Abstract
Background
Autoimmune diseases encompass a broad range of illnesses with a variety of underlying causes, some of which are known and some of which remain elusive.
Objective
The focus of this review will be on describing the development of a new type of therapy that could potentially treat T cell-mediated autoimmune diseases. Unlike traditional therapies, which have primarily focused on suppressing T cells directly, targeting the step of antigen presentation may allow a less toxic therapy in which autoimmunity is lessened without compromising the entire immune system. This review will outline the science behind the development of the therapy, the roles of dendritic cells in generating autoimmune disease, and the function of the FLT3 receptor in this process.
Keywords: autoimmune disease, dendritic cell, FLT3, signal transduction
1. Role of dendritic cells in autoimmunity
Dendritic cells (DCs) are a specialized cell of the immune system that serve as a link between the innate and adaptive immune system and have an extremely potent capacity to activate naive T cells [1]. While T cells are generally thought to be the mediators of many autoimmune diseases (Table 1), they receive instruction on when to become activated by DCs. Thus, targeting the step of DC activation of T cells is an upstream step at which potential intervention may take place. In addition, the interaction between DCs and T cells not only instructs the T cells on activation but also on the characteristics and differentiation of T cells; interaction with DCs and the cytokines produced by DCs can drive T cells towards these phenotypes. Targeting the differentiation of T cells such that they are skewed away from a particular phenotype would be one possibly selective way of downregulating an autoimmune response while keeping helpful immune responses intact. While the exact nature of DCs responsible for generating autoreactive responses remains controversial, more data have been generated that implicate the potential for DCs to contribute to pathology. DCs provide an attractive alternative target in that the specific antigen does not need to be identified, issues such as epitope spreading are avoided, and it may be possible to bias the response away from an autoimmune response but leave beneficial immune responses intact.
Table 1.
Known or suspected T cell-mediated autoimmune diseases.
| Multiple sclerosis |
| Type 1 diabetes |
| Psoriasis |
| Systemic lupus erythematosus |
| Rheumatoid arthritis |
| Sjögren’s syndrome |
| Crohn’s disease |
| Myasthenia gravis |
| Dermatomyositis |
| Addison’s disease |
| Grave’s disease |
| Pernicious anemia |
| Primary biliary cirrhosis |
| Scleroderma |
| Hashimoto’s thyroiditis |
| Uveitis |
| Vitiligo |
Initially, the inflammatory profile of TH1 cells (those that secrete IFN-γ) implicated these cells in the pathogenesis of autoimmune disease and they became a major target for therapy development. Many T-cell autoimmune diseases, including psoriasis [2–4], EAE (experimental allergic encephalomyelitis) and MS (multiple sclerosis) [5–7], arthritis [8,9], Crohn’s disease [10,11] and type 1 diabetes [12–14] have been shown to have an increase in TH 1 cells and IL-12, which were both associated with both the generation and severity of disease. IL-12 and IFN-γ are necessary for the generation of TH 1 responses. On a molecular level, T-bet was shown to be necessary for the generation of IFN-γ and considered to be a ‘master regulator’ of TH1 generation [15,16], and thus became a new type of target for inhibition.
IL-12 is a heterodimer, made up of two components, known as p40 and p35 subsets. It was later discovered that the p40 subset is shared with another cytokine, IL-23, which is made up of p40 and a second component, p19 [17]. IL-23, which is produced by DCs, is important for driving T cells to differentiate along the TH17 pathway, which derived their name from their secretion of IL-17. Interestingly, although ROR-α and ROR-γ have been identified as transcription factors that drive cells towards TH17 [18,19], T-bet may also be involved in the generation of the autoimmune responses of the TH17 subset [20]. Additional studies showed that this more recently defined subset of T cells was necessary for either onset or maintenance of a number of disease models [21]. In addition, in several case studies, these cells have been identified in patients with autoimmune diseases [22–24]. For the future, targeting this cell type selectively may lead to the generation of less toxic and more specific therapies.
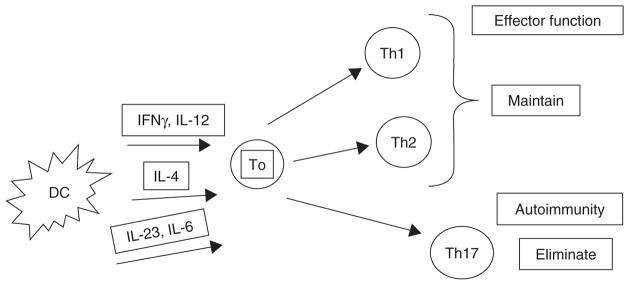
As mentioned, DCs are thought to stimulate the differentiation of T cells into TH17 in part because of their secretion of cytokines, notably IL-23, TGF-β, and IL-6 [25]. Thus, preventing the differentiation into TH17 cells by blocking this step may be one avenue by which such a goal could be achieved (Figure 1).
Figure 1.
Manipulating DC function as a means to maintain beneficial immune responses while eliminating harmful ones.
Another important barrier to successful autoimmune disease therapy is the issue of antigen identification and specificity. As T cells are specific for an antigen, targeting the T cells requires not only that the antigen be identified but also that the antigen remain stable. In a disease model for MS and EAE, as well as other autoimmune diseases, a number of reports have identified the occurrence of epitope spreading [26,27], which is the process by which T cells that have a different specificity from the original antigenic epitope become mediators of disease. Thus, in effect, T cells with the original specificity could be eliminated, but disease would not be improved, because of the emergence of T cells with specificity for new epitopes. In addition, tolerance to the newly-identified epitopes has been shown to improve disease outcome, further indicating the relevance of this event in disease progression.
2. FLT3 signaling
FLT3 is a receptor tyrosine kinase that was originally reported in 1991 as a murine gene with similarities to fms, kit, and pdgfr. It was thus named as fms-like tyrosine kinase [28]. It was shown to have a relatively restricted expression in hematopoietic stem and progenitor cells [29,30]. This restricted expression led to the study of this gene as an important contributor to stem cell survival, and the human form of FLT3 was cloned shortly thereafter and was shown to be important for bone marrow stem cell survival [31]. Its relevance in disease was investigated in 1992, with the report that 92% of human acute myelogenous leukemia cells expressed high levels of FLT3 message [32]; and further in 1996, with the finding of high-level protein expression in AML and B-lineage leukemias [33]. Its function as a strong growth stimulus thus had the potential for a significantly negative outcome, which was found to be the case with acute myeloid leukaemia (AML).
The role of FLT3 in DC development was first reported in 1996, with both in vivo and in vitro studies showing that treatment of progenitor cells or mice with FLT3L led to the generation of DCs [34,35] and that mice that are deficient in FLT3L have a profound defect in the generation of DCs [36]. With the notion that stimulation through FLT3 could generate DCs, studies were undertaken in which FLT3L was investigated as an immunostimulatory antitumor agent and found to produce significant anticancer effects [37]. This initial report was followed by multiple others showing that FLT3L, either alone or in combination with other agents, elicited antitumor effects [38–40]. Thus, the immunostimulatory capacity of stimulation through FLT3 was fairly clearly demonstrated; although, in some models, limitations were observed [41,42].
3. Development of FLT3 inhibitors
The findings described above that demonstrated a high level of expression of FLT3 in leukemias led to further discoveries that constitutive activation of FLT3 was occurring through mutation in some forms of leukemia. As a form of molecular targeted therapy, a number of small-molecule FLT3 tyrosine kinase inhibitors (TKI) were developed, and continue to be developed, for the treatment of AML in particular [43–46]. These molecules include CEP-701/lestaurtinib, MLN 518/CT53518/tandutinib [47,48], PKC412 [49,50], SU11248/sunitinib [51,52], BAY43-9006/sorafenib [53,54] and SU5614 [55], among others. As signaling through the receptor leads to kinase activation, these small compounds prevent signal transduction by competitively inhibiting the binding of ATP to the receptor’s active site. These types of drugs are potentially appealing for the treatment of autoimmune disease, since they are often administered orally, which would provide a significant advantage over currently available treatments; in addition, several of them have already been tested in clinical trials. Thus, much of the pharmacokinetic and toxicology information is already available.
As inhibitors of signal transduction pathways have varying degrees of specificity, many agents are multi-kinase inhibitors, and the targeting of these additional pathways should also be considered. In some instances, this may contribute to a therapeutic effect, if an additional target also contributes to pathology.
4. FLT3 inhibition as an approach to autoimmunity
The role of FLT3 in generating DCs has been shown through results of studies demonstrating that mice deficient in FLT3L have markedly reduced numbers of DCs and that mice that have been administered FLT3L have dramatically increased numbers of DCs. While some currently used therapies have effects on DCs, the rational targeting of DCs as opposed to T cells is a relatively recent concept.
While these and other studies indicated that FLT3 expression on progenitor cells was necessary for the development of DCs, receptor expression on mature DCs had not been reported until two studies have showed that mature steady-state DCs retained expression of FLT3 [56,57] and, importantly, that the receptor was activated upon exposure to ligand [57]. The activation of FLT3 on mature cells was an important consideration, since inhibition of signaling would only produce an effect if the mature cells maintained signaling through this receptor. Of note, expression was maintained on DCs derived from common myeloid progenitors as well as from common lymphoid progenitors [56], indicating that most, if not all, DC subsets would be potential targets for this class of agents.
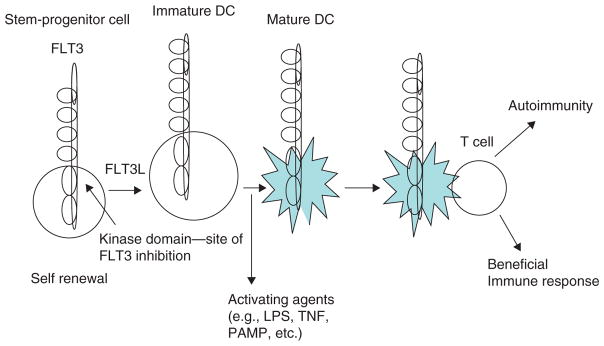
While treatment with FLT3 inhibitors is likely to lower the number of DCs through its actions on progenitors, DCs also appear to rely on ongoing signaling through FLT3, since inhibition produced apoptosis in a significant fraction of the more mature DCs. These findings are significant in that in order for FLT3 inhibition to have an effect on ongoing disease, presumably mature DCs would need to be somewhat dependent on this pathway (Figure 2).
Figure 2.
FLT3 signaling as a target for DCs.
Two separate studies have now reported results that suggest the possibility of developing FLT3 inhibition for the treatment of autoimmunity. In one study, development of type I interferon producing DCs (classically plasmacytoid DCs, pDCs) was inhibited in culture by treatment with SU11657 [58]. Further, in vivo treatment of mice produced a phenotype that was strikingly similar to that reported for the FLT3-deficient mice. In consideration for clinical use, there were additional implications of some of the results. First, the effect was reversible in vivo in that numbers of DCs returned to normal after discontinuation of therapy. Second, the effect of FLT3 inhibition on repopulation of bone marrow stem/progenitor cells was not affected. The second parameter was measured both in vitro for colony-forming capacity, and in vivo for hematopoietic reconstitution ability. Neither of these was dramatically reduced after treatment, indicating that no serious toxicities should be expected from usage of this class of agents [58].
In another study, the FLT3 inhibitor CEP-701 was used with similar results in terms of decreasing the in vivo populations of DCs and, further, decreasing an autoimmune response. Both pDCs and cDCs were decreased after in vivo administration of CEP-701, as well as NK cells; but no changes in mature B- and total T-cell number were observed. However, a decrease in expansion of autoreactive T cells was observed, and further, in the model system for multiple sclerosis and EAE, mice that had established disease showed a significant improvement in the course of disease after treatment with CEP-701. No major toxicity was observed, and the mice were able to ward off a Listeria infection in a manner similar to control counterparts, indicating that no severe gross immunosuppresion was present [57]. One possible mechanism for the downregulation in the effector response is that even activated T cells rely on continued co-stimulation to varying degrees; thus, it may be that inhibiting an ongoing T-cell response via inhibition of DCs will prove to be effective. Since DCs are also important to maintaining tolerance, it is possible that the reverse effect might have occurred, i.e., worse autoimmune disease; however, this was not the case in these experiments. Two significant advantages to this approach are that, in theory, it would be applicable to all T cell-mediated autoimmune diseases, since there is no antigen specificity required; and oral bioavailability and Phase II data in humans would make it easy to rapidly begin clinical testing once appropriate preclinical data are obtained.
5. Alternative approaches
Traditional approaches to treating autoimmune disease have primarily focused on downregulating immune responses nonspecifically. Drugs and biologics may act by decreasing the numbers of lymphocytes or subsets of lymphocytes, decreasing the activity of lymphocytes, altering traffic patterns of T cells, or shifting the phenotype of T cells. In the early 1980s, ciclosporin A was shown to suppress both organ rejection and autoimmunity. While this approach has had some degree of efficacy, it carries with it significant toxicity, due in a large part to its high degree of nonspecificity.
Cyclophosphamide is an alkylating agent that must be metabolized in vivo by the cytochrome P450 family in order to generate an active metabolite. Methotrexate (N-10-methylaminopterin) was developed in the 1940s as an antagonist for folic acid. Its mechanism of action is probably due to its competitive inhibition of dihydrofolate reductase, which leads to defects in the synthesis of DNA. It has been shown to have a number of toxic effects on the immune system, including inhibition of T-cell proliferation and activity as well as several off-target toxicities, including liver, gut, and brain.
The notion of using a B cell-targeted therapy in auto-immune disease was lent some support by a case report showing improvement in a patient with psoriasis who was being treated with an anti-CD20 antibody (rituximab) [59]. Since that time, trials have been undertaken to assess the efficacy of this approach; and in rheumatoid arthritis and systemic lupus erythematosus (SLE), it has shown some positive effects. It is currently under evaluation for MS, but no human trial data are yet available. Possible mechanisms of action of this agent include decreasing antigen presentation and decreased production of cytokines that drive inflammation and/or autoimmunity (e.g., IL-6), as well as eventual depletion of Ig.
Etanercept, a soluble tumor necrosis family receptor, has been tested in a number of autoimmune diseases, including psoriasis [60]. As TNF is a hallmark inflammatory cytokine and its upregulation has been noted in many autoimmune/inflammatory conditions, it might be expected that this approach would be applicable to most autoimmune diseases. However, while treatment of patients with psoriasis yielded positive clinical outcomes [61], there were reports of new-onset MS in at least one patient, and a trial testing this agent in MS was stopped prematurely due to worsening of disease. Thus, it can be difficult to predict the results that agents will generate under different circumstances.
Another cytokine that has been directly targeted is IL-12, which is required for driving TH1 responses and is necessary for generating T-cell responses against infectious agents; thus it is possible that inhibiting its function may lead to an undesirable level of immunosuppression, potentially resulting in infections. However, there are preclinical data that strongly support its role in TH1-mediated diseases. In addition, since it shares the p40 chain with IL-23, which has been strongly implicated in the generation of TH-IL-17 cells found in autoimmunity, this approach does have significant potential. Again, a limitation may be found in that TH1 cells may be required to fight off infection, which leads to the concern of an unacceptably high level of nonspecific immunosuppression. Encouragingly, one trial in plaque psoriasis reports an improvement of symptoms as well as a high level of tolerability [62].
Early reports of effects of statins on autoimmune disease were mixed, with an induction of SLE [63] and an improvement of symptoms in EAE [64–66], as well as in other diseases such as inflammatory arthritis [67] and others [68,69]. These drugs appear to have anti-inflammatory properties as well as effects on T-cell tolerance [70], modulating TH1:TH2 ratios [71] and cell migration [69].
Blocking migration of cells into inflamed tissue via targeting alpha 4 integrin is currently an approved therapy for MS and Crohn’s disease, and is being investigated as a therapy for other autoimmune diseases [72,73]; but fatal side effects related to JC viral infection in MS have limited its widespread application.
Another approach to blocking lymphocyte migration by limiting egress from the lymph node has been tested using FTY720, a sphingosine receptor agonist that is currently in Phase III trials for MS [74].
6. Expert opinion
Autoimmune diseases are absolutely in need of new types of therapies. The toxicities and lack of effectiveness for many of the currently available therapies produce significant limitations on their use. As most autoimmune diseases are chronic, developing therapies that could be used intermittently for long-term therapy is one goal. Targeting antigen presentation of self components is a new avenue that is being developed.
One challenge of decreasing autoimmune responses is discovering methods to selectively inhibit autoreactivity without generally suppressing the immune system. Targeting FLT3 is a potentially advantageous approach, in that it targets a signaling pathway that is expressed in antigen-presenting cells but not in mature B or T cells. This selectivity provides a theoretical advantage in two distinct considerations. First, the cells that are specific for an autoantigen are difficult to target specifically, as the antigen may either be unknown or may mutate over time. By targeting the antigen presentation, this limitation is bypassed. Second, since mature B and T cells are not direct targets, it may be possible to eliminate an auto-immune response without destroying existing B and T cells, which may help to decrease the common side effect of gross immunosuppression as a result of auto-immune therapy. In addition, the ability to administer some of these agents orally would present a distinct advantage for patients.
Acknowledgments
KA Whartenby is supported by NCI 111989. D Small is supported by NCI-CA70970 and the Kyle Haydock Professorship. PA Calabresi is supported by RO1-NS41435 and the National MS Society TR 3760-A-3.
Bibliography
Papers of special note have been highlighted as either of interest
• or of considerable interest
•• to readers.
- 1.Mellman I, Steinman RM. Dendritic cells: specialized and regulated antigen processing machines. Cell. 2001;106:255–8. doi: 10.1016/s0092-8674(01)00449-4. [DOI] [PubMed] [Google Scholar]
- 2.Schlaak JF, Buslau M, Jochum W, et al. T cells involved in psoriasis vulgaris belong to the Th1 subset. J Invest Dermatol. 1994;102:145–9. doi: 10.1111/1523-1747.ep12371752. [DOI] [PubMed] [Google Scholar]
- 3.Mollison KW, Fey TA, Gauvin DM, et al. A macrolactam inhibitor of T helper type 1 and T helper type 2 cytokine biosynthesis for topical treatment of inflammatory skin diseases. J Invest Dermatol. 1999;112(5):729–38. doi: 10.1046/j.1523-1747.1999.00567.x. [DOI] [PubMed] [Google Scholar]
- 4.Nestle FO, Turka LA, Nickoloff BJ. Characterization of dermal dendritic cells in psoriasis. Autostimulation of T lymphocytes and induction of Th1 type cytokines. J Clin Invest. 1994;94:202–9. doi: 10.1172/JCI117308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ando DG, Clayton J, Kono D, et al. Encephalitogenic T cells in the B10. PL model of experimental allergic encephalomyelitis (EAE) are of the Th-1 lymphokine subtype. Cell Immunol. 1989;124:132–43. doi: 10.1016/0008-8749(89)90117-2. [DOI] [PubMed] [Google Scholar]
- 6.Baron JL, Madri JA, Ruddle NH, et al. Surface expression of alpha 4 integrin by CD4 T cells is required for their entry into brain parenchyma. J Exp Med. 1993;177:57–68. doi: 10.1084/jem.177.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Voskuhl RR, Martin R, Bergman C, et al. T helper 1 (Th1) functional phenotype of human myelin basic protein-specific T lymphocytes. Autoimmunity. 1993;15:137–43. doi: 10.3109/08916939309043888. [DOI] [PubMed] [Google Scholar]
- 8.Joosten LA, Lubberts E, Helsen MM, van den Berg WB. Dual role of IL-12 in early and late stages of murine collagen type II arthritis. J Immunol. 1997;159:4094–102. [PubMed] [Google Scholar]
- 9.Matsumoto T, Ametani A, Hachimura S, et al. Intranasal administration of denatured type II collagen and its fragments can delay the onset of collagen-induced arthritis. Clin Immunol Immunopathol. 1998;88:70–9. doi: 10.1006/clin.1998.4521. [DOI] [PubMed] [Google Scholar]
- 10.Neurath MF, Fuss I, Kelsall BL, et al. Antibodies to interleukin 12 abrogate established experimental colitis in mice. J Exp Med. 1995;182:1281–90. doi: 10.1084/jem.182.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parronchi P, Romagnani P, Annunziato F, et al. Type 1 T-helper cell predominance and interleukin-12 expression in the gut of patients with Crohn’s disease. Am J Pathol. 1997;150(3):823–32. [PMC free article] [PubMed] [Google Scholar]
- 12.Jun HS, Yoon CS, Zbytnuik L, et al. The role of macrophages in T cell-mediated autoimmune diabetes in nonobese diabetic mice. J Exp Med. 1999;189(2):347–58. doi: 10.1084/jem.189.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Faulkner-Jones BE, Dempsey-Collier M, Mandel TE, Harrison LC. Both TH1 and TH2 cytokine mRNAs are expressed in the NOD mouse pancreas in vivo. Autoimmunity. 1996;23(2):99–110. doi: 10.3109/08916939608995333. [DOI] [PubMed] [Google Scholar]
- 14.Trembleau S, Germann T, Gately MK, Adorini L. The role of IL-12 in the induction of organ-specific autoimmune diseases. Immunol Today. 1995;16(8):383–6. doi: 10.1016/0167-5699(95)80006-9. [DOI] [PubMed] [Google Scholar]
- 15.Szabo SJ, Kim ST, Costa GL, et al. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100(6):655–69. doi: 10.1016/s0092-8674(00)80702-3. [DOI] [PubMed] [Google Scholar]
- 16.Szabo SJ, Sullivan BM, Stemmann C, et al. Distinct effects of T-bet in TH1 lineage commitment and IFN-gamma production in CD4 and CD8 T cells. Science. 2002;295(5553):338–42. doi: 10.1126/science.1065543. [DOI] [PubMed] [Google Scholar]
- 17.Trinchieri G, Pflanz S, Kastelein RA. The IL-12 family of heterodimeric cytokines: new players in the regulation of T cell responses. Immunity. 2003;19(5):641–4. doi: 10.1016/s1074-7613(03)00296-6. [DOI] [PubMed] [Google Scholar]
- 18.Ivanov II, McKenzie BS, Zhou L, et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126(6):1121–33. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 19.Yang XO, Pappu BP, Nurieva R, et al. T helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR alpha and ROR gamma. Immunity. 2008;28(1):29–39. doi: 10.1016/j.immuni.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gocke AR, Cravens PD, Ben LH, et al. T-bet regulates the fate of Th1 and Th17 lymphocytes in autoimmunity. J Immunol. 2007;178(3):1341–8. doi: 10.4049/jimmunol.178.3.1341. [DOI] [PubMed] [Google Scholar]
- 21••.Cua DJ, Sherlock J, Chen Y, et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421(6924):744–8. doi: 10.1038/nature01355. This paper demonstrated that IL-23 was critical to EAE pathology. [DOI] [PubMed] [Google Scholar]
- 22.Amadi-Obi A, Yu CR, Liu X, et al. TH17 cells contribute to uveitis and scleritis and are expanded by IL-2 and inhibited by IL-27/STAT1. Nature Med. 2007;13(6):711–8. doi: 10.1038/nm1585. [DOI] [PubMed] [Google Scholar]
- 23.Mangini AJ, Lafyatis R, Van Seventer JM. Type I interferons inhibition of inflammatory T helper cell responses in systemic lupus erythematosus. Ann NY Acad Sci. 2007;1108:11–23. doi: 10.1196/annals.1422.002. [DOI] [PubMed] [Google Scholar]
- 24.Yamada H, Nakashima Y, Okazaki K, et al. Th1 but not Th17 cells predominate in the joints of patients with rheumatoid arthritis. Ann Rheum Dis. 2007 doi: 10.1136/ard.2007.080341. [DOI] [PubMed] [Google Scholar]
- 25.Kimura A, Naka T, Kishimoto T. IL-6-dependent and -independent pathways in the development of interleukin 17-producing T helper cells. Proc Natl Acad Sci USA. 2007;104(29):12099–104. doi: 10.1073/pnas.0705268104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26••.McMahon EJ, Bailey SL, Castenada CV, et al. Epitope spreading initiates in the CNS in two mouse models of multiple sclerosis. Nature Med. 2005;11(3):335–9. doi: 10.1038/nm1202. This study showed the importance of sites of initiation of epitope spreading, which is a process that limits the effectiveness of many T cell-based therapies. [DOI] [PubMed] [Google Scholar]
- 27.Fairley JA, Woodley DT, Chen M, et al. A patient with both bullous pemphigoid and epidermolysis bullosa acquisita: an example of intermolecular epitope spreading. J Am Acad Dermatol. 2004;51(1):118–22. doi: 10.1016/j.jaad.2003.12.033. [DOI] [PubMed] [Google Scholar]
- 28.Rosnet O, Marchetto S, deLapeyriere O, Birnbaum D. Murine Flt3, a gene encoding a novel tyrosine kinase receptor of the PDGFR/CSF1R family. Oncogene. 1991;6(9):1641–50. [PubMed] [Google Scholar]
- 29.Matthews W, Jordan CT, Wiegand GW, et al. A receptor tyrosine kinase specific to hematopoietic stem and progenitor cell-enriched populations. Cell. 1991;65(7):1143–52. doi: 10.1016/0092-8674(91)90010-v. [DOI] [PubMed] [Google Scholar]
- 30.Rosnet O, Mattei MG, Marchetto S, Birnbaum D. Isolation and chromosomal localization of a novel FMS-like tyrosine kinase gene. Genomics. 1991;9(2):380–5. doi: 10.1016/0888-7543(91)90270-o. [DOI] [PubMed] [Google Scholar]
- 31.Small D, Levenstein M, Kim E, et al. STK-1, the human homolog of Flk-2/Flt-3, is selectively expressed in CD34+ human bone marrow cells and is involved in the proliferation of early progenitor/stem cells. Proc Natl Acad Sci USA. 1994;91(2):459–63. doi: 10.1073/pnas.91.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Birg F, Courcoul M, Rosnet O, et al. Expression of the FMS/KIT-like gene FLT3 in human acute leukemias of the myeloid and lymphoid lineages. Blood. 1992;80(10):2584–93. [PubMed] [Google Scholar]
- 33.Carow CE, Levenstein M, Kaufmann SH, et al. Expression of the hematopoietic growth factor receptor FLT3 (STK-1/Flk2) in human leukemias. Blood. 1996;87(3):1089–96. [PubMed] [Google Scholar]
- 34.Saunders D, Lucas K, Ismaili J, et al. Dendritic cell development in culture from thymic precursor cells in the absence of granulocyte/macrophage colony-stimulating factor. J Exp Med. 1996;184(6):2185–96. doi: 10.1084/jem.184.6.2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35•.Maraskovsky E, Brasel K, Teepe M, et al. Dramatic increase in the numbers of functionally mature dendritic cells in Flt3 ligand-treated mice: multiple dendritic cell subpopulations identified. J Exp Med. 1996;184(5):1953–62. doi: 10.1084/jem.184.5.1953. This study demonstrated that it was possible to use FLT3 ligand to generate large numbers of DCs in vivo. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36•.McKenna HJ, Stocking KL, Miller RE, et al. Mice lacking flt3 ligand have deficient hematopoiesis affecting hematopoietic progenitor cells, dendritic cells, and natural killer cells. Blood. 2000;95(11):3489–97. This study showed the importance of FLT3 in DC development by showing that mice deficient in ligand had reduced numbers of DCs. [PubMed] [Google Scholar]
- 37.Lynch DH, Andreasen A, Maraskovsky E, et al. Flt3 ligand induces tumor regression and antitumor immune responses in vivo. Nature Med. 1997;3(6):625–31. doi: 10.1038/nm0697-625. [DOI] [PubMed] [Google Scholar]
- 38.Borges L, Miller RE, Jones J, et al. Synergistic action of fms-like tyrosine kinase 3 ligand and CD40 ligand in the induction of dendritic cells and generation of antitumor immunity in vivo. J Immunol. 1999;163(3):1289–97. [PubMed] [Google Scholar]
- 39.Sivanandham M, Stavropoulos CI, Kim EM, et al. Therapeutic effect of colon tumor cells expressing FLT-3 ligand plus systemic IL-2 in mice with syngeneic colon cancer. Cancer Immunol Immunother. 2002;51(2):63–71. doi: 10.1007/s00262-001-0256-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Neal ZC, Sondel PM, Bates MK, et al. Flt3-L gene therapy enhances immunocytokine-mediated antitumor effects and induces long-term memory. Cancer Immunol Immunother. 2007;56(11):1765–74. doi: 10.1007/s00262-007-0320-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ciavarra RP, Brown RR, Holterman DA, et al. Impact of the tumor microenvironment on host infiltrating cells and the efficacy of flt3-ligand combination immunotherapy evaluated in a treatment model of mouse prostate cancer. Cancer Immunol Immunother. 2003;52(9):535–45. doi: 10.1007/s00262-003-0383-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ciavarra RP, Holterman DA, Brown RR, et al. Prostate tumor microenvironment alters immune cells and prevents long-term survival in an orthotopic mouse model following flt3-ligand/CD40-ligand immunotherapy. J Immunother. 2004;27(1):13–26. doi: 10.1097/00002371-200401000-00002. [DOI] [PubMed] [Google Scholar]
- 43.Tse KF, Novelli E, Civin CI, et al. Inhibition of FLT3-mediated transformation by use of a tyrosine kinase inhibitor. Leukemia. 2001;15(7):1001–10. doi: 10.1038/sj.leu.2402199. [DOI] [PubMed] [Google Scholar]
- 44•.Levis M, Allebach J, Tse KF, et al. A FLT3-targeted tyrosine kinase inhibitor is cytotoxic to leukemia cells in vitro and in vivo. Blood. 2002;99(11):3885–91. doi: 10.1182/blood.v99.11.3885. This study demonstrated that FLT3 inhibitors could kill leukemia cells that constitutively expressed FLT3. Further, treatment of mice bearing FLT3 mutant tumors with FLT3 inhibitors prolonged survival. [DOI] [PubMed] [Google Scholar]
- 45.Levis M, Small D. Novel FLT3 tyrosine kinase inhibitors. Exp Opin Investig Drugs. 2003;12(12):1951–62. doi: 10.1517/13543784.12.12.1951. [DOI] [PubMed] [Google Scholar]
- 46.Levis M, Small D. FLT3: ITDoes matter in leukemia. Leukemia. 2003;179:1738–52. doi: 10.1038/sj.leu.2403099. [DOI] [PubMed] [Google Scholar]
- 47.DeAngelo DJ, Stone RM, Heaney ML, et al. Phase 1 clinical results with tandutinib (MLN518), a novel FLT3 antagonist, in patients with acute myelogenous leukemia or high-risk myelodysplastic syndrome: safety, pharmacokinetics, and pharmacodynamics. Blood. 2006;108(12):3674–81. doi: 10.1182/blood-2006-02-005702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kelly LM, Yu JC, Boulton CL, et al. CT53518, a novel selective FLT3 antagonist for the treatment of acute myelogenous leukemia (AML) Cancer Cell. 2002;1(5):421–32. doi: 10.1016/s1535-6108(02)00070-3. [DOI] [PubMed] [Google Scholar]
- 49.Armstrong SA, Kung AL, Mabon ME, et al. Inhibition of FLT3 in MLL. Validation of a therapeutic target identified by gene expression based classification. Cancer Cell. 2003;3(2):173–83. doi: 10.1016/s1535-6108(03)00003-5. [DOI] [PubMed] [Google Scholar]
- 50.Weisberg E, Boulton C, Kelly LM, et al. Inhibition of mutant FLT3 receptors in leukemia cells by the small molecule tyrosine kinase inhibitor PKC412. Cancer Cell. 2002;1(5):433–43. doi: 10.1016/s1535-6108(02)00069-7. [DOI] [PubMed] [Google Scholar]
- 51.O’Farrell AM, Abrams TJ, Yuen HA, et al. SU11248 is a novel FLT3 tyrosine kinase inhibitor with potent activity in vitro and in vivo. Blood. 2003;101(9):3597–605. doi: 10.1182/blood-2002-07-2307. [DOI] [PubMed] [Google Scholar]
- 52.O’Farrell AM, Foran JM, Fiedler W, et al. An innovative Phase I clinical study demonstrates inhibition of FLT3 phosphorylation by SU11248 in acute myeloid leukemia patients. Clin Cancer Res. 2003;9(15):5465–76. [PubMed] [Google Scholar]
- 53.Auclair D, Miller D, Yatsula V, et al. Antitumor activity of sorafenib in FLT3-driven leukemic cells. Leukemia. 2007;21(3):439–45. doi: 10.1038/sj.leu.2404508. [DOI] [PubMed] [Google Scholar]
- 54.Zhang W, Konopleva M, Shi YX, et al. Mutant FLT3: a direct target of sorafenib in acute myelogenous leukemia. J Natl Cancer Inst. 2008;100(3):184–98. doi: 10.1093/jnci/djm328. [DOI] [PubMed] [Google Scholar]
- 55.Yee KW, O’Farrell AM, Smolich BD, et al. SU5416 and SU5614 inhibit kinase activity of wild-type and mutant FLT3 receptor tyrosine kinase. Blood. 2002;100(8):2941–9. doi: 10.1182/blood-2002-02-0531. [DOI] [PubMed] [Google Scholar]
- 56.Karsunky H, Merad M, Cozzio A, et al. Flt3 ligand regulates dendritic cell development from Flt3+ lymphoid and myeloid-committed progenitors to Flt3+ dendritic cells in vivo. J Exp Med. 2003;198(2):305–13. doi: 10.1084/jem.20030323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57••.Whartenby KA, Calabresi PA, McCadden E, et al. Inhibition of FLT3 signaling targets DCs to ameliorate autoimmune disease. Proc Natl Acad Sci USA. 2005;102(46):16741–6. doi: 10.1073/pnas.0506088102. This study showed that FLT3 inhibition therapy could improve the outcome of disease in mice with the autoimmune model EAE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tussiwand R, Onai N, Mazzucchelli L, Manz MG. Inhibition of natural type I IFN-producing and dendritic cell development by a small molecule receptor tyrosine kinase inhibitor with Flt3 affinity. J Immunol. 2005;175(6):3674–80. doi: 10.4049/jimmunol.175.6.3674. [DOI] [PubMed] [Google Scholar]
- 59.Singh F, Weinberg JM. Partial remission of psoriasis following rituximab therapy for non-Hodgkin lymphoma. Cutis. 2005;76(3):186–8. [PubMed] [Google Scholar]
- 60.Paller AS, Siegfried EC, Langley RG, et al. Etanercept treatment for children and adolescents with plaque psoriasis. N Engl J Med. 2008;358(3):241–1. doi: 10.1056/NEJMoa066886. [DOI] [PubMed] [Google Scholar]
- 61.Romero-Mate A, Garcia-Donoso C, Cordoba-Guijarro S. Efficacy and safety of etanercept in psoriasis/psoriatic arthritis: an updated review. Am J Clin Dermatol. 2007;8(3):143–55. doi: 10.2165/00128071-200708030-00002. [DOI] [PubMed] [Google Scholar]
- 62.Kimball AB, Gordon KB, Langley RG, et al. Safety and efficacy of ABT-874, a fully human interleukin 12/23 monoclonal antibody, in the treatment of moderate to severe chronic plaque psoriasis: results of a randomized, placebo-controlled, phase 2 trial. Arch Dermatol. 2008;144(2):200–7. doi: 10.1001/archdermatol.2007.63. [DOI] [PubMed] [Google Scholar]
- 63.Ahmad S. Lovastatin-induced lupus erythematosus. Arch Intern Med. 1991;151(8):1667–8. [PubMed] [Google Scholar]
- 64.Stanislaus R, Pahan K, Singh AK, Singh I. Amelioration of experimental allergic encephalomyelitis in Lewis rats by lovastatin. Neuroscience Lett. 1999;269(2):71–4. doi: 10.1016/s0304-3940(99)00414-0. [DOI] [PubMed] [Google Scholar]
- 65.Stanislaus R, Singh AK, Singh I. Lovastatin treatment decreases mononuclear cell infiltration into the CNS of Lewis rats with experimental allergic encephalomyelitis. J Neurosci Res. 2001;66(2):155–62. doi: 10.1002/jnr.1207. [DOI] [PubMed] [Google Scholar]
- 66.Youssef S, Stuve O, Patarroyo JC, et al. The HMG-CoA reductase inhibitor, atorvastatin, promotes a Th2 bias and reverses paralysis in central nervous system autoimmune disease. Nature. 2002;420(6911):78–84. doi: 10.1038/nature01158. [DOI] [PubMed] [Google Scholar]
- 67.Leung BP, Sattar N, Crilly A, et al. A novel anti-inflammatory role for simvastatin in inflammatory arthritis. J Immunol. 2003;170(3):1524–30. doi: 10.4049/jimmunol.170.3.1524. [DOI] [PubMed] [Google Scholar]
- 68.Aprahamian T, Bonegio R, Rizzo J, et al. Simvastatin treatment ameliorates autoimmune disease associated with accelerated atherosclerosis in a murine lupus model. J Immunol. 2006;177(5):3028–34. doi: 10.4049/jimmunol.177.5.3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gegg ME, Harry R, Hankey D, et al. Suppression of autoimmune retinal disease by lovastatin does not require Th2 cytokine induction. J Immunol. 2005;174(4):2327–35. doi: 10.4049/jimmunol.174.4.2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Waiczies S, Prozorovski T, Infante-Duarte C, et al. Atorvastatin induces T cell anergy via phosphorylation of ERK1. J Immunol. 2005;174(9):5630–5. doi: 10.4049/jimmunol.174.9.5630. [DOI] [PubMed] [Google Scholar]
- 71.Liu W, Li WM, Gao C, Sun NL. Effects of atorvastatin on the Th1/Th2 polarization of ongoing experimental autoimmune myocarditis in Lewis rats. J Autoimmun. 2005;25(4):258–63. doi: 10.1016/j.jaut.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 72.Sandborn WJ, Colombel JF, Enns R, et al. Natalizumab induction and maintenance therapy for Crohn’s disease. N Engl J Med. 2005;353(18):1912–25. doi: 10.1056/NEJMoa043335. [DOI] [PubMed] [Google Scholar]
- 73.Targan SR, Feagan BG, Fedorak RN, et al. Natalizumab for the treatment of active Crohn’s disease: results of the ENCORE Trial. Gastroenterology. 2007;132(5):1672–83. doi: 10.1053/j.gastro.2007.03.024. [DOI] [PubMed] [Google Scholar]
- 74.Kappos L, Antel J, Comi G, et al. Oral fingolimod (FTY720) for relapsing multiple sclerosis. N Engl J Med. 2006;355(11):1124–40. doi: 10.1056/NEJMoa052643. [DOI] [PubMed] [Google Scholar]