Abstract
Aim
Cardiac magnetic resonance (CMR) can visualize inflammatory tissue changes in acute myocarditis. Several quantitative image-derived parameters have been described to enhance the diagnostic value of CMR, but no direct comparison of these techniques is available.
Methods and results
A total of 34 patients with suspected acute myocarditis and 50 control subjects underwent CMR. CMR protocol included quantitative assessment of T1 relaxation times using modified Look-Locker inversion recovery (MOLLI) and shortened MOLLI (ShMOLLI) acquisition schemes, extracellular volume fraction (ECV), T2 relaxation times, and longitudinal strain. Established Lake-Louise criteria (LLC) consisting of T2-weighted signal intensity ratio (T2-ratio), early gadolinium enhancement ratio (EGEr), and late gadolinium enhancement (LGE) were assessed. Receiver operating characteristics analysis was performed to compare diagnostic performance. Areas under the curve of native T1 (MOLLI: 0.95; ShMOLLI: 0.92) and T2 relaxation times (0.92) were higher compared with those of the other CMR parameters (T2-ratio: 0.71, EGEr: 0.71, LGE: 0.87, LLC: 0.90, ECV MOLLI: 0.77, ECV ShMOLLI: 0.80, longitudinal strain: 0.83). Combined with LGE, each native mapping technique outperformed the diagnostic performance of LLC (P < 0.01, respectively). A combination of native parameters (T1, T2, and longitudinal strain) significantly increased the diagnostic performance of CMR compared with LLC without need of contrast media application (0.99 vs. 0.90; P = 0.008).
Conclusion
In patients suspected of having acute myocarditis, diagnostic performance of CMR can be improved by implementation of quantitative CMR parameters. Especially, native mapping techniques have the potential to replace current LLC.
ClinicalTrials.gov number
Keywords: Myocarditis, Inflammation, Magnetic resonance imaging, Mapping, Diagnosis
Introduction
Acute myocarditis is an important differential diagnosis in patients presenting with acute chest pain and elevated troponin levels, who have unobstructed coronary arteries at invasive catheterization.1 As an important cause of cardiac morbidity and mortality, it accounts for up to 20% of deaths in adults younger than 40 years.2 In addition, dilated cardiomyopathy may result from chronic inflammatory activation in patients with inadequate immune response.3
Cardiac magnetic resonance (CMR) can characterize inflammatory myocardial tissue changes and is increasingly used in patients with clinically suspected myocarditis.4 CMR diagnosis of myocarditis is made if two of the three ‘Lake-Louise’ criteria (LLC) are met: (i) increased myocardial early gadolinium enhancement ratio (EGEr) or absolute myocardial enhancement of ≥45% on T1-wheighted images, (ii) increased myocardial signal intensity on T2-weighted images (increased T2-ratio or regional increase in T2 signal intensity), and (iii) non-ischaemic lesions at late gadolinium enhancement (LGE) imaging.5 However, correct image interpretation of qualitative LLC depends on the direct visualization of the inflammatory myocardial involvement (e.g. myocardial oedema and/or necrosis) or on the signal intensities of a reference tissue,5–8 hindering the correct assessment of diffuse inflammatory changes. In this regard, quantitative CMR approaches such as CMR mapping techniques directly yielding absolute T1 and T2 relaxation times may prove to be beneficial, as they are independent from the signal intensities of a reference tissue. Recent studies provided evidence that myocardial T1 and T2 mapping and T1-derived extracellular volume fraction (ECV) can significantly improve the diagnostic accuracy of CMR compared with conventional single or combined LLC.4,9–12 However, to date different acquisition strategies have been applied for myocardial T1 mapping in acute myocarditis, e.g. modified Look-Locker inversion recovery (MOLLI) and shortened MOLLI (ShMOLLI) acquisition schemes.4,10,13 In addition, quantitative longitudinal strain in echocardiography has been shown to detect even minor alterations in left ventricular function in patients suspected of having acute myocarditis allowing for reliable discrimination between health and disease.14
While each of these studies showed that new CMR techniques might be beneficial for diagnosing acute myocarditis, there is a special need for comparative studies, which directly compare each of the established and new techniques within a single CMR protocol. Therefore, we prospectively evaluated the diagnostic performance of CMR in patients suspected of having acute myocarditis by using a comprehensive CMR approach including the assessment of the LLC, different pre- and post-contrast T1 mapping schemes, T2 mapping, and longitudinal myocardial strain analysis.
Methods
The institutional review committee approved the study, and all subjects gave informed consent prior to CMR. The study population of this prospective study consisted of patients with suspected acute myocarditis and control subjects. The diagnosis of acute myocarditis was made solely on the basis of clinical observation.4 This clinical evidence presented the reference standard against which the diagnostic performance of CMR parameters was tested. Patients with suspected myocarditis had: (i) acute chest pain, (ii) evidence of acute myocardial injury [electrocardiogram (ECG) changes and/or elevated troponin serum levels], and (iii) a history of viral infection during the last few weeks with elevated serum markers indicating infectious disease (e.g. C-reactive protein). Coronary artery disease was ruled out before CMR by means of invasive cardiac catheterization. Exclusion criteria included contraindications to CMR, previous myocardial infarction, previous myocarditis, and other medical history of cardiac disease. The diagnosis of acute myocarditis was made on the basis of clinical and laboratory observation only, and CMR results were not taken into consideration.
Healthy volunteers and outpatients referred for non-specific thoracic pain underwent CMR as controls. All control subjects had unremarkable CMR results without structural abnormalities, no medical history of cardiac disease, no cardiac risk factors, and normal ECG results. In outpatients referred for non-specific thoracic pain, a detailed diagnostic workup and clinical follow-up was unremarkable, without signs of cardiac disease.
Cardiac magnetic resonance
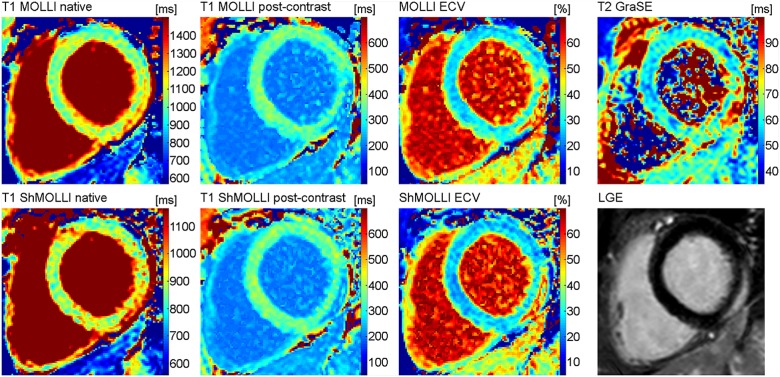
All scans were performed on a 1.5 Tesla CMR system (Ingenia 1.5T, Philips Healthcare, Best, The Netherlands). A 32-channel torso coil with digital interface was used for signal reception. For functional analysis, ECG-gated steady-state free precession cine images were obtained including short axis (SA), vertical long axis (VLA), and horizontal long axis (HLA) stacks. The SA stack covered the whole left ventricle. Oedema-sensitive black blood T2-weighted short-tau inversion recovery (STIR) sequences were performed in SA and VLA orientation. To correct for torso coil-related signal inhomogeneities, an inherent signal intensity correction algorithm (‘Constant LEvel AppeaRance’-CLEAR) was used. Early gadolinium enhancement was assessed using transverse free-breathing fast spin echo T1-weighted images, which were acquired in three identical slices both before and after intravenous injection of a double-dose bolus of 0.2 mmol/kg of body weight of gadobutrol (Gadovist, Bayer Healthcare, Leverkusen, Germany) using the body coil for signal reception. For LGE imaging, segmented inversion recovery gradient-echo sequences in SA, VLA, and HLA orientation were performed. Optimal inversion time was determined by using the Look-Locker technique.15 Breath hold T1 and T2 mapping were performed in end-diastole in SA orientation. Three SA slices were obtained (basal, mid-ventricular, and apical). For myocardial T1 mapping, 3(3)3(3)5 MOLLI16 and 5(1)1(1) ShMOLLI17 acquisition schemes were used. MOLLI and ShMOLLI maps were performed before and 10–12 min after contrast administration (Figure 1). For myocardial T2 mapping, an optimized 6-echo gradient spin echo (GraSE) sequence was used as previously described.18 Quality assessment of relaxation maps was performed directly after image acquisition. If necessary, maps with motion artefacts were directly repeated until maps with sufficient image quality were obtained. T1 MOLLI and T2 maps were reconstructed directly on the imager console after image acquisition. T1 ShMOLLI maps were reconstructed offline using a dedicated plugin for the OsiriX DICOM viewer software (Pixmeo, Geneva, Switzerland), in which exponential fitting with a maximum likelihood estimator was used to calculate the T1 maps. The following fit model was used for the magnitude data: |(A + B) × exp(−TI/T1*)|.
Figure 1.
Typical inflammatory lesion at the subepicardium of the inferolateral wall (basal section) in a 37-year-old male with acute myocarditis. The composition of pictures exemplarily illustrates the alterations of different quantitative tissue parameters in acute oedematous lesions.
A Rician noise distribution was assumed due to the magnitude operation. A ‘Look-Locker’ correction was performed to calculate T1 based on the fit parameters T1*, A, and B.
Detailed sequence parameters are given in the Supplementary data online, Table S1.
Image analysis
Two physicians with 2 (J.A.L.) and 10 (C.P.N.) years of experience in CMR analysed the data and performed the measurements. Readers were blinded to the patient information. Cardiac functional analysis was performed offline using dedicated software (ViewForum, Philips Healthcare). Papillary muscles were included in the left ventricular cavity volume. The presence of focal myocardial oedema on T2 STIR and/or non-ischaemic lesions on LGE images was visually assessed by consensus agreement of the two readers. T2-ratio for the presence of global myocardial oedema and EGEr for the presence of inflammation-induced hyperaemia were calculated as recommended for the assessment of the LLC.5–7 Myocardial T1 and T2 relaxation times were extracted from the relaxation maps by using freely available software (Segment, version 1.9, R2783; http://segment.heiberg.se).19 Endocardial and epicardial borders were carefully contoured to exclude epicardial fat, blood pool, and pericardial effusion from analysis. Maps were analysed by a segmental approach.20 Haematocrit-corrected segmental ECV values were calculated separately for pre- and post-contrast MOLLI and ShMOLLI T1 values as previously described.4,21 Longitudinal strain was derived from HLA cine datasets using the feature tracking technique.22 CMR feature tracking strain analysis was performed using dedicated software (Diogenes, TomTec, Unterschleissheim, Germany).
Statistical analysis
Statistical analysis was performed using SAS 9.2 (SAS Institute, Inc., Cary, NC, USA). Patient characteristics are presented as mean ± standard deviation or as absolute frequency. Continuous variables between the two groups were compared using the unpaired Student's t-test. Correlation analysis was performed using Spearman's rank correlation coefficient. Diagnostic performance of single continuous variables, qualitative LGE, and derived combination scores of continuous variables was analysed by plotting receiver operation characteristics (ROCs) and comparing the area under the ROCs. Areas under the ROCs were compared as previously described.23 Cut-off values were chosen by maximizing the reclassification accuracy for the single predictive variables and scores, and (reclassification) sensitivity, specificity, and accuracy were calculated. For the combination of single predictive variables, scores were derived based on logistic regression analysis. The level of statistical significance was set to P < 0.05.
Results
Population characteristics
A total of 84 subjects were included in this study (34 patients with acute myocarditis and 50 control subjects). Mean time from admission to CMR was 2.63 ± 1.93 days. Clinical characteristics in myocarditis and control subjects are given in Table 1.
Table 1.
Clinical characteristics in myocarditis and control subjects
| Parameter | Myocarditis group (n = 34) | Control group (n = 50) | P-value |
|---|---|---|---|
| Age (years) | 44.9 ± 18.7 | 39.2 ± 17.2 | 0.163 |
| Male | 17 (50.0) | 30 (60.0) | 0.365 |
| Heart rate (beats/min) | 70.4 ± 15.2 | 65.7 ± 12.7 | 0.140 |
| Body mass index (kg/m²) | 26.4 ± 5.9 | 24.7 ± 4.2 | 0.137 |
| Left ventricular ejection fraction (%) | 55.5 ± 11.4 | 60.9 ± 3.6 | 0.002 |
| LVESVi (ml/m²) | 71.4 ± 15.0 | 72.7 ± 12.7 | 0.693 |
| Interventricular septal thickness (mm) | 10.3 ± 1.9 | 9.2 ± 1.7 | 0.010 |
| Troponin I (ng/mL) | 4.7 ± 6.4 | Below detection limit | |
| White blood cell count (10³/µL) | 9.9 ± 3.6 | 6.8 ± 1.8 | <0.001 |
| C-reactive protein (mg/L) | 47.7 ± 49.4 | 1.2 ± 0.9 | 0.001 |
| Haematocrit (%) | 37.6 ± 6.6 | 40.7 ± 4.1 | 0.011 |
Numbers are given as mean ± standard deviation or as absolute frequency with percentages in parentheses.
LVESVi, left ventricular end-systolic volume index.
CMR results
T2-ratio (1.84 ± 0.48 vs. 1.57 ± 0.27; P = 0.002) and EGEr (3.32 ± 2.04 vs. 2.26 ± 1.98; P = 0.021) were significantly higher in myocarditis subjects than in control subjects. Non-ischaemic LGE was found in 25/34 (73.5%) of all myocarditis patients. Myocardial T1 and T2 relaxation times were significantly prolonged in the myocarditis group compared with the control group (MOLLI: 1048.6 ± 51.9 vs. 966.9 ± 27.8 ms, ShMOLLI: 887.1 ± 37.2 vs. 831.4 ± 26.9 ms, T2 GraSE: 60.43 ± 7.47 vs. 52.42 ± 2.56 ms; P < 0.001, respectively). Segmental T1, T2, and ECV data are given in the Supplementary data online, Tables S2–S4. Four per cent (272/6720) of all segments had to be excluded from segmental analysis of T1 and T2 relaxation maps due to off-resonance or motion artefacts. Global longitudinal strain was significantly reduced in the myocarditis group compared with the control group (−15.59 ± 4.97% vs. −21.03 ± 2.95%; P ≤ 0.001). All CMR parameters evaluated are given in Table 2. There was a significant correlation between native T1 relaxation times and T2 relaxation times (MOLLI T1 vs. T2: r = 0.664; P < 0.001 and ShMOLLI T1 vs. T2: r = 0.621; P < 0.001). Longitudinal strain showed good association with native T1 MOLLI (r = 0.503; P < 0.001), native T1 ShMOLLI (r = 0.457; P < 0.001), and T2 (r = 0.423; P < 0.001). No significant correlation was found between longitudinal strain and ECV measures (P > 0.05, respectively).
Table 2.
CMR characteristics in myocarditis and control subjects
| Parameter | Myocarditis group (n = 34) | Control group (n = 50) | P-value |
|---|---|---|---|
| T2-ratio | 1.84 ± 0.48 | 1.57 ± 0.27 | 0.002 |
| EGEr | 3.32 ± 2.04 | 2.26 ± 1.98 | 0.021 |
| LGE | 25 (73.5) | 0 (0.0) | <0.001 |
| Regional myocardial oedema | 24 (70.6) | 0 (0.0) | <0.001 |
| MOLLI T1 native (ms) | 1048.6 ± 51.9 | 966.9 ± 27.8 | <0.001 |
| MOLLI ECV (%) | 34.47 ± 8.52 | 27.68 ± 5.82 | <0.001 |
| ShMOLLI T1 native (ms) | 887.1 ± 37.2 | 831.4 ± 26.9 | <0.001 |
| ShMOLLI ECV (%) | 32.60 ± 8.51 | 25.33 ± 4.46 | <0.001 |
| T2 GraSE (ms) | 60.43 ± 7.47 | 52.42 ± 2.56 | <0.001 |
| MOLLI T1 skeletal muscle (ms) | 880.0 ± 87.8 | 831.4 ± 56.5 | 0.003 |
| Longitudinal strain (%) | −15.59 ± 4.97 | −21.03 ± 2.95 | <0.001 |
Numbers are given as mean ± standard deviation or as absolute frequency with percentages in parentheses.
EGEr, early gadolinium enhancement ratio; LGE, late gadolinium enhancement; MOLLI, modified Look-Locker inversion recovery; ECV, extracellular volume fraction; ShMOLLI, shortened modified Look-Locker inversion recovery; GraSE, gradient spin echo.
Diagnostic performance of single CMR parameters
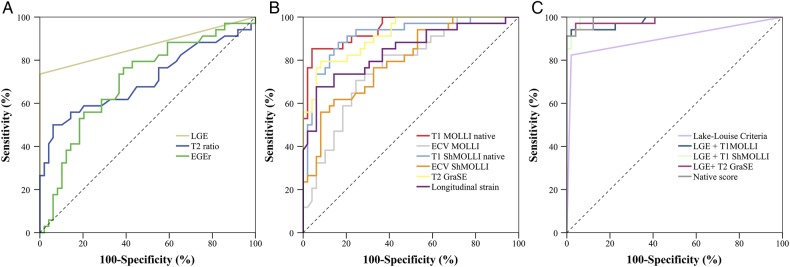
Cut-off values for CMR parameters are given in Table 3. Native global T1 and T2 values showed an excellent diagnostic performance with area under the curve (AUC) values of 0.95 (MOLLI), 0.92 (ShMOLLI), and 0.92 (T2). All three parameters showed significantly higher AUC values compared with T2-ratio (0.71), EGEr (0.71), ECV MOLLI (0.77), and ECV ShMOLLI (0.80) (P < 0.05, respectively). LGE (0.87) and longitudinal strain (0.83) showed comparable AUCs (P > 0.05, respectively). A comparison matrix for differences in the AUC between all tested single CMR parameters is provided in the Supplementary data online, Table S5.
Table 3.
Diagnostic performance of different CMR parameters for diagnosis of acute myocarditis
| Parameter | Sensitivity | Specificity | Accuracy | PPV | NPV |
|---|---|---|---|---|---|
| Lake-Louise parameter | |||||
| T2-ratio (≥1.9) | 50 | 94 | 76 | 85 | 73 |
| EGEr (≥1.95) | 77 | 62 | 67 | 58 | 80 |
| LGE (±) | 74 | 100 | 89 | 100 | 85 |
| Quantitative parameter | |||||
| T1 MOLLI native (≥1000 ms) | 85 | 96 | 92 | 94 | 90 |
| T1 ShMOLLI native (≥852 ms) | 88 | 84 | 86 | 79 | 91 |
| ECV MOLLI (≥28.8%) | 70 | 76 | 74 | 67 | 79 |
| ECV ShMOLLI (≥30.0%) | 57 | 92 | 77 | 83 | 75 |
| T2 GraSE (≥55.9 ms) | 79 | 92 | 87 | 87 | 87 |
| Longitudinal strain (≤−15.7%) | 44 | 96 | 75 | 88 | 72 |
| Combinations | |||||
| Lake-Louise criteria | 82 | 98 | 92 | 97 | 89 |
| LGE + T1 MOLLI native | 94 | 98 | 96 | 94 | 98 |
| LGE + T1 ShMOLLI native | 100 | 94 | 96 | 92 | 100 |
| LGE + T2 GraSE | 97 | 96 | 96 | 100 | 94 |
| T1 MOLLI native + T2 GraSE + longitudinal strain | 91 | 100 | 96 | 100 | 94 |
Data are percentages. Cut-off values are given in parentheses after each parameter.
EGEr, early gadolinium enhancement ratio; LGE, late gadolinium enhancement; GraSE, gradient spin echo; MOLLI, modified Look-Locker inversion recovery; ShMOLLI, shortened modified Look-Locker inversion recovery; ECV, extracellular volume fraction.
Diagnostic performance of combined CMR parameters
The LLC yielded an AUC of 0.90 (Figure 2), which was superior compared with T2-ratio and EGEr alone (P < 0.01, respectively). However, no significant differences could be found when compared with single native quantitative parameters (P > 0.05, respectively). Using the combined LLC, CMR in our study population yielded a sensitivity of 82%, a specificity of 98%, a diagnostic accuracy of 92%, a positive predictive value (PPV) of 97%, and a negative predictive value (NPV) of 89% (Table 3). T1 MOLLI alone yielded comparable values with a sensitivity of 85%, a specificity of 96%, a diagnostic accuracy of 92%, a PPV of 94%, and a NPV of 90%. Diagnostic performance of CMR could be significantly enhanced when native T1 and T2 relaxation times were combined with LGE (AUC for T1 MOLLI plus LGE: 0.98, T1 ShMOLLI plus LGE: 0.99, T2 GraSE plus LGE: 0.99). Those scores were superior to those yielded by using conventional LLC alone (P = 0.008, 0.006, and 0.008, respectively). A combination of non-contrast parameters (T1 MOLLI, T2 GraSE, and longitudinal strain) yielded an excellent diagnostic performance with an AUC of 0.99, which was significantly higher compared with the AUC of the LLC (AUC: 0.90; P = 0.008).
Figure 2.
Graphs show receiver operating characteristic curves for (A) single Lake-Louise criteria: LGE (AUC: 0.87), T2-ratio (AUC: 0.71), and EGEr (AUC: 0.71); (B) additional quantitative parameters: T1 MOLLI native (AUC: 0.95), ECV MOLLI (AUC: 0.77), T1 ShMOLLI native (AUC: 0.92), ECV ShMOLLI (AUC: 0.80), T2 GraSE (AUC: 0.92), and longitudinal strain (AUC: 0.83); and (C) for different scores: Lake-Louise criteria (AUC: 0.90), LGE + T1 MOLLI (AUC: 0.98), LGE + T1 ShMOLLI (AUC: 0.99), LGE + T2 GraSE (AUC: 0.99), and native score (T1 MOLLI native + T2 GraSE + longitudinal strain) (AUC: 0.99). EGEr, early gadolinium enhancement ratio; LGE, late gadolinium enhancement; GraSE, gradient spin echo; MOLLI, modified Look-Locker inversion recovery; ShMOLLI, shortened modified Look-Locker inversion recovery; ECV, extracellular volume fraction.
Discussion
In this prospective study, we evaluated the diagnostic value of CMR in patients suspected of having acute myocarditis by using a comprehensive CMR approach, which included established CMR parameters of myocardial inflammation and several quantitative parameters. The major findings of this study are as follows: (i) CMR offers a variety of qualitative, semi-quantitative, and quantitative techniques, which allow for safe and reliable diagnosis of acute inflammatory myocardial alterations; (ii) native T1 and T2 mapping techniques provide the best diagnostic performance of all single CMR parameters evaluated; (iii) diagnostic performance of native T1 and T2 mapping are comparable with the diagnostic performance of established LLC; and (iv) when combined with LGE, native T1 and T2 mapping techniques improve the diagnostic performance of CMR significantly compared with LLC.
Lake-Louise criteria
With a sensitivity of 82% and a specificity 98%, we found similar values for the LLC as in an earlier reported cohort of acute myocarditis, where a sensitivity of 76% and a specificity of 96% have been reported.6 However, although LLC represent a well-accepted diagnostic approach, the individual components of the criteria suffer from specific technical limitations:
EGEr is technically limited by low reproducibility and susceptibility to artefacts.24 In addition, depending on slice orientation, myocardial segments not covered by CMR imaging are not available for reading and analysis, potentially limiting the diagnostic performance in case of focal instead of global myocarditis.4
Black blood T2-weighted images often suffer from signal intensity inhomogeneities, which can obscure myocardial oedema. In addition, image interpretation can be hindered further in cases of global myocardial oedema (i.e. when no normal signal intensity from healthy myocardium is present).25 Interpretation of T2-ratio and EGEr may also be hampered by coexisting myositis as both approaches are based on relative comparison of signal to skeletal muscle.26 The clinical relevance of this limitation is underlined by higher T1 relaxation times of skeletal muscle in myocarditis subjects in comparison with control subjects found in our study (Table 2), indicating that coexisting myositis was present in some cases. All of these reasons may be responsible for the relatively low diagnostic performance of T2-ratio and EGEr found in our datasets.
LGE alone provided the best diagnostic performance of all LLC with a perfect specificity to identify patients with acute myocarditis. However, diagnostic performance of LGE is known to be limited due to a modest sensitivity, which was found to be 74% in our study population. In pooled datasets, an even lower sensitivity of 59% has been reported.5 Early changes observed during acute myocarditis, such as expansion of intracellular compartments, which occurs before myocyte necrosis, does not increase extracellular space. As LGE specifically targets the extracellular space, sensitivity might be hampered early in the course of acute myocarditis with its normal extracellular space but intracellular oedema. Thus, there is a particular need for excellent discriminators between health and disease in LGE-negative subjects with possible diffuse myocardial injury.
T1 and T2 mapping
Native myocardial T1 relaxations times using the MOLLI and ShMOLLI acquisition schemes were significantly higher in patients with acute myocarditis compared with control subjects. Both schemes yielded an excellent diagnostic performance with AUC values of 0.95 and 0.92. These values are in good agreement with two previous studies, in which patients in the acute stage of myocarditis had been included. Ferreira et al. reported an AUC of 0.95 for native T1 mapping using the ShMOLLI scheme.9 In another study, an AUC for native T1 mapping of 0.94 using the MOLLI scheme had been reported.4 Previous studies and our results indicate that native T1 mapping provides an excellent diagnostic performance in patients suspected of having acute myocarditis regardless of the employed imaging technique/acquisition scheme. However, absolute T1 values vary depending on the employed CMR sequence and algorithm of T1 calculation,27–29 necessitating reference values for the specific scanner and sequence before the technique may be introduced into clinical routine. Then, T1 mapping can play out its strengths as a significant prolongation of T1 relaxation times goes along with cellular oedema, increased extracellular space, and myocyte necrosis, which all commonly occur in patients with acute myocarditis.4,5,30
Diagnostic accuracies of ECV have been inferior to native T1 mapping, which is concordant with the results of a previous study.4 Comparable with LGE, ECV calculation targets enlargement of the extracellular space by measuring the proportion of the extracellular space. Therefore, diagnostic performance might be hampered in the early course of disease, when ECV can still be normal. This might explain that ECV provides a similar diagnostic accuracy compared with LLC when used in patients with a more subacute clinical presentation.10 However, an advantage of ECV is that it is independent of MR imaging field strength and partially compensates for site-specific factors such as the T1 mapping sequence.31,32
Interestingly, T2 mapping18 yielded an equivalent diagnostic performance with an AUC of 0.92 when compared with native T1 mapping. While elevated myocardial T2 values in inflamed myocardium have already been described,11 the diagnostic value of myocardial T2 mapping has not yet been assessed within a multiparametric approach. According to our results, T2 mapping showed a diagnostic accuracy of 87%, whereas standard T2-weighted imaging showed only a diagnostic accuracy of 76%. A possible explanation for this increase in diagnostic accuracy of T2 mapping compared with standard T2-weighted imaging may be an improved detection of diffuse inflammation by T2 mapping in patients with coexisting myositis or diffuse myocarditis.18
Moreover, in a more subacute/chronic presentation, a diagnostic accuracy of only 63% has been reported for myocardial T2 mapping.10 Therefore, myocardial T2 mapping may be also useful as discriminator between acute and subacute/chronic myocardial inflammation.
Strain analysis
According to our datasets global longitudinal strain was significantly reduced in myocarditis subjects compared with control subjects. The AUC for global longitudinal strain was 0.83, suggesting that the technique can provide incremental diagnostic information in suspected acute myocarditis. In a retrospective echocardiographic cohort, a sensitivity and specificity for longitudinal strain of 78 and 93% had been reported, respectively.14 In our cohort, the specificity was equivalent with 96%, but sensitivity was substantially lower with only 44%, suggesting that wall motion abnormalities in our collective were less pronounced. This is supported by the fact that mean left ventricular ejection fraction was still preserved in the myocarditis group. We also found significant correlations between longitudinal strain and native parametric measures, suggesting that functional parameters are directly associated with inflammatory changes of the myocardium and therefore may be useful for patient follow-up.
Diagnostic performance of combined CMR parameters
Although global native T1 and T2 relaxation time alone already yielded excellent diagnostic accuracies, these can further be improved by combining them with assessment of LGE. With these combinations, diagnostic accuracies of 96% could be achieved. These results are in good agreement with a previous study at 3 Tesla, where combination of native T1 relaxation times with LGE achieved an equivalent diagnostic accuracy of 96%.4 Furthermore, a diagnostic accuracy of 96% could also be achieved by combining the three non-contrast enhanced parameters native T1 MOLLI, T2 GraSE, and longitudinal strain. Using this diagnostic approach, more patients with acute myocardial injury could be detected in our study, which were otherwise missed by standard LLC. This may be of special importance for evaluation of myocarditis in patients with contraindications for the use of gadolinium-based contrast agents.
Limitations
This study was performed by using clinical validation for patients suspected of having acute myocarditis. A systematic endomyocardial biopsy as a reference standard was not performed as a low sensitivity for excluding myocarditis has been reported for this method.33 Instead, the presence of acute myocarditis was defined by combining typical clinical features, exclusion of coronary artery disease, and elevated biomarkers as reported previously in multiple cardiac MR validation studies.4,6,9,10 Alterations of myocardial T1 and T2 relaxation times are non-specific and can be caused by fibrosis and oedema of various aetiologies,4,11,18,34 leaving e.g. a possible diagnostic uncertainty in the differentiation of acute and chronic myocarditis, and potentially pre-existing cardiac disease in the patient group. Therefore, quantitative mapping—like semi-quantitative—techniques may only be used within the appropriate clinical context. The significant positive correlation of T1 and T2 values in the setting of acute myocarditis in the present study indicates that elevation in T1 relaxation times was mostly triggered due to myocardial oedema. In this regard, it is of note that the time interval between onset of symptoms and CMR may have impact on the clinical utility of T1 and T2 parametric mapping techniques, as they both directly quantify myocardial oedema, which is more pronounced in the acute stage of the disease.30 Therefore, the results of this study are valid for the acute stage of myocarditis, and caution must be exercised when transferring them to subacute or even chronic setting. Because of the small sample size and the use of reclassification methods, further prospective studies are necessary to substantiate the results of this study.
Conclusion
In patients suspected of having acute myocarditis, a comprehensive, multiparametric CMR approach allows for a reliable discrimination between healthy and diseased patients. As native mapping techniques provide high diagnostic accuracies for the diagnosis of acute inflammatory myocardial changes and represent a direct measure for myocardial oedema, we recommend an implementation of these techniques into routine protocols. With its high specificity, LGE imaging represents another essential part of myocarditis CMR. A combination of native T1 relaxation times, T2 relaxation times, and longitudinal strain measures may serve as an alternative to the LLC for assessing patients with suspected acute myocarditis. Further research on a wider applicability with different T1 mapping techniques is warranted.
Supplementary data
Supplementary data are available at European Journal—cardiovascular Imaging online.
Acknowledgement
We thank Dr B. Schnackenburg for his support and invaluable advice.
Conflict of interest: C.S. and J.G. are employees of Philips Research (Hamburg, Germany).
References
- 1.Baccouche H, Mahrholdt H, Meinhardt G, Merher R, Voehringer M, Hill S, et al. Diagnostic synergy of non-invasive cardiovascular magnetic resonance and invasive endomyocardial biopsy in troponin-positive patients without coronary artery disease. Eur Heart J 2009;30:2869–79. [DOI] [PubMed] [Google Scholar]
- 2.Drory Y, Turetz Y, Hiss Y, Lev B, Fisman EZ, Pines A, et al. Sudden unexpected death in persons less than 40 years of age. Am J Cardiol 1991;68:1388–92. [DOI] [PubMed] [Google Scholar]
- 3.Liu PP, Mason JW. Advances in the understanding of myocarditis. Circulation 2001;104:1076–82. [DOI] [PubMed] [Google Scholar]
- 4.Luetkens JA, Doerner J, Thomas DK, Dabir D, Gieseke J, Sprinkart AM, et al. Acute myocarditis: multiparametric cardiac MR imaging. Radiology 2014;273:383–92. [DOI] [PubMed] [Google Scholar]
- 5.Friedrich MG, Sechtem U, Schulz-Menger J, Holmvang G, Alakija P, Cooper LT, et al. Cardiovascular magnetic resonance in myocarditis: A JACC white paper. J Am Coll Cardiol 2009;53:1475–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abdel-Aty H, Boye P, Zagrosek A, Wassmuth R, Kumar A, Messroghli D, et al. Diagnostic performance of cardiovascular magnetic resonance in patients with suspected acute myocarditis: comparison of different approaches. J Am Coll Cardiol 2005;45:1815–22. [DOI] [PubMed] [Google Scholar]
- 7.Friedrich MG, Strohm O, Schulz-Menger J, Marciniak H, Luft FC, Dietz R. Contrast media-enhanced magnetic resonance imaging visualizes myocardial changes in the course of viral myocarditis. Circulation 1998;97:1802–9. [DOI] [PubMed] [Google Scholar]
- 8.Gutberlet M, Spors B, Thoma T, Bertram H, Denecke T, Felix R, et al. Suspected chronic myocarditis at cardiac MR: diagnostic accuracy and association with immunohistologically detected inflammation and viral persistence. Radiology 2008;246:401–9. [DOI] [PubMed] [Google Scholar]
- 9.Ferreira VM, Piechnik SK, Dall'Armellina E, Karamitsos TD, Francis JM, Ntusi N, et al. T(1) mapping for the diagnosis of acute myocarditis using CMR: comparison to T2-weighted and late gadolinium enhanced imaging. JACC Cardiovasc Imaging 2013;6:1048–58. [DOI] [PubMed] [Google Scholar]
- 10.Radunski UK, Lund GK, Stehning C, Schnackenburg B, Bohnen S, Adam G, et al. CMR in patients with severe myocarditis: diagnostic value of quantitative tissue markers including extracellular volume imaging. JACC Cardiovasc Imaging 2014;7:667–75. [DOI] [PubMed] [Google Scholar]
- 11.Thavendiranathan P, Walls M, Giri S, Verhaert D, Rajagopalan S, Moore S, et al. Improved detection of myocardial involvement in acute inflammatory cardiomyopathies using T2 mapping. Circ Cardiovasc Imaging 2012;5:102–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hinojar R, Foote L, Arroyo Ucar E, Jackson T, Jabbour A, Yu CY, et al. Native T1 in discrimination of acute and convalescent stages in patients with clinical diagnosis of myocarditis: a proposed diagnostic algorithm using CMR. JACC Cardiovasc Imaging 2015;8:37–46. [DOI] [PubMed] [Google Scholar]
- 13.Ferreira VM, Piechnik SK, Dall'Armellina E, Karamitsos TD, Francis JM, Ntusi N, et al. Native T1-mapping detects the location, extent and patterns of acute myocarditis without the need for gadolinium contrast agents. J Cardiovasc Magn Reson 2014;16:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hsiao JF, Koshino Y, Bonnichsen CR, Yu Y, Miller FA, Jr, Pellikka PA, et al. Speckle tracking echocardiography in acute myocarditis. Int J Cardiovasc Imaging 2013;29:275–84. [DOI] [PubMed] [Google Scholar]
- 15.Look DC, Locker DR. Time saving in measurement of NMR and EPR relaxation times. Rev Sci Instrum 1970;41:250–1. [Google Scholar]
- 16.Messroghli DR, Radjenovic A, Kozerke S, Higgins DM, Sivananthan MU, Ridgway JP. Modified Look-Locker inversion recovery (MOLLI) for high-resolution T1 mapping of the heart. Magn Reson Med 2004;52:141–6. [DOI] [PubMed] [Google Scholar]
- 17.Piechnik SK, Ferreira VM, Dall'Armellina E, Cochlin LE, Greiser A, Neubauer S, et al. Shortened modified Look-Locker inversion recovery (ShMOLLI) for clinical myocardial T1-mapping at 1.5 and 3T within a 9 heartbeat breathhold. J Cardiovasc Magn Reson 2010;12:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sprinkart A, Luetkens J, Traber F, Doerner J, Gieseke J, Schnackenburg B, et al. Gradient spin echo (GraSE) imaging for fast myocardial T2 mapping. J Cardiovasc Magn Reson 2015;17:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heiberg E, Sjogren J, Ugander M, Carlsson M, Engblom H, Arheden H. Design and validation of segment—freely available software for cardiovascular image analysis. BMC Med Imaging 2010;10:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Int J Cardiovasc Imaging 2002;18:539–42. [PubMed] [Google Scholar]
- 21.Flett AS, Hayward MP, Ashworth MT, Hansen MS, Taylor AM, Elliott PM, et al. Equilibrium contrast cardiovascular magnetic resonance for the measurement of diffuse myocardial fibrosis: preliminary validation in humans. Circulation 2010;122:138–44. [DOI] [PubMed] [Google Scholar]
- 22.Kuetting D, Sprinkart AM, Doerner J, Schild H, Thomas D. Comparison of magnetic resonance feature tracking with harmonic phase imaging analysis (CSPAMM) for assessment of global and regional diastolic function. Eur J Radiol 2015;84:100–7. [DOI] [PubMed] [Google Scholar]
- 23.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 1988;44:837–45. [PubMed] [Google Scholar]
- 24.Schulz-Menger J, Bluemke DA, Bremerich J, Flamm SD, Fogel MA, Friedrich MG, et al. Standardized image interpretation and post processing in cardiovascular magnetic resonance: Society for Cardiovascular Magnetic Resonance (SCMR) board of trustees task force on standardized post processing. J Cardiovasc Magn Reson 2013;15:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eitel I, Friedrich MG. T2-weighted cardiovascular magnetic resonance in acute cardiac disease. J Cardiovasc Magn Reson 2011;13:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laissy JP, Messin B, Varenne O, Iung B, Karila-Cohen D, Schouman-Claeys E, et al. MRI of acute myocarditis: a comprehensive approach based on various imaging sequences. Chest 2002;122:1638–48. [DOI] [PubMed] [Google Scholar]
- 27.Moon JC, Messroghli DR, Kellman P, Piechnik SK, Robson MD, Ugander M, et al. Myocardial T1 mapping and extracellular volume quantification: a Society for Cardiovascular Magnetic Resonance (SCMR) and CMR Working Group of the European Society of Cardiology consensus statement. J Cardiovasc Magn Reson 2013;15:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salerno M, Kramer CM. Advances in parametric mapping with CMR imaging. JACC Cardiovasc Imaging 2013;6:806–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kellman P, Hansen MS. T1-mapping in the heart: accuracy and precision. J Cardiovasc Magn Reson 2014;16:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kindermann I, Barth C, Mahfoud F, Ukena C, Lenski M, Yilmaz A, et al. Update on myocarditis. J Am Coll Cardiol 2012;59:779–92. [DOI] [PubMed] [Google Scholar]
- 31.Kawel N, Nacif M, Zavodni A, Jones J, Liu S, Sibley CT, et al. T1 mapping of the myocardium: intra-individual assessment of the effect of field strength, cardiac cycle and variation by myocardial region. J Cardiovasc Magn Reson 2012;14:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fontana M, White SK, Banypersad SM, Sado DM, Maestrini V, Flett AS, et al. Comparison of T1 mapping techniques for ECV quantification. histological validation and reproducibility of ShMOLLI versus multibreath-hold T1 quantification equilibrium contrast CMR. J Cardiovasc Magn Reson 2012;14:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hauck AJ, Kearney DL, Edwards WD. Evaluation of postmortem endomyocardial biopsy specimens from 38 patients with lymphocytic myocarditis: implications for role of sampling error. Mayo Clin Proc 1989;64:1235–45. [DOI] [PubMed] [Google Scholar]
- 34.Dass S, Suttie JJ, Piechnik SK, Ferreira VM, Holloway CJ, Banerjee R, et al. Myocardial tissue characterization using magnetic resonance noncontrast t1 mapping in hypertrophic and dilated cardiomyopathy. Circ Cardiovasc Imaging 2012;5:726–33. [DOI] [PubMed] [Google Scholar]