Abstract
Aims
Changes in electrical activation sequence are known to affect the timing of cardiac mechanical events. We aim to demonstrate that these also modify global properties of the intraventricular blood flow pattern. We also explore whether such global changes present a relationship with clinical outcome.
Methods and results
We investigated 30 heart failure patients followed up after cardiac resynchronization therapy (CRT). All subjects underwent echocardiography before implant and at follow-up after 6+ months. Left ventricular mechanics was investigated at follow-up during active CRT and was repeated after a temporary interruption <5 min later. Strain analysis, performed by speckle tracking, was used to assess the entity of contraction (global longitudinal strain) and its synchronicity (standard deviation of time to peak of radial strain). Intraventricular fluid dynamics, by echographic particle image velocimetry, was used to evaluate the directional distribution of global momentum associated with blood motion. The discontinuation of CRT pacing reflects into a reduction of deformation synchrony and into the deviation of blood flow momentum from the base–apex orientation with the development of transversal flow-mediated haemodynamic forces. The deviation of flow momentum presents a significant correlation with the degree of volumetric reduction after CRT.
Conclusion
Changes in electrical activation alter the orientation of blood flow momentum. The long-term CRT outcome correlates with the degree of re-alignment of haemodynamic forces. These preliminary results suggest that flow orientation could be used for optimizing the biventricular pacing setting. However, larger prospective studies are needed to confirm this hypothesis.
Keywords: cardiac fluid mechanics, echo-PIV, haemodynamic forces, cardiac mechanics, electrical activation
Introduction
Numerous recent studies about left ventricular (LV) fluid dynamics demonstrated the existence of a relationship between LV flow and cardiac function.1–4 Based on these and numerous other works, a consensus has grown about the potential relevance of blood motion to the heart physiology,5–8 in particular to the modulation of adverse clinical outcome in heart failure patients.9
Intraventricular blood motion is characterized by the formation of vortices,10,11 which are fundamental performers in fluid dynamics with a marked, intrinsic instability that gives rise to rapid accelerations, deviations, and sharp fluctuations of pressure and shear stress. Modifying the fine balance between LV vortices and cardiac tissue may lead to large differences in terms of energetic and dynamical interaction between blood and tissue.12
The sequence of electrical activation was previously shown to influence the timing of mechanical contraction events.13,14 It is therefore natural to assume that changes in electrical activation also affect the development of intraventricular flow. However, due to the nature of blood motion, a contraction event at one time reflects on the organization intraventricular flow also at subsequent times. Therefore, modifications in contraction timings are expected to reflect globally in the overall development of the flow pattern.
We hypothesize that changes in the sequence of electrical activation affect global dynamic properties of intraventricular vortex flow. To test this hypothesis, we considered patients who underwent cardiac synchronization therapy (CRT) and investigated the changes in mechanical function after the sudden interruption of biventricular pacing. These changes are also compared with the effectiveness of the pacing therapy.
Methods
Clinical procedure
We studied 30 heart failure patients with dilated LV that were implanted with biventricular pacemaker and were followed up after not <6 months to assess the response to CRT by echocardiography. This is an observational study where patients originally underwent the normal routine at the clinical institution when they were admitted to CRT. The CRT implant was performed according to the guidelines in use and by means of standard procedure.15 Three transvenous pacing leads were inserted: one in right atrium and another in the septal or apical endocardial sites of the right ventricle. A coronary sinus lead was positioned on the LV free wall through coronary sinus tributary veins.
After implantation, patients returned for regular in-hospital clinic visits every 6 months, and the clinical data were collected in the hospital databases. Inclusion criteria were non-ischaemic and non-valvular dilated cardiomyopathy, sinus rhythm with spontaneous atrioventricular conduction, age >18 years, and ability to understand and sign the informed consent. Exclusion criteria were persistent and/or chronic atrial fibrillation, severe renal insufficiency (creatinine clearance <30 mL/min), acute coronary syndrome, cardiac insufficiency acute or of advanced grade (NYHA IV), severe either pulmonary hypertension or chronic obstructive pulmonary disease, uncontrolled systemic hypertension, and pacemaker dependence. This study was performed conform to the declaration of Helsinki under the approval of our Institution's Ethical Committee for the protection of human subjects.
Recruitment was performed from June 2013 to June 2014; at the time of the scheduled in-hospital visit, we selected 36 patients who met the criteria. Of the 36 selected patients, 6 were excluded from the study: 1 patient refused the informed consent, 2 patients did not have available baseline clinical data, and for 3 were not available baseline echocardiographic data. All enrolled patients were in optimal medical therapy with an effective CRT device stimulation >95% (defined as the ratio of the amount of biventricular pacing over the pacing time). During the follow-up period, if it was considered necessary, the device setting (AV delay and/or VV delay) was optimized: this was performed at least 6 months before the examination for the present study. The clinical characteristics of the study population are shown in Table 1. The LV functions are then evaluated at baseline (CRT-ON) and during a temporarily discontinuation (CRT-OFF), lasting <5 min.
Table 1.
Demographic and clinical characteristics of the study population
| Variable | Idiopathic DCM (n = 30) |
|---|---|
| Age (years) | 58 ± 11 |
| Male/female | 26/4 (86.7%) |
| Heart rate (bpm) | 61.4 ± 7.2 |
| QRS duration (ms) | 151.6 ± 16.9 |
| Systolic BP (mmHg) | 110 ± 10 |
| Diastolic BP (mmHg) | 72 ± 10 |
| Body surface area (m2) | 1.99 ± 0.02 |
| NYHA functional class III/IV | 29 (97%)/1 (3%) |
| Hypertension | 18 (60%) |
| Diabetes | 9 (30%) |
| COPD | 9 (30%) |
| Hyperlidaemia | 10 (33.3%) |
| b-Blockade (%) | 30 (100%) |
| Loop diuretics (%) | 29 (96.7%) |
| ACE inhibitors (%) | 24 (80%) |
| K-sparing agents (%) | 20 (66.7%) |
| Digitalis | 14 (46.7%) |
| Ivabradine | 7 (23.3%) |
| Antiarrhythmic drugs | 6 (20%) |
| Ca-antagonists | 4 (13.3%) |
| Antiplatelet/anticoagulants | 29 (96.7%) |
| Statins | 16 (53.3%) |
DCM, dilated cardiomyopathy; NYHA, New York Heart Association; ACE, angiotensin-converting enzyme; BP, blood pressure; COPD, chronic obstructive pulmonary disease.
Echocardiography
All subjects underwent echocardiographic examination with Siemens SC2000 equipment (Siemens Ultrasound, Mountain View, CA, USA). Two-dimensional (2D) B-mode apical two-chamber and four-chamber views were recorded for volume and strain quantification. Volumes were computed by biplane Simpson method. Two-dimensional strain parameters were computed by speckle tracking software included in the echograph (VVI 3.0.1.5, Siemens Ultrasound). Global longitudinal strain (GLS), averaging the values in the 12 segments, is reported as a measure of systolic function, and the standard deviation of the time to peak of radial/transverse strain out of 12 segments (SDTTS) is reported as a measure of synchronicity.13,14
Two-dimensional B-mode apical three-chamber view with infusion of contrast agent was recorded for the evaluation of intra-cavity blood motion. The contrast study was carried on by infusing a slow bolus of 1 mL of SonoVue (Bracco Imaging SpA, Milano, Italy) followed by rapid infusion of normal saline. Three cardiac cycles were digitally acquired using second tissue harmonic at mechanical index ∼0.4. The ultrasound beam was focused at the LV base to have uniform insonation on the contrast bubble region. The scan field was optimized to contain the entire LV and ensure high frame rates (70–90 Hz). The video sequences were recorded during the washout of the contrast agent when the diluted bubbles adequately display the typical swirling motion of intraventricular blood flow.16 The clips captured in this phase of the contrast study were processed by particle imaging velocimetry (echo-PIV); this is a post-processing technique that allows tracking micro bubbles over two subsequent frames: the distance travelled from one frame to the next, divided by the time interval, is the velocity vector.16–19 Echo-PIV velocity estimation and post-processing were performed through a dedicated software (Hyperflow ver. 6.5.3.2, AMID SRL, Sulmona, Italy).
Dynamic flow properties are assessed by computing the rate of change of flow momentum20 integrated in the whole LV chamber. This global momentum rate represents the haemodynamic force globally exchanged, at every time instant, between blood and surrounding tissue. The directional distribution of global momentum during the entire heart cycle is summarized in terms of a polar histogram (magnitude-weighted frequency along very specific direction, such as the one used for wind description, see Figure 2 below). This polar image gives a synthetic picture of the overall haemodynamic forces associated with intraventricular blood motion, in particular identifying whether they are aligned along the base–apex direction, in compliance with the emptying–filling process, or they deviate by developing non-physiological transversal components. For the sake of quantification, a single flow angle parameter, φ, indicating the dominant orientation of the haemodynamic forces, is evaluated (sin2φ = ∑F × sin2θ/∑F, where F and θ are the magnitude and orientation of the force, and the summation is extended to all frames). This parameter ranges from zero, when flow force is predominantly along the base–apex direction, up to 90° when it becomes transversal.
Figure 2.
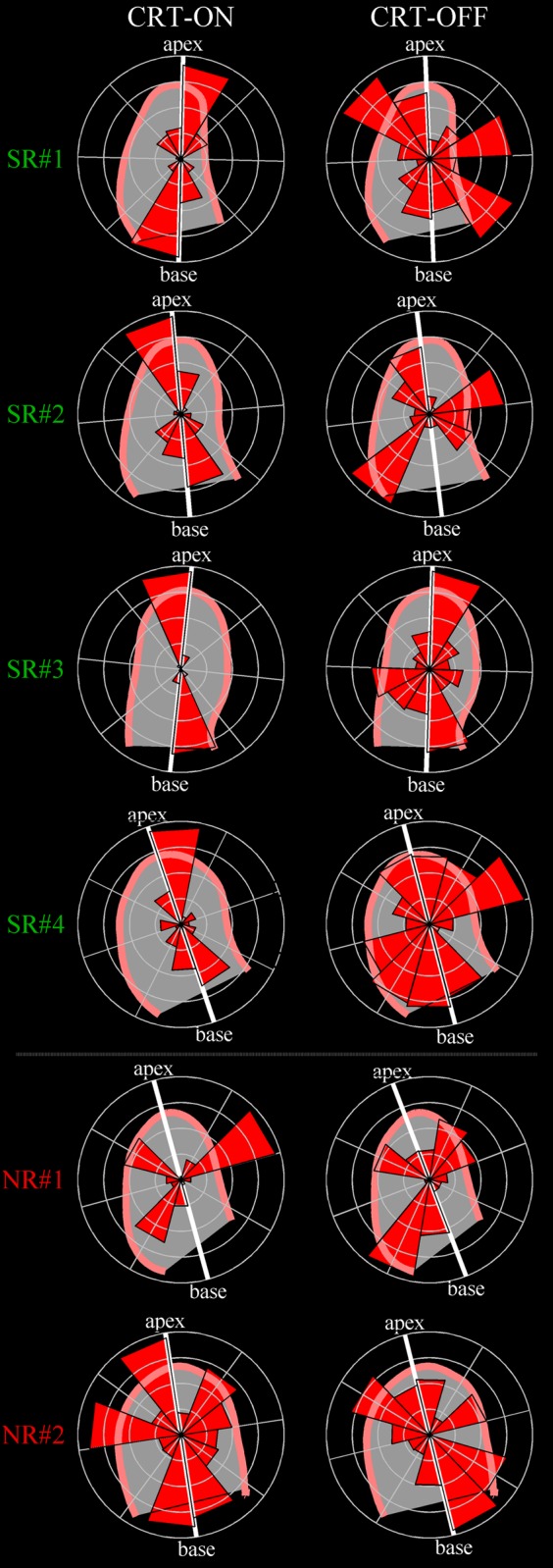
Directional distribution of flow momentum in SR and NR patients. Polar histograms show the orientation and relative magnitude of blood-induced intraventricular forces in four examples of SR patients and two examples of NRs. The left column shows the flow forces distribution under normal condition with CRT active (CRT-ON); the right column reports the same representation during temporary CRT deactivation (CRT-OFF). SR patients present a longitudinal alignment of haemodynamic forces that is lost when the therapeutic support is discontinued. Differently, NRs do not display a preferable longitudinal orientation either during active or inactive synchronization therapy.
In addition, the dissipation of kinetic energy integrated during the heartbeat was computed as an index of cardiac efficiency.21
Statistical analysis
The values of the cardiac mechanics parameters, and of their changes from active electrical pacing (CRT-ON) to temporary deactivation (CRT-OFF), were expressed as average ± standard deviation. The degree of dispersion about the mean was expressed by the coefficient of variation, CV, defined as the ratio of standard deviation to the average; dispersion was considered significant when CV > 1. The comparisons between parameters measured on all the patients during CRT-ON and CRT-OFF were performed by a two-tailed paired Student t-test with significance level of P = 0.01.
Mechanical variables, and their changes from CRT-ON to CRT-OFF, were correlated with measures of the degree of effectiveness of the electrical pacing therapy. The latter was expressed by the relative reduction, ΔEDV and ΔESV, of end-diastolic volume (EDV) and end-systolic volume (ESV), where the prefix Δ indicates the changes from pre-implant to follow-up, and by the value of ejection fraction (EF) measured at follow-up or its increase in ΔEF. Correlations were graded by the linear correlation coefficient r with significance threshold, |r|>0.6. Statistical processing was performed with MatLab (Natick, MA, USA; MatLab R2014a, ver. 8.3.0.532 with Statistics Toolbox R2014a).
The statistical analysis was integrated by a comparison at the individual level in two well-differentiated subgroups corresponding to super-responder (SR) and non-responder patients (NR). Both subgroups were selected from patients with critical pre-implant condition defined as EDV > 200 mL, ESV > 160 mL, EF < 30%; the SR subgroup was characterized by high therapeutic outcome ΔEDV > 40%, ΔESV > 40%, and by the value of EF measured at follow-up EF > 40%. On the opposite end, the NR subgroup was defined by ineffective outcome ΔEDV < 15%, ΔESV < 15%, and EF < 30%.
Reproducibility of flow analysis
Inter-operator variability of flow momentum quantification was evaluated by repeating evaluation on the same images by a second operator, blind to the previous results, on all 30 cases both CRT-ON and CRT-OFF. Repeatability of flow analysis was also evaluated with respect to a second acquisitions, performed by the same operator at time distance <60 min. This was limited because of the need of infusion of contrast agent, to five subjects, both CRT-ON and CRT-OFF.
Differences were described in terms of average ± standard deviation; statistical comparison was also verified by a paired two-tailed Student t-test with significance level assumed P = 0.1. Inter-observer agreement was presented using intra-class correlation coefficient (ICC). Bland–Altman analysis was also performed to verify the absence of systematic bias.
Results
Table 2 reports the echocardiographic measurements of LV volumes pre-implant (PRE-CRT) and at follow-up (POST-CRT). On average, the therapy produced a reduction of LV volumes and an increase of EF and GLS, whereas stroke volume and SDTTS were not significantly modified.
Table 2.
Echocardiographic LV measurements pre-implant (PRE-CRT) and at post-implant follow-up (POST-CRT)
| PRE-CRT | POST-CRT | P | |
|---|---|---|---|
| Ejection fraction (%) | 23.6 ± 7.2 | 39.2 ± 11.9 | 0.0001 |
| Stroke volume (mL) | 51.6 ± 22 | 53.8 ± 15 | NS |
| End-diastolic volume (mL) | 227.8 ± 94 | 151.7 ± 94 | 0.0001 |
| End-systolic volume (mL) | 176.1 ± 84 | 101.8 ± 99 | 0.0001 |
| GLS (%) | −7.7 ± 2.9 | −12 ± 3.2 | 0.0001 |
| SDTTS (ms) | 148.7 ± 48.8 | 145 ± 40.1 | NS |
GLS, global longitudinal strain; SDTTS, standard deviation of time to peak of transverse strain.
Table 3 reports the values of the clinical, mechanical, and flow parameters evaluated during CRT-ON and CRT-OFF. All the clinical parameters, volumetric, mitral inflow, or aortic outflow, did not show significant modifications in the brief period from CRT-ON to CRT-OFF. The modifications of LV mechanical parameters were non-significant in terms of global contraction (GLS: P = 0.43), while they were significant for the synchrony of contraction (SDTTS: P = 0.001). Flow displayed a significant change of momentum orientation (φ: P < 0.0001) and non-significant alteration of energetic dissipation.
Table 3.
Parameters evaluated during active electrical pacing (CRT-ON) and temporary deactivation (CRT-OFF) expressed as average ± standard deviation, and significance (P-value) of statistical comparison by a two-tailed paired Student t-test (significance level P = 0.01)
| CRT-ON | CRT-OFF | P | |
|---|---|---|---|
| Heart rate (bpm) | 61.4 ± 7.2 | 60.5 ± 9.7 | NS |
| Ejection fraction (%) | 39.2 ± 12 | 37.7 ± 11 | NS |
| End-diastolic volume (mL) | 151.7 ± 94 | 161.5 ± 96 | NS |
| End-systolic volume (mL) | 101.8 ± 99 | 106.7 ± 86 | NS |
| Mitral E/Vp ratio | 1.78 ± 0.7 | 1.83 ± 0.7 | NS |
| Mitral E/A ratio | 0.90 ± 0.37 | 0.82 ± 0.28 | NS |
| Mitral E deceleration time (ms) | 215.1 ± 58 | 202 ± 54 | NS |
| VTI mitral inflow (m) | 0.227 ± 0.18 | 0.182 ± 0.03 | NS |
| Diastolic filling time (ms) | 546.9 ± 102 | 517.5 ± 158 | NS |
| VTI aorta outflow (m) | 0.24 ± 0.05 | 0.24 ± 0.06 | NS |
| Systolic ejection time (ms) | 302.1 ± 33 | 301.6 ± 36 | NS |
| E/e′ (septal) | 9.7 ± 2.1 | 10.0 ± 2.0 | NS |
| E/e′ (lateral) | 8.7 ± 3.5 | 9.1 ± 3.2 | NS |
| GLS (%) | −12 ± 3 | −12 ± 3 | NS |
| SDTTS (ms) | 145 ± 40 | 175 ± 43 | 0.001 |
| Flow momentum orientation φ (°) | 34.7 ± 6.5 | 43.3 ± 6.1 | 0.0001 |
| Flow energy dissipation (−) | 0.432 ± 0.47 | 0.346 ± 0.14 | NS |
E, mitral flow E wave peak velocity, evaluated by PW Doppler; Vp, mitral flow propagation velocity assessed by color M-Mode Doppler; A, mitral flow A wave peak velocity, evaluated by PW Doppler; VTI, velocity time integral; GLS, global longitudinal strain; SDTTS, standard deviation of time to peak of transverse strain.
The change of a parameter from CRT-ON to CRT-OFF is briefly indicated by a prefix δ, to differentiate them from the long-term clinical changes, from pre-implant to follow-up indicated by the prefix Δ.
Change in tissue synchronicity (δSDTTS = SDTTSOFF – SDTTSON = 36 ± 54 ms) was positive on average, indicating a systematic reduction when pacing was active, with significant variability described CV = 1.5. Differences in flow angle (δφ = φOFF – φON = 8.6 ± 9.4°) was positive on average, indicating a systematic increase of longitudinal alignment when pacing was active, with significant variability (CV = 1.1). A careful look at the values of δφ shows that they were uniformly distributed from small (with a few negative) values to large positive ones.
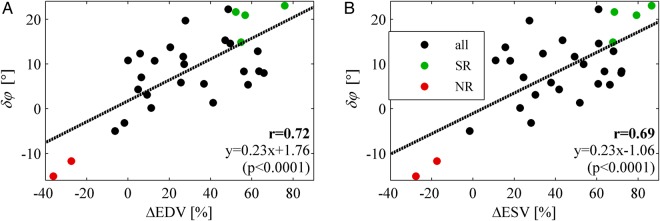
The correlation coefficients between the changes of mechanical variables (δGLS, δSDTTS, and δφ) and measures of the degree of effectiveness of the electrical pacing therapy (ΔEDV, ΔESV, EF, and ΔEF) are reported in Table 4. A significant correlation was found between volumetric reductions (reverse remodelling) and modification of the flow momentum angle δφ (ΔEDV: r = 0.72; ΔESV: r = 0.69), as shown graphically in Figure 1. This correlation resulted to be mainly ascribed to a significant correlation between flow momentum angle φ at CRT-ON and volumetric reductions (ΔEDV: r = 0.69; ΔESV: r = 0.65). No other significant correlation was found, with the exception of that between GLS and EF that represented a confirmation in the present data of their geometric relationship.22
Table 4.
Correlation coefficient, |r|, between changes from CRT-ON to CRT-OFF of mechanical parameters (GLS, SDTTS, φ) and measures of the degree of effectiveness of the therapy (ΔEDV, ΔESV, EF, ΔEF)
| ΔEDV | ΔESV | EF | ΔEF | |
|---|---|---|---|---|
| GLSOFF – GLSON (δGLS) | 0.05 | 0.02 | 0.09 | 0.25 |
| SDTTSOFF – SDTTSON (δSDTTS) | 0.07 | 0.10 | 0.01 | 0.03 |
| φOFF – φON (δφ) | 0.72 | 0.69 | 0.32 | 0.31 |
| GLSON | 0.24 | 0.43 | 0.61 | 0.37 |
| GLSOFF | 0.20 | 0.41 | 0.65 | 0.50 |
| SDTTSON | 0.06 | 0.17 | 0.26 | 0.21 |
| SDTTSOFF | 0.03 | 0.03 | 0.22 | 0.16 |
| φON | 0.69 | 0.65 | 0.20 | 0.10 |
| φOFF | 0.35 | 0.35 | 0.27 | 0.37 |
ΔEDV, relative reduction of end-diastolic volume; ΔESV, relative reduction of end-systolic volume; EF, value of ejection fraction at follow-up; ΔEF, increase of EF; GLS, global longitudinal strain; SDTTS, standard deviation of time to peak of transverse strain; φ, flow momentum angle. Significant correlation (r > 0.6) are indicated in bold.
Figure 1.
Correlation between flow orientation changes and volumetric response. Correlation between change in the orientation of flow momentum from CRT-ON to CRT-OFF (δφ = φOFF – φON) and relative volumetric reduction from pre-implant to follow-up of (A) end-diastolic volume (ΔEDV = EDVpost− EDVpre) and (B) end-systolic volume (ΔESV = ESVpost− ESVpre). The coloured green and red points indicate, respectively, the individual cases of SR and NR patients analysed in the text and in the subsequent pictures.
Individual polar graphs of momentum distribution in all SR patients are shown in Figure 2. All SR patients with active CRT (CRT-ON) presented a distribution of intraventricular haemodynamic forces predominantly aligned along the base-to-apex direction; when the CRT was discontinued (CRT-OFF), the flow momentum deviated by developing components along the transversal directions during various phases of the heartbeat. On the same figure, in contrast to SR patients, individual polar graphs of momentum distribution in NR patients are also shown. NR patients did not display any dominance of flow momentum along predefined directions either during CRT-ON or CRT-OFF.
The changes in intraventricular dynamics with pacing can be verified by comparing Supplementary data online, Movies 1 and 2, that report the velocity field and pressure gradient for one SR patients in CRT-ON and CRT-OFF, respectively. Blood flow displays minor changes when pacing is deactivated, although a careful inspection permits to notice sharper turns of the rotating flow at the onset of systole as well as deviations of the mitral jet during diastole. Nevertheless, these small changes, which represent accelerative events, are associated with deviations of the intraventricular forces from the longitudinal direction.
Reproducibility results
Inter-operator differences in flow analysis resulted non-significant (φ1 = 39.5 ± 7.8°, φ2 = 38.8 ± 7.9°, φ1– φ2 = 0.7 ± 4.0°; P = 0.18); they showed an intra-class correlation coefficient 0.87 (95% confidence interval 0.79–0.92). Repeatability of flow results from different recordings showed non-significant differences (φ1 = 39.0 ± 6.6°, φ2 = 39.3 ± 5.5°, φ1– φ2 = –0.3 ± 3.5°; P = 0.80); they showed an intra-class correlation coefficient 0.83 (95% confidence interval 0.55–0.95). Bland–Altman graphs reported in Figure 3 showed good agreement and the absence of systematic bias.
Figure 3.
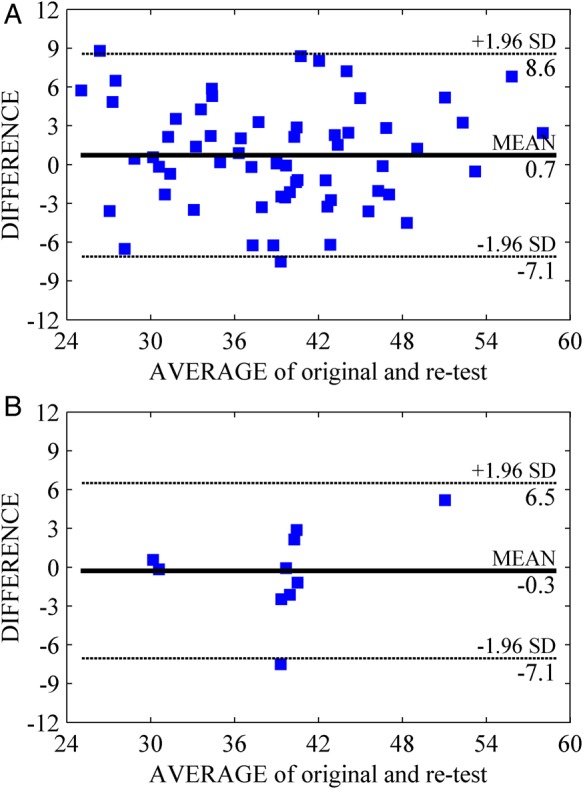
Bland–Altman analysis of inter-observer agreement for the momentum orientation angle. Inter-operator agreement (A) in the repeated flow analysis of same recording (60 cases) and (B) in the flow analysis of second acquisitions (10 recordings).
Discussion
The electrical activation sequence is known to directly affect the timing of the myocardial deformation events.23,24 In addition, it was previously shown by echo-PIV that it also influences flow timings: it was demonstrated that the deactivation of CRT in heart failure patients immediately led to a significant time delay in the vortex formation process affecting diastolic filling.25 The present results provide evidence, for the first time, that changes in electric activation alter absolute flow dynamics properties. The modification of flow-induced haemodynamic forces is presumably a consequence of the variation of time sequence of myocardial contraction–relaxation events; this study shows for the first time how the combination of these timings' modifications leads to a macroscopic change in terms of global deviation of flow momentum and orientation of haemodynamic forces. Moreover, the pacing-induced longitudinal alignment of flow forces appears proportional to the degree of response to the resynchronization therapy. SR patients appear very sensible to pacing that produces a longitudinal realignment of intraventricular forces; in contrast, in NR patients, forces are not realigned indicating a lack of effectiveness of the pacing stimulation.
Indeed, it was previously suggested that fluid dynamics represents a sort of coupling between systole and diastole without a sharp separation between them9; this is because flow properties at one instant depend on the combination of mechanical events during previous time.
A previous experimental study in pigs' heart revealed the presence of changes in intracavitary flow induced by pacing.17 The present study did not evidence large qualitative alterations of the LV vortex pattern. Careful retrospective visual inspection (as shown in Supplementary data online, Movies 1 and 2, for example) permitted to notice some minor differences during brief phases, such as a slight displacement of the diastolic jet or a little sharper flow bends during the systolic ejection. However, these small kinematic changes reflected into macroscopic alterations of blood flow momentum. In this work, the clinical interpretation of LV flow is moved from the description of vortex geometry or velocity characteristics (that are about kinematics) to a description of dynamic properties concerned with the action of forces (i.e. momentum), which are the foundation for understanding how blood flow influences the tissue. These results also show that the electrical activation does not reflect in an immediate alteration of kinetic energy dissipation. This observation agrees with previous results, suggesting that modifications of energetic properties develop progressively during different stages of LV dysfunction.21
The observed changes in the orientation of flow momentum evidence an increasing longitudinal alignment in correspondence of an increasing volumetric response to CRT. This suggests that the electric changes provided by the therapy are more effective when they reflect into haemodynamic modifications that improve the longitudinal orientation of flow forces.
Literature provides strong evidence that LV mechanics has a fundamental effect on CRT:13,14 basal dyssynchrony26 and acute resynchronization of wall motion after CRT implantation24 have been described as response predictors. However, equating CRT effectiveness through measurements of regional mechanics could be limiting.27 Indeed, the normal regional motion is not perfectly synchronous or uniform, and minor departures at an early stage are difficult to recognize. Moreover, measures of tissue synchrony, like SDTTS, have to rely on segmental measurements of tissue motion whose differentiation can be technically inaccurate. The analysis of intraventricular flow momentum may provide a global measure to recognize whether the tissue deformation develops in a co-ordinate manner. The present study is certainly preliminary in nature; however, it suggests that a biventricular pacing setting optimization accounting for flow momentum alignment may represent a natural comprehensive solution for global tissue synchronization. This is a conjecture based on the present limited population study; although conceptually sound, it still requires a careful verification in a large prospective study.
The present result indicates that reverse remodelling could be associated with a longitudinal alignment of blood momentum; conversely, a blood motion arrangement presenting transversal intraventricular forces is associated with lack of response. This observation conjectures the existence, at least within the limited specific population investigated here, of a relationship between the qualities of intraventricular vortex dynamics and longer term geometrical adaptation of the myocardial structure.12 This could be related to the development of local, short-lasting accelerations, not easily detectable in terms of tissue displacement, that translate in vortex instabilities and, in global terms, in a misalignment of the intraventricular forces with the generation of sharp untimed tissue stresses. It can be speculated that LV endothelial cells are able to sense the vorticity and loading conditions via shear changes (mechano-sensing), transforming any abnormal condition into adaptive responses (mechano-transduction).28,29
Haemodynamic forces are known to participate in the development of the embryonic heart.30,31 In the same perspective, it is natural to expect that they could also participate to the development of the grown heart. LV flow momentum analysis could integrate existing predictive models for detecting condition leading to LV remodelling or predicting long-term outcome.12
Limitations
This is a preliminary study on a limited number of patients that should be confirmed or refined in larger populations. Flow imaging technology is also new and susceptible to improvements. First, blood motion is three-dimensional, and assessment is here performed on a single scan plane. It is assumed here that anomalous tissue contraction on any individual segment influences the entire blood motion whose change can be detected, at least partially, on the single scan plane. This assumption is plausible but not tested, and 3D blood flow analysis will have to be considered when technology is available. Secondly, the echo-PIV technique presents a limited accuracy: for this reason the study used only global values that present a better technical reliability and was designed to compare variations within the same patient and not absolute figures.
Conclusion
Changes in the timing of electrical activation sequence reflect on the orientation of blood flow momentum and on the forces globally exchanged between blood and myocardium. The degree of volumetric response to CRT correlates with the orientation of flow-induced haemodynamic forces. This suggests that flow orientation represent a new gage of favourable response to CRT and could be used for optimizing the biventricular pacing setting. However, larger prospective studies are needed to confirm this hypothesis.
Supplementary data
Supplementary data are available at European Heart Journal—Cardiovascular Imaging online.
Conflict of interest: None declared.
Funding
G.P. acknowledges funding from Italian Government project (Progetti di Rilevante Interesse Nazionale) PRIN 2012, Prot. N. 2012HMR7CF_002.
References
- 1.Carlhäll CJ, Bolger AF. Passing strange. Circ Heart Fail 2010;3:326–31. [DOI] [PubMed] [Google Scholar]
- 2.Son JW, Park WJ, Choi JH, Houle H, Vannan MA, Hong GR, et al. Abnormal left ventricular vortex flow patterns in association with left ventricular apical thrombus formation in patients with anterior myocardial infarction. Circ J 2012;76:2640–6. [DOI] [PubMed] [Google Scholar]
- 3.Mangual JO, De Luca A, Kraigher-Krainer E, Toncelli L, Shah A, Solomon S, et al. Comparative numerical study on left ventricular fluid dynamics after dilated cardiomyopathy. J Biomech 2013;46:1611–7. [DOI] [PubMed] [Google Scholar]
- 4.Gharib M, Rambod E, Kheradvar A, Sahn DJ, Dabiri JO. Optimal vortex formation as an index of cardiac health. Proc Natl Acad Sci USA 2006;103:6305–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Markl M, Kilner PJ, Ebbers T. Comprehensive 4D velocity mapping of the heart and great vessels by cardiovascular magnetic resonance. J Cardiovasc Magn Reson 2011;13:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sengupta PP, Pedrizzetti G, Kilner P, Kheradvar A, Ebbers T, Frazer A, et al. Emerging trends in clinical assessment of cardiovascular fluid dynamics. J Am Coll Cardiol Imaging 2012;5:305–16. [DOI] [PubMed] [Google Scholar]
- 7.Rodriguez Munoz D, Markl M, Moya JL, Barker A, Fernandez-Golfin C, Lancellotti P, et al. Intracardiac flow visualization: current status and future directions. Eur Heart J Cardiovasc Imaging 2013;14:1029–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hong GR, KIm M, Pedrizzetti G, Vannan MA. Current clinical application of intracardiac flow analysis using echocardiography. J Cardiovascular Ultrasound 2013;21:155–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abe H, Caracciolo G, Kheradvar A, Pedrizzetti G, Khandheria BK, Narula J, et al. Left ventricular efficiency in heart failure is related to the intracavitary vortex strength during isovolumic contraction. Eur Heart J Cardiovasc Imaging 2013;14:1049–60. [DOI] [PubMed] [Google Scholar]
- 10.Kilner PJ, Yang GZ, Wilkes AJ, Mohiaddin RH, Firmin DN, Yacoub MH. Asymmetric redirection of flow through the heart. Nature 2000;404:759–61. [DOI] [PubMed] [Google Scholar]
- 11.Pedrizzetti G, Domenichini F. Nature optimizes the swirling flow in the human left ventricle. Phys Rev Lett 2005;95:108101. [DOI] [PubMed] [Google Scholar]
- 12.Pedrizzetti G, La Canna G, Alfieri O, Tonti G. The vortex - an early predictor of cardiovascular outcome? Nat Rev Cardiol 2014;11:545–53. [DOI] [PubMed] [Google Scholar]
- 13.Knappe D, Pouleur AC, Shah AM, Cheng S, Uno H, Bourgoun M, et al. Dyssynchrony, contractile function, and response to cardiac resynchronization therapy. Circ Heart Failure 2011;4:433–40. [DOI] [PubMed] [Google Scholar]
- 14.Pouleur AC, Knappe D, Shah AM, Uno H, Bourgoun M, Foster E, et al. Relationship between improvement in left ventricular dyssynchrony and contractile function and clinical outcome with cardiac resynchronization therapy: the MADIT-CRT trial. Eur Heart J 2011;32:1720–9. [DOI] [PubMed] [Google Scholar]
- 15.Brignole M, Auricchio A, Baron-Esquivias G, Bordachar P, Boriani G, Breithardt OA, et al. 2013 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy: The Task Force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). Developed in collaboration with the European Heart Rhythm Association (EHRA). Europace 2013;15:1070–118. [DOI] [PubMed] [Google Scholar]
- 16.Gao H, Claus P, Amzulescu MS, Stankovic I, D'Hooge J, Voigt JU. How to optimize intracardiac blood flow tracking by echocardiographic particle image velocimetry? Exploring the influence of data acquisition using computer-generated data sets. Eur Heart J Cardiovasc Imaging 2012;13:490–9. [DOI] [PubMed] [Google Scholar]
- 17.Sengupta PP, Khandheria BK, Korinek J, Jahangir A, Yoshifuku S, Milosevic I, et al. Left Ventricular isovolumic flow sequence during sinus and paced rhythms. J Am Coll Cardiol 2007;49:899–908. [DOI] [PubMed] [Google Scholar]
- 18.Hong GR, Pedrizzetti G, Tonti G, Li P, Wei Z, Kim JK, et al. Characterization and quantification of vortex flow in the human left ventricle by contrast echocardiography using vector particle image velocimetry. J Am Coll Cardiol Imaging 2008;1:705–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kheradvar A, Houle H, Pedrizzetti G, Tonti G, Belcik T, Ashraf M, et al. Echographic particle image velocimetry: a novel technique for quantification of left ventricular blood vorticity pattern. J Am Soc Echocardiogr 2010;23:86–94. [DOI] [PubMed] [Google Scholar]
- 20.Pedrizzetti G, Martiniello AR, Bianchi V, D'Onofrio A, Caso P, Tonti G. Cardiac fluid dynamics anticipates heart adaptation. J Biomech 2015;48:388–91. [DOI] [PubMed] [Google Scholar]
- 21.Agati L, Cimino S, Tonti G, Cicogna F, Petronilli V, Pedrizzetti G. Quantitative analysis of intraventricular blood flow dynamics by echocardiographic particle image velocimetry in patients with acute myocardial infarction at different stages of left ventricular dysfunction. Eur Heart J Cardiovasc Imaging 2014;15:1203–12. [DOI] [PubMed] [Google Scholar]
- 22.Pedrizzetti G, Mangual J, Tonti G. On the Relationship between global longitudinal strain and ejection fraction. J Biomech 2014;47:746–9. [DOI] [PubMed] [Google Scholar]
- 23.Sengupta PP, Khandheria BK, Korinek J, Wang J, Jahangir A, Seward JB, et al. Apex-to-base dispersion in regional timing of left ventricular shortening and lengthening. J Am Coll Cardiol 2006;47:163–72. [DOI] [PubMed] [Google Scholar]
- 24.Kydd AC, Khan FZ, O'Halloran D, Pugh PJ, Virdee MS, Dutka DP. Radial strain delay based on segmental timing and strain amplitude predicts left ventricular reverse remodeling and survival after cardiac resynchronization therapy. Circ Cardiovasc Imaging 2013;6:177–84. [DOI] [PubMed] [Google Scholar]
- 25.Goliasch G, Goscinska-Bis K, Caracciolo G, Nakabo A, Smolka G, Pedrizzetti G, et al. CRT improves left ventricular filling dynamics: insights from echocardiographic particle imaging velocimetry. J Am Coll Cardiol Imaging 2013;6:704–13. [DOI] [PubMed] [Google Scholar]
- 26.Bleeker GB, Mollema SA, Holman ER, Van de Veire N, Ypenburg C, Boersma E, et al. Left ventricular resynchronization is mandatory for response to cardiac resynchronization therapy: analysis in patients with echocardiographic evidence of left ventricular dyssynchrony at baseline. Circulation 2007;116:1440–8. [DOI] [PubMed] [Google Scholar]
- 27.Kirn B, Jansen A, Bracke F, van Gelder B, Arts T, Prinzen FW. Mechanical discoordination rather than dyssynchrony predicts reverse remodeling upon cardiac resynchronization. Am J Physiol Heart Circ Physiol 2008;295:H640–6. [DOI] [PubMed] [Google Scholar]
- 28.Friedrich O, Wagner S, Battle AR, Schürmann S, Martinac B. Mechano-regulation of the beating heart at the cellular level e mechanosensitive channels in normal and diseased heart. Prog Biophys Mol Biol 2012;110:226–38. [DOI] [PubMed] [Google Scholar]
- 29.Farge E. Mechanotransduction in development. Curr Top Dev Biol 2011;95:243–65. [DOI] [PubMed] [Google Scholar]
- 30.Hove JR, Koster RW, Forouhar AS, Bolton GA, Fraser SE, Gharib M. Intracardiac fluid forces are an essential epigenetic factor for embryonic cardiogenesis. Nature 2003;421:172–7. [DOI] [PubMed] [Google Scholar]
- 31.Freund JB, Goetz JG, Hill KL, Vermot J. Fluid flows and forces in development: functions, features and biophysical principles. Development 2012;139:1229–45. [DOI] [PMC free article] [PubMed] [Google Scholar]