Abstract
Aims
The aim of this prospective study was to use left ventricular global longitudinal strain (LV-GLS) as a non-invasive tool for the monitoring of graft function in relation to acute cellular rejection (ACR) during the first year after heart transplantation (HTX).
Methods and results
The study population consisted of 36 patients undergoing HTX from November 2010 until October 2013. Patients were followed by comprehensive echocardiography and biopsies at 2 weeks and 1, 3, 6, and 12 months after HTX. ACRs were classified based on the ISHLT classification (0R–3R). Patients were divided into two groups according to the presence of one or more episodes of biopsy proven ≥grade 2R ACR during follow-up. We found that LV-GLS and tricuspid annular plane systolic excursion (TAPSE) were significantly related to ACR burden in a linear regression model. The absolute difference in LV-GLS between patients in the ACR group (−14.4%) and patients in the ACR-free group (−16.8%) was −2.4% (P < 0.01) 12 months after HTX. In the ACR group, patients' LV-GLS did not improve between 1 and 12 months, whereas an improvement of −2.9% was seen in the ACR-free group in this period (P < 0.01). The two groups appeared not to differ in terms of diastolic Doppler parameters or LV ejection fraction, but TAPSE was 15.3 ± 2.8 mm in the ACR-free group vs. 13.2 ± 2.1 mm ACR group, P < 0.05, 12 months after HTX.
Conclusion
Gradual improvement of longitudinal LV and RV function was seen within the first year after HTX, but the degree of recovery was strongly influenced by ACR episodes.
Keywords: heart transplantation, rejection, cardiac allograft vasculopathy, global longitudinal systolic function, speckle tracking
Introduction
In the first post-operative year after heart transplantation (HTX), the main causes of death are acute graft failure, acute cellular rejection (ACR), and infection.1 Approximately two-thirds of the patients experience ACR episodes during the first year after HTX.2,3 ACR is associated with the development of cardiac allograft vasculopathy (CAV), and the outcome is often poor.2,3 Standard surveillance of graft function after HTX includes measurement of left ventricular (LV) ejection fraction (EF) and diastolic function by trans-mitral Doppler flow analysis and routine myocardial biopsy. Clinical experience shows that LVEF often presents itself within the normal range during rejection and severe CAV, which suggests that LVEF is an inappropriate parameter for graft function surveillance.4,5 It is well known from several other cardiac diseases that early stages of fibrosis and oedema often affect the subendocardial muscle fibres of the myocardium which leads to impaired longitudinal myocardial function.6 Monitoring of the global LV longitudinal deformation could therefore be a more appropriate method for graft function surveillance. 2D-speckle tracking echocardiography (2D-STE) with measurement of global longitudinal strain (GLS) is known to be a more sensitive tool for detection of early, subtle changes of myocardial function than LVEF. Furthermore, 2D-STE has several methodological advantages, such as low inter-observer variability and less angle dependence than tissue Doppler imaging.6 LV-GLS has been proven to yield important prognostic information when measured in the first post-operative weeks after HTX.7 In addition, lack of improvement of longitudinal strain on myocardial segmental level within 3 months after HTX has also been associated with adverse outcome.8 LV-GLS has previously been shown to be impaired in stable HTX patients,9–11 but little is known about the serial changes of GLS after HTX and the relation of such changes to ACR during first year after HTX.
The aim of the present prospective study was to use 2D-STE to evaluate changes in longitudinal graft function and implications of ACRs during the first year after HTX.
Methods
Study population
The study population consisted of all patients who underwent HTX at Aarhus University Hospital, Skejby, Denmark, between November 2010 and October 2013. Thirty-six patients operated by bicaval technique were consecutively followed with comprehensive echocardiographic examination within 2 weeks after HTX and 1, 3, 6, and 12 months after HTX. One patient was examined at baseline and after 1 year only due to severe neurological deficits after cerebral haemorrhage in the acute phase after HTX.
Patients were divided into two groups according to their incidence of ACR within the first year after HTX:
ACR-free group: no treatment-demanding (≥2R) ACR episodes during follow-up
ACR group: at least one episode of ≥2R ACR during follow-up
Echocardiography
We used a commercially available ultrasound system (Vivid 9, GE Healthcare, Horten, Norway) with a 3.5 MHz phased array transducer (M5S).
From a parasternal view, M-mode measurements included septal and posterior wall thickness; the end-diastolic and the end-systolic diameter of LV and RV were registered.
From an apical view, two-dimensional LVEF measurements were based on end-systolic/diastolic LV volumes; the biplane method of discs was used.12
Peak systolic mitral annular velocities (S′) and tissue tracking (TT) were estimated in EchoPAC from the tissue velocity image as an average of the septal, lateral, anterior, and posterior mitral annulus velocities. LV-GLS was obtained from frame-by-frame tracking of speckle patterns throughout the left-sided myocardium from three standard apical 2D cine-loops (four-chamber, two-chamber, and apical long axis) using greyscale harmonic imaging. The frame rate for speckle tracking images was adjusted to 60–90 frames/s. The speckle region of interest was manually adjusted for optimal tracking results of both endocardium and epicardium. Prior to the tracking, the opening and closing characteristics of the aorta valve were defined by the event timing function in a five-chamber view using continuous wave Doppler in the aortic valve. We excluded segments with unacceptably low tracking quality due to poor image acquisition or artifacts. LV-GLS13 was calculated by the software as the average longitudinal systolic strain of 17 myocardial segments14 at peak negative value in systole. EchoPAC allowed calculation of LV-GLS when tracking quality was adequate in at least five of six segments in each view. In case of two projections with good image quality for speckle tracking, exclusion of up to three segments in the last projection was accepted. We then calculated the average of the remaining segments and used this as a measure for the projection. Subsequently, LV-GLS was manually calculated as the average of all three projections. Circumferential strain was obtained from six segments in a short-axis view of LV at the level of the papillary muscles.
A single investigator (T.S.C.), blinded to the clinical data, analysed the data off-line using dedicated software (EchoPAC PC SW-Only, 113, GE Healthcare, Milwaukee, WI, USA).
Our group has previously reported on the repeatability of LV-GLS and found very low intra- and inter-observer variation in a HTX population15
Endomyocardial biopsy
Biopsies were taken by a standard local hospital procedure using the internal jugular or femoral vein. Patients underwent routine biopsies the first year after HTX. Biopsies were scheduled weekly during the first 6 weeks, every 2 weeks up to 3 months, then monthly up to 6 month, and every 2 months for the rest of the first post-operative year. We treated all ACRs classified as ≥grade 2R with intravenous methylprednisolone, 1 g for 3 days, and basal oral immunosuppression was adjusted if necessary.
All biopsies were analysed by an experienced cardiac pathologist who was blinded to echocardiographic results and CAG. ACR was histopathologically graded according to the classification of the International Society of Heart and Lung Transplantation (ISHLT) (1R-3R).16 We calculated a total rejection score as previously described:3
All biopsies were histopathologically assessed for antibody-mediated rejection. Immunological assessment and interpretation of antibodies (Luminex analysis) were performed in case of clinically or histopathologically suspected rejection.
Angiography and cardiac allograft vasculopathy
We assessed CAV by CAG performed 12 months after HTX in all patients. All CAGs were reviewed by an experienced cardiologist blinded to clinical status, echocardiography, and biopsies. CAV was classified using the guidelines of the ISHLT.17
Statistics
Continuous data conforming to a normal distribution are presented as mean ± standard deviation (SD), and categorical data are presented as absolute values with percentages. We used one-way analysis of variance (ANOVA) when comparing continuous variables measured at baseline and 1, 3, 6, and 12 months after HTX. Repeated measures in each subject were taken into account in the ANOVA test. We used unpaired t-test to compare continuous valuables between the ACR group and ACR-free group. Histograms and Q–Q plots were used to check continuous values for normal distribution. We used linear regression model when comparing continuous variables and predicted value and residuals to check the regression models. Clinically relevant parameters and parameters that appeared to be statistically significant in the univariate analysis were entered into the multivariate model. P-values were two-tailed; values <0.05 were considered significant. We used a standard statistical software package (STATA/IC 12, StataCorp LP, College Station, TX, USA).
Results
We included 36 HTX patients (66.7% men) from November 2010 until October 2013. Thirty-four patients (94.4%) survived the first year after HTX, one patient died suddenly 320 days after HTX, and one patient died from severe pneumonia 125 days after HTX. Sixteen patients (44%) had at least one episode of moderate 2R rejection during follow-up. No patients had antibody-mediated rejection during follow-up.
Table 1 displays changes in demographics of the 36 included patients during the first year. The use of ACE inhibitors (P < 0.0001) and aspirin (P < 0.01) increased significantly, whereas the use of diuretics decreased significantly during follow-up (P < 0.0001). Six patients (18%) had angiographic signs of CAV after 12 months. Four patients had severe stenosis (CAV2-3) and two patients had CAV1. We found no correlation between CAV and total number of 1R (P = 0.61) or 2R (P = 0.83) rejection episodes during follow-up.
Table 1.
Patient characteristics during follow-up
| Baseline (n =36) | 1 month (n = 35) | 3 months (n = 34) | 6 months (n = 34) | 12 months (n = 34) | ANOVA, P | |
|---|---|---|---|---|---|---|
| Donor age (years) | 44.3 ± 13.0 | |||||
| Age at HTX (years) | 45.48 | |||||
| Reason for transplantation | ||||||
| Cardiomyopathy, n (%) | 19 (53) | |||||
| Ischaemic heart disease, n (%) | 12 (33) | |||||
| Other, n (%) | 5 (14) | |||||
| Gender mismatch, n (%) | 7 (19) | |||||
| Pre-HTX PVR (wood unit) | 1.8 ± 1.2 | |||||
| BMI | 25.2 ± 5.1 | |||||
| Weight, kg | 78.5 ± 19.8 | 77.3 ± 18.6 | 77.6 ± 19.6 | 80.3 ± 19.2 | 79.7 ± 23.2 | 0.0001* |
| Diabetes, n (%) | 6 (16.7) | 5 (14.3) | 6 (17.6) | 6 (17.6) | 6 (17.6) | 0.73 |
| Hypertension, n (%) | 21 (58.3) | 21 (60.0) | 23 (67.6) | 26 (76.5) | 25 (73.5) | <0.01* |
| Hypercholesterolaemia, n (%) | 32 (88.9) | 32 (91.4) | 31 (91.1) | 31 (91.1) | 30 (88.2) | 1.00 |
| Medication | ||||||
| Prednisolone, n (%) | 36 (100) | 35 (100) | 34 (100) | 33 (97.1) | 31 (91.1) | <0.05* |
| Cyclosporine, n (%) | 6 (16.7) | 6 (17.1) | 4 (11.8) | 6 (17.6) | 5 (14.7) | 0.62 |
| Tacrolimus, n (%) | 29 (80.6) | 28 (80.0) | 28 (82.4) | 28 (82.4) | 29 (85.3) | 0.84 |
| Mycophenolate, n (%) | 36 (100) | 35 (100) | 33 (97.1) | 32 (94.1) | 27 (81.8) | <0.001* |
| Everolimus, n (%) | 4 (11.1) | 4 (11.4) | 4 (11.8) | 7 (20.6) | 8 (23.5) | 0.23 |
| Statins, n (%) | 30 (83.3) | 30 (85.7) | 31 (91.2) | 31 (91.2) | 31 (91.2) | 0.71 |
| ACE/ATII inhibitor, n (%) | 6 (16.7) | 11 (31.4) | 18 (52.9) | 23 (67.6) | 25 (73.5) | <0.0001* |
| Furosemide or bumetanide, n (%) | 20 (55.6) | 19 (54.3) | 13 (38.2) | 8 (23.5) | 6 (17.6) | <0.0001* |
| Thiazide, n (%) | 3 (8.3) | 3 (8.6) | 4 (11.8) | 3 (8.8) | 3 (8.8) | 0.91 |
| Calcium channel blocker, n (%) | 11 (30.6) | 11 (31.4) | 9 (26.5) | 10 (29.4) | 11 (32.4) | 0.93 |
| Aspirin, n (%) | 6 (16.7) | 7 (20.0) | 8 (23.5) | 10 (29.4) | 11 (32.4) | <0.01* |
| Biochemistry | ||||||
| Creatinine, µmol/L | 218.3 ± 195.4 | 158.3 ± 121.9 | 118.8 ± 79.3 | 103.2 ± 42.3 | 105.8 ± 73.9 | <0.0001* |
| Haemoglobin, mmol/L | 6.6 ± 0.8 | 6.9 ± 0.8 | 7.1 ± 1.1 | 7.8 ± 0.9 | 8.0 ± 0.8 | <0.0001* |
| Total cholesterol, mmol/L | 4.2 ± 1.0 | 4.8 ± 1.1 | 4.9 ± 1.3 | 5.0 ± 1.5 | 4.9 ± 1.5 | <0.01* |
| s-tacrolimus, µg/L | 7.6 ± 3.2 | 9.9 ± 3.3 | 9.1 ± 3.2 | 8.0 ± 2.9 | 7.0 ± 2.2 | <0.01* |
| s-everolimus, µg/L | 2.4 ± 0.8 | 3.7 ± 1.6 | 7.3 ± 4.5 | 3.8 ± 0.9 | 4.6 ± 0.7 | 0.06 |
Data are presented as absolute number and present or mean ± standard deviation.
PVR, pulmonary vascular resistance; BMI, body mass index.
*P < 0.05.
Table 2 displays changes in the echocardiographic parameters of the 36 included patients during the first year. LV mass was unaffected the first month after HTX and decreased significantly afterwards (P < 0.0001). This decrease was mediated by decreasing wall thickness. LVEF was within normal range (60.4 ± 8.3%) at baseline and significantly increased the first month after HTX. Thereafter, LVEF remained unaffected. Fractional shortening (FS) remained unaffected and within normal range after HTX (P = 0.33). The magnitude of LV-GLS increased from −11.8 ± 3.2% at baseline to −15.3 ± 2.3% 3 months after HTX (P < 0.0001), and thereafter, LV-GLS was unchanged. We saw the same pattern in all myocardial layers. Tissue Doppler parameters of long-axis function, tissue tracking, and S′ likewise improved significantly within 3 months after HTX and stabilized afterwards. LV circumferential strain did not improve significantly after HTX.
Table 2.
Echocardiographic parameters during follow-up
| Baseline (n =36) | 1 month (n =35) | 3 months (n =34) | 6 months (n =34) | 12 months (n =34) | P-value | |
|---|---|---|---|---|---|---|
| SBP (mmHg) | 128.8 ± 15.0 | 135.9 ± 20.3 | 135.3 ± 18.1 | 138.6 ± 15.7 | 133.0 ± 18.4 | 0.37 |
| DBP (mmHg) | 76.6 ± 12.2 | 82.6 ± 11.0 | 83.6 ± 15.2 | 84.5 ± 13.1 | 82.2 ± 9.6 | <0.05* |
| Heart rate (bpm) | 85.5 ± 13.6 | 84.3 ± 12.6 | 83.5 ± 10.4 | 86.5 ± 12.3 | 86.9 ± 11.2 | 0.45 |
| Traditional LV parameters | ||||||
| FS (%) | 36.3 ± 6.9 | 37.6 ± 6.7 | 38.2 ± 4.8 | 36.1 ± 4.6 | 36.9 ± 5.1 | 0.33 |
| LV-mass | 186.0 ± 46.0 | 192.4 ± 46.3 | 175.0 ± 41.5 | 163.6 ± 48.2 | 159.5 ± 48.3 | <0.0001* |
| IVS (mm) | 11.3 ± 1.8 | 11.4 ± 1.9 | 10.5 ± 1.6 | 10.4 ± 2.0 | 10.0 ± 1.7 | <0.0001* |
| PW (mm) | 11.2 ± 1.9 | 11.5 ± 1.8 | 10.6 ± 1.4 | 10.3 ± 1.2 | 9.6 ± 1.6 | <0.0001* |
| LVEF | 60.4 ± 8.3 | 64.8 ± 5.9 | 65.6 ± 6.4 | 63.7 ± 5.8 | 64.7 ± 6.4 | <0.001* |
| EDV (mL) | 92.1 ± 26.2 | 99.9 ± 26.9 | 102.1 ± 25.0 | 100.4 ± 26.7 | 97.7 ± 27.2 | 0.12 |
| ESV (mL) | 36.1 ± 11.9 | 35.4 ± 11.8 | 35.8 ± 12.3 | 37.1 ± 13.7 | 35.2 ± 14.9 | 0.90 |
| 2D-STE LV | ||||||
| LV GLS ENDO (%) | −14.8 ± 3.8 | −17.6 ± 3.2 | −18.7 ± 2.7 | −18.5 ± 3.0 | −18.9 ± 2.9 | <0.0001* |
| LV GLS MID (%) | −11.8 ± 3.2 | −14.2 ± 2.9 | −15.3 ± 2.3 | −15.2 ± 2.5 | −15.7 ± 2.6 | <0.0001* |
| LV GLS EPI (%) | −9.6 ± 3.1 | −11.5 ± 2.6 | −12.6 ± 2.0 | −12.6 ± 2.2 | −13.3 ± 2.3 | <0.0001* |
| TTP (ms) | 63.7 ± 25.2 | 51.0 ± 17.9 | 44.9 ± 14.7 | 42.9 ± 14.7 | 43.2 ± 14.5 | <0.0001* |
| GCS ENDO (%) | −29.1 ± 7.0 | −30.3 ± 5.7 | −29.1 ± 6.0 | −29.9 ± 5.3 | −30.0 ± 6.6 | 0.81 |
| GCS MID (%) | −19.4 ± 4.8 | −20.3 ± 4.0 | −19.4 ± 4.0 | −20.0 ± 4.0 | −20.5 ± 4.4 | 0.64 |
| GCS EPI (%) | −13.4 ± 3.8 | −14.0 ± 3.2 | −13.2 ± 3.0 | −13.6 ± 3.3 | −14.0 ± 3.4 | 0.61 |
| Tissue Doppler LV | ||||||
| TT (mm) | 6.6 ± 2.0 | 8.1 ± 2.2 | 9.5 ± 1.6 | 9.6 ± 2.1 | 9.7 ± 1.7 | <0.0001* |
| S′ (cm/s) | 5.1 ± 1.7 | 5.7 ± 1.0 | 6.4 ± 1.5 | 6.3 ± 1.5 | 6.2 ± 1.3 | <0.0001* |
| Diastole | ||||||
| E/e′ (ratio) | 15.3 ± 5.9 | 12.6 ± 4.9 | 9.9 ± 4.4 | 8.3 ± 2.9 | 8.2 ± 2.6 | <0.0001* |
| E/A (ratio) | 2.3 ± 0.8 | 2.0 ± 0.7 | 1.8 ± 0.7 | 1.6 ± 0.5 | 1.6 ± 0.5 | <0.0001* |
| E-dec (ms) | 163.6 ± 30.3 | 161.7 ± 35.2 | 167.9 ± 38.1 | 169.1 ± 30.2 | 172.2 ± 34.1 | 0.30 |
| IVRT (ms) | 59.8 ± 16.4 | 65.6 ± 16.9 | 65.0 ± 15.0 | 66.6 ± 12.4 | 69.5 ± 19.2 | <0.05* |
| LA volume (mL) | 69.4 ± 28.2 | 84.8 ± 35.0 | 71.3 ± 29.5 | 71.5 ± 32.2 | 71.1 ± 28.2 | <0.001* |
| Traditional RV parameters | ||||||
| RV diameter (mm) | 30.3 ± 6.2 | 29.8 ± 7.4 | 30.3 ± 6.7 | 30.9 ± 6.4 | 30.7 ± 5.3 | 0.54 |
| TAPSE (mm) | 12.1 ± 3.2 | 13.6 ± 3.0 | 14.5 ± 3.4 | 14.1 ± 3.2 | 14.4 ± 2.7 | <0.0001* |
| TI gradient (mmHg) | 26.1 ± 7.0 | 25.4 ± 6.9 | 22.4 ± 6.7 | 23.5 ± 5.2 | 22.7 ± 3.7 | 0.10 |
Data are presented as mean ± standard deviation.
SBP, systolic blood pressure; DBP, diastolic blood pressure; FS, fractional shortening; LVEF, left ventricle ejection fraction; EDV, end-diastolic volume; ESV, end-systolic volume; GLS, global longitudinal strain; ENDO, endocardium; EPI, epicardium; TTP, time to peak; GCS, global circumferential strain; TT, tissue tracking; S′, peak systolic mitral annular velocities; E-dec, E-deceleration time; IVRT, isovolumetric relaxation time; LA, left atrium; RV, right ventricle; TAPSE, tricuspid annular plane systolic excursion; TI, tricuspid insufficiency.
*P < 0.05.
Parameters of LV myocardial relaxation, E/e′-ratio, E/A ratio, E-deceleration time (e-dec), and isovolumetric relaxation time (IVRT) revealed a significant, restrictive filling pattern at baseline but normalized within the first 6 months after HTX.
RV longitudinal function increased significantly within 3 months after HTX and stabilized afterwards. Tricuspid annular plane systolic excursion (TAPSE) increased from 11.8 ± 3.3 to 14.3 ± 3.3 mm (P < 0.0001). We found no correlation between pre-HTX pulmonary vascular resistance (PVR) and baseline TAPSE [β1 = −0.02 (95% CI: −1.8 to 1.2), P = 0.68] or between the tricuspid regurgitation gradient and baseline TAPSE [β1 = 7.0 (95% CI: −1.3 to 15.4), P = 0.09].
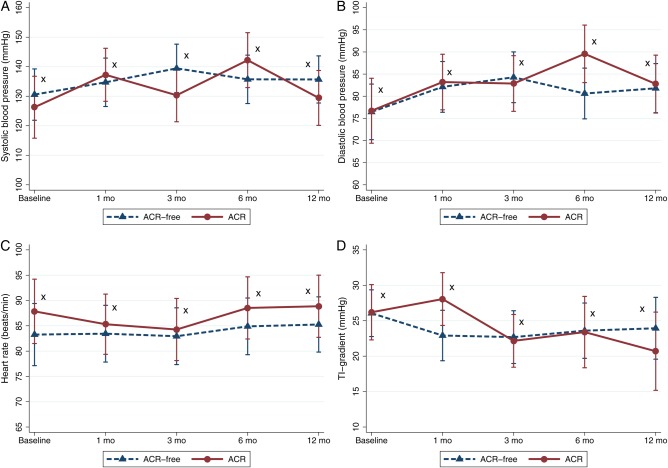
We found no difference between the ACR and the ACR-free groups 12 months after HTX regarding recipient or donor age, blood pressure, heart rate, co-morbidity (diabetes, hypertension, and hypercholesterolaemia), medication, and biochemistry; see Figure 1. As expected, patients in the ACR group had more biopsies taken (15.7 ± 2.6 vs. 13.2 ± 3.2, P < 0.05) and a higher number of biopsies showing 1R (6.8 ± 3.5 vs. 4.4 ± 2.3, P < 0.05) and 2R rejection (1.6 ± 0.6 vs. 0, P < 0.0001). In addition, signs of ischaemic transport damage were seen on average in 1.3 ± 1.3 biopsies in the ACR group vs. 0.6 ± 0.8 (P = 0.06) biopsies in the ACR-free group. Pre-HTX PVR did not differ significantly between the ACR and ACR-free group (1.5 ± 0.6 vs. 2.1 ± 1.6, P = 0.17).
Figure 1.
Margin plots with 95% confidence interval in reference to time after HTX in the ACR group and the ACR-free group. (A) Systolic blood pressure, (B) diastolic blood pressure, (C) heart rate, (D) tricuspid regurgitation gradient. *P > 0.05 comparing the ACR group and the ACR-free group.
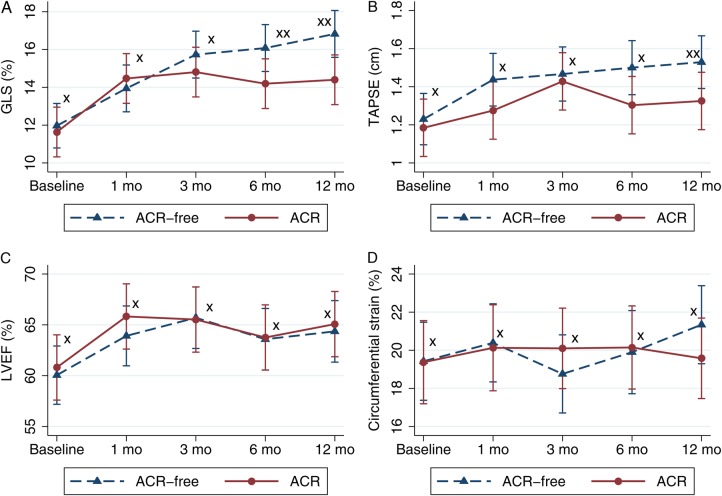
Table 3 shows various echocardiographic parameters of the ACR and the ACR-free group. LV-GLS after 12 months was −16.8 ± 2.2% in the ACR-free group and −14.4 ± 2.4% in the ACR group (P < 0.01). Serial assessment showed increasing GLS in both groups. ΔGLS 0–12 months was −4.6 ± 1.2% in the ACR-free group and −2.8 ± 2.7% in the ACR group (P = 0.19). However, ΔGLS 1–12 months was −2.9 ± 3.5% in the ACR-free group and 0.1 ± 2.4 in the ACR group (P < 0.01); see Figure 2.
Table 3.
Echocardiographic parameters comparing ACR with ACR-free group
| ACR-free group (n =20) | ACR group (n =16) | P-value | |
|---|---|---|---|
| LV-parameters | |||
| LV-GLS 12 months (%) | −16.8 ± 2.2 | −14.4 ± 2.4 | <0.01* |
| ΔLV-GLS 0–12 months | −4.6 ± 1.2 | −2.8 ± 2.7 | 0.19 |
| ΔLV-GLS 1–12 months | −2.9 ± 3.5 | 0.1 ± 2.4 | <0.01* |
| LV-ENDO-GLS 12 months (%) | −20.1 ± 2.7 | −17.5 ± 2.8 | <0.05* |
| ΔLV-ENDO-GLS 0–12 months | −4.1 ± 5.0 | −3.0 ± 2.9 | 0.44 |
| ΔLV-ENDO-GLS 1–12 months | −2.6 ± 3.9 | 0.4 ± 2.5 | <0.05* |
| LV-TTP 12 months (ms) | 39.1 ± 10.8 | 48.3 ± 16.5 | 0.06 |
| ΔLV-TTP 0–12 months | −19.8 ± 22.5 | −19.9 ± 26.1 | 0.99 |
| ΔLV-TTP 1–12 months | −11.7 ± 16.0 | −1.8 ± 15.0 | 0.08 |
| LV-GCS 12 months (%) | −21.3 ± 5.3 | −19.6 ± 3.2 | 0.26 |
| ΔLV-GCS 0–12 months | −2.1 ± 5.0 | 0.2 ± 5.2 | 0.23 |
| ΔLV-GCS 1–12 months | −1.2 ± 5.8 | 0.3 ± 4.4 | 0.44 |
| LVEF 12 months (%) | 64.4 ± 5.9 | 65.1 ± 7.2 | 0.75 |
| ΔLVEF 0–12 months | 3.4 ± 10.8 | 4.3 ± 7.5 | 0.79 |
| ΔLVEF 1–12 months | 0.2 ± 5.9 | −0.8 ± 5.8 | 0.64 |
| RV-parameters | |||
| TAPSE 12 months (mm) | 15.3 ± 2.8 | 13.2 ± 2.1 | <0.05* |
| ΔTAPSE 0–12 months | 3.2 ± 3.8 | 1.4 ± 1.3 | 0.08 |
| ΔTAPSE 1–12 months | 1.2 ± 4.3 | 0.5 ± 2.5 | 0.57 |
Data are presented as mean ± standard deviation.
LV, left ventricle; GLS, global longitudinal strain; ENDO, endocardium; TTP, time to peak; GCS, global circumferential strain; LVEF, left ventricular ejection fraction; RV, right ventricle; TAPSE, tricuspid annular plane systolic excursion.
*P < 0.05.
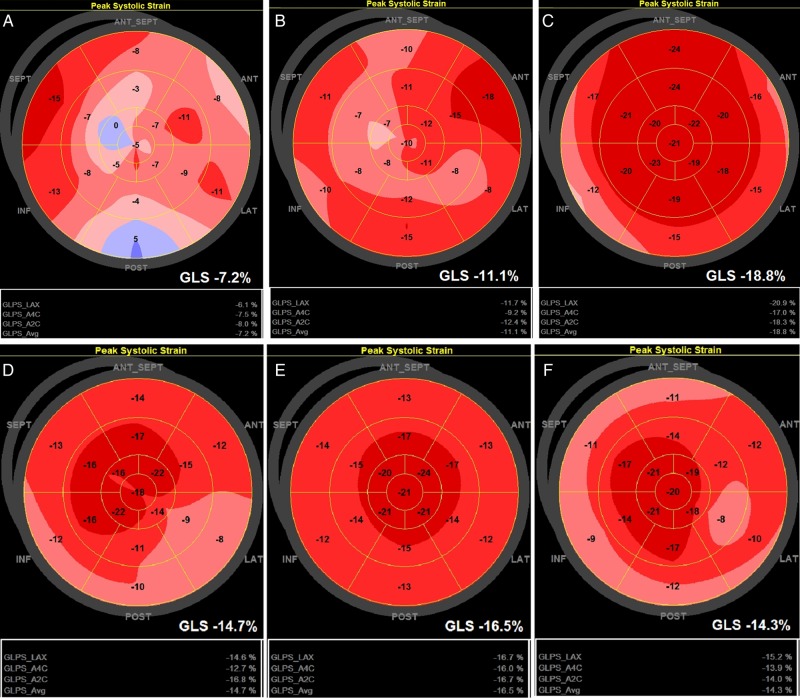
Figure 2.
Bulls eye 17-segment model of global longitudinal strain. (A–C) HTX Patient I: four 1R rejections and 0 ≥ 2R rejections during follow-up. (A) Baseline; (B) 1 month after HTX; and (C) 12 months after HTX. Normal epicardial vessels at coronary angiography 12 months after HTX. (D–F) HTX Patient II: eight 1R rejections and two 2R rejections (after 5 weeks and after 6 months) during follow-up. (D) Baseline; (E) 1 month after HTX; and (F) 12 months after HTX. Normal epicardial vessels at coronary angiography 12 months after HTX.
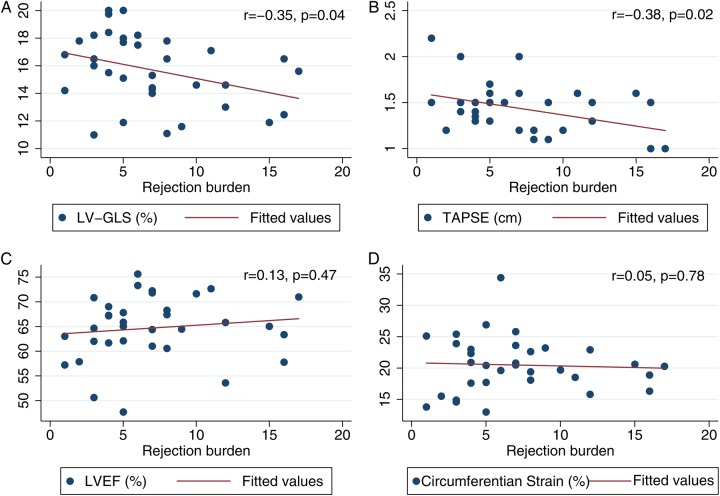
We found a significant correlation between LV-GLS after 12 months and the total rejection score (r = −0.35, P < 0.05). As demonstrated by multivariate analysis, after 12 months LV-GLS was significantly correlated with ACR group after adjustment for CAV, ischaemic transport damage, gender, donor age, blood pressure (systolic and diastolic), heart rate, serum haemoglobin, body mass index, LVEF, LV mass, hypertension, and diabetes [β1 = −1.8 (95% CI: −3.5;−0.1), P < 0.05]. The results of univariate and multivariate analysis of the ACR groups are shown in Table 4. We found no difference in circumferential deformation or LVEF between the ACR and ACR-free group; see Figure 3. In addition, we found no significant difference in diastolic function between the ACR and the ACR-free group 12 months after HTX (E/A ratio: 1.6 ± 0.5 vs. 1.7 ± 0.5, P = 0.47; E/e′: 8.4 ± 2.4 vs. 8.1 ± 2.8, P = 0.78; e-dec: 173.7 ± 20.8 vs. 171.0 ± 42.3 ms, P = 0.83; IVRT: 74.9 ± 16.8 vs. 65.1 ± 20.2 ms, P = 0.14). In a regression model, we found a non-significant trend towards correlation between ΔGLS and ΔE/e′ (r = −0.22, P = 0.23).
Table 4.
Univariate and multivariate analysis of relation of confounders to ACR group
| Univariate analysis | P-value | Multivariable analysis | P-value | |
|---|---|---|---|---|
| Odds ratio (95% CI) | Odds ratio (95% CI) | |||
| Confounders | ||||
| Gender | 0.71 (0.18–2.87) | 0.64 | 0.32 (0.02–4.28) | 0.39 |
| Diabetes | 1.33 (0.23–7.80) | 0.75 | 1.22 (0.09–16.77) | 0.88 |
| Ischaemic transport damage | 1.94 (0.94–4.02) | 0.07 | 3.02 (0.77–11.86) | 0.11 |
| Weight | 1.00 (0.97–1.03) | 0.96 | 1.02 (0.94–1.11) | 0.62 |
| CAV | 0.54 (0.08–3.40) | 0.51 | 0.16 (0.01–3.04) | 0.23 |
| SBP | 0.98 (0.94–1.02) | 0.33 | 0.97 (0.91–1.03) | 0.30 |
| DBP | 1.01 (0.94–1.09) | 0.78 | 1.09 (0.94–1.26) | 0.26 |
| HR | 1.03 (0.97–1.10) | 0.35 | 1.04 (0.92–1.17) | 0.52 |
| LV mass | 1.00 (0.99–1.01) | 0.93 | 1.00 (0.97–1.03) | 0.88 |
| LVEF | 1.02 (0.91–1.13) | 0.74 | 0.98 (0.85–1.13) | 0.80 |
| LV and RV long-axis function | ||||
| LV-GLS | 1.55 (1.08–2.22) | <0.05* | 4.11 (1.03–16.3) | <0.05* |
| TAPSE | 0.70 (0.50–0.98) | <0.05* | 0.23 (0.07–0.81) | <0.05* |
CAV, coronary allograft vasculopathy; SBP, systolic blood pressure; DBP, diastolic blood pressure; HR, heart rate; LV, left ventricle; LVEF, left ventricular ejection fraction; GLS, global longitudinal strain; TAPSE, tricuspid annular plane systolic excursion.
*P < 0.05.
Figure 3.
Margin plots with 95% confidence interval in reference to time after HTX in the ACR group and the ACR-free group. (A) Left ventricular global longitudinal strain, (B) tricuspid annular plane systolic excursion, (C) left ventricular ejection fraction, (D) left ventricular global circumferential strain. *P > 0.05 comparing the ACR group and the ACR-free group. **P < 0.05 comparing the ACR group and the ACR-free group.
RV longitudinal function was significantly lower in the ACR group than in the ACR-free group. TAPSE 12 months after HTX was 15.3 ± 2.8 mm in the ACR-free group vs. 13.2 ± 2.1 mm in the ACR group, P < 0.05. In addition, RV longitudinal function was significantly correlated with total rejection score (TAPSE: r = −0.38, P < 0.05); see Figure 4.
Figure 4.
Scatter plots with regression lines for (A) left ventricular global longitudinal strain 12 months after HTX; (B) tricuspid annular plane systolic excursion 12 months after HTX; (C) left ventricular ejection fraction 12 months after HTX; (D) left ventricular global circumferential strain 12 months after HTX in reference to total rejection score within first year after HTX.
Discussion
The main findings of this study are that despite an overall significant recovery of LV deformation and RV function, the occurrence of ACR impairs the improvement of LV GLS and TAPSE during the first year after HTX. Longitudinal systolic LV and RV function 12 months after HTX, measured by LV-GLS and TAPSE, correlated significantly with the rejection burden, whereas traditional measures of systolic function, such as LVEF, did not correlate with the rejection burden. We found an absolute difference of −2.4% in LV-GLS between the ACR-free group (−16.8) and the ACR group (−14.4%) 12 months after HTX. The results remained significant after adjustment for potential confounders in a multivariate analysis. All longitudinal myocardial layers showed recovery after HTX, whereas the circumferential function remained within the normal range.
We found significant LV myocardial relaxation and deformation abnormality at baseline with elevated E/e′ ratio and severely reduced LV-GLS. Despite normal LVEF, ∼60% the patients were treated with diuretics. In our experience, the clinical condition of patients early after HTX is often characterized by oedema, dyspnoea, reduced exercise capacity, and pleural effusion. These findings are traditionally interpreted as RV failure, graft transport injury, and operational sequelae. In accordance with this interpretation, we found TAPSE to be significantly reduced at baseline. However, we also demonstrated that LV longitudinal deformation is significantly affected (despite normal LVEF) which leads to signs of elevated LV filling pressures as indicated by E/e'. Monitoring of the RV and the LV longitudinal function therefore seems relevant and may be used to guide the pharmacological treatment.
Our results corroborate those of other studies that found reduced longitudinal myocardial function, measured by LV-GLS, in stable HTX patients compared with normal subjects9–11 and during ACR.15,18 Several factors, such as the surgical procedure, ischaemic transport damage, LV remodelling, rejections, hypertension, fibrosis due to immunosuppressive treatment, and impaired micro- and macrovascular perfusion potentially affect the longitudinal myocardial function in HTX patients. Despite the presence of several possible confounders, our study demonstrates that ACR are major contributors to the impaired LV and RV longitudinal function seen in otherwise stable HTX patients during the first year after HTX. Interestingly, we found a gradual improvement of LV and RV longitudinal function in the ACR-free group during the 12-month follow-up period. This indicates that remodelling after HTX is an extended process that involves all longitudinal myocardial layers.
Other parameters remained unaffected during follow-up. Hence, we found no change in circumferential deformation, LVEF, and FS in the ACR group. The absence of any change in these parameters together with the high inter- and intra-observer variability limits their use in the overall monitoring of graft function with respect to ACR. LV-GLS is an important prognostic marker in the early7 and the intermediate phase (within 24 months) after transplantation.8 Eleid et al.8 found speckle tracking to be helpful in estimating the burden of LV dysfunction in HTX patients where LV dysfunction evolved independently of ACR episodes. However, in their study, longitudinal strain was obtained solely from a four-chamber view (six segments), whereas we used a 17-LV-segment global assessment. Our results suggest that the global assessment of LV-GLS is more appropriate for graft monitoring, and assessment of LV-GLS seems to be a highly valuable addition to LV-EF and diastolic Doppler evaluation of LV function. LV-GLS should therefore be considered for graft function monitoring of HTX patients.
The demonstrated correlation between impaired longitudinal LV and RV function and ACR in our study may have several explanations. Both the ACR group and the ACR-free group showed significant improvement in systolic and diastolic function within the first month after HTX. Afterwards, the remodelling process in the ACR group did not increase GLS any further. Previous studies demonstrated an increased risk of CAV development in patients with a high rejection burden, which suggests that an immune-mediated cause of vasculopathy is at play.3 Longitudinal myocardial function is dependent on both the epicardial vessels and the microvascular system. Hiemann etal.19 demonstrated that microvascular dysfunction is very common in HTX patients and that this dysfunction is associated with an adverse prognosis. Inflammation-induced, impaired microvascular perfusion may be triggered by severe or repeated ACRs which results in impaired longitudinal myocardial deformation. Finally, patients with severe or repeated ACR may have received a higher dose of immunosuppressive treatment in periods after HTX, which is associated with myocardial fibrosis.20,21
Previous studies have described impaired longitudinal function of RV measured by TAPSE and tissue Doppler in stable HTX patients.22–24 Interestingly, D'Andrea A et al. found that RV-EF measured with 3D echocardiography in HTX patients without previous ACRs to be within the normal range despite a reduced longitudinal function, which indicates that geometrical rather than functional changes are responsible for the reduced long-axis function of RV. However, our results show that the long-axis function of RV is significantly affected by ACR. RV systolic function is often reduced in the early phase after HTX which causes clinical problems with oedema and renal impairment. Clinical recovery is seen within weeks, but the function of RV is difficult to assess in the clinical settings, and no single parameter describes RV systolic function in HTX patients. More studies are needed to evaluate the overall function of RV in HTX patients.
Limitations
We acknowledge a number of limitations to this study. It reflects the experience of a single centre with a relatively small cohort of patients. We did not evaluate the microvascular perfusion or the degree of cardiac fibrosis, which could be relevant to assess the cause of impaired long-axis function in this population.
We did not assess the physical capacity of the HTX patients, and more studies are needed to clarify the clinical role of impaired TAPSE seen in our study.
Conclusion
Gradual recovery of LV and RV longitudinal myocardial function was seen through the first year after HTX. All LV longitudinal myocardial layers improved during follow-up. However, the degree of LV and RV recovery was significantly affected by the occurrence of ACR. In contrast to traditional measures such as LVEF and LV diastolic Doppler parameters, LV-GLS and TAPSE significantly correlated with rejection burden and therefore seem to be more sensitive tools for graft monitoring.
Acknowledgements
Lene Lindencrone Konrad and Bente Mortensen are acknowledged for their highly appreciated assistance with echocardiographies and Else Marie Tram for her valuable assistance with data collection.
Conflict of interest: None declared.
References
- 1.Stehlik J, Edwards LB, Kucheryavaya AY, Benden C, Christie JD, Dipchand AI, et al. The Registry of the International Society for Heart and Lung Transplantation: 29th official adult heart transplant report–2012. J Heart Lung Transplant 2012;31:1052–64. [DOI] [PubMed] [Google Scholar]
- 2.Patel JK, Kittleson M, Kobashigawa JA. Cardiac allograft rejection. Surgeon 2011;9:160–7. [DOI] [PubMed] [Google Scholar]
- 3.Raichlin E, Edwards BS, Kremers WK, Clavell AL, Rodeheffer RJ, Frantz RP, et al. Acute cellular rejection and the subsequent development of allograft vasculopathy after cardiac transplantation. J Heart Lung Transplant 2009;28:320–7. [DOI] [PubMed] [Google Scholar]
- 4.Clemmensen TS, Eiskjaer H, Kofoed-Nielsen PB, Hoyer S, Poulsen SH. Case of acute graft failure during suspected humoral rejection with preserved ejection fraction, but severely reduced longitudinal deformation detected by 2D-speckle tracking. Case Rep Transplant 2014;2014:173589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clemmensen TS, Logstrup BB, Eiskjaer H, Poulsen SH. Evaluation of longitudinal myocardial deformation by 2-dimensional speckle-tracking echocardiography in heart transplant recipients: relation to coronary allograft vasculopathy. J Heart Lung Transplant 2015;34:195–203. [DOI] [PubMed] [Google Scholar]
- 6.Dandel M, Hetzer R. Echocardiographic strain and strain rate imaging--clinical applications. Int J Cardiol 2009;132:11–24. [DOI] [PubMed] [Google Scholar]
- 7.Sarvari SI, Gjesdal O, Gude E, Arora S, Andreassen AK, Gullestad L, et al. Early postoperative left ventricular function by echocardiographic strain is a predictor of 1-year mortality in heart transplant recipients. J Am Soc Echocardiogr 2012;25:1007–14. [DOI] [PubMed] [Google Scholar]
- 8.Eleid MF, Caracciolo G, Cho EJ, Scott RL, Steidley DE, Wilansky S, et al. Natural history of left ventricular mechanics in transplanted hearts: relationships with clinical variables and genetic expression profiles of allograft rejection. JACC Cardiovasc Imaging 2010;3:989–1000. [DOI] [PubMed] [Google Scholar]
- 9.Saleh HK, Villarraga HR, Kane GC, Pereira NL, Raichlin E, Yu Y, et al. Normal left ventricular mechanical function and synchrony values by speckle-tracking echocardiography in the transplanted heart with normal ejection fraction. J Heart Lung Transplant 2011;30:652–8. [DOI] [PubMed] [Google Scholar]
- 10.Syeda B, Hofer P, Pichler P, Vertesich M, Bergler-Klein J, Roedler S, et al. Two-dimensional speckle-tracking strain echocardiography in long-term heart transplant patients: a study comparing deformation parameters and ejection fraction derived from echocardiography and multislice computed tomography. Eur J Echocardiogr 2011;12:490–6. [DOI] [PubMed] [Google Scholar]
- 11.Pichler P, Binder T, Hofer P, Bergler-Klein J, Goliasch G, Lajic N, et al. Two-dimensional speckle tracking echocardiography in heart transplant patients: three-year follow-up of deformation parameters and ejection fraction derived from transthoracic echocardiography. Eur Heart J Cardiovasc Imaging 2012;13:181–6. [DOI] [PubMed] [Google Scholar]
- 12.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Recommendations for chamber quantification. Eur J Echocardiogr 2006;7:79–108. [DOI] [PubMed] [Google Scholar]
- 13.Reisner SA, Lysyansky P, Agmon Y, Mutlak D, Lessick J, Friedman Z. Global longitudinal strain: a novel index of left ventricular systolic function. J Am Soc Echocardiogr 2004;17:630–3. [DOI] [PubMed] [Google Scholar]
- 14.Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation 2002;105:539–42. [DOI] [PubMed] [Google Scholar]
- 15.Clemmensen TS, Logstrup BB, Eiskjaer H, Poulsen SH. Changes in longitudinal myocardial deformation during acute cardiac rejection: the clinical role of two-dimensional speckle-tracking echocardiography. J Am Soc Echocardiogr 2015;28:330–9. [DOI] [PubMed] [Google Scholar]
- 16.Stewart S, Winters GL, Fishbein MC, Tazelaar HD, Kobashigawa J, Abrams J, et al. Revision of the 1990 working formulation for the standardization of nomenclature in the diagnosis of heart rejection. J Heart Lung Transplant 2005;24:1710–20. [DOI] [PubMed] [Google Scholar]
- 17.Mehra MR, Crespo-Leiro MG, Dipchand A, Ensminger SM, Hiemann NE, Kobashigawa JA, et al. International Society for Heart and Lung Transplantation working formulation of a standardized nomenclature for cardiac allograft vasculopathy-2010. J Heart Lung Transplant 2010;29:717–27. [DOI] [PubMed] [Google Scholar]
- 18.Sera F, Kato TS, Farr M, Russo C, Jin Z, Marboe CC, et al. Left ventricular longitudinal strain by speckle-tracking echocardiography is associated with treatment-requiring cardiac allograft rejection. J Card Fail 2014;20:359–64. [DOI] [PubMed] [Google Scholar]
- 19.Hiemann NE, Wellnhofer E, Knosalla C, Lehmkuhl HB, Stein J, Hetzer R, et al. Prognostic impact of microvasculopathy on survival after heart transplantation: evidence from 9713 endomyocardial biopsies. Circulation 2007;116:1274–82. [DOI] [PubMed] [Google Scholar]
- 20.Hiemann NE, Wellnhofer E, Lehmkuhl HB, Knosalla C, Hetzer R, Meyer R. Everolimus prevents endomyocardial remodeling after heart transplantation. Transplantation 2011;92:1165–72. [DOI] [PubMed] [Google Scholar]
- 21.Gramley F, Lorenzen J, Pezzella F, Kettering K, Himmrich E, Plumhans C, et al. Hypoxia and myocardial remodeling in human cardiac allografts: a time-course study. J Heart Lung Transplant 2009;28:1119–26. [DOI] [PubMed] [Google Scholar]
- 22.D'Andrea A, Riegler L, Nunziata L, Scarafile R, Gravino R, Salerno G, et al. Right heart morphology and function in heart transplantation recipients. J Cardiovasc Med (Hagerstown) 2013;14:648–58. [DOI] [PubMed] [Google Scholar]
- 23.Goland S, Siegel RJ, Burton K, De Robertis MA, Rafique A, Schwarz E, et al. Changes in left and right ventricular function of donor hearts during the first year after heart transplantation. Heart 2011;97:1681–6. [DOI] [PubMed] [Google Scholar]
- 24.Lunze FI, Colan SD, Gauvreau K, Chen MH, Perez-Atayde AR, Blume ED, et al. Cardiac allograft function during the first year after transplantation in rejection-free children and young adults. Circ Cardiovasc Imaging 2012;5:756–64. [DOI] [PubMed] [Google Scholar]