Abstract
Aims
The left ventricular global function index (LVGFI) is a novel indicator of left ventricular performance. Its prognostic value in patients after ST-segment elevation myocardial infarction (STEMI) is unknown. We sought to evaluate the prognostic significance of LVGFI measured by cardiovascular magnetic resonance (CMR) imaging after STEMI.
Methods and results
Two hundred eligible STEMI patients (56 ± 11 years, 16% female) revascularized by primary percutaneous coronary intervention were followed-up for 3.1 [2–4.1] years for major adverse cardiac events (MACE). MACE was defined as a composite of death, non-fatal myocardial re-infarction, and new congestive heart failure. All patients underwent CMR imaging within 2 [2–4] days after STEMI. Late enhancement and cine images were acquired to assess myocardial injury as well as myocardial function, including LVGFI. Patients suffering a MACE event (n = 20, 10%) had a significantly lower LVGFI (P = 0.001). In Kaplan–Meier analysis, a decreased LVGFI was associated with a reduced MACE-free survival (P < 0.001). Multivariate Cox regression analysis revealed a decreased LVGFI as a predictor for MACE [hazard ratio = 4.79, 95% confidence interval (CI) 1.46–15.67, P = 0.010] after adjusting for microvascular obstruction, left ventricular mass, and multivessel disease. In receiver operating characteristic analysis, LVGFI was a strong predictor for MACE (area under the curve = 0.73, CI 0.61–0.85). However, c-statistics revealed that LVGFI does not provide incremental prognostic information over left ventricular ejection fraction (LVEF) (P = 0.38).
Conclusion
LVGFI assessed by CMR is a strong predictor of MACE within 3 years after first STEMI. A superior predictive value as compared with LVEF was not found in this study.
Keywords: Left ventricular global function index, Myocardial infarction, Magnetic resonance imaging, Prognosis
Introduction
After ST-segment elevation myocardial infarction (STEMI), individualized risk stratification is one of the key issues in optimizing patient treatment. Several functional and morphological parameters of the left ventricle (LV) are available to estimate the risk of poor outcome.1–7 In today's clinical routine, left ventricular ejection fraction (LVEF) is the most commonly used parameter for monitoring cardiac performance, and has great impact on therapeutic decision-making.8 Nevertheless, the use of LVEF has various limitations including the missing information on LV size and mass as well as on LV diastolic function. In line, the area under the curve (AUC) of LVEF to predict outcome after STEMI is described to be ∼0.7–0.8.4,9,10 Therefore, further improvements of LV parameters for the prognostic evaluation after STEMI are still desirable for clinicians.11 Several additional markers of cardiac performance might provide incremental information for prognostification.7,11,12 Especially LV mass and size were described to be independent predictors of increased morbidity and mortality after acute myocardial infarction (AMI).5–7 These echocardiographic studies showed that AMI patients who suffered major adverse cardiac events (MACE) in follow-up had more abnormal early LV remodelling (higher end-systolic and end-diastolic volumes and higher LV mass) compared with patients who did not suffer MACE.6,7 Consequently, a comprehensive marker reflecting both global systolic function and early LV remodelling might add incremental prognostic information over LVEF. The left ventricular global function index (LVGFI) was recently proposed as such a novel measure of LV performance.13,14 The formula for the calculation of the LVGFI combines LV stroke-, end-systolic, and end-diastolic volumes, and LV mass. Importantly, these parameters can be easily obtained with high accuracy in daily clinical practice. In 5004 healthy participants of the Multi-Ethnic Study of Atherosclerosis (MESA) cohort, LVGFI was independently associated with the incidence of cardiovascular events during a mean follow-up period of 7.2 years. LVGFI was superior to LVEF and LV mass index in predicting cardiovascular-related study endpoints.13 We have previously shown the relation of LVGFI with infarct characteristics after acute STEMI.14 However, the prognostic significance of LVGFI on clinical events after STEMI is unknown.
Therefore, the aim of the study was to extent our previous findings by conducting a clinical follow-up to investigate the prognostic value of LVGFI, as assessed by cardiac magnetic resonance (CMR) imaging, in patients with STEMI. Furthermore, the predictive power of LVGFI and LVEF was compared.
Methods
Study population
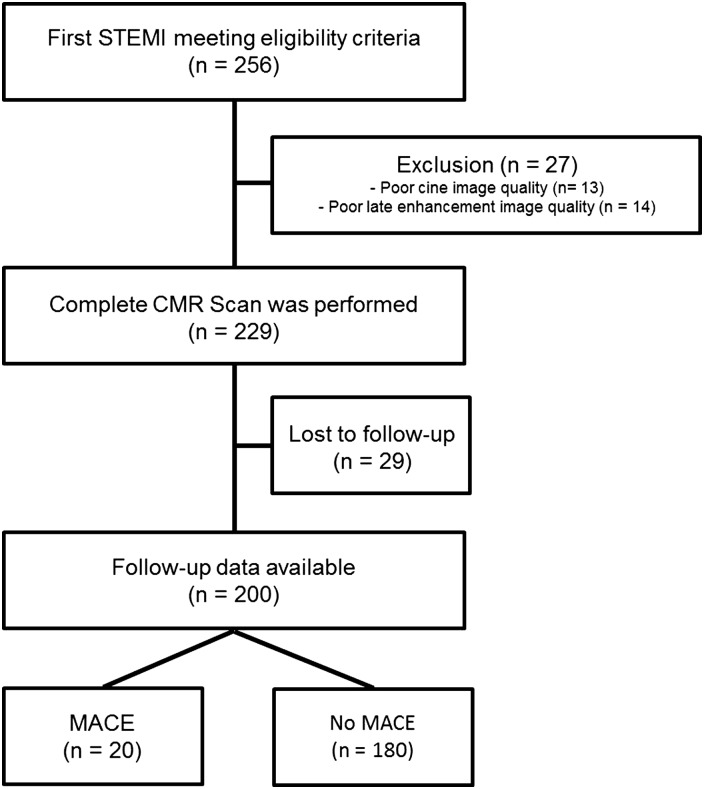
Patients who were admitted to hospital with first STEMI and treated with percutaneous coronary intervention (PCI) were recruited to this single-center, observational study if they met the eligibility criteria. Diagnosis of STEMI was made according to the redefined ESC/ACC committee criteria.15 Eligibility criteria were successful PCI (defined as TIMI flow three post-PCI), Killip class ≤2 on admission, no contraindication for CMR examination, and onset of symptoms <24 h before PCI. As shown in Figure 1, out of 256 STEMI patients included in the study, 229 (89.4%) underwent a complete CMR scan. Thirteen patients were excluded from analysis because of poor cine image quality, and 14 patients for reasons of poor late enhancement image quality. Of those 229 patients, 29 (12.7%) were excluded because of missing follow-up data despite at least four separate attempts to contact them. Thus, for analysis, data of 200 STEMI patients were available. Demographic data and clinical profile of patients were acquired with the help of a standardized questionnaire during hospitalization for STEMI. Blood samples for routine creatine kinase analysis were collected as previously reported.16
Figure 1.
Flow diagram of study patients. STEMI, ST-segment elevation myocardial infarction; CMR, cardiovascular magnetic resonance imaging; MACE, major adverse cardiac events.
The study complies with the Declaration of Helsinki and the local ethics committee approved it. Written informed consent was obtained from participants.
Study endpoints and follow-up
Follow-up data were obtained through telephone contact with the patients or a close relative using a standardized questionnaire by cardiologists blinded to CMR data. All declared endpoints were carefully checked by reviewing corresponding medical records. The primary endpoint of this study was the incidence of MACE, defined as a composite of death, myocardial re-infarction and new congestive heart failure. Outcome was the time from the date of PCI until the first event of the primary endpoint occurring after treatment for the index event and discharge from hospital. Death included all-cause mortality. Myocardial re-infarction was deemed to be present if the redefined ESC/ACC committee criteria were met.15 New congestive heart failure was defined as a first episode of cardiac decompensation requiring medical attention (including intravenous diuretics). The secondary endpoint was each MACE outcome assessed as an individual endpoint. If more than one MACE occurred during study follow-up, the most severe endpoint (death > new congestive heart failure > non-fatal myocardial re-infarction) was selected for primary endpoint analysis.
Cardiac magnetic resonance
All patients underwent contrast-enhanced CMR within the first week after the index event. CMR scans were performed on a 1.5-T AVANTO-scanner (Siemens, Erlangen, Germany) according to a standardized protocol previously described.17,18
Evaluation of images was performed manually using standard software (ARGUS, Siemens). In brief, cine CMR images in short axis (11 slices) were acquired using breath-hold, retrospective electrocardiogram-triggered trueFISP bright blood sequences. The LVGFI was determined according to the formula published by Mewton et al.:13
| (1) |
where LVSV is the left ventricular stroke volume and LVGV is the left ventricular global volume. The left ventricular global volume was calculated according to the following formula:
| (2) |
where LVEDV is the left ventricular end-diastolic volume, and LVESV the left ventricular end-systolic volume.
Infarct size (IS) and microvascular obstruction were determined from late enhancement images as described previously.19 ‘Hyperenhancement’ was defined using a threshold of +5 standard deviation as previously stated.20,21 Microvascular obstruction was defined as a persisting area of hypoenhancement, surrounded by enhanced myocardium.22
Statistical analysis
Continuous, normally distributed variables (Shapiro–Wilk test) are expressed as mean ± standard deviation, not normally distributed continuous variables as median with interquartile range. Categorical variables are displayed as the number and percentage. Student's t-test, ANOVA, and Mann–Whitney U test were used to determine differences in continuous variables between groups. χ2 test was used to compare categorical variables between groups. Pearson or Spearman ρ correlations were calculated as appropriate. Outcome functions were estimated using Kaplan–Meier graphs, and groups were compared using the log-rank test. Univariate and multivariate Cox regression analysis was applied to identify parameters associated with outcome. Only variables with a P < 0.05 in univariate analysis were entered in multivariate models. LVGFI, LVEF, LVESV, and IS were used as dichotomized categorical variables for outcome analysis. They were dichotomized according to optimized cut-off values (LVGFI = 29%, LVEF = 51%, IS = 15.6%, LVESV = 88 mL), which were calculated by receiver operating characteristic (ROC) analysis using the following formula:
LVESV and LVEF were not entered into the multivariate model together with LVGFI, because of a collinear correlation (LVESV and LVGFI: r = −0.677, P < 0.001; LVEF and LVGFI: r = 0.882, P < 0.001).23 The predictive value of LVGFI and other CMR parameters was assessed using ROC analyses. The potential incremental information of LVGFI over LVEF for the prediction of MACE was assessed with c-statistics. C-statistic results were compared as described previously by DeLong et al.24 All statistical tests were two-tailed, and a P-value <0.05 was considered statistically significant. Statistical analysis was performed with SPSS 22.0 (IBM, Armonk, NY, USA) and MedCalc Version 15.2.2 (MedCalc Software bvba, Ostend, Belgium).
Results
Patient characteristics and CMR parameters
Baseline characteristics as well as CMR parameters of the study cohort and their relation with MACE are shown in Table 1. In the total study population, the median LVGFI was 32 [28–37]%. LVGFI was strongly correlated with LVEF (r = 0.882, P < 0.001), moderately with LVESV (r = −0.678, P < 0.001), LVMM (r = −0.482, P < 0.001) and only weakly with LVEDV (r = −0.280, P < 0.001). Furthermore, an inverse association between LVGFI and IS was found (r = −0.436, P < 0.001). Patients with microvascular obstruction (MVO) showed a significantly lower LVGFI compared with patients with no MVO (30 [26–35] % vs. 36 [30–42] %, P < 0.001). The mean follow-up period was 2.9 ± 1.5 years with the median being 3.1 [2.0–4.1] years. During follow-up, 20 MACE events (10%) were documented, including 5 deaths (2.5%), 11 new congestive heart failure events (5.5%), and 10 non-fatal myocardial re-infarctions (5.0%). Out of these, four patients (2.0%) suffered two and one (0.5%) patient suffered three MACE events. Diabetic patients showed a trend for an increased risk for MACE (P = 0.070). Patients with MACE trended to have more likely three-vessel disease (P = 0.074).
Table 1.
Baseline patient characteristics of all patients as well as those with and without MACE
| All patients (n = 200) | No MACE (n = 180) | MACE (n = 20) | P-value | |
|---|---|---|---|---|
| Age, years | 56 ± 11 | 56 ± 11 | 57 ± 14 | 0.959 |
| Female Gender, n (%) | 31 (15.5) | 28 (15.6) | 3 (15.0) | 0.948 |
| Body mass index, kg/m2 | 26 ± 3 | 26 ± 3 | 25 ± 4 | 0.262 |
| Cardiovascular risk factors | ||||
| Hypertension, n (%) | 118 (59) | 106 (59) | 12 (60) | 0.924 |
| Diabetes mellitus, n (%) | 18 (9) | 14 (8) | 4 (20) | 0.070 |
| Current smoker, n (%) | 106 (53) | 94 (52) | 12 (60) | 0.509 |
| Hypercholesterolemia, n (%) | 132 (66) | 118 (66) | 14 (70) | 0.691 |
| Family history for AMI, n (%) | 49 (25) | 46 (26) | 3 (15) | 0.298 |
| Pain-to-balloon time, minutes | 192 (128–276) | 193 (128–296) | 178 (130–249) | 0.556 |
| Time from STEMI to CMR, days | 2 (2–4) | 2 (2–4) | 2 (2–4) | 0.893 |
| Infarct-related artery | 0.295 | |||
| Right coronary artery, n (%) | 93 (46) | 87 (48) | 6 (30) | |
| Left anterior descending artery, n (%) | 83 (42) | 72 (40) | 11 (55) | |
| Left circumflex coronary artery, n (%) | 24 (12) | 21 (12) | 3 (15) | |
| Number of diseased vessels | 0.074 | |||
| 1, n (%) | 118 (60) | 106 (60) | 12 (60) | |
| 2, n (%) | 56 (29) | 53 (30) | 3 (15) | |
| 3, n (%) | 22 (11) | 17 (10) | 5 (25) | |
| Peak CK, U/L | 1906 (1041–3309) | 1840 (988–3100) | 3416 (1259–7522) | 0.033 |
| CMR variables | ||||
| LV mass index, g/m² | 71 (62–80) | 70 (62–80) | 76 (67–89) | 0.072 |
| Infarct mass, % of MM | 14 (6–23) | 13 (6–22) | 18 (7–29) | 0.261 |
| Microvascular obstruction, n (%) | 113 (56) | 96 (53) | 17 (85) | 0.007 |
| LVEDV, mL | 154 (127–172) | 153 (127–170) | 164 (116–179) | 0.460 |
| LVESV, mL | 72 (53–90) | 70 (52–83) | 92 (66–110) | 0.014 |
| LVEF, % | 53 (46–59) | 54 (47–59) | 44 (37–51) | <0.001 |
| LVGFI, % | 32 (28–37) | 33 (28–38) | 27 (21–29) | 0.001 |
MACE, major adverse cardiac events; AMI, acute myocardial infarction; STEMI, ST-segment elevation myocardial infarction; CMR, cardiovascular magnetic resonance imaging; CK, creatine kinase; LV, left ventricular; MM, myocardial mass; LVEDV, left ventricular end-diastolic volume; LVESV, left ventricular end-systolic volume; LVEF, left ventricular ejection fraction; LVGFI, left ventricular global function index.
Significant differences (P ≤ 0.05) are highlighted in bold.
Primary endpoint
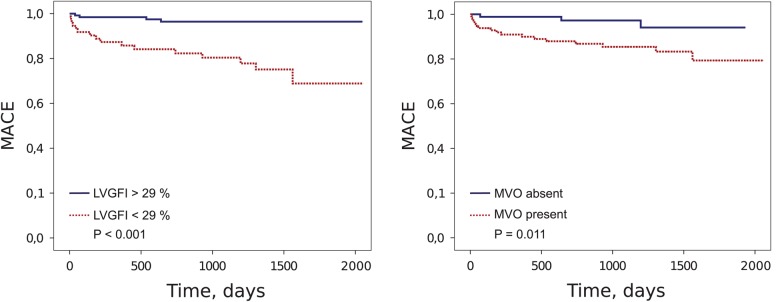
Patients suffering a MACE event had significantly higher LVESV, and lower LVEF as well as LVGFI (P = 0.014, P < 0.001, P = 0.001, respectively). The IS, LVEDV, and LV mass index were also higher in patients with MACE, but the differences did not reach significance (all P > 0.05). Patients with MACE were more likely to have MVO (P = 0.007). Kaplan–Meier analysis showed that MACE rate was significantly higher in patients with reduced LVGFI (LVGFI < 29%, log-rank P < 0.001), reduced LVEF (LVEF < 51%, log-rank P < 0.001), increased LVESV (LVESV > 88 mL, log-rank P < 0.001) and in patients in whom MVO was present (log-rank P = 0.011) (Figure 2). In ROC analysis, LVGFI showed a high AUC for the prediction of MACE [AUC = 0.73, confidence interval (CI) 0.61–0.85] (Figure 3). The optimal LVGFI cut-off for the prediction of MACE with a sensitivity of 80% and a specificity of 68% was 29%. The AUC obtained with LVEF was 0.74 (CI 0.61–0.87). The optimal cut-off for LVEF was 51% (sensitivity = 80%, specificity = 63%). The AUC of LV mass index was 0.62 (CI 0.49–0.75). IS as well as LVESV had predictive value for the primary endpoint (AUC = 0.58, CI 0.43–0.72 and AUC = 0.67, CI 0.53–0.81, respectively). Multivariate Cox regression analysis, revealed LVGFI as a predictor of MACE [hazard ratio (HR) = 4.79, 95% CI 1.46 to 15.67, P = 0.010] (Table 2), when omitting collinear variables. When, instead of LVGFI, LVEF was included into the same multivariate model, a HR of 3.36 (95% CI 1.02 to 11.08, P = 0.046) for the prediction of MACE was observed. However, after inclusion of both LVEF and LVGFI, LVGFI was no longer an independent predictor of MACE (P = 0.11). Accordingly, the inclusion of LVGFI in addition to LVEF resulted in a small, but non-significant increase of c-statistics from 0.739 (CI 0.612–0.866) to 0.741 (CI 0.615–0.868, P = 0.38), thus demonstrating that LVGFI has no incremental prognostic value over LVEF.
Figure 2.
Kaplan–Meier analysis. Kaplan–Meier curves showing the risk of MACE, stratified by LVGFI and the presence of MVO. LVGFI was dichotomized according to the optimized cut-off value (LVGFI = 29%), which was calculated by ROC analysis. MACE, major adverse cardiac events; LVGFI, left ventricular global function index; MVO, microvascular obstruction; ROC, receiver operating characteristic.
Figure 3.
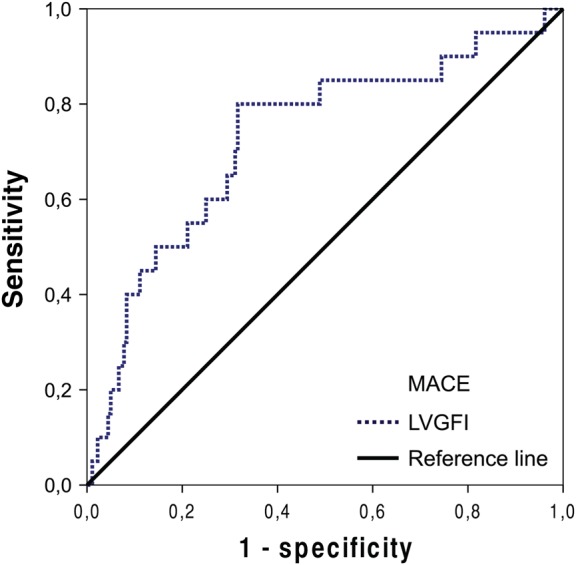
ROC analysis. ROC curve for the prognostic value of LVGFI for the occurrence of MACE at follow-up (AUC = 0.73, CI 0.61–0.85). ROC, receiver operating characteristics; LVGFI, left ventricular global function index; MACE, major adverse cardiac events; AUC, area under the curve.
Table 2.
Univariable and multivariable Cox regression model for MACE
| Univariable analysis |
Multivariable analysis |
|||
|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Age above 65 years | 1.20 (0.44–3.30) | 0.728 | – | |
| Male Gender | 1.05 (0.31–3.60) | 0.933 | – | |
| Hypertension | 1.04 (0.42–2.54) | 0.938 | – | |
| Current smoker | 1.26 (0.52–3.10) | 0.609 | – | |
| Hypercholesterolemia | 1.02 (0.39–2.67) | 0.962 | – | |
| Diabetes mellitus | 2.56 (0.86–7.67) | 0.093 | – | |
| Multivessel disease | 2.97 (1.07–8.22) | 0.036 | 1.32 (0.72–2.44) | 0.369 |
| Peak CK, U/L | 1.59 (0.65–3.89) | 0.312 | – | |
| LV mass index, g/m² | 2.72 (1.13–6.54) | 0.026 | 1.60 (0.61–4.19) | 0.336 |
| Infarct mass, % of MM | 2.05 (0.84–5.03) | 0.116 | – | |
| Microvascular obstruction | 4.28 (1.25–14.59) | 0.020 | 2.96 (0.85–10.29) | 0.088 |
| LVEDV, mL | 2.29 (0.93–5.61) | 0.071 | – | |
| LVESV, mL | 4.34 (1.77–10.65) | 0.001 | Not included | |
| LVEF, % | 5.86 (1.96–17.52) | <0.001 | Not included | |
| LVGFI, % | 7.16 (2.39–21.45) | <0.001 | 4.79 (1.46–15.67) | 0.010 |
In this model, collinear variables (LVESV and LVEF) were not included in multivariate analysis. Of note, LVGFI was not independently associated with MACE after adjusting for these variables (P > 0.05).
MACE, major adverse cardiac events; HR, hazard ratio; CI, confidence interval; CK, creatine kinase; LV, left ventricular; MM, myocardial mass; LVEDV, left ventricular end-diastolic volume; LVESV, left ventricular end-systolic volume; LVEF, left ventricular ejection fraction; LVGFI, left ventricular global function index.
Significant variables (P ≤ 0.05) are highlighted in bold.
Secondary endpoints
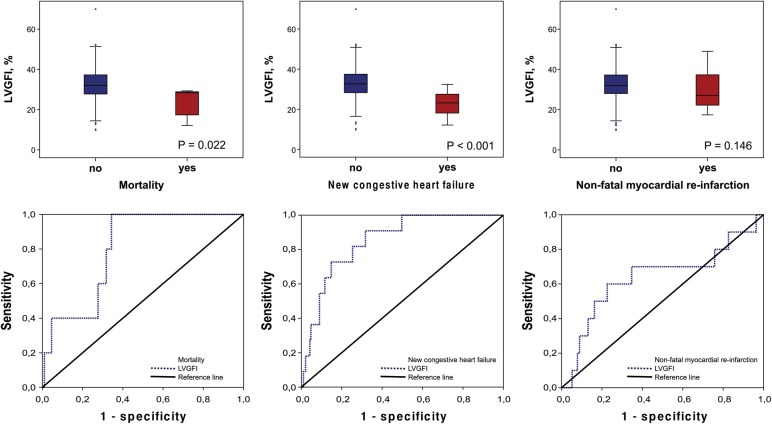
Five patients (2.5%) died during study follow-up. Four patients (2.0%) died from cardiovascular causes, whereas one patient (0.5%) died from a non-cardiovascular cause. Non-survivors and patients with new congestive heart failure (n = 11) had a significantly pronounced myocardial dysfunction as assessed by LVGFI (28 [15–29] % vs. 32 [28–37] %, P = 0.022 and 23 [17–28] % vs. 33 [28–37] %, P < 0.001, respectively). Patients who experienced non-fatal myocardial re-infarction (n = 10) showed no significant difference in LVGFI (27 [22–38] % vs. 32 [28–37] %, P = 0.147) (Figure 4). Kaplan–Meier calculation revealed a significantly reduced survival time for patients with reduced LVGFI as compared with patients with high LVGFI (log-rank P = 0.010) (Figure 5). ROC curves for the predictive value of LVGFI for each secondary endpoint are shown in Figure 4. LVGFI was a strong predictor of mortality and new congestive heart failure (AUC = 0.80, CI 0.67–0.93, and AUC = 0.85, CI 0.76–0.94, respectively). The predictive power of LVGFI for non-fatal myocardial re-infarction was only weak (AUC = 0.64, CI 0.43–0.85). LVEF showed a similar predictive power for the secondary endpoints compared with LVGFI (data not shown).
Figure 4.
Box plots and ROC analysis. Box plots of LVGFI according to the occurrence of the secondary endpoints (mortality, new congestive heart failure and non-fatal myocardial re-infarction). Corresponding ROC curves for the predictive value of LVGFI for the secondary endpoints (mortality: AUC = 0.80, CI 0.67–0.93; new congestive heart failure: AUC = 0.85, CI 0.76–0.94); Non-fatal myocardial re-infarction: AUC = 0.64, CI 0.43–0.85). LVGFI, left ventricular global function index; ROC, receiver operating characteristic; AUC, area under the curve.
Figure 5.
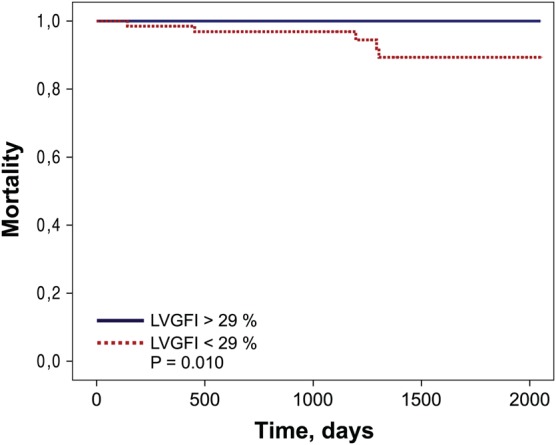
Kaplan–Meier analysis. Kaplan–Meier curves showing the risk of death, stratified by LVGFI. LVGFI, left ventricular global function index.
Discussion
This is the first study to evaluate the prognostic value of LVGFI in patients after acute STEMI. The main findings of the present study are: (i) the LVGFI determined by CMR early after uncomplicated STEMI is a powerful predictor of MACE at long-term follow-up (3.1 years); (ii) LVGFI was strongly predictive for mortality and incident congestive heart failure, but it was only a weak predictor for recurrent non-fatal myocardial infarction; (iii) the predictive value of LVGFI and LVEF was similar and the combination of both did not improve the prognostic value of either marker alone.
Multiple trials showed that CMR parameters of myocardial function and injury are important determinants of poor prognosis after STEMI.21,25,26 Furthermore, echocardiographic studies have shown that patients with increased LV mass and abnormal LV geometry are at an increased risk for morbidity and mortality after myocardial infarction.6,7 Despite this knowledge, studies investigating novel parameters potentially improving the prediction of outcome are still necessary. Mewton et al.13 proposed the LVGFI as such a new and promising parameter for predicting adverse outcome in healthy individuals. The LVGFI is the first approach that comprehensively incorporates information on LV size and mass as well as on global systolic function, thus possibly providing additional prognostic information over LVEF. Indeed, in the study by Mewton et al., LVGFI was shown to be a powerful predictor of hard cardiovascular events including mortality. LVGFI was more robust in the prediction of clinical endpoints as compared with LVEF. This superior predictive value was explained by the fact that LVEF does not incorporate important adverse outcome predictors such as LV mass.6 To our knowledge, this is the first study investigating the prognostic value of LVGFI in patients after STEMI. The results of our investigation on a STEMI population of 200 patients are in part consistent with these earlier findings. We found LVGFI to be a strong predictor for MACE and mortality over a median follow-up of 3.1 years. Therefore, our findings suggest that assessment of LVGFI can be used to risk-stratify patients following STEMI. However, in this population, a stronger correlation coefficient between LVGFI and LVEF (r = 0.88) was observed as compared with the previously published study by Mewton et al. (r = 0.67). This association indicates a strong similarity between these two indexes after acute STEMI. Consequently, both the LVGFI and LVEF showed a similar predictive power for the primary and secondary endpoints. Furthermore, c-statistics confirmed that LVGFI does not provide incremental prognostic information over LVEF when assessed in the acute phase after STEMI. These intriguing observations might be explained by several reasons. CMR was performed in median 2 days after the index event. Significant concentric or eccentric hypertrophy is unlikely to occur within the first days after reperfused STEMI. Several months thereafter, however, the occurrence of chronic maladaptive transformation processes (remodelling) is common.27,28 Moreover, previous studies suggesting a potential incremental value of LV size and mass used echocardiography as imaging method,6,7 which is less accurate than CMR.19 Importantly, none of the used echocardiographic methods described to measure LV mass have been validated in the post-AMI setting.6 The VALIANT study, included only patients with LVEF < 35% on echocardiography performed up to 10 days after AMI, making generalization to a broader group of AMI patients difficult.6 The strong correlation between LVGFI and LVEF in our study indicates that differences in LV mass play only a small role acutely in our population. Indeed, LV mass index was only a weak predictor of outcome in our study (AUC 0.62) that was significant only in univariate but not in multivariate regression analysis. One might therefore speculate that LVGFI might add significant prognostic information in the chronic stage after STEMI. To answer these questions, further well-designed longitudinal investigations are necessary.
Microvascular obstruction, a phenomenon caused by microcirculatory perfusion defects after restoration of epicardial blood flow,29 is present in >50% of STEMI patients treated with PCI.30 Consistent with these data, MVO was present in 56% of this study population. In a large (n = 1025) international meta-analysis of pooled individual patient data by van Kranenburg et al.,30 MVO was shown to be a strong predictor of adverse outcome after STEMI (median follow-up of 12 (4–21) months). Interestingly, this study also showed that IS is not independently associated with MACE or cardiac death. In line with this study, we found that patients with MVO had a significantly higher risk for MACE, whereas IS was not associated with outcome. Therefore, the results of our analysis further underline the prognostic significance of MVO. The mean follow-up time of 3.1 years in the present study is longer as compared with almost all CMR trials published previously.3,21,25,26,30,31 Hence, our findings highlight the long-term predictive value of MVO for hard clinical endpoints.
A limitation of this study is that it was conducted on patients with first-time STEMI who were successfully reperfused by p-PCI. Unstable patients or patients with severe heart failure during hospitalization for the index event (Killip class > 2) were not included. Although the characteristics of our patient group as well as the incidence of outcome are comparable with other large CMR studies,21 our results are not generalizable to other STEMI populations, such as for instance, to patients treated with thrombolytic therapy or patients presenting with cardiogenic shock. Another limitation is the lost to follow-up rate of ∼12%, which is however well below the generally accepted cut-off of 20% for outcome studies.32 Moreover, the identified prognostic variables were not significantly different between patients with and without follow-up.
In conclusion, the LVGFI is a strong predictor of MACE and death at 3 years following PCI for acute STEMI. A superior predictive value as compared with LVEF was not found in this study.
Funding
This study was supported by grants from the Austrian Society of Cardiology to S.J.R., H.J.F. and G.K. and by the funding programme of Medical University of Innsbruck, MUI-START, project 2013042016 to G.K.
Conflict of interest: None declared.
References
- 1.Solomon SD, Anavekar N, Skali H, McMurray JJ, Swedberg K, Yusuf S, et al. Influence of ejection fraction on cardiovascular outcomes in a broad spectrum of heart failure patients. Circulation 2005;112:3738–44. [DOI] [PubMed] [Google Scholar]
- 2.Burns RJ, Gibbons RJ, Yi Q, Roberts RS, Miller TD, Schaer GL, et al. The relationships of left ventricular ejection fraction, end-systolic volume index and infarct size to six-month mortality after hospital discharge following myocardial infarction treated by thrombolysis. J Am Coll Cardiol 2002;39:30–6. [DOI] [PubMed] [Google Scholar]
- 3.Wu E, Ortiz JT, Tejedor P, Lee DC, Bucciarelli-Ducci C, Kansal P, et al. Infarct size by contrast enhanced cardiac magnetic resonance is a stronger predictor of outcomes than left ventricular ejection fraction or end-systolic volume index: prospective cohort study. Heart 2008;94:730–6. [DOI] [PubMed] [Google Scholar]
- 4.de Waha S, Eitel I, Desch S, Fuernau G, Lurz P, Stiermaier T, et al. Prognosis after ST-elevation myocardial infarction: a study on cardiac magnetic resonance imaging versus clinical routine. Trials 2014;15:249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bolognese L, Dellavesa P, Rossi L, Sarasso G, Bongo AS, Scianaro MC. Prognostic value of left ventricular mass in uncomplicated acute myocardial infarction and one-vessel coronary artery disease. Am J Cardiol 1994;73:1–5. [DOI] [PubMed] [Google Scholar]
- 6.Verma A, Meris A, Skali H, Ghali JK, Arnold JM, Bourgoun M, et al. Prognostic implications of left ventricular mass and geometry following myocardial infarction: the VALIANT (VALsartan In Acute myocardial iNfarcTion) Echocardiographic Study. JACC Cardiovasc Imaging 2008;1:582–91. [DOI] [PubMed] [Google Scholar]
- 7.Dokainish H, Rajaram M, Prabhakaran D, Afzal R, Orlandini A, Staszewsky L, et al. Incremental value of left ventricular systolic and diastolic function to determine outcome in patients with acute ST-segment elevation myocardial infarction: the echocardiographic substudy of the OASIS-6 trial. Echocardiography 2014;31:569–78. [DOI] [PubMed] [Google Scholar]
- 8.Steg PG, James SK, Atar D, Badano LP, Blomstrom-Lundqvist C, Borger MA, et al. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J 2012;33:2569–619. [DOI] [PubMed] [Google Scholar]
- 9.Larose E, Rodes-Cabau J, Pibarot P, Rinfret S, Proulx G, Nguyen CM, et al. Predicting late myocardial recovery and outcomes in the early hours of ST-segment elevation myocardial infarction traditional measures compared with microvascular obstruction, salvaged myocardium, and necrosis characteristics by cardiovascular magnetic resonance. J Am Coll Cardiol 2010;55:2459–69. [DOI] [PubMed] [Google Scholar]
- 10.Hall TS, Hallen J, Krucoff MW, Roe MT, Brennan DM, Agewall S, et al. Cardiac troponin I for prediction of clinical outcomes and cardiac function through 3-month follow-up after primary percutaneous coronary intervention for ST-segment elevation myocardial infarction. Am Heart J 2015;169:257–65. [DOI] [PubMed] [Google Scholar]
- 11.Dagres N, Hindricks G. Risk stratification after myocardial infarction: is left ventricular ejection fraction enough to prevent sudden cardiac death? Eur Heart J 2013;34:1964–71. [DOI] [PubMed] [Google Scholar]
- 12.Mollema SA, Nucifora G, Bax JJ. Prognostic value of echocardiography after acute myocardial infarction. Heart 2009;95:1732–45. [DOI] [PubMed] [Google Scholar]
- 13.Mewton N, Opdahl A, Choi EY, Almeida AL, Kawel N, Wu CO, et al. Left ventricular global function index by magnetic resonance imaging—a novel marker for assessment of cardiac performance for the prediction of cardiovascular events: the multi-ethnic study of atherosclerosis. Hypertension 2013;61:770–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reinstadler SJ, Klug G, Feistritzer HJ, Mayr A, Kofler M, Aschauer A, et al. Left ventricular global function index: Relation with infarct characteristics and left ventricular ejection fraction after STEMI. Int J Cardiol 2014;175:579–81. [DOI] [PubMed] [Google Scholar]
- 15.Alpert JS, Thygesen K, Antman E, Bassand JP. Myocardial infarction redefined—a consensus document of The Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction. J Am Coll Cardiol 2000;36:959–69. [DOI] [PubMed] [Google Scholar]
- 16.Reinstadler SJ, Klug G, Feistritzer HJ, Mayr A, Harrasser B, Mair J, et al. Association of copeptin with myocardial infarct size and myocardial function after ST segment elevation myocardial infarction. Heart 2013;99:1525–29. [DOI] [PubMed] [Google Scholar]
- 17.Klug G, Trieb T, Schocke M, Nocker M, Skalla E, Mayr A, et al. Quantification of regional functional improvement of infarcted myocardium after primary PTCA by contrast-enhanced magnetic resonance imaging. J Magn Reson Imaging 2009;29:298–304. [DOI] [PubMed] [Google Scholar]
- 18.Reinstadler SJ, Klug G, Feistritzer HJ, Mayr A, Bader K, Mair J, et al. Relation of plasma adiponectin levels and aortic stiffness after acute ST-segment elevation myocardial infarction. Eur Heart J Acute Cardiovasc Care 2014;3:10–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klug G, Metzler B. Assessing myocardial recovery following ST-segment elevation myocardial infarction: short- and long-term perspectives using cardiovascular magnetic resonance. Expert Rev Cardiovasc Ther 2013;11:203–19. [DOI] [PubMed] [Google Scholar]
- 20.Bondarenko O, Beek AM, Hofman MB, Kuhl HP, Twisk JW, van Dockum WG, et al. Standardizing the definition of hyperenhancement in the quantitative assessment of infarct size and myocardial viability using delayed contrast-enhanced CMR. J Cardiovasc Magn Resonan 2005;7:481–5. [DOI] [PubMed] [Google Scholar]
- 21.Eitel I, de Waha S, Wohrle J, Fuernau G, Lurz P, Pauschinger M, et al. Comprehensive prognosis assessment by CMR imaging after ST-segment elevation myocardial infarction. J Am Coll Cardiol 2014;64:1217–26. [DOI] [PubMed] [Google Scholar]
- 22.Klug G, Mayr A, Schenk S, Esterhammer R, Schocke M, Nocker M, et al. Prognostic value at 5 years of microvascular obstruction after acute myocardial infarction assessed by cardiovascular magnetic resonance. J Cardiovasc Magn Reson 2012;14:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hosmer DW, Lemeshow S. Applied Survival Analysis: Regression Modeling of Time to Event Data. New York: Wiley; 1999. ISBN: 0-471-15410–5. [Google Scholar]
- 24.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 1988;44:837–45. [PubMed] [Google Scholar]
- 25.Bodi V, Sanchis J, Nunez J, Mainar L, Lopez-Lereu MP, Monmeneu JV, et al. Prognostic value of a comprehensive cardiac magnetic resonance assessment soon after a first ST-segment elevation myocardial infarction. JACC Cardiovasc Imaging 2009;2:835–42. [DOI] [PubMed] [Google Scholar]
- 26.de Waha S, Desch S, Eitel I, Fuernau G, Zachrau J, Leuschner A, et al. Impact of early vs. late microvascular obstruction assessed by magnetic resonance imaging on long-term outcome after ST-elevation myocardial infarction: a comparison with traditional prognostic markers. Eur Heart J 2010;31:2660–8. [DOI] [PubMed] [Google Scholar]
- 27.Cohn JN, Ferrari R, Sharpe N. Cardiac remodeling—concepts and clinical implications: a consensus paper from an international forum on cardiac remodeling. Behalf of an International Forum on Cardiac Remodeling. J Am Coll Cardiol 2000;35:569–82. [DOI] [PubMed] [Google Scholar]
- 28.Pokorney SD, Rodriguez JF, Ortiz JT, Lee DC, Bonow RO, Wu E. Infarct healing is a dynamic process following acute myocardial infarction. J Cardiovasc Magn Reson 2012;14:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu KC. CMR of microvascular obstruction and hemorrhage in myocardial infarction. J Cardiovasc Magn Reson 2012;14:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Kranenburg M, Magro M, Thiele H, de Waha S, Eitel I, Cochet A, et al. Prognostic value of microvascular obstruction and infarct size, as measured by CMR in STEMI patients. JACC Cardiovasc Imaging 2014;7:930–9. [DOI] [PubMed] [Google Scholar]
- 31.Hombach V, Grebe O, Merkle N, Waldenmaier S, Hoher M, Kochs M, et al. Sequelae of acute myocardial infarction regarding cardiac structure and function and their prognostic significance as assessed by magnetic resonance imaging. Eur Heart J 2005;26:549–57. [DOI] [PubMed] [Google Scholar]
- 32.Fewtrell MS, Kennedy K, Singhal A, Martin RM, Ness A, Hadders-Algra M, et al. How much loss to follow-up is acceptable in long-term randomised trials and prospective studies? Arch Dis Childh 2008;93:458–61. [DOI] [PubMed] [Google Scholar]