Abstract
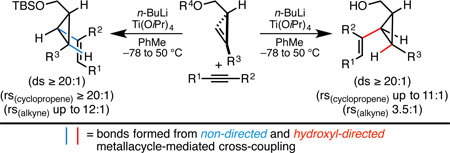
Stereodivergent metallacycle-mediated cross-coupling reactions are described for the synthesis of densely functionalized vinylcyclopropanes from the union of alkynes with cyclopropenes. Strategies explored include hydroxyl-directed and non-directed processes, with the latter of these delivering vinylcyclopropanes with exquisite levels of regio- and stereoselectivity. Challenges inherent to these coupling reactions include diastereoselectivity (with respect to the cyclopropene) and regioselectivity with respect to both coupling partners).
Graphical Abstract
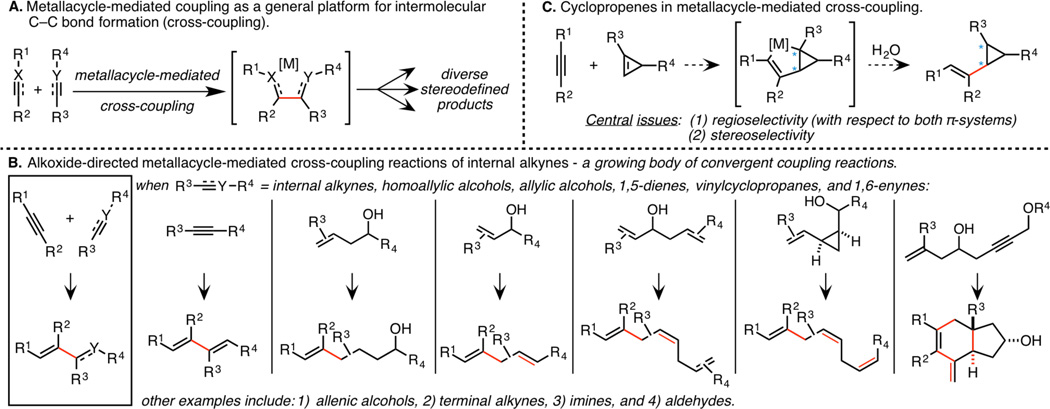
Convergent coupling by way of site and stereoselective C–C bond formation is arguably among the most powerful strategies for enhancing efficiency in complex molecule synthesis, particularly in the context of natural product synthesis.1 While this significance is widely appreciated, few classes of reactivity have proven to be of broad utility for such bond construction (i.e., nucleophilic addition to polarized π-bonds, cycloaddition, and metal-catalyzed cross-coupling).2 With the goal of establishing a class of reactions capable of realizing complementary bond-forming processes to these well established strategies, we have focused on controlling the course of metallacycle-mediated cross-coupling (Scheme 1A).2 To date, alkoxide-directed strategies for the union of internal alkynes with a range of other π-systems have been quite successful, with examples including a variety of alkenes, enynes, terminal and internal alkynes, allenes, and carbonyl systems (Scheme 1B).3 Our recent studies have been focused on expanding substrate scope within this class of reactivity to further develop its broad utility in stereoselective synthesis. Here, we target the development of coupling chemistry capable of delivering highly substituted and stereodefined vinylcyclopropanes – structural motifs that are of great value in stereoselective synthesis (Scheme 1C).4 Our efforts have resulted in the realization of stereodivergent strategies for alkyne–cyclopropene coupling that deliver vinylcyclopropanes containing a 1,2,3-trisubstituted cyclopropane motif.
Scheme 1.
Introduction to metallacycle-mediated cross-coupling and its potential utility for the convergent synthesis of vinylcyclopropanes.
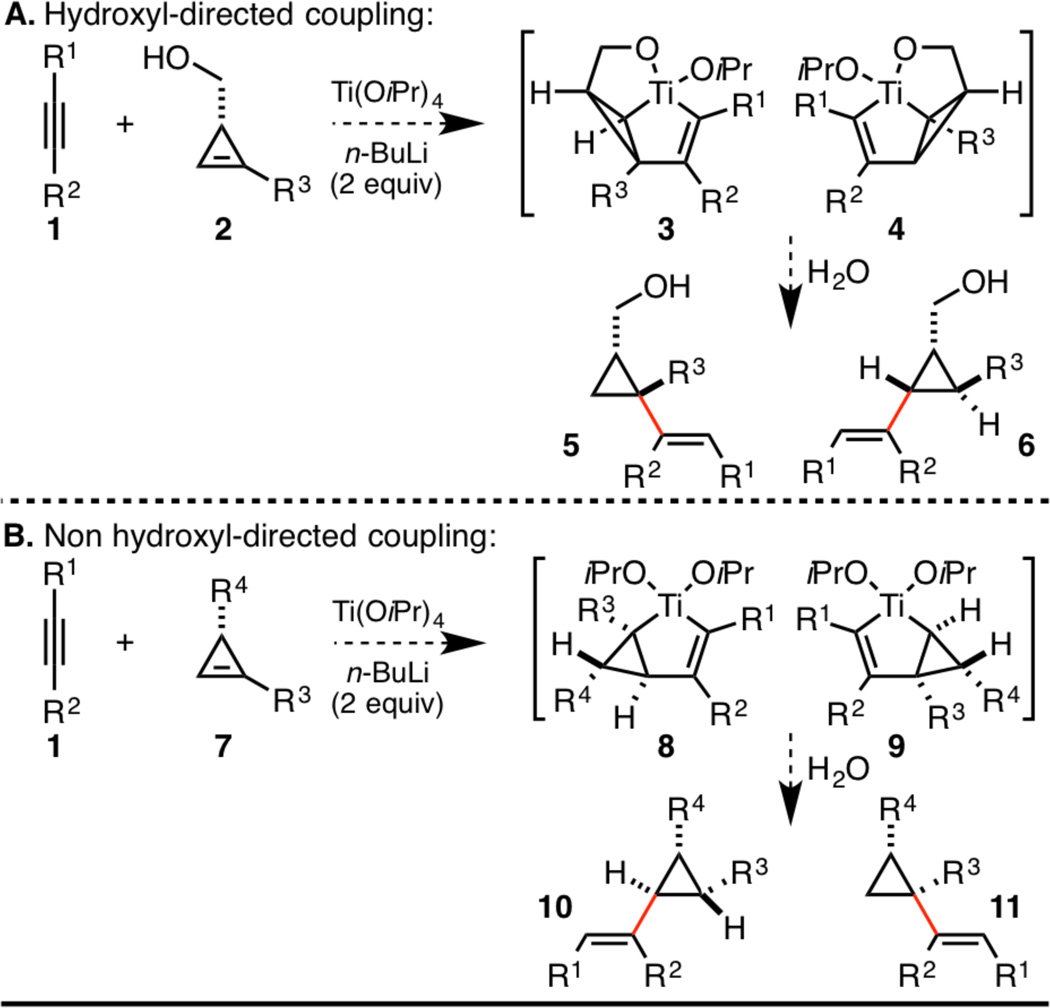
Investigations began by contemplating the course of alkoxide-directed coupling between internal alkynes 1 and hydroxymethyl-substituted cyclopropenes 2 (Scheme 2A).5 While confident that the stereoselectivity of this process would be quite high, with C–C bond formation occurring syn-to the hydroxymethyl substituent, the regioselectivity with which such a process would functionalize the cyclopropene was less clear. Alternatively, given the high reactivity of the cyclopropene double bond, we expected that it may be possible to conduct the coupling reaction in the absence of a directing group.6 As illustrated in Scheme 2B, it was expected that the coupling reaction would proceed under steric control, with C–C bond formation occurring anti- to R4. While stereoselectivity was thought to be readily controlled, regioselectivity would remain a concern for this non hydroxyl-directed reaction.
Scheme 2.
Strategies for cyclopropene–alkyne coupling.
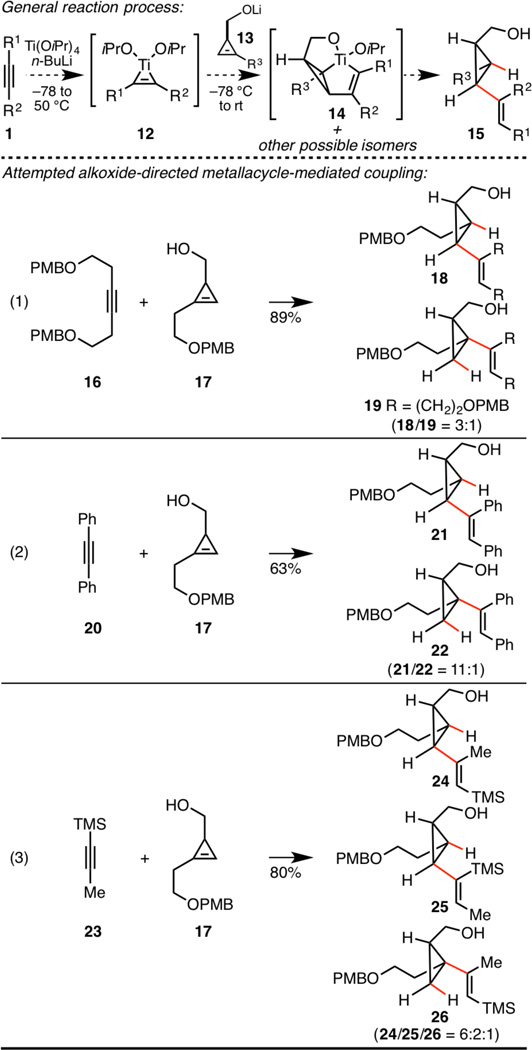
Our studies began by exploring the feasibility of hydroxyl-directed metallacycle-mediated coupling between hydroxymethyl-substituted cyclopropenes and internal alkynes. As depicted at the top of Scheme 3, the general reaction process was designed to proceed by initial formation of the Ti–alkyne complex 12 and subsequent addition of the Li-alkoxide of cyclopropene 13. Rapid and reversible ligand exchange would then ensue to provide a transient mixed titanate ester capable of undergoing intramolecular carbometalation en route to 14. Finally, protonation of the organometallic intermediate would generate the substituted vinylcyclopropane 15 – a process that was anticipated to proceed with retention of configuration.
Scheme 3.
Alkoxide-directed cyclopropene–alkyne coupling.
Treatment of symmetrical alkyne 16 with the combination of Ti(OiPr)4 and n-BuLi,7 followed by addition of the lithium alkoxide of 17 and protonation, led to vinylcyclopropane products in 89% yield and with very high levels of stereoselection – all coupled products had the vinyl and hydroxymethyl substitutents syn- about the cyclopropane core (eq 1). Interestingly, this coupling reaction proceeded with 3:1 regioselectivity in favor of the 1,2,3-trisubstituted cyclopropane 18 – a product that is typically the minor isomer in related carbometalation chemistry of cyclopropenes.8 As illustrated in eq 2, use of diphenylacetylene led to vinylcyclopropenes with similar stereoselection, in this case with much higher levels of regioselectivity (11:1) – again in favor of the 1,2,3-trisubstituted cyclopropane 21.
While excited to observe regio- and stereocontrol in this new alkoxide-directed metallacycle-mediated cross-coupling, the use of an unsymmetrical alkyne (23) in a coupling reaction with cyclopropene 17 led to dampened levels of enthusiasm (eq 3). Here, while stereoselection remained high, the vinylcyclopropane products were produced with low levels of regioselectivity (24/25/26 = 6:2:1). Regioselectivity with respect to cyclopropene functionalization was reasonable (24 + 25/26 = 8:1) but regioselectivity with respect to alkyne functionalization was only 3.5:1 (24 + 26/25).9
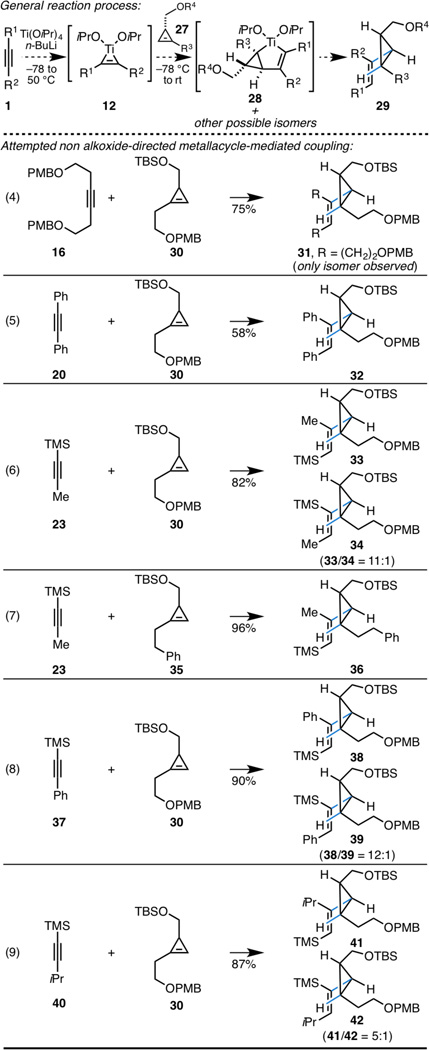
Moving on, we wondered whether the high reactivity of the cyclopropene π-system would be sufficient to enable non-directed alkyne–“alkene” coupling – it is well appreciated that in the absence of a directing group substituted alkenes are sluggish to react in metallacycle-mediated coupling chemistry.6 As depicted in Scheme 4 (eq 4), non alkoxide-directed coupling of alkyne 16 with cyclopropene 30 proceeds in 75% yield, and delivers the 1,2,3-trisubsituted cyclopropene 31 as the only observed regio- and stereoisomer. Similar success was seen in the coupling of diphenylacetylene 20 with 30, delivering 32 in 58% yield (eq 5).
Scheme 4.
Non alkoxide-directed cyclopropene–alkyne coupling.
Distinct from earlier attempts to employ unsymmetrical alkynes in coupling reactions with cyclopropenes (eq 3, Scheme 3), union of TMS-propyne 23 with 30 proceeded in 82% yield, and delivered an 11:1 mixture of isomeric vinylcyclopropanes (eq 6). Here, regioselectivity with respect to the cyclopropene remained very high (no regioisomer was observed), with the minor product being derived from C–C bond formation occurring at the TMS-containing carbon of the alkyne 34. To explore the potential role of the PMB ether of 30 in the regiochemical course of cyclopropene functionalization,10 we explored a substrate lacking this motif. As illustrated in eq 7 of Scheme 4, reaction of TMS-propyne 23 with cyclopropene 35 delivered the vinylcyclopropane product 36 in 96% yield - no evidence was found for the production of regio- or stereoisomeric products in this coupling reaction.
Moving on to explore substrate scope, TMS-phenylacetylene 37 and the α-branched alkyne 40 could be coupled to 30 in high yield (eqs 8 and 9). Here, regioselectivity with respect to alkyne functionalization varied from 12:1 in the case of 37 to 5:1 with the α-branched coupling partner 40.
The observation that this coupling reaction proceeds in the absence of an alkoxide directing group led us to further consider the unique reactivity of cyclopropenes in metallacycle-mediated cross-coupling. While unstrained 1,2-disubstituted alkenes have not proven to be exceptionally useful for the formation of titanacyclopropanes, we thought that the strained π-unsaturation resident in the cyclopropene could offer distinct character of potential utility in metallacycle-mediated cross-coupling.11
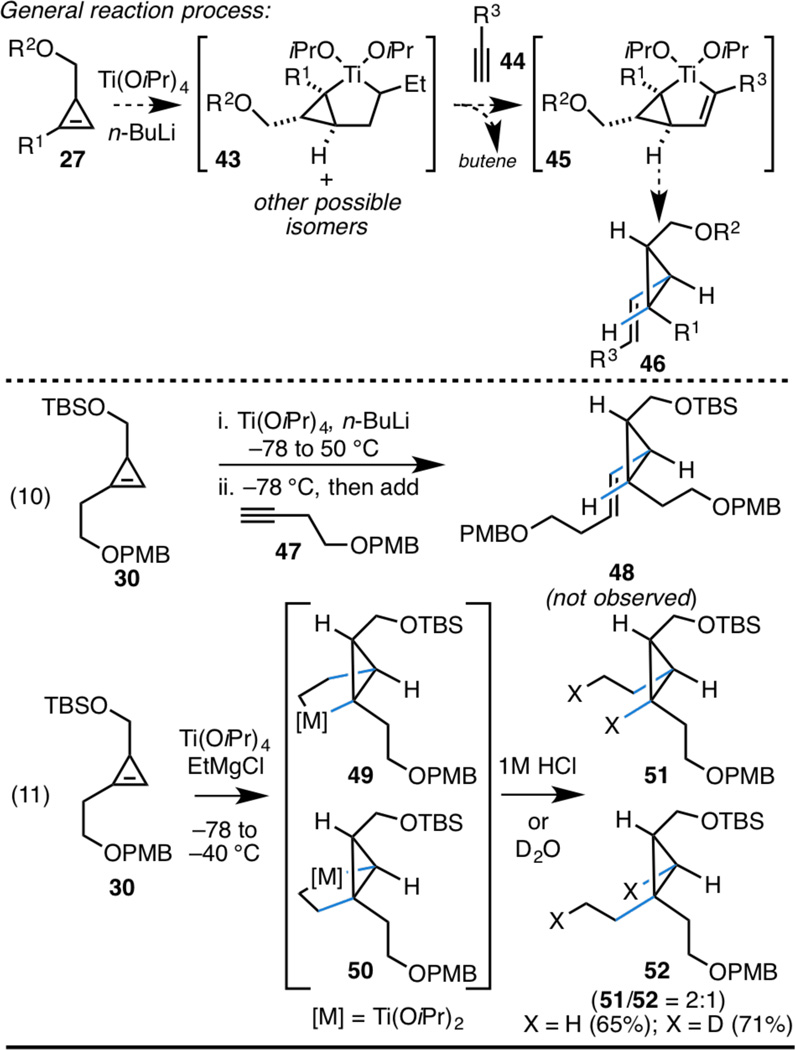
As illustrated in the top portion of Scheme 5, we expected that treatment of a cyclopropene with Ti(OiPr)4 and n-BuLi could result in the formation of an unusually stable saturated metallacyclopentane 43 – this stability being thought to derive from the expected difficulty of either regenerating the cyclopropene (accompanied by formation of a Ti-butene complex) or accessing the strained Ti-cyclopropene complex (with loss of butene).12 We suspected that heating 43 in the presence of a terminal alkyne may result in ligand exchange, with loss of butene and formation of a fused bicyclic titanacyclopentene 45.13 Protonation would then deliver the vinylcyclopropane product possessing a disubstituted alkene 46. Unfortuantely, attempted union of cyclopropene 30 with alkyne 47 was not successful (eq 10, Scheme 5). Efforts to explore this lack of desired reactivity led to the experiment depicted in equation 11 (Scheme 5). Here, treatment of cyclopropene 30 with Ti(OiPr)4 and EtMgCl was directly followed by quenching with a proton source. As illustrated, quenching with 1N HCl led to a 2:1 mixture of the ethylated cyclopropanes 51 and 52 in 65% yield. To confirm that this alkylative process was proceeding through the intermediacy of fused bicyclic metallacy-clopentane intermediates 49 and 50, the reaction was also quenched with D2O. In this latter process, the dideuterated products (51 and 52; X = D) were generated.
Scheme 5.
Attempted cyclopropene–terminal alkyne coupling.
While we were not successful in realizing a coupling reaction of a cyclopropene with a terminal alkyne, we speculate that such a process may be successful by initial formation of a Cp2Zr-alkyne complex by Buchwald’s method,14 followed by exposure to the cyclopropene coupling partner.
Overall, we report the use of unsymmetrically substituted cyclopropenes in metallacycle-mediated cross-coupling and define concise and convergent paths to the synthesis of stereodefined vinylcyclopropanes. We demonstrate that both alkoxide-directed and non-directed metallacycle-mediated cross-coupling reactions of cyclopropenes with alkynes proceed with exquisite levels of diastereoselection, and generally favor the formation of 1,2,3-trisubsituted cyclopropane products – an isomer not typically observed in carbometalation reactions of cyclopropenes. In addition, we have observed unusual stability of titanacyclopentanes formed by exposure of cyclopropenes to Ti(OiPr)4/2RMgCl.
Supplementary Material
Acknowledgments
We thank the NIH NIGMS (GM080266) for financial support.
Footnotes
ASSOCIATED CONTENT
Supporting Information
Procedures and spectroscopic data. This material is available free of charge via the Internet at http://pubs.acs.org.
Author Contributions
The manuscript was written through contributions of both authors. / All authors have given approval to the final version of the manuscript.
REFERENCES
- 1.Corey EJ, Cheng X-M. The Logic of Chemical Synthesis. New York: Wiley; 1989. [Google Scholar]
- 2.(a) Reichard HA, McLaughlin M, Chen MZ, Micalizio GC. Eur. J. Org. Chem. 2010:391–409. doi: 10.1002/ejoc.200901094. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Reichard HA, Micalizio GC. Chem. Sci. 2011;2:573–589. doi: 10.1039/C0SC00394H. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Micalizio GC, Hale SB. Acc. Chem. Res. 2015;48:663–673. doi: 10.1021/ar500408e. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Micalizio GC. Early Transition Metal-Mediated Reductive Coupling Reactions. In: Molander GA, Knochel P, editors. Comprehensive Organic Synthesis 2nd Edition. Vol. 5. Oxford: Elsevier; 2014. pp. 1660–1737. [Google Scholar]
- 3.(a) Ryan J, Micalizio GC. J. Am. Chem. Soc. 2006;128:2764–2765. doi: 10.1021/ja057352w. [DOI] [PubMed] [Google Scholar]; (b) Reichard HA, Micalizio GC. Angew. Chem. Int. Ed. 2007;46:1440–1443. doi: 10.1002/anie.200603515. [DOI] [PubMed] [Google Scholar]; (c) Kolundzic F, Micalizio GC. J. Am. Chem. Soc. 2007;129:15112–15113. doi: 10.1021/ja075678u. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) McLaughlin M, Takahashi M, Micalizio GC. Angew. Chem. Int. Ed. 2007;46:3912–3914. doi: 10.1002/anie.200605060. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Shimp HL, Micalizio GC. Chem. Commun. 2007:4531–4533. doi: 10.1039/b708256h. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Shimp HL, Hare A, McLaughlin M, Micalizio GC. Tetrahedron. 2008;64:6831–6837. doi: 10.1016/j.tet.2008.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Perez LJ, Shimp HL, Micalizio GC. J. Org. Chem. 2009;74:7211–7219. doi: 10.1021/jo901451c. [DOI] [PubMed] [Google Scholar]; (h) Macklin TK, Micalizio GC. Nat. Chem. 2010;2:638–643. doi: 10.1038/nchem.665. [DOI] [PMC free article] [PubMed] [Google Scholar]; (i) Diez PS, Micalizio GC. J. Am. Chem. Soc. 2010;132:9576–9578. doi: 10.1021/ja103836h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.(a) Rubin M, Rubina M, Gevorgyan V. Chem. Rev. 2007;107:3117–3179. doi: 10.1021/cr050988l. [DOI] [PubMed] [Google Scholar]; (b) Jiao L, Yu Z-X. J. Org. Chem. 2013;78:6842–6848. doi: 10.1021/jo400609w. [DOI] [PubMed] [Google Scholar]; (c) Hudlicky T, Reed JW. Angew. Chem. Int. Ed. 2010;49:4864–4876. doi: 10.1002/anie.200906001. [DOI] [PubMed] [Google Scholar]; (d) Baldwin JE. Chem. Rev. 2013;103:1197–1212. doi: 10.1021/cr010020z. [DOI] [PubMed] [Google Scholar]; (e) Ylijoki KEO, Stryker JM. Chem. Rev. 2013;113:2244–2266. doi: 10.1021/cr300087g. [DOI] [PubMed] [Google Scholar]; (f) Wender PA, Takahashi H, Witulski B. J. Am. Chem. Soc. 1995;117:4720–4721. [Google Scholar]; (g) Trost BM, Toste FD, Shen H. J. Am. Chem. Soc. 2000;122:2379–2380. [Google Scholar]
- 5. Petiniot N, Anciaux AJ, Noels AF, Hubert AJ, Teyssié Ph. Tetrahedron Lett. 1978;19:1239–1242. For a recent catalytic enantioselective variant, see: Lou Y, Remarchuk TP, Corey EJ. J. Am. Chem. Soc. 2005;127:14223–14230. doi: 10.1021/ja052254w.
- 6.Carbometalation of electronically unactivated and substituted double bonds is a substantial challenge due to issues related to achieving the desired reactivity and controlling regio- and stereoselectivity. For a discussion and recent examples, see refs 2b and 2d.
- 7. Obora Y, Moriya H, Tokunaga M, Tsuji Y. Chem. Commun. 2003:2820–2821. doi: 10.1039/b310565b. Rassadin VA, Six Y. Tetrahedron. 2014;70:787–794. For use of n-BuLi en route to Ti–imine complexes, see: Tarselli MA, Micalizio GC. Org. Lett. 2009;11:4596–4599. doi: 10.1021/ol901870n.
- 8. Araki S, Nakano H, Subburaj K, Hirashita T, Shibutani K, Yamamura H, Kawai M, Butsugan Y. Tetrahedron Lett. 1998;39:6327–6330. Araki S, Shiraki F, Tanaka T, Nakano H, Subburaj K, Hirashita T, Yamamura H, Kawai M. Chem. Eur. J. 2001;7:2784–2790. doi: 10.1002/1521-3765(20010702)7:13<2784::aid-chem2784>3.0.co;2-a. Liao L, Fox JM. J. Am. Chem. Soc. 2002;124:14322–14323. doi: 10.1021/ja0278234. Yan N, Liu X, Fox JM. J. Org. Chem. 2008;73:563–568. doi: 10.1021/jo702176x. Didier D, Delaye P-O, Simaan M, Island B, Eppe G, Eijsberg H, Kleiner A, Knochel P, Marek I. Chem. Eur. J. 2014;20:1038–1048. doi: 10.1002/chem.201303569. Simaan M, Delaye P-O, Shi M, Marek I. Angew. Chem. Int. Ed. 2015;54:12345–12348. doi: 10.1002/anie.201412440. For a recent review, see: Marek I, Simaan S, Masarwa A. Angew. Chem. Int. Ed. 2007;46:7364–7376. doi: 10.1002/anie.200604774.
- 9.Dampened levels of regioselectivity have also been observed in coupling reactions between TMS-alkynes and allenes. See ref 3f.
- 10.Tanaka R, Yuza A, Watai W, Suzuki D, Takayama Y, Sato F, Urabe H. J. Am. Chem. Soc. 2005;127:7774–7780. doi: 10.1021/ja050261e. [DOI] [PubMed] [Google Scholar]
- 11.Ti(OiPr)4/2-RMet-mediated coupling reactions of terminal alkynes typically introduce the alkyne after initial formation of a Ti-complex of a different π-system (i.e., internal alkyne or imine).
- 12.Mashima K, Takaya H. Organometallics. 1985;4:1464–1466. [Google Scholar]
- 13.(a) McDermott JX, Whitesides GM. J. Am. Chem. Soc. 1974;96:947–948. [Google Scholar]; (b) McDermott JX, Wilson ME, Whitesides GM. J. Am. Chem. Soc. 1976;98:6529–6536. [Google Scholar]; (c) Grubbs RH, Miyashita A. J. Chem. Soc. Chem. Comm. 1977:864–865. [Google Scholar]; (d) Grubbs RH, Miyashita A. J. Am. Chem. Soc. 1978;100:1300–1302. [Google Scholar]
- 14.Buchwald SL, Watson BT. J. Am. Chem. Soc. 1987;109:2544–2546. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.