any β-sheet content (Fig. 4 A, 7 A), which appears to be non-toxic (Fig. 5 A, D). Since some interactions between α-syn and binding partners are not revealed in chemical shift changes, we also analysed for changes to resonance intensities, which can sometimes reveal interactions that are otherwise missed. In this case, neither chemical shifts nor intensities changed. Nevertheless, it remains possible that a very weak interaction occurs that is missed by both measures, as indeed was the case in the analysis of Hsp70 binding by Dedmon et al. (Dedmon, Christodoulou, Wilson, & Dobson, 2005). The NMR data suggests that if any interaction is present with monomeric α-syn, it is a very weak and transient one. When combined with the size exclusion data, we conclude that the *predominant* interaction of Rb1 is with oligomeric α-syn rather than with the monomeric protein. These findings are in accordance with previous studies indicating that some polyphenolic compounds, such as baicalein, curcumin and epigallocatechin gallate induce the formation of soluble, nontoxic oligomers of α-syn or Aβ (Porat et al., 2006).
Amyloid fibrils formed by aged α-syn are rich in β-sheet content and binding of these aggregates to the cell membrane results in membrane permeabilization and a resultant alteration in calcium homeostasis, which can cause cytotoxicity (El-Agnaf et al., 1998; Pieri, Madiona, Bousset, & Melki, 2012; Reynolds et al., 2011). Indeed, the fluorescence pattern of α-syn aggregates that we observed in cultured BE(2)-M17 cells exposed to aggregated α-syn alone is suggestive of the localization of these aggregates to the plasma membrane, while the cells lost their characteristic neuronal shape as they appeared rounded and unhealthy. Treatment of the cells with α-syn aged in the presence of Gn Rb1, which did not result in α-syn fibrillation, appeared healthy without the accumulation of α-syn at the cell membranes, whereas exposure to α-syn aged in the presence of Rg1 or Rg3, which were poor inhibitors of α-syn fibrillation, led to the formation of membrane-bound aggregates, and the cells appeared unhealthy and rounded. This observation is in accordance with the results obtained from the MTT assay, wherein cells treated with aged α-syn alone or in the presence of Rg1 or Rg3 displayed cytotoxicity, which was not observed when the cells were exposed to α-syn aged in the presence of Rb1. Since Rb1 was found to inhibit α-syn fibrillation and stabilize soluble, non-toxic oligomers without a β-sheet content (Fig. 3), we hypothesize that the neuroprotective role of Rb1 stems from its ability to inhibit the formation of mature amyloid fibrils containing β-sheet structures and thereby prevent plasma membrane disruption in neuroblastoma cells. However, we cannot exclude the possibility that the anti-oxidant properties (Y. H. Xie et al., 2007) and the anti-inflammatory activity (Wang et al., 2011) of Gn Rb1 may also contribute to its neuroprotective effect. Interestingly, Gn Rb1 has previously been shown to be a much more potent anti-oxidant than Rg1, while Rg3 was shown to promote the free radical-induced heamolysis in human red blood cells (Liu et al., 2003).
In conclusion, from the three tested ginsenosides, Rb1 was shown for the first time to inhibit α-syn fibrillation and toxicity in vitro and to be able to disaggregate preformed fibrils and block the α-syn seeded polymerization possibly by binding and stabilizing non-toxic α-syn oligomers without β-sheet content. The next step is to assess its effect on PD animal models. Gn Rb1 could thus represent the starting point for designing new molecules that in turn could be used as new drugs for the treatment of PD and related disorders.
Highlights.
For the first time, the effect of the most Ginsenosides, namely Rg1, Rg3 and Rb1, on α-syn aggregation and toxicity was determined.
Only Rb1 was shown to be a potent inhibitor of α-syn fibrillation and toxicity.
Rb1 exhibited a strong ability to disaggregate preformed fibrils and to inhibit the seeded polymerization of α-syn.
Rb1 was found to stabilize soluble non-toxic oligomers with no β-sheet content that were susceptible to proteinase K digestion.
Acknowledgments
Funding Sources
This work is supported by grant from Sheikh Hamdan Bin Rashid Al Maktoum Award for Medical Sciences (Dubai, UAE; Grant MRG-23/2005-2006). This study was made possible by NPRP grant 4-1371-1-223 from the Qatar National Research Fund (a member of Qatar Foundation). The statements made herein are solely the responsibility of the author[s]. DE is also supported by NIH/NIA grant AG019391. MA was supported by United Arab Emirates University- PhD scholarship.
ABBREVIATIONS
- α-syn
α-synuclein
- PD
Parkinson's disease
- Aβ
beta amyloid
- LBs
Lewy bodies
- LNs
Lewy neuritis
- Gn
Ginsenoside
- GST
Glutathion S-transferase
- IPTG
sopropyl β-D-1-thiogalactopyranoside
- DTT
Dithiothreitol
- SDS
sodium dodecyl sulfate
- PBS
phosphate buffered saline
- Th-T
Thioflavin-T
- MTT
3-(4, 5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- TEM
transmission electron microscope
- WB
western blot
- PK
proteinase K
- NMR
nuclear magnetic resonance
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
REFERENCES
- Ardah MT, Paleologou KE, Lv G, Abul Khair SB, Kazim AS, Minhas ST, El-Agnaf OM. Structure activity relationship of phenolic acid inhibitors of α-synuclein fibril formation and toxicity. Front Aging Neurosci. 2014;6:197. doi: 10.3389/fnagi.2014.00197. doi: 10.3389/fnagi.2014.00197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attele AS, Wu JA, Yuan CS. Ginseng pharmacology: multiple constituents and multiple actions. Biochem Pharmacol. 1999;58(11):1685–1693. doi: 10.1016/s0006-2952(99)00212-9. [DOI] [PubMed] [Google Scholar]
- Baba M, Nakajo S, Tu PH, Tomita T, Nakaya K, Lee VM, Iwatsubo T. Aggregation of alpha-synuclein in Lewy bodies of sporadic Parkinson's disease and dementia with Lewy bodies. Am J Pathol. 1998;152(4):879–884. [PMC free article] [PubMed] [Google Scholar]
- Bussell R, Eliezer D. A structural and functional role for 11-mer repeats in alpha-synuclein and other exchangeable lipid binding proteins. J Mol Biol. 2003;329(4):763–778. doi: 10.1016/s0022-2836(03)00520-5. [DOI] [PubMed] [Google Scholar]
- Caruana M, Högen T, Levin J, Hillmer A, Giese A, Vassallo N. Inhibition and disaggregation of α-synuclein oligomers by natural polyphenolic compounds. FEBS Lett. 2011;585(8):1113–1120. doi: 10.1016/j.febslet.2011.03.046. doi: 10.1016/j.febslet.2011.03.046. [DOI] [PubMed] [Google Scholar]
- Chen F, Eckman EA, Eckman CB. Reductions in levels of the Alzheimer's amyloid beta peptide after oral administration of ginsenosides. FASEB J. 2006;20(8):1269–1271. doi: 10.1096/fj.05-5530fje. doi: 10.1096/fj.05-5530fje. [DOI] [PubMed] [Google Scholar]
- Chen RJ, Chung TY, Li FY, Lin NH, Tzen JT. Effect of sugar positions in ginsenosides and their inhibitory potency on Na+/K+-ATPase activity. Acta Pharmacol Sin. 2009;30(1):61–69. doi: 10.1038/aps.2008.6. doi: 10.1038/aps.2008.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho IH. Effects of Panax ginseng in Neurodegenerative Diseases. J Ginseng Res. 2012;36(4):342–353. doi: 10.5142/jgr.2012.36.4.342. doi: 10.5142/jgr.2012.36.4.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway KA, Lee SJ, Rochet JC, Ding TT, Williamson RE, Lansbury PT., Jr. Acceleration of oligomerization, not fibrillization, is a shared property of both alpha-synuclein mutations linked to early-onset Parkinson's disease: implications for pathogenesis and therapy. Proc Natl Acad Sci U S A. 2000;97(2):571–576. doi: 10.1073/pnas.97.2.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedmon MM, Christodoulou J, Wilson MR, Dobson CM. Heat shock protein 70 inhibits alpha-synuclein fibril formation via preferential binding to prefibrillar species. J Biol Chem. 2005;280(15):14733–14740. doi: 10.1074/jbc.M413024200. doi: 10.1074/jbc.M413024200. [DOI] [PubMed] [Google Scholar]
- Di Giovanni S, Eleuteri S, Paleologou KE, Yin G, Zweckstetter M, Carrupt PA, Lashuel HA. Entacapone and tolcapone, two catechol O-methyltransferase inhibitors, block fibril formation of alpha-synuclein and beta-amyloid and protect against amyloid-induced toxicity. J Biol Chem. 2010;285(20):14941–14954. doi: 10.1074/jbc.M109.080390. doi: M109.080390 [pii] 10.1074/jbc.M109.080390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Monte DA. The environment and Parkinson's disease: is the nigrostriatal system preferentially targeted by neurotoxins? Lancet Neurol. 2003;2(9):531–538. doi: 10.1016/s1474-4422(03)00501-5. [DOI] [PubMed] [Google Scholar]
- Dorsey ER, Constantinescu R, Thompson JP, Biglan KM, Holloway RG, Kieburtz K, Tanner CM. Projected number of people with Parkinson disease in the most populous nations, 2005 through 2030. Neurology. 2007;68(5):384–386. doi: 10.1212/01.wnl.0000247740.47667.03. doi: 10.1212/01.wnl.0000247740.47667.03. [DOI] [PubMed] [Google Scholar]
- El-Agnaf OM, Jakes R, Curran MD, Middleton D, Ingenito R, Bianchi E, Wallace A. Aggregates from mutant and wild-type alpha-synuclein proteins and NAC peptide induce apoptotic cell death in human neuroblastoma cells by formation of beta-sheet and amyloid-like filaments. FEBS Lett. 1998;440(1-2):71–75. doi: 10.1016/s0014-5793(98)01418-5. doi: S0014-5793(98)01418-5 [pii] [DOI] [PubMed] [Google Scholar]
- El-Agnaf OM, Walsh DM, Allsop D. Lancet Neurol. Vol. 2. England: 2003. Soluble oligomers for the diagnosis of neurodegenerative diseases; pp. 461–462. [DOI] [PubMed] [Google Scholar]
- Eliezer D, Kutluay E, Bussell R, Browne G. Conformational properties of alpha-synuclein in its free and lipid-associated states. J Mol Biol. 2001;307(4):1061–1073. doi: 10.1006/jmbi.2001.4538. doi: 10.1006/jmbi.2001.4538. [DOI] [PubMed] [Google Scholar]
- Findeis MA. Approaches to discovery and characterization of inhibitors of amyloid beta-peptide polymerization. Biochim Biophys Acta. 2000;1502(1):76–84. doi: 10.1016/s0925-4439(00)00034-x. [DOI] [PubMed] [Google Scholar]
- Galvin JE, Lee VM, Schmidt ML, Tu PH, Iwatsubo T, Trojanowski JQ. Pathobiology of the Lewy body. Adv Neurol. 1999;80:313–324. [PubMed] [Google Scholar]
- Goedert M, Spillantini MG, Del Tredici K, Braak H. 100 years of Lewy pathology. Nat Rev Neurol. 2013;9(1):13–24. doi: 10.1038/nrneurol.2012.242. doi: 10.1038/nrneurol.2012.242. [DOI] [PubMed] [Google Scholar]
- Groenning M. Binding mode of Thioflavin T and other molecular probes in the context of amyloid fibrils-current status. J Chem Biol. 2010;3(1):1–18. doi: 10.1007/s12154-009-0027-5. doi: 10.1007/s12154-009-0027-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper JD, Lansbury PT. Models of amyloid seeding in Alzheimer's disease and scrapie: mechanistic truths and physiological consequences of the time-dependent solubility of amyloid proteins. Annu Rev Biochem. 1997;66:385–407. doi: 10.1146/annurev.biochem.66.1.385. doi: 10.1146/annurev.biochem.66.1.385. [DOI] [PubMed] [Google Scholar]
- Harper JD, Wong SS, Lieber CM, Lansbury PT. Assembly of A beta amyloid protofibrils: an in vitro model for a possible early event in Alzheimer's disease. Biochemistry. 1999;38(28):8972–8980. doi: 10.1021/bi9904149. doi: 10.1021/bi9904149. [DOI] [PubMed] [Google Scholar]
- He P, Li P, Hua Q, Liu Y, Staufenbiel M, Li R, Shen Y. Chronic administration of anti-stroke herbal medicine TongLuoJiuNao reduces amyloidogenic processing of amyloid precursor protein in a mouse model of Alzheimer's disease. PLoS One. 2013;8(3):e58181. doi: 10.1371/journal.pone.0058181. doi: 10.1371/journal.pone.0058181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im DS, Nah SY. Yin and Yang of ginseng pharmacology: ginsenosides vs gintonin. Acta Pharmacol Sin. 2013;34(11):1367–1373. doi: 10.1038/aps.2013.100. doi: 10.1038/aps.2013.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrett JT, Lansbury PT. Amyloid fibril formation requires a chemically discriminating nucleation event: studies of an amyloidogenic sequence from the bacterial protein OsmB. Biochemistry. 1992;31(49):12345–12352. doi: 10.1021/bi00164a008. [DOI] [PubMed] [Google Scholar]
- Jarrett JT, Lansbury PT. Seeding “one-dimensional crystallization” of amyloid: a pathogenic mechanism in Alzheimer's disease and scrapie? Cell. 1993;73(6):1055–1058. doi: 10.1016/0092-8674(93)90635-4. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Kim P, Shin CY. A comprehensive review of the therapeutic and pharmacological effects of ginseng and ginsenosides in central nervous system. J Ginseng Res. 2013;37(1):8–29. doi: 10.5142/jgr.2013.37.8. doi: 10.5142/jgr.2013.37.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Cho SY, Lee JH, Jeong SM, Yoon IS, Lee BH, Nah SY. Neuroprotective effects of ginsenoside Rg3 against homocysteine-induced excitotoxicity in rat hippocampus. Brain Res. 2007;1136(1):190–199. doi: 10.1016/j.brainres.2006.12.047. doi: 10.1016/j.brainres.2006.12.047. [DOI] [PubMed] [Google Scholar]
- Liu ZQ, Luo XY, Liu GZ, Chen YP, Wang ZC, Sun YX. In vitro study of the relationship between the structure of ginsenoside and its antioxidative or prooxidative activity in free radical induced hemolysis of human erythrocytes. J Agric Food Chem. 2003;51(9):2555–2558. doi: 10.1021/jf026228i. doi: 10.1021/jf026228i. [DOI] [PubMed] [Google Scholar]
- Luk KC, Kehm V, Carroll J, Zhang B, O'Brien P, Trojanowski JQ, Lee VM. Pathological α-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science. 2012;338(6109):949–953. doi: 10.1126/science.1227157. doi: 10.1126/science.1227157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lü JM, Yao Q, Chen C. Ginseng compounds: an update on their molecular mechanisms and medical applications. Curr Vasc Pharmacol. 2009;7(3):293–302. doi: 10.2174/157016109788340767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nag SA, Qin JJ, Wang W, Wang MH, Wang H, Zhang R. Ginsenosides as Anticancer Agents: In vitro and in vivo Activities, Structure-Activity Relationships, and Molecular Mechanisms of Action. Front Pharmacol. 2012;3:25. doi: 10.3389/fphar.2012.00025. doi: 10.3389/fphar.2012.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obeso JA, Rodríguez-Oroz MC, Benitez-Temino B, Blesa FJ, Guridi J, Marin C, Rodriguez M. Functional organization of the basal ganglia: therapeutic implications for Parkinson's disease. Mov Disord. 2008;23(Suppl 3):S548–559. doi: 10.1002/mds.22062. doi: 10.1002/mds.22062. [DOI] [PubMed] [Google Scholar]
- Pieri L, Madiona K, Bousset L, Melki R. Fibrillar α-synuclein and huntingtin exon 1 assemblies are toxic to the cells. Biophys J. 2012;102(12):2894–2905. doi: 10.1016/j.bpj.2012.04.050. doi: 10.1016/j.bpj.2012.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popovich DG, Kitts DD. Structure-function relationship exists for ginsenosides in reducing cell proliferation and inducing apoptosis in the human leukemia (THP-1) cell line. Arch Biochem Biophys. 2002;406(1):1–8. doi: 10.1016/s0003-9861(02)00398-3. [DOI] [PubMed] [Google Scholar]
- Porat Y, Abramowitz A, Gazit E. Inhibition of amyloid fibril formation by polyphenols: structural similarity and aromatic interactions as a common inhibition mechanism. Chem Biol Drug Des. 2006;67(1):27–37. doi: 10.1111/j.1747-0285.2005.00318.x. doi: 10.1111/j.1747-0285.2005.00318.x. [DOI] [PubMed] [Google Scholar]
- Qi LW, Wang CZ, Yuan CS. American ginseng: potential structure-function relationship in cancer chemoprevention. Biochem Pharmacol. 2010;80(7):947–954. doi: 10.1016/j.bcp.2010.06.023. doi: 10.1016/j.bcp.2010.06.023. [DOI] [PubMed] [Google Scholar]
- Qian YH, Han H, Hu XD, Shi LL. Protective effect of ginsenoside Rb1 on beta-amyloid protein(1-42)-induced neurotoxicity in cortical neurons. Neurol Res. 2009;31(7):663–667. doi: 10.1179/174313209X385572. doi: 10.1179/174313209X385572. [DOI] [PubMed] [Google Scholar]
- Radad K, Moldzio R, Rausch WD. Ginsenosides and their CNS targets. CNS Neurosci Ther. 2011;17(6):761–768. doi: 10.1111/j.1755-5949.2010.00208.x. doi: 10.1111/j.1755-5949.2010.00208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds NP, Soragni A, Rabe M, Verdes D, Liverani E, Handschin S, Seeger S. Mechanism of membrane interaction and disruption by α-synuclein. J Am Chem Soc. 2011;133(48):19366–19375. doi: 10.1021/ja2029848. doi: 10.1021/ja2029848. [DOI] [PubMed] [Google Scholar]
- Shi YQ, Huang TW, Chen LM, Pan XD, Zhang J, Zhu YG, Chen XC. Ginsenoside Rg1 attenuates amyloid-beta content, regulates PKA/CREB activity, and improves cognitive performance in SAMP8 mice. J Alzheimers Dis. 2010;19(3):977–989. doi: 10.3233/JAD-2010-1296. doi: 10.3233/JAD-2010-1296. [DOI] [PubMed] [Google Scholar]
- Spillantini MG, Crowther RA, Jakes R, Hasegawa M, Goedert M. alpha-Synuclein in filamentous inclusions of Lewy bodies from Parkinson's disease and dementia with lewy bodies. Proc Natl Acad Sci U S A. 1998;95(11):6469–6473. doi: 10.1073/pnas.95.11.6469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997;388(6645):839–840. doi: 10.1038/42166. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- Sun H, Yang Z, Ye Y. Structure and biological activity of protopanaxatriol-type saponins from the roots of Panax notoginseng. Int Immunopharmacol. 2006;6(1):14–25. doi: 10.1016/j.intimp.2005.07.003. doi: 10.1016/j.intimp.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Tohda C, Matsumoto N, Zou K, Meselhy MR, Komatsu K. Abeta(25-35)-induced memory impairment, axonal atrophy, and synaptic loss are ameliorated by M1, A metabolite of protopanaxadiol-type saponins. Neuropsychopharmacology. 2004;29(5):860–868. doi: 10.1038/sj.npp.1300388. doi: 10.1038/sj.npp.1300388. [DOI] [PubMed] [Google Scholar]
- Uversky VN, Lee HJ, Li J, Fink AL, Lee SJ. Stabilization of partially folded conformation during alpha-synuclein oligomerization in both purified and cytosolic preparations. J Biol Chem. 2001;276(47):43495–43498. doi: 10.1074/jbc.C100551200. doi: 10.1074/jbc.C100551200. [DOI] [PubMed] [Google Scholar]
- Volpicelli-Daley LA, Luk KC, Patel TP, Tanik SA, Riddle DM, Stieber A, Lee VM. Exogenous α-synuclein fibrils induce Lewy body pathology leading to synaptic dysfunction and neuron death. Neuron. 2011;72(1):57–71. doi: 10.1016/j.neuron.2011.08.033. doi: 10.1016/j.neuron.2011.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Liu J, Zhang Z, Bi P, Qi Z, Zhang C. Anti-neuroinflammation effect of ginsenoside Rbl in a rat model of Alzheimer disease. Neurosci Lett. 2011;487(1):70–72. doi: 10.1016/j.neulet.2010.09.076. doi: 10.1016/j.neulet.2010.09.076. [DOI] [PubMed] [Google Scholar]
- Wee JJ, P. K, Chung A-S. Biological activities of ginseng and its application to human health. 2nd edition ed. CRC Press; Boca Raton (FL): 2011. [PubMed] [Google Scholar]
- Weinreb PH, Zhen W, Poon AW, Conway KA, Lansbury PT. NACP, a protein implicated in Alzheimer's disease and learning, is natively unfolded. Biochemistry. 1996;35(43):13709–13715. doi: 10.1021/bi961799n. doi: bi961799n [pii] 10.1021/bi961799n. [DOI] [PubMed] [Google Scholar]
- Winner B, Jappelli R, Maji SK, Desplats PA, Boyer L, Aigner S, Riek R. Proc Natl Acad Sci U S A. Vol. 108. United States: 2011. In vivo demonstration that alpha-synuclein oligomers are toxic; pp. 4194–4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X, Wang HT, Li CL, Gao XH, Ding JL, Zhao HH, Lu YL. Ginsenoside Rb1 protects PC12 cells against β-amyloid-induced cell injury. Mol Med Rep. 2010;3(4):635–639. doi: 10.3892/mmr_00000308. doi: 10.3892/mmr_00000308. [DOI] [PubMed] [Google Scholar]
- Xie YH, Chen XC, Zhang J, Huang TW, Song JQ, Fang YX, Lin ZY. [Ginsenoside Rb1 attenuates beta-amyloid peptide(25-35) -induced hyperphosphorylation of tau protein through CDK5 signal pathway]. Yao Xue Xue Bao. 2007;42(8):828–832. [PubMed] [Google Scholar]