Abstract
Background
Previous studies showed that patients with takotsubo cardiomyopathy had a higher long-term mortality rate than the general population and the incidence of in-hospital complications was higher in takotsubo cardiomyopathy with than without right ventricular (RV) involvement. This study was performed to investigate the long-term prognostic impact of RV involvement in takotsubo cardiomyopathy.
Methods and results
The clinical data of 113 patients (72.7 ± 11.4 years old, 84 females) with takotsubo cardiomyopathy were studied retrospectively. The patients were divided into two groups according to the presence (biventricular group, n = 21, 18.6%) or absence (classical group, n = 92, 81.4%) of RV involvement assessed by initial echocardiography. The end point was a composite of all-cause death, re-hospitalization due to heart failure, and recurrence of takotsubo cardiomyopathy. The in-hospital mortality rate was significantly higher in the biventricular group than the classical group (14.3 vs. 1.1%, respectively, P = 0.02). Kaplan–Meier analysis indicated a significantly lower event-free survival rate in the biventricular group than the classical group (log-rank, P < 0.001). On multivariate analysis, RV involvement was the only independent predictor of the end point (HR: 2.73, P = 0.026).
Conclusion
The rates of in-hospital and long-term events were significantly higher in takotsubo cardiomyopathy with than without RV involvement, and RV involvement was the independent predictor of the poor prognosis.
Keywords: cardiomyopathy, echocardiography, prognosis
Introduction
Takotsubo cardiomyopathy is a unique syndrome characterized by transient left ventricular (LV) apical ballooning, which mimics acute coronary syndrome.1 Although it has been recognized as a self-limiting and benign disease,2,3 recent studies have suggested that some critical complications, such as acute heart failure,4,5 ventricular arrhythmia,6 LV outflow tract obstruction,7 mitral regurgitation,8 and cardiac rupture,9,10 may occur in takotsubo cardiomyopathy. In one large population-based study, the in-hospital mortality rate reached 4.2%.11 Furthermore, long-term observational studies suggested that the long-term mortality rate of takotsubo cardiomyopathy is higher than that in the general population.12,13
Takotsubo cardiomyopathy with right ventricular (RV) involvement has been reported as a new variant observed in about one-fifth to one-third of takotsubo cardiomyopathy cases.14,15 Previous studies suggested that the incidence rate of in-hospital complications may be higher in cases of takotsubo cardiomyopathy with than without RV involvement.14,15 However, there have been no studies of the long-term prognosis of takotsubo cardiomyopathy with RV involvement. The present study was performed to investigate the prognostic impact of RV involvement in takotsubo cardiomyopathy.
Methods
A total of 128 patients with takotsubo cardiomyopathy were admitted to Kawasaki Medical School Hospital between October 1999 and June 2013. The diagnosis of takotsubo cardiomyopathy was based on the Mayo Clinic criteria: (i) transient hypokinesis, akinesis, or dyskinesis in the LV mid-segment with or without apical involvement; regional wall motion abnormalities extending beyond a single epicardial vascular distribution; and frequently, but not always, a stressful trigger; (ii) the absence of obstructive coronary disease or angiographic evidence of acute plaque rupture; (iii) new ECG abnormalities (ST-segment elevation and/or T-wave inversion) or modest elevation of cardiac troponin; and (iv) the absence of pheochromocytoma and myocarditis.16 Stressful trigger was defined as emotional or physical pressure which causes a tension or strain beyond daily routine. The patients were divided into two groups based on the presence (biventricular group) or absence (classical group) of RV involvement, defined by akinesis or dyskinesis of the RV free wall with or without apical involvement assessed by echocardiography on admission.
Past medical history was determined by chart review. Hypertension, dyslipidaemia, and diabetes mellitus were diagnosed according to the following criteria: hypertension was defined as blood pressure >140/90 mmHg or current medication; hyperlipidaemia was defined as total cholesterol level >220 mg/dL, triglyceride level >150 mg/dL, or current medication; diabetes mellitus was defined as fasting plasma glucose >120 mg/dL, haemoglobin A1c >6.5%, or current medication.
The study protocol was approved by the Ethics Committee of Kawasaki Medical School. This study was performed in compliance with the Declaration of Helsinki with regard to investigations in human subjects.
Echocardiography
All patients with takotsubo cardiomyopathy underwent 2D and Doppler echocardiographic examinations within 12 h of admission. LV end-diastolic diameter (LVDd), LV end-systolic diameter (LVDs) and left atrial diameter (LAD) were measured in parasternal long-axis view. LV ejection fraction (LVEF) was calculated using the biplane modified Simpson method. As mentioned above, RV involvement was defined by the presence of akinesis or dyskinesis of the RV free wall with sparing of the RV basal segments with or without apical involvement. As right heart parameters, the RV basal diameter, mid-cavity diameter, longitudinal diameter, end-diastolic area, end-systolic area, fractional area change (FAC), and diameter of the inferior vena cava (IVC) were measured according to the published guidelines.17 Systolic pulmonary artery pressure was estimated from the peak tricuspid regurgitant jet velocity.17 The prevalence of moderate or severe valvular heart disease was assessed in clinically standard manner.18 Follow-up echocardiography was performed to confirm the improvement of the wall motion abnormalities.
Clinical outcome
As in-hospital events, ventricular arrhythmia, cardiac rupture, use of non-invasive positive-pressure ventilation, intubation, use of a temporary pacemaker, use of inotropic agent, and in-hospital death were assessed based on chart review. Killip classification of acute heart failure was assessed by chest X-ray on admission. The end point of this study, a composite of all-cause death, re-hospitalization due to heart failure and recurrence of takotsubo cardiomyopathy were assessed based on chart review and/or telephone interview.
Statistical analysis
Data are presented as means ± SD for continuous variables with a normal distribution, median (interquartile range) for continuous variables with a non-normal distribution, and as frequency (%) for categorical variables. The Kolmogorov–Smirnov test was used to assess normal distribution. Student's t-test and the Mann–Whitney U-test were used to compare continuous variables with normal and non-normal distributions, respectively. The χ2-test or Fisher's exact test was used to compare categorical variables. The log-rank test was used to compare the survival curves between the biventricular group and the classical group. The Cox univariate regression hazard model was used to identify the factors associated with the primary end point. Factors with P < 0.10 on univariate analysis were entered into the Cox multivariate regression hazard model to define independent risk factors for the end point. A stepwise akaike's information criterion method was used for Cox multivariate analysis. Statistical analysis was performed with SPSS (version 21.0; SPSS Inc., Chicago, IL) and EZR (version 1.24; Saitama Medical Center, Jichi Medical University, Saitama, Japan). EZR is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria). More precisely, it is a modified version of R Commander designed to add statistical functions frequently used in biostatistics.19 In all analyses, P < 0.05 was taken to indicate statistical significance.
Results
Patient characteristics
Fifteen of the 128 consecutive patients treated during the study period were excluded because of insufficient echocardiographic images. We retrospectively studied clinical and echocardiographic data of 113 patients (72.7 ± 11.4 years old, 83 female). These patients were divided into two groups according to the presence (biventricular group, n = 21, 18.6%, 16 female) or absence (classical group, n = 92, 81.4%, 68 female) of RV involvement assessed by initial echocardiography. Table 1 shows patient background and Table 2 shows echocardiographic data on admission for the two groups, respectively. Compared with the classical group, the patients in the biventricular group had a higher prevalence of heart failure history, higher log brain natriuretic peptide level, lower LVEF and greater LV size. Among the right heart parameters, FAC was significantly lower and RV cavity was significantly larger in the biventricular group than in the classical group. There was no significant difference in estimated systolic pulmonary artery pressure between the two groups. Follow-up echocardiography was performed in 17 (81.0%) cases in the biventricular group and 77 (83.7%) in the classical group and revealed that wall motion abnormalities were completely recovered in all the cases. There was no significant difference in the length of time required for complete recovery between two groups (15 [10–22] vs. 18 [10–30] days, respectively, P = 0.79).
Table 1.
Patient characteristics
| Classical group (n = 92) | Biventricular group (n = 21) | P-value | |
|---|---|---|---|
| Age, years | 71.8 ± 11.7 | 76.9 ± 9.4 | 0.066 |
| Female gender | 68 (73.9) | 16 (76.2) | >0.99 |
| BSA | 1.44 ± 0.17 | 1.47 ± 0.15 | 0.548 |
| Chest pain | 40 (43.5) | 10 (47.6) | 0.810 |
| Type of stress | 0.942 | ||
| Emotional | 30 (32.6) | 7 (33.3) | |
| Physical | 51 (55.4) | 11 (52.4) | |
| None | 11 (12.0) | 3 (14.3) | |
| In-hospital occurrence | 23 (25.0) | 3 (14.3) | 0.395 |
| Onset to echocardiography time | 0.835 | ||
| 0–12 h | 34 (37.0) | 7 (33.3) | |
| 12–24 h | 14 (15.2) | 2 (9.5) | |
| >24 h | 19 (20.7) | 6 (28.6) | |
| Unknown | 25(27.2) | 6 (28.6) | |
| Systolic BP, mmHg | 132.6 ± 30.9 | 137 ± 31.7 | 0.529 |
| Diastolic BP, mmHg | 73.2 ± 18.4 | 78.4 ± 20.8 | 0.254 |
| Medical history | |||
| Atrial fibrillation | 4 (4.3) | 1 (4.8) | >0.99 |
| Heart failure | 3 (3.3) | 6 (28.6) | 0.001 |
| COPD | 7 (7.6) | 2 (9.5) | 0.673 |
| Dyslipidaemia | 25 (27.2) | 2 (9.5) | 0.098 |
| Diabetes mellitus | 23 (25.0) | 3 (14.3) | 0.395 |
| Dialysis | 9 (9.8) | 0 (0.0) | 0.206 |
| Hypertension | 60 (65.2) | 14 (66.7) | >0.99 |
| Smoking | 22 (23.9) | 5 (23.8) | >0.99 |
| Past histroy of malignancy | 18 (19.6) | 3 (14.3) | 0.760 |
| Drugs on admission | |||
| ACE inhibitor/ARB | 30 (32.6) | 4 (19.0) | 0.295 |
| Beta-blocker | 6 (6.5) | 2 (9.5) | 0.640 |
| Laboratory data | |||
| Log BNP | 2.38 ± 0.64 | 2.79 ± 0.59 | 0.018 |
| Troponin T, pg/nL | 0.39 ± 0.50 | 0.47 ± 0.38 | 0.566 |
| eGFR, mL/min/1.73 m2 | 69.9 ± 38.6 | 46.6 ± 20.6 | 0.010 |
BP, blood pressure; COPD, chronic obstructive pulmonary disease; ACE, angiotensin-converting enzyme; ARB, angiotensin II receptor antagonist; BNP, brain natriuretic peptide; eGFR, estimated glomerular filtration rate.
Table 2.
Echocardiographic data
| Classical group (n = 92) | Biventricular group (n = 21) | P-value | |
|---|---|---|---|
| LVDd, cm | 4.46 ± 0.61 | 4.84 ± 0.64 | 0.012 |
| LVDs, cm | 3.08 ± 0.68 | 3.64 ± 0.95 | 0.002 |
| LVEDV, mL | 85.8 ± 28.5 | 92.8 ± 29.1 | 0.421 |
| LVESV, mL | 44.4 ± 18.6 | 51.6 ± 19.2 | 0.189 |
| LVEF, % | 47.2 ± 9.9 | 37.9 ± 15.3 | 0.001 |
| LAD, cm | 3.49 ± 0.75 | 3.77 ± 0.48 | 0.121 |
| TMF pattern | 0.610 | ||
| Normal | 1 (1.1) | 0 (0.0) | |
| Abnormal relaxation | 64 (72.7) | 13 (65.0) | |
| Pseudo normal | 14 (15.9) | 3 (15.0) | |
| Restrictive | 9 (10.2) | 4 (20.0) | |
| Valvular heart disease (moderate or severe) | |||
| AS | 2 (2.2) | 1 (4.8) | 0.464 |
| AR | 4 (4.3) | 2 (9.5) | 0.309 |
| MR | 11 (12.0) | 6 (28.6) | 0.085 |
| TR | 8 (8.7) | 4 (19.0) | 0.231 |
| Systolic PAP, mmHg | 36.0 ± 10.8 | 40.1 ± 13.7 | 0.187 |
| RV basal diameter, cm | 2.50 ± 0.35 | 2.99 ± 0.50 | <0.001 |
| RV mid-cavity diameter, cm | 2.02 ± 0.38 | 2.49 ± 0.64 | <0.001 |
| RV longitudinal diameter, cm | 6.57 ± 0.71 | 7.02 ± 0.99 | 0.018 |
| RV end-diastolic area, cm2 | 13.6 ± 2.8 | 17.8 ± 6.3 | <0.001 |
| RV end-systolic area, cm2 | 7.7 ± 1.8 | 12.4 ± 5.7 | <0.001 |
| RV FAC, % | 43.3 ± 5.9 | 32.5 ± 12.6 | <0.001 |
| IVC diameter, cm | 1.46 ± 0.43 | 1.79 ± 0.38 | 0.004 |
| Left ventricular thrombus | 3 (3.3) | 0 (0.0) | >0.99 |
| Absence of LV apical ballooning | 19 (20.7) | 2 (9.5) | 0.354 |
| SAM | 8 (8.7) | 3 (14.3) | 0.426 |
| Pericardial effusion | 34 (37.0) | 9 (42.9) | 0.627 |
LVDd, left ventricular end-diastolic diameter; LVDs, left ventricular end-systolic diameter; LVEDV, left ventricular end-diastolic volume; LVESV, left ventricular end-systolic volume; LVEF, left ventricular ejection fraction; LAD, left atrial diameter; TMF, trans-mitral flow; AS, aortic stenosis; AR, aortic regurgitation; MR, mitral regurgitation; TR, tricuspid regurgitation; PAP, pulmonary arterial pressure; RV, right ventricular; FAC, fractional area change; IVC, inferior vena cava; SAM, systolic anterior motion of the mitral valve.
In-hospital events
Table 3 shows a comparison of the prevalence of in-hospital events between the two groups. The biventricular group had significantly higher prevalence rates of severe heart failure defined as Killip class ≥3 than the classical group and intubation. The rate of admission to the intensive care unit was higher in the biventricular group than the classical group. Three patients in the biventricular group and one patient in the classical group died during the initial period of hospitalization (14.3 vs. 1.1%, respectively, P = 0.02); in the biventricular group, one patient died of cardiac rupture, one died of intractable ventricular fibrillation, and the other died of the primary disease (malignant lymphoma), while one patient in the classical group died of lung cancer.
Table 3.
In-hospital events
| Classical group (n = 92) | Biventricular group (n = 21) | P-value | |
|---|---|---|---|
| Acute heart failure, Killip class | <0.001 | ||
| 1 | 63 (68.5) | 4 (9.0) | |
| 2 | 13 (14.1) | 5 (23.8) | |
| 3 | 15 (16.3) | 11 (52.4) | |
| 4 | 1 (1.1) | 1 (4.8) | |
| Ventricular tachycardia/fibrillation | 1 (1.1) | 2 (9.5) | 0.088 |
| Cardiac rupture | 0 (0.0) | 1 (4.8) | 0.186 |
| NPPV | 1 (1.1) | 0 (0.0) | >0.99 |
| Intubation | 9 (9.8) | 9 (42.9) | 0.001 |
| Temporary pacemaker | 0 (0.0) | 2 (9.5) | 0.033 |
| Use of inotropic agent | 24 (26.1) | 16 (76.1) | <0.001 |
| IABP | 1 (1.1) | 0 (0.0) | >0.99 |
| VA-ECMO | 0 (0.0) | 1 (4.8) | 0.186 |
| Hospital length of stay, days | 20 [10, 31] | 19 [8, 25] | 0.372 |
| Admission to the ICU | 14 (15.4) | 14 (66.7) | <0.001 |
| In-hospital death | 1 (1.1) | 3 (14.3) | 0.020 |
NPPV, non-invasive positive-pressure ventilation; IABP, intraaortic balloon pumping; VA-ECMO, venoarterial extracorporeal membrane oxygenation; ICU, intensive care unit.
Long-term prognosis
There was no difference in follow-up period between the biventricular group and the classical group (median 596 [98–1724] vs. 709 [132–1404] days, respectively, P = 0.642). Over a median follow-up of 709 (106–1660) days, 24 patients in the whole cohort (21.2%) had the composite end point. Kaplan–Meier analysis indicated that the event-free survival rate was significantly lower in the biventricular group than in the classical group (Figure 1). There were two cardiac deaths, five non-cardiac deaths, three cases of re-hospitalization due to heart failure, and one recurrence of takotsubo cardiomyopathy in the biventricular group, while there was one cardiac death, 11 non-cardiac deaths, and one re-hospitalization due to heart failure in the classical group (Figure 2).
Figure 1.
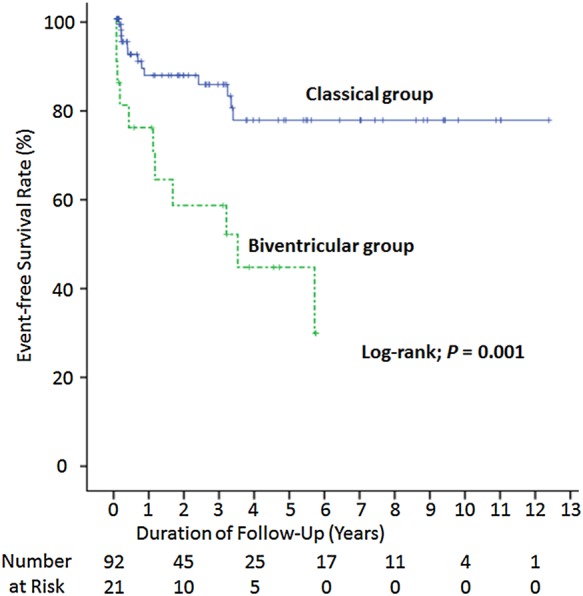
Kaplan–Meier curve for the composite end point. The rate of event-free survival was significantly lower in the biventricular group than the classical group.
Figure 2.
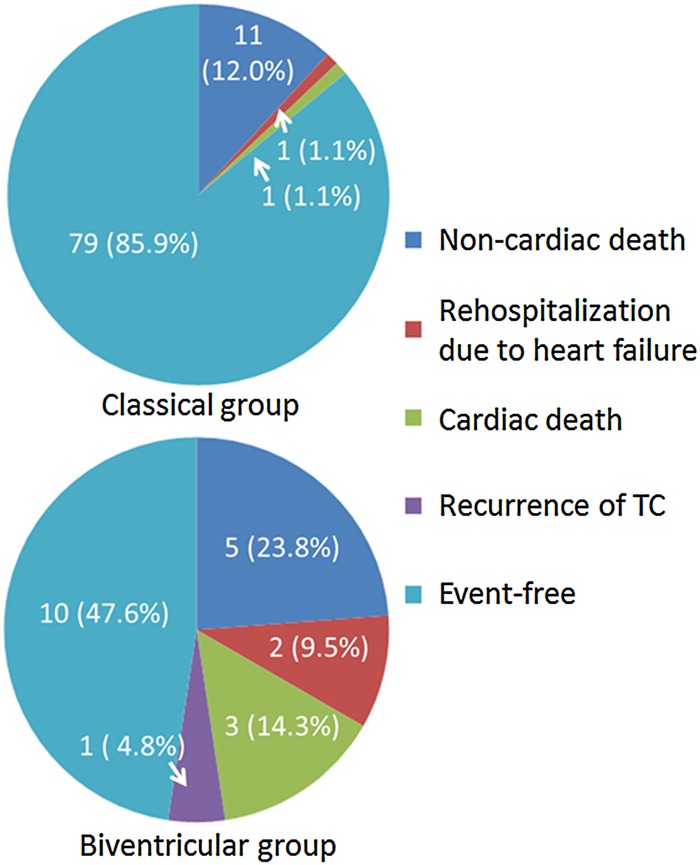
Details of long-term prognosis. All types of adverse events were more frequently documented in the biventricular group than the classical group.
On Cox univariate analysis, age (hazard ratio [HR]: 1.05, 95% CI: 1.001–1.092, P = 0.046), severe heart failure (HR: 2.474, 95% CI: 1.097–5.58, P = 0.029), low LVEF (HR: 0.964, 95% CI: 0.966–0.993, P = 0.014), low FAC (HR: 0.966, 95% CI: 0.936–0.993, P = 0.014), and the presence of RV involvement (HR: 3.72, 95% CI: 1.67–8.32, P = 0.001) were associated with the end point. No other baseline factors, including history of heart failure (HR: 1.92, 95% CI: 0.54–6.80, P = 0.311), were associated with the composite end point. On Cox multivariate analysis, RV involvement was the only independent predictor of the end point (HR: 2.73, 95% CI: 1.13–6.62, P = 0.026; Table 4).
Table 4.
Multivariate analysis for the primary end point
| Univariate analysis |
Multivariate analysis |
|||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | |
| Age | 1.05 | 1.00–1.09 | 0.046 | |||
| Killip ≥ 3 | 2.47 | 1.10–5.58 | 0.029 | |||
| RV involvement | 3.72 | 1.67–8.32 | 0.001 | 2.73 | 1.13–6.62 | 0.026 |
| RV FAC | 0.97 | 0.93–1.00 | 0.061 | |||
| Absence of LV apical ballooning | 0.16 | 0.02–1.20 | 0.074 | 0.17 | 0.02–1.27 | 0.084 |
| LVEF | 0.96 | 0.94–0.99 | 0.014 | 0.97 | 0.95–1.00 | 0.109 |
HR, hazard ratio; BNP, brain natriuretic peptide; RV, right ventricular; LVEF, left ventricular ejection fraction.
Discussion
We performed a retrospective clinical investigation in 113 consecutive takotsubo cardiomyopathy patients, and showed that (i) RV involvement in takotsubo cardiomyopathy is not uncommon (18.6%); (ii) the in-hospital morbidity and mortality rates were significantly higher in takotsubo cardiomyopathy with than without RV involvement; and (iii) the long-term prognosis was poorer in cases of takotsubo cardiomyopathy with than without RV involvement.
Although takotsubo cardiomyopathy was originally named after its unique wall motion abnormality localized in the LV apical wall, called LV apical ballooning, some variant wall motion abnormalities, such as mid-ventricular ballooning20 and RV involvement, have been reported in takotsubo cardiomyopathy. Takotsubo cardiomyopathy with RV involvement was first reported in 200021 and had been recognized as a relatively rare form of the disease until 2006 when Elesber et al. reported this as a variant observed in about one-third of patients with takotsubo cardiomyopathy.14 Several subsequent studies using echocardiography or cardiac magnetic resonance for assessment of RV wall motion showed that RV involvement in takotsubo cardiomyopathy is not uncommon, with a prevalence of 14.5–36.0%.13,14,22,23 In our series using echocardiography, the prevalence of RV involvement was 18.6%, which was reasonably consistent with previous reports.
Takotsubo cardiomyopathy has been classified as a transient and benign disease.2,3 However, recent studies indicated that some critical complications and sudden death may occur in takotsubo cardiomyopathy.24 In a study of 25 patients, Elesber et al. reported higher prevalence rates of in-hospital complications, including acute heart failure, in takotsubo cardiomyopathy with RV involvement.14 Our study in a larger population (n = 113) also showed a higher prevalence of in-hospital complications in takotsubo cardiomyopathy with RV involvement consistent with the previous study in a smaller cohort. In both of these studies, LVEF was lower in takotsubo cardiomyopathy with than without RV involvement. The lower LVEF (depressed LV function) may be responsible for the higher incidence of left-sided heart failure or pulmonary congestion in takotsubo cardiomyopathy with RV involvement. In addition, depressed RV function may reduce LV preload, and further facilitate low-output syndrome. Therefore, it is reasonable that in-hospital mortality and morbidity were higher in patients with RV involvement.
In this study, even after discharge, both cardiac events and non-cardiac deaths were more frequently documented in cases of takotsubo cardiomyopathy with RV involvement. Many previous studies have shown that RV dysfunction is a powerful predictor of poor prognosis in coronary heart disease,25 congenital heart disease,26 and heart failure.27 A previous study using cardiac magnetic resonance showed that cardiac oedema and inflammation were observed in the acute phase in takotsubo cardiomyopathy and partially persisted even after LVEF had fully recovered.28 Although this study evaluated only LV function, it is possible that the oedema and inflammation develop similarly in the RV, may persist for a long time, and may be associated with late cardiac events.
Interestingly, the rate of non-cardiac death was also higher in takotsubo cardiomyopathy with than without RV involvement. A previous long-term observational study indicated that the underlying critical illness rather than the severity of LV dysfunction was a predictor of poor prognosis in takotsubo cardiomyopathy.13 Recently, isolated RV takotsubo cardiomyopathy has been reported as a variant of takotsubo cardiomyopathy.29–31 This new variant suggests that RV involvement represents specific pathological significance in takotsubo cardiomyopathy and is not the result of marked ventricular dysfunction (including both LV and RV). Taken together, our data suggest that RV involvement may be associated with more critical underlying conditions than classical takotsubo cardiomyopathy. Therefore, RV involvement in takotsubo cardiomyopathy may suggest the presence of severe comorbid underlying conditions, which could contribute to the subsequent non-cardiac death. These hypotheses must be validated in future studies in larger cohorts.
Study limitations
Our study had some limitations. First, this was a single-centre retrospective observational study including patients admitted over a 15-year period. In our series including patients since 1999, RV wall motion may not have been examined systematically in all cases until RV involvement was reported as a common variant in 2006. Therefore, the incidence of RV involvement may have been underestimated. However, the overall prevalence of RV involvement in our population (18.6%) was consistent with previous reports (14.5–34.0%)13,14,22,23 and the prevalence was similar before (5 of 28, 17.9%) and after 2006 (16 of 85, 18.8%). In this study, we did not routinely perform serial magnetic resonance imaging. Therefore, the impact of the remained oedema and inflammation in the convalescent phase on long-term prognosis remains to be determined. Finally, because the clinical outcome was assessed based on telephone interview in some cases, the detailed cause of death was uncertain.
Conclusions
RV involvement is not uncommon among patients with takotsubo cardiomyopathy. The rates of in-hospital morbidity and mortality as well as long-term events were higher in takotsubo cardiomyopathy with than without RV involvement and RV involvement was the only independent predictor of the poor prognosis. These results provide a new and important insight into the prognosis of takotsubo cardiomyopathy. RV wall motion should be carefully assessed to detect this high-risk population of patients with takotsubo cardiomyopathy.
Conflict of interest: none declared.
References
- 1.Kume T, Akasaka T, Kawamoto T, Yoshitani H, Watanabe N, Neishi Y, et al. Assessment of coronary microcirculation in patients with takotsubo-like left ventricular dysfunction. Circ J 2005;69:934–9. [DOI] [PubMed] [Google Scholar]
- 2.Bybee KA, Kara T, Prasad A, Lerman A, Barsness GW, Wright RS, et al. Systematic review: transient left ventricular apical ballooning: a syndrome that mimics st-segment elevation myocardial infarction. Ann Intern Med 2004;141:858–65. [DOI] [PubMed] [Google Scholar]
- 3.Cacciotti L, Passaseo I, Marazzi G, Camastra G, Campolongo G, Beni S, et al. Observational study on takotsubo-like cardiomyopathy: clinical features, diagnosis, prognosis and follow-up. BMJ Open 2012;2:e001–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akashi YJ, Goldstein DS, Barbaro G, Ueyama T. Takotsubo cardiomyopathy: a new form of acute, reversible heart failure. Circulation 2008;118:2754–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Madhavan M, Rihal CS, Lerman A, Prasad A. Acute heart failure in apical ballooning syndrome (takotsubo/stress cardiomyopathy): clinical correlates and mayo clinic risk score. J Am Coll Cardiol 2011;57:1400–1. [DOI] [PubMed] [Google Scholar]
- 6.Madias JE. Is takotsubo syndrome one of the causes of sudden cardiac death? PACE 2013;36:793–4. [DOI] [PubMed] [Google Scholar]
- 7.El Mahmoud R, Mansencal N, Pilliere R, Leyer F, Abbou N, Michaud P, et al. Prevalence and characteristics of left ventricular outflow tract obstruction in tako-tsubo syndrome. Am Heart J 2008;156:543–8. [DOI] [PubMed] [Google Scholar]
- 8.Parodi G, Del Pace S, Salvadori C, Carrabba N, Olivotto I, Gensini GF, et al. Left ventricular apical ballooning syndrome as a novel cause of acute mitral regurgitation. J Am Coll Cardiol 2007;50:647–9. [DOI] [PubMed] [Google Scholar]
- 9.Kurisu S, Inoue I. Cardiac rupture in tako-tsubo cardiomyopathy with persistent st-segment elevation. Int J Cardiol. 2012;158:e5–6. [DOI] [PubMed] [Google Scholar]
- 10.Yamada R, Watanabe N, Kume T, Kawamoto T, Okahashi N, Wada N, et al. Left ventricular rupture associated with takotsubo-like left ventricular dysfunction (apical ballooning). J Echocardiogr 2006;4:59–62. [Google Scholar]
- 11.Brinjikji W, El-Sayed AM, Salka S. In-hospital mortality among patients with takotsubo cardiomyopathy: a study of the national inpatient sample 2008 to 2009. Am Heart J 2012;164:215–21. [DOI] [PubMed] [Google Scholar]
- 12.Sharkey SW, Windenburg DC, Lesser JR, Maron MS, Hauser RG, Lesser JN, et al. Natural history and expansive clinical profile of stress (tako-tsubo) cardiomyopathy. J Am Coll Cardiol 2010;55:333–41. [DOI] [PubMed] [Google Scholar]
- 13.Parodi G, Bellandi B, Del Pace S, Barchielli A, Zampini L, Velluzzi S, et al. Natural history of tako-tsubo cardiomyopathy. Chest 2011;139:887–92. [DOI] [PubMed] [Google Scholar]
- 14.Elesber AA, Prasad A, Bybee KA, Valeti U, Motiei A, Lerman A, et al. Transient cardiac apical ballooning syndrome: prevalence and clinical implications of right ventricular involvement. J Am Coll Cardiol 2006;47:1082–3. [DOI] [PubMed] [Google Scholar]
- 15.Haghi D, Athanasiadis A, Papavassiliu T, Suselbeck T, Fluechter S, Mahrholdt H, et al. Right ventricular involvement in takotsubo cardiomyopathy. Eur Heart J 2006;27:2433–9. [DOI] [PubMed] [Google Scholar]
- 16.Prasad A, Lerman A, Rihal CS. Apical ballooning syndrome (tako-tsubo or stress cardiomyopathy): a mimic of acute myocardial infarction. Am Heart J 2008;155:408–17. [DOI] [PubMed] [Google Scholar]
- 17.Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr 2010;23:685–713. quiz 786–8. [DOI] [PubMed] [Google Scholar]
- 18.Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP, Guyton RA, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014;129:e521–643. [DOI] [PubMed] [Google Scholar]
- 19.Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone marrow transplantation 2013;48:452–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hurst RT, Askew JW, Reuss CS, Lee RW, Sweeney JP, Fortuin FD, et al. Transient midventricular ballooning syndrome: a new variant. J Am Coll Cardiol 2006;48:579–83. [DOI] [PubMed] [Google Scholar]
- 21.Nyui N, Yamanaka O, Nakayama R, Sawano M, Kawai S. ‘Tako-tsubo’ transient ventricular dysfunction: a case report. Jpn Circ J 2000;64:715–9. [DOI] [PubMed] [Google Scholar]
- 22.Citro R, Rigo F, D'Andrea A, Ciampi Q, Parodi G, Provenza G, et al. Echocardiographic correlates of acute heart failure, cardiogenic shock, and in-hospital mortality in tako-tsubo cardiomyopathy. JACC Cardiovasc Imaging 2014;7:119–29. [DOI] [PubMed] [Google Scholar]
- 23.Eitel I, Von Knobelsdorff-Brenkenhoff F, Bernhardt P, Carbone I, Muellerleile K, Aldrovandi A, et al. Clinical characteristics and cardiovascular magnetic resonance findings in stress (takotsubo) cardiomyopathy. JAMA 2011;306:277–86. [DOI] [PubMed] [Google Scholar]
- 24.Liang JJ, Cha YM, Oh JK, Prasad A. Sudden cardiac death: an increasingly recognized presentation of apical ballooning syndrome (takotsubo cardiomyopathy). Heart & Lung 2013;42:270–2. [DOI] [PubMed] [Google Scholar]
- 25.Polak JF, Holman BL, Wynne J, Colucci WS. Right ventricular ejection fraction: an indicator of increased mortality in patients with congestive heart failure associated with coronary artery disease. J Am Coll Cardiol 1983;2:217–24. [DOI] [PubMed] [Google Scholar]
- 26.Fuster V, McGoon DC, Kennedy MA, Ritter DG, Kirklin JW. Long-term evaluation (12 to 22 years) of open heart surgery for tetralogy of fallot. Am J Cardiol 1980;46:635–42. [DOI] [PubMed] [Google Scholar]
- 27.Di Salvo TG, Mathier M, Semigran MJ, Dec GW. Preserved right ventricular ejection fraction predicts exercise capacity and survival in advanced heart failure. J Am Coll Cardiol 1995;25:1143–53. [DOI] [PubMed] [Google Scholar]
- 28.Neil C, Nguyen TH, Kucia A, Crouch B, Sverdlov A, Chirkov Y, et al. Slowly resolving global myocardial inflammation/oedema in Tako-Tsubo cardiomyopathy: evidence from T2-weighted cardiac MRI. Heart 2012;98:1278–84. [DOI] [PubMed] [Google Scholar]
- 29.Mrdovic I, Kostic J, Perunicic J, Asanin M, Vasiljevic Z, Ostojic M. Right ventricular takotsubo cardiomyopathy. J Am Coll Cardiol 2010;55:1751. [DOI] [PubMed] [Google Scholar]
- 30.Stahli BE, Ruschitzka F, Enseleit F. Isolated right ventricular ballooning syndrome: a new variant of transient cardiomyopathy. Eur Heart J 2011;32:1821. [DOI] [PubMed] [Google Scholar]
- 31.Kagiyama N, Okura H, Kume T, Hayashida A, Yoshida K. Isolated right ventricular takotsubo cardiomyopathy. Eur Heart J Cardiovasc Imaging 2015;16:285. [DOI] [PubMed] [Google Scholar]


