Abstract
After spinal cord injury (SCI), disruption of blood–spinal cord barrier (BSCB) elicits blood cell infiltration such as neutrophils and macrophages, contributing to permanent neurological disability. Previous studies show that epidermal growth factor (EGF) produces potent neuroprotective effects in SCI models. However, little is known that whether EGF contributes to the integrity of BSCB. The present study is performed to explore the mechanism of BSCB permeability changes which are induced by EGF treatment after SCI in rats. In this study, we demonstrate that EGF administration inhibits the disruption of BSCB permeability and improves the locomotor activity in SCI model rats. Inhibition of the PI3K/Akt pathways by a specific inhibitor, LY294002, suppresses EGF‐induced Rac1 activation as well as tight junction (TJ) and adherens junction (AJ) expression. Furthermore, the protective effect of EGF on BSCB is related to the activation of Rac1 both in vivo and in vitro. Blockade of Rac1 activation with Rac1 siRNA downregulates EGF‐induced TJ and AJ proteins expression in endothelial cells. Taken together, our results indicate that EGF treatment preserves BSCB integrity and improves functional recovery after SCI via PI3K‐Akt‐Rac1 signalling pathway.
Keywords: blood–spinal cord barrier, epidermal growth factor, Rac1, PI3K/Akt, spinal cord injury
Introduction
Spinal cord injury (SCI), a serious health problem that usually initiates permanent disability, leads to direct vascular damage and a cascade of events that adjust the permeability of the blood–spinal cord barrier (BSCB) 1, 2. The BSCB is the functional equality of the blood‐brain barrier (BBB), offering a specialized microenvironment for the cellular constituents of the spinal cord. The barrier function of BSCB is based on the specialized system of non‐fenestrated endothelial cells and their accessory structures, including the basement membrane, pericytes and astrocytic end feet processes, which play a protective and regulatory role for the spinal cord parenchyma 3. Previous studies show that the disruption of BSCB participates in the pathophysiological processes of SCI, for example, spinal cord oedema and secondary nerve injury. The disruption of BSCB causes inflammation and blood infiltration and then engenders neurotoxic products that compromise neuronal and synaptic functions and causes the ‘programmed death’ of glia and neurons, in result, it leads to permanent neurological deficits 4, 5. Recently, it reports that the disruption of BSCB is correlated with increased mortality after intravascular therapy, and improvements in the BSCB function can significantly diminish secondary nerve injury 6. In general, it suggests that early recovery of BSCB plays a vital role in the treatment of SCI.
Rac1, a member of small GTPases, is well‐characterized in the Rho family 7. Intracellularly, the GTP‐bound form of Rac1 performs a mutual effect on downstream effectors that operate multiple cellular processes, including membrane trafficking 8, gene transcription 9, the formation and maintenance of cell–cell junctions and the establishment of epithelial barriers 10, 11. For example, the small GTPases Rac1 regulates adherens junction (AJ) function in epithelial cells and participates in the formation of the epithelial permeability barrier in human airway epithelial cells 12, 13, 14. Daniels et al. also reports that the small GTPases Rac1 has effect on the regulation of endothelial permeability and tight junction (TJ) formation by using an in vitro BBB model 15. In addition, it is well‐established that the PI3K/Akt pathway is required for the stability of barrier function. A recent study shows that miR‐21 regulates intestinal epithelial TJ (Occludin, Claudin‐1) permeability through PTEN/PI3K/Akt signalling pathway 16. In the retina, activation of the PI3K/AKT pathway is involved in the expression of ZO1 and Occludin levels, which are synthesized by Pigment Epithelium‐Derived Factor Peptide 17. Gunduz et al. also reports that insulin stabilizes endothelial barrier function via Rac1 activation induced by PI3K/Akt 18. According to studies above, we find that PI3K/Akt and Rac1 are involved in regulating barrier permeability, however, the role of PI3K/Akt and Rac1 on BSCB after SCI is unclear.
As a widely expressed protein, epidermal growth factor (EGF) has the ability to coordinate different aspects of cell proliferation, growth, differentiation and morpho‐functional maintenance via a more complex signal transduction system. Epidermal growth factor is a neurotrophic factor that promotes survival and protraction of midbrain dopaminergic neurons 19, 20, 21. After SCI in rats, EGF can improve functional recovery by promoting the division, differentiation and migration of a large number of ependymal cells, including endogenous neural precursor cells and atrocities 22. Although EGF shows protective effects on SCI 23, 24, its influence on the BSCB and underlying signalling pathway after SCI remains unclear.
In this study, we demonstrate that EGF administration attenuates secondary SCI, specifically by preserving endothelial TJ and AJ; therefore it attenuates neurofunctional deficits in the rat subjected to SCI. Furthermore, EGF improves the permeability of BSCB by enhancing TJ and AJ proteins expression through activation of the PI3K/Akt/Rac1 pathway.
Materials and methods
Spinal cord injury
The adult female Sprague–Dawley rats (220–250 g) were obtained from the Animal Center of the Chinese Academy of Sciences. All animal experiments were conformed to the Guide for the Care and Use of Laboratory Animals from the National Institutes of Health and were approved by the Animal Care and Use Committee of Wenzhou University. All animals were housed in standard temperature conditions with 12 hrs light/dark cycle and fed with food and water. Rats were anaesthetized with 10% chloralic hydras (3.5 ml/kg, i.p.) and a laminectomy was performed at the T9 level, exposing the cord beneath without disrupting the dura. The exposed spinal cord was subjected to moderate contusion injury (150 kdyn force with no dwell time) using an Infinite Horizon Impact Device. The sham‐operated group rats underwent a T9 laminectomy without contusion injury. Postoperative care included manual urinary bladder emptying per 12 hrs until the return of bladder function and the administration of cefazolin sodium (50 mg/kg, i.p.).
Drug treatment
Epidermal growth factor (Sigma‐Aldrich, St. Louis, MO, USA) dissolved in 0.9% NaCl (60 μg/kg) was injected subcutaneously near the back wound after SCI and treated once a day for 1 week for behavioural test or for indicated time‐points for other experiments. PI3K inhibitor LY294002 (Sigma‐Aldrich) were dissolved in 25% dimethylsulphoxide solution. A total volume of 5 μl (50 nmol/kg) solution was injected into the spinal cord via intrathecal injection in 5 min. For the sham‐operated group rats, they received no pharmacological treatment.
Cell culture
Human brain microvascular endothelial cells (HBMECs) were purchased from ScienCell Research Laboratories (ScienCell Research Laboratories, San Diego, CA, USA). Cells were cultured in endothelial cell medium (ScienCell Research Laboratories) and incubated in a humidified atmosphere contain 5% CO2 at 37°C. Cells were pretreated for 2 hrs with EGF (100 ng/ml), EGF compound with LY294002 (20 μM). All experiments were performed in triplicate.
Behavioural tests
Examination of functional deficits after SCI was conducted as previously described 25. The Basso, Beattie, and Bresnahan (BBB) scores were assessed in an open field scale by two blinded independent examiners at 14 days post‐operation. Briefly, the BBB locomotion rating scale scores range from 0 points (complete paralysis) to 21 points (normal locomotion). The scale was based on the natural progression of locomotion recovery in rats with thoracic SCI.
Western blot analysis
For protein analysis in vivo, the protein extraction was homogenized in a modified RIPA buffer (50 mM Tris–HCl, 1% NP‐40, 20 mM DTT, 150 mM NaCl, pH = 7.4) containing protease inhibitor cocktail (10 μl/ml; GE Healthcare Biosciences, PA, Little Chalfont, UK). The complex was then centrifuged at 11,792 g, and the supernatant was obtained for protein assay. For protein analysis in vitro, HBMECs were lysed in RIPA buffer [25 mM Tris–HCl (pH 7.6), 150 mM NaCl, 1% Nonidet P‐40, 1% sodium deoxycholate and 0.1% SDS] with protease and phosphatase inhibitors. The extracts above were quantified with bicinchoninic acid reagents (Thermo, Rockford, IL, USA). The equivalent of 50 μg protein was separated using 12% gel and then transferred onto a PVDF membrane (Bio‐Rad, Hercules, CA, USA). The membrane was blocked with 5% non‐fat milk in TBS with 0.05% Tween 20 for 1 hr, then incubated with following antibody solutions: p120‐Catenin, beta‐Catenin, Occludin, Claudin‐5, p‐Akt, Akt, Rac1, GAPDH. The membranes were washed with TBS three times and incubated with secondary antibodies for 2 hrs at room temperature. Signals were visualized using the ChemiDicTM XRS + Imaging System (Bio‐Rad), and band densities were quantified with Image J software. Results were expressed as a relative density ratio, normalized to the value of the Sham or Control group. Anti‐ p120‐Catenin, β‐Catenin, Rac1 were purchased from Abcam (Cambridge, UK), other antibodies were from Santa (Santa Cruz Biotechnology, Santa Cruz, CA, USA).
Haematoxylin and eosin staining
The rats were anesthetized with 10% chloralic hydras (3.5 ml/kg, i.p.), then perfused with 0.9% NaCl, followed by 4% paraformaldehyde in 0.01 M PBS (pH = 7.4) at 7 days after surgery. The spinal cords from the T7–T10 level around the lesion epicentre were excised, transverse paraffin sections (5 mm thick) were mounted on poly‐l‐lysine‐coated slides for histopathological examination by haematoxylin and eosin staining.
Immunofluorescence staining
The sections were incubated with 5% bovine serum albumin (BSA) for 1 hr at room temperature and then incubated overnight at 4°C with primary antibodies in blocking buffer (Claudin‐5, Santa Cruz Biotechnology; Occludin, Santa Cruz Biotechnology; CD31, Santa Cruz Biotechnology). Then the cords were separately incubated with secondary antibody (Alexa Fluor 488‐conjugated anti‐IgG, Abcam; Texas red‐conjugated anti‐IgG, Santa Cruz Biotechnology). The nuclei were stained with Hoechst 33258 (0.25 mg/ml) dye (Beyotime Institute of Biotechnology, Shanghai, China). For cells, grown on 14 × 14 mm microscopic glass were washed with ice‐cold PBS, fixed with 4% paraformaldehyde for 30 min., then washed with ice‐cold PBS, and blocked in 5% BSA for 1 hr. Then cells were incubated with anti‐p120‐Catennin (Abcam), anti‐beta‐Catenin (Abcam), anti‐Occludin (Santa Cruz Biotechnology), anti‐Claudin‐5 (Santa Cruz Biotechnology) diluted in 1% BSA at 4°C overnight. Cells were washed with PBS followed by incubation with Alexa Fluor 488‐conjugated anti‐IgG or Texas red‐conjugated anti‐IgG secondary antibodies for 1 hr at room temperature. After washing with PBS, the nuclei were stained with Hoechst 33258 (0.25 mg/ml) dye for 7 min., washed with PBS. At last, cells were added with Antifade Mounting Medium (Beyotime Institute of Biotechnology).
Pull‐down assays
GTP‐bound active form of Rac1 was detected by Active Rac1 Pull‐Down and Detection Kit (Thermo Scientific, Mass, Waltham, USA) according to the manufacturer's protocol. Extracts of HBMECs were incubated at 4°C for 60 min. with GST‐human Pak1‐PBD, Bound proteins were mixed with 1 part β‐mercaptoethanol to 20 parts 2× SDS Sample Buffer and boiled for 5 min. GTP‐bound Rac1 were detected by Western blotting with the anti‐Rac1 antibody.
Small interfering RNA
Rac1 expression was silenced by transfection of small interfering RNA (siRNA). Human brain microvascular endothelial cells were transfected with 100 pmol of Rac1 siRNA (Bioneer, Daejeon, Korea) in serum‐free medium mixed with lipofectamine 2000 (Life Technologies, Carlsbad, CA, USA) according to the manufacturer's instructions. Four hours after transfection, HBMECs were incubated in a medium containing 5% foetal bovine serum for 24 hrs.
Evaluation of BSCB permeability
Evans blue dye assays
At 1 day after SCI, rats were injected with 2% Evans blue dye (EB; Sigma‐Aldrich, 2 ml/kg) solution in saline intravenously into the tail vein. Two hours after injection, rats were anesthetized with 10% chloralic hydras (3.5 ml/kg, i.p), then perfused with 0.9% normal saline. The injured spinal cord tissues of EB were weighed and immersed in N,N′‐dimethylformamide (JinSan, Wenzhou, China) at 50°C for 72 hrs. The optical density of the supernatant was examined with enzyme‐labelled meter (at an excitation wavelength of 620 nm and an emission wavelength of 680 nm). Dye in samples was determined as μg/g of tissue from a standard curve plotted using known amounts of dye 26. Rats were fixed by perfusion with 4% paraformaldehyde at 2 hrs after EB injection. The spinal cord tissues were cut into 20 μm thickness at −20°C using frozensection machine, then the sections were observed.
FITC‐dextran assays
At 1 day after SCI, rats were injected with 2% FITC‐dextran (MW 70 kD, 4 mg/kg; Sigma‐Aldrich) solution in PBS intravenously into the tail vein. Two hours after injection, rats were anesthetized with 10% chloralic hydras (3.5 ml/kg, i.p), then perfused with 0.9% normal saline. The injured spinal cord tissues of FITC‐dextran were weighed and homogenized in PBS, and centrifuged. The optical density of the supernatant was examined (at an excitation wavelength of 493 nm and an emission wavelength of 517 nm).
Paracellular permeability assay
Human brain microvascular endothelial cells were seeded on Transwell permeable supports (PET membrane 24‐well cell culture inserts with 0.4 μm pore size; Corelle; Corning Life Sciences, Corning, New York, USA) at a density of 1 × 105 cells/well in 200 μl medium overnight, and subjected to oxygen‐glucose deprivation (OGD) for 10 hrs, then cells were incubated with FITC‐dextran (1 mg/ml) in medium for another 2 hrs OGD. Thereafter, FITC‐dextran passed through the Transwell (in the lower chambers) was determined by using enzyme‐labelled meter at an excitation wavelength of 493 nm and an emission wavelength of 517 nm.
Statistical analysis
Data are expressed as the mean ± S.E.M. Statistical significance was determined with Student's t‐test when there were two experimental groups. For more than two groups, statistical evaluation of the data was performed with the one‐way anova test, Tukey's multiple comparison is used as a post hoc analysis.
Results
EGF improves the functional recovery and attenuates BSCB disruption after SCI
After SCI, rats were immediately treated with EGF and further treated once a day for 1 week. Functional recovery was then estimated using BBB scores for locomotion. As a result, EGF treatment significantly increased the locomotor activity 3–14 days after injury, compared with that observed in SCI group (Fig. 1A). Meanwhile, we examined the effect of EGF on BSCB permeability at 1 day after SCI by Evan's Blue assay. As shown in Figure 1B, the amount of Evan's Blue dye extravasation had a marked increase compared with the Sham group after SCI, which implies BSCB disruption. Furthermore, EGF treatment significantly reduced the amount of Evan's Blue dye extravasation at 1 day after SCI when compared with SCI group (Fig. 1C). The fluorescence of Evan's Blue in the injured spinal cord (at 1 day) was higher than Sham group, and EGF significantly reduced the fluorescence intensity (Fig. 1D). Traumatic SCI also triggers immediate mechanical damage. According to the experiment, Sham group showed integrated infrastructures, clear boundary between grey and white matters, morphologically normal neurons with clear profile, polygonal perikaryon and round nucleus. By contrast, in the SCI group, spinal cord has cavities, lacked of clear infrastructures. The number of normal neurons was apparently reduced. In contrast to SCI group, the EGF treatment group showed more normal neurons (Fig. 1E and F). These data indicate that EGF improves the functional recovery and inhibits the BSCB permeability after SCI.
Figure 1.
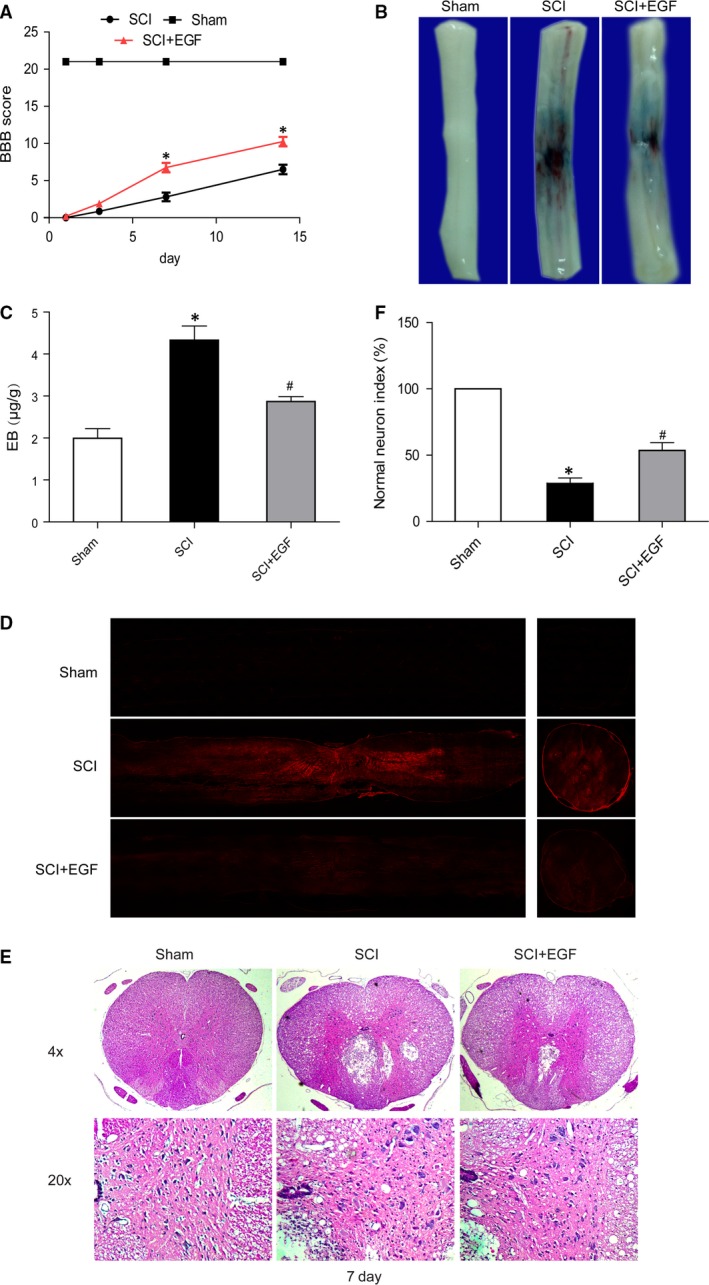
Epidermal growth factor improves the functional recovery and attenuates BSCB disruption after SCI. (A) The Basso, Beattie and Bresnahan (BBB) scores, *represents P < 0.01 versus the SCI group, n = 5. (B) Representative whole spinal cords show that Evan's Blue dye permeabilized into injury spinal cord at 1 day (n = 4/group). (C) Quantification of the amount of Evan's Blue at 1 day (μg/g), *represents P < 0.01 versus the Sham group, #represents P < 0.05 versus the SCI group. (D) Representative confocal images of Evans Blue Dye extravasation at 1 day after SCI. (E) H and E staining at 7 day. (F) A quantitative analysis of normal neurons in haematoxylin and eosin staining, *represents P < 0.01 versus the Sham group, #represents P < 0.01 versus the SCI group.
EGF administration prevents the loss of TJ and AJ after SCI
It is well known that TJ and AJ in the endothelial cells of blood vessels are involved in the integrity of BBB or BSCB 3. We examined the alterations of SCI‐induced TJ and AJ proteins and the effect of EGF on these alterations by western blot. As shown in Figure 2A, the levels of TJ (Occludin, Claudin‐5) and AJ (p120‐Catenin, β‐Catenin) were decreased at 1 day after SCI. Furthermore, EGF significantly attenuated the decrease in TJ and AJ levels at 1 day after injury compared with SCI group (Fig. 2B and C). Double labelling immunofluorescence also showed that the fluorescence intensity of Occludin or Claudin‐5 and CD31 immunoreactivity was decreased after SCI compared to the Sham group, and EGF treatment attenuated the decrease in its intensity (Fig. 2D and E). These data indicate that EGF prevents BSCB disruption by inhibiting degradation of AJ and TJ proteins after SCI.
Figure 2.
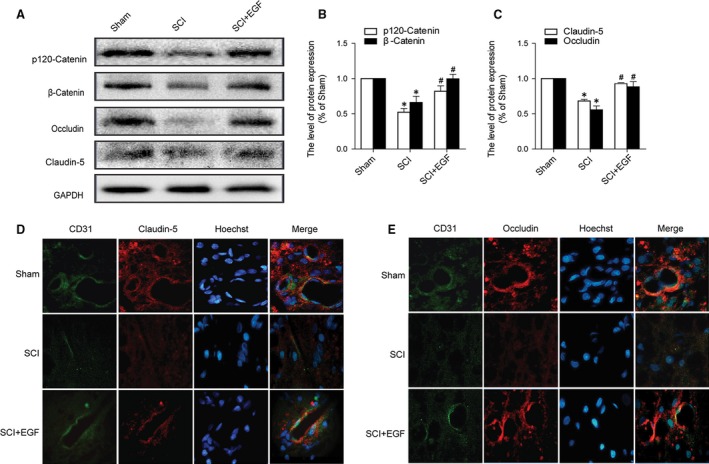
EGF administration prevents the degradation of TJ and AJ proteins after SCI. (A) Protein expressions of p120‐Catenin, β‐Catenin, Occludin and Claudin‐5 in the spinal cord segment at the contusion epicentre. GAPDH was used as the loading control and for band density normalization. (B and C) The optical density analysis of p120‐Catenin, β‐Catenin, Occludin and Claudin‐5 protein, *represents P < 0.05 versus the Sham group, #represents P < 0.05 versus the SCI group, n = 5. (D and E) Double immunofluorescence shows that TJ and AJ proteins colocalize in CD31 (endothelial cell marker)‐positive blood vessels in the Sham, SCI rat and SCI rat treated with EGF groups.
PI3K inhibition reverses the protective effect of EGF on BSCB
To evaluate whether PI3K was involved in the protection by EGF, PI3K inhibitor LY294002 was co‐administered with EGF. LY294002 alone has no toxic and devastating effect on BSCB (Fig. S1). Evans Blue dye extravasation was examined at 24 hrs after SCI. The results of the EGF+LY group were going to compare with those of the Sham, SCI, and SCI+EGF group, respectively. LY294002 co‐administration reversed the protection caused by EGF as shown by the results in the Evans Blue dye (Fig. 3A), Evans Blue extravasation assay (Fig. 3B). EGF+LY group performed significantly more dye extravasation than animals that received EGF treatment. Furthermore, the animals treated with EGF+LY had significantly increased the fluorescence intensity than EGF group in the injured spinal cord (at 1 day). Meanwhile, Western blot analyses were performed with the spinal cord of animals at 24 hrs after SCI and subsequent co‐administration of EGF and LY294002. As shown in Figure 3, EGF treatment significantly increased the protein level of p‐Akt, comparing to both the Sham and the SCI group. The PI3K inhibitor LY294002 (EGF+ LY) reversed this treatment effect (Fig. 3D and G). In addition, GTP‐Rac1/Total‐Rac1‐ratio was increased significantly in the EGF group compared to the SCI group, but decreased in the EGF+LY group (Fig. 3D and H). Meanwhile the ratios of p120‐Catenin, β‐Catenin, Occludin and Claudin‐5 were significantly decreased in the EGF+LY group (Fig. 3D–F). These data imply that PI3K inhibition (LY294002) blocks the protective effect of EGF on BSCB after SCI.
Figure 3.
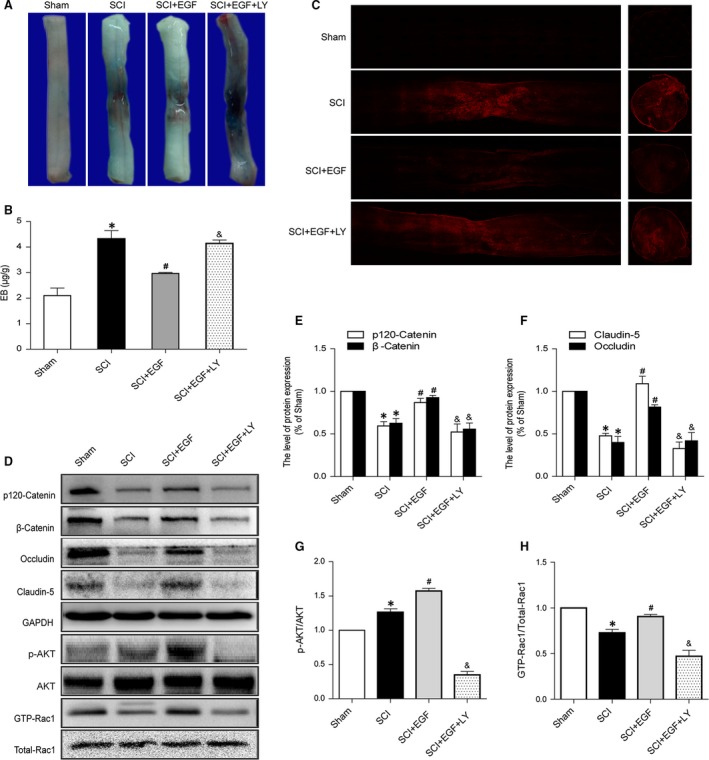
PI3K inhibitor LY294002 attenuates the protective effect of EGF on BSCB after SCI. (A) Representative whole spinal cords show that Evan's Blue dye permeabilized into injury spinal cord at 1 day (n = 4/group). (B) Quantification of the amount of Evan's Blue at 1 day (μg/g). (C) Representative confocal images of Evans Blue Dye extravasation at 1 day of Sham, SCI group, SCI rat treated with EGF group and SCI rat treated with EGF and LY294002 group. (D) Protein expressions of p120‐Catenin, β‐Catenin, Occludin, Claudin‐5, p‐Akt, Akt, GTP‐Rac1 and Total‐Rac1 in the spinal cord segment at the contusion epicentre. (E and F) The optical density analysis of p120‐Catenin, β‐Catenin, Occludin and Claudin‐5 protein. (G and H) The optical density analysis of p‐Akt/Akt and GTP‐Rac1/Total‐Rac1 proteins. *represents P < 0.05 versus the Sham group, #represents P < 0.05 versus the SCI group, & represents P < 0.05 versus the SCI+EGF group, n = 5.
PI3K inhibition attenuates the protective effect of EGF on junction protein in endothelial cells after oxygen‐glucose deprivation
To further confirm the potential mechanism of EGF mediated regulation TJ and AJ in vitro, we applied OGD to HBMECs. Paracellular permeability assay of FITC‐dextran were measured to evaluate whether the inhibition of PI3K would affect the permeability of BSCB. LY294002 alone has no toxic effect in endothelial cells (Fig. S1). As shown in Figure 4A, the permeability values to FITC–dextran were reduced in EGF group compared with the OGD group. However, LY294002 co‐administration increased the permeability. Western blot analyses were performed with the HBMECs at 12 hrs after OGD. As shown in Figure 4, the levels of TJ (Occludin, Claudin‐5) and AJ (p120‐Catenin, β‐Catenin) decreased at 12 hrs after OGD. However, the decrease in TJ (Occludin, Claudin‐5) and AJ (p120‐Catenin, β‐Catenin) level after OGD was notably reversed by EGF treatment. The group treated with EGF significantly increased the protein level of p‐Akt, comparing to both the Control and the OGD group. In addition, GTP‐Rac1/Total‐Rac1 ratio was significantly increased in the EGF group as compared to the OGD group. However, LY294002 administration evidently decreased the protein level of p‐Akt as well as the GTP‐Rac1/Total‐Rac1 ratio, the levels of TJ (Occludin, Claudin‐5) and AJ (p120‐Catenin, β‐Catenin) were also significantly decreased in the EGF+LY group. Cell immunofluorescence also showed that the fluorescence intensity of TJ (Occludin, Claudin‐5) and AJ (p120‐Catenin, β‐Catenin) decreased after OGD as compared to the Control group, and EGF treatment attenuated the decrease in its intensity. However, LY294002 administration significantly decreased the fluorescence intensity (Fig. 4G and H). These data indicate that PI3K inhibition reverses the protective effect of EGF on TJ and AJ in endothelial cell after OGD.
Figure 4.
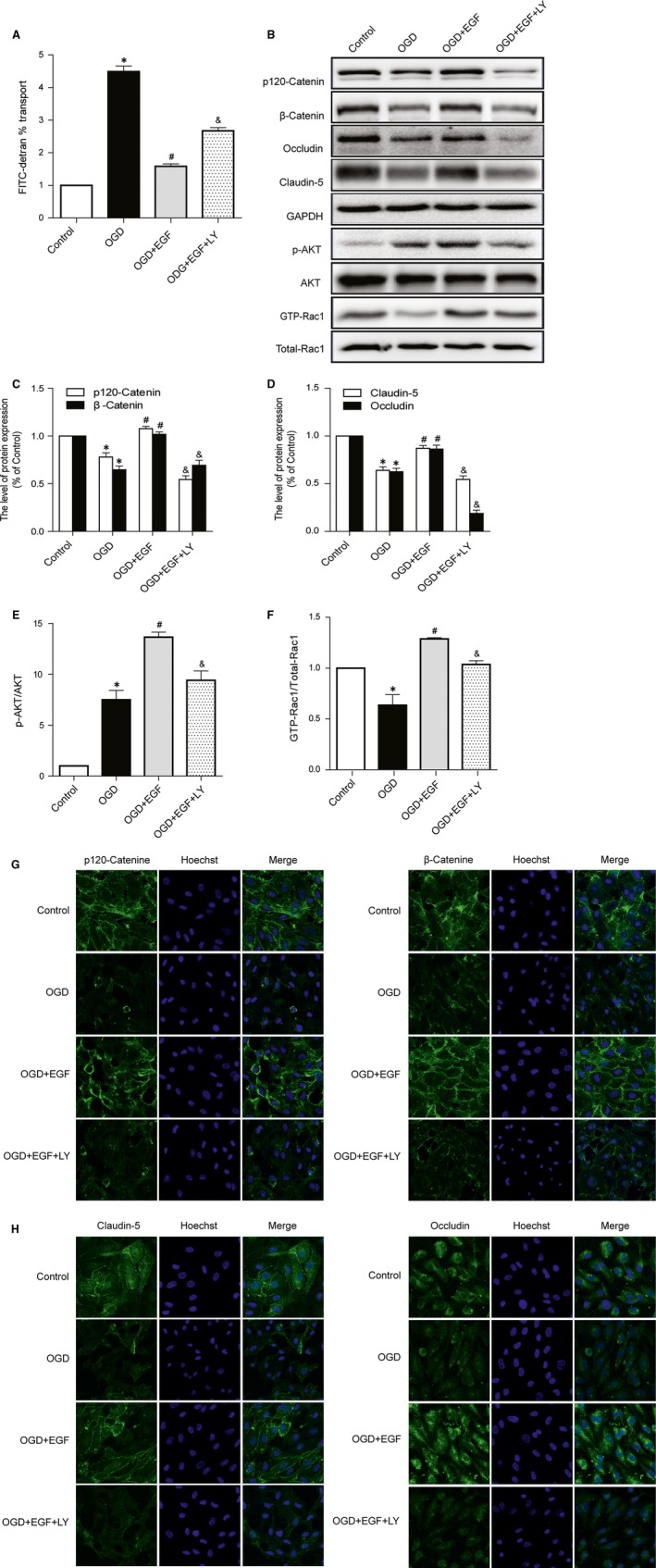
PI3K inhibitor LY294002 attenuates the protective effect that EGF inhibits the loss of TJ and AJ in Human brain microvascular endothelial cells after OGD. (A) Evaluation of paracellular permeability by FITC–dextran. (B) Protein expressions of p120‐Catenin, β‐Catenin, Occludin, Claudin‐5, p‐Akt, Akt, GTP‐Rac1 and Total‐Rac1. (C and D) The optical density analysis of p120‐Catenin, β‐Catenin, Occludin and Claudin‐5 protein. (E and F) The optical density analysis of p‐Akt/Akt and GTP‐Rac1/Total‐Rac1 proteins. (G and H) Immunofluorescence staining of TJ and AJ protein. Nucleus (blue) was labelled with Hoechst. *represents P < 0.05 versus the Control group, #represents P < 0.05 versus the OGD group, & represents P < 0.05 versus the OGD + EGF group.
Rac1 activation is required for EGF‐induced TJ and AJ proteins expression
To evaluate whether Rac1 was involved in the protection by EGF on TJ and AJ, we administrated Rac1 siRNA in endothelial cells. In vitro delivery of siRNA targeting Rac1 inhibited Rac1 expression and activity of Rac1 (Fig. 5A and B). Meantime, the expression of AJ and TJ proteins were much lower in Rac1 silence than Rac1 activation (Fig. 5A, C and D). In addition, the protective effect of EGF on TJ and AJ also significantly inhibited by silencing Rac1. Cell immunofluorescence also showed that the fluorescence intensity of TJ and AJ was decreased after silencing Rac1 (Fig. 5E and F). These data imply that Rac1 activation is necessary for the protective effect of EGF on TJ and AJ in endothelial cells.
Figure 5.
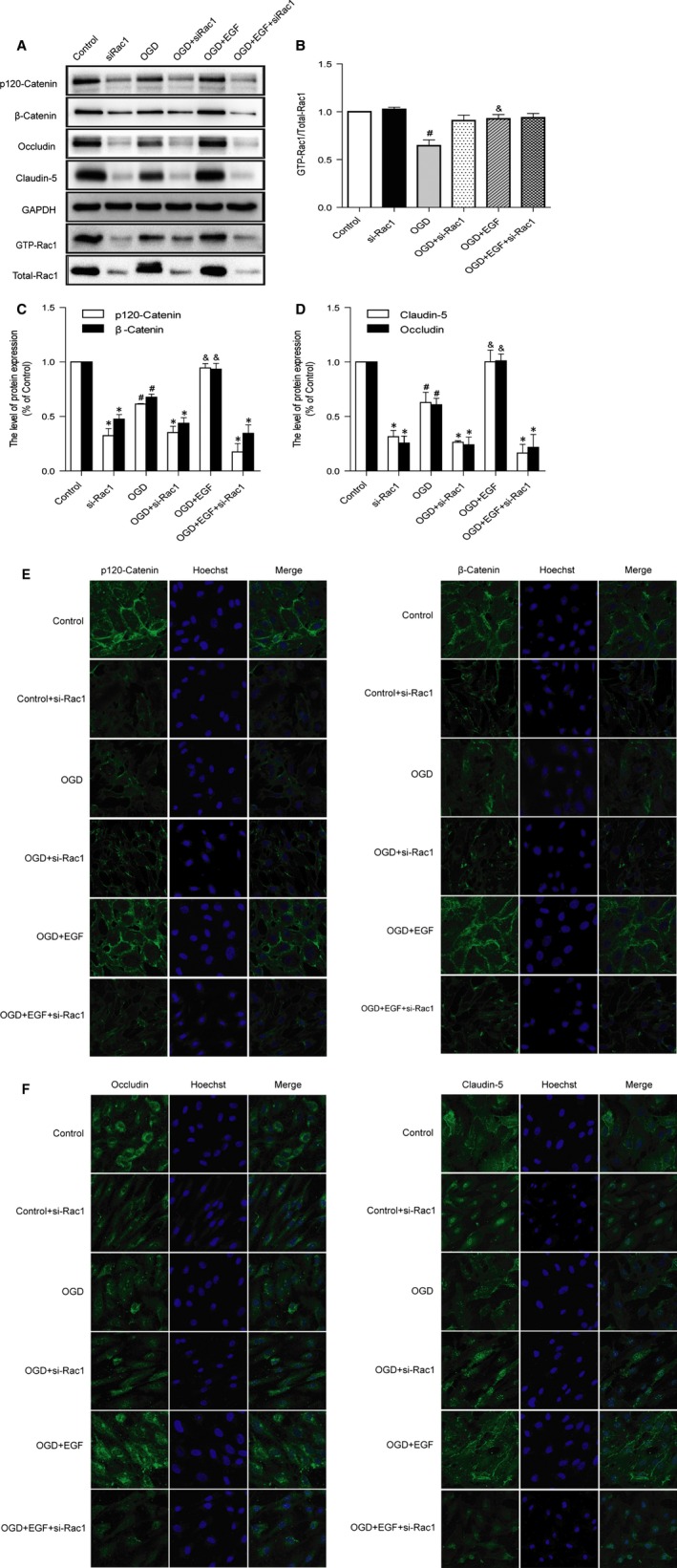
Rac1 activation is required for EGF‐induced TJ and AJ proteins expression. (A) Rac1 silencing inhibits the protein expressions of p120‐Catenin, β‐Catenin, Occludin, Claudin‐5. (B–D) The optical density analysis of p120‐Catenin, β‐Catenin, Occludin, Claudin‐5 and GTP‐Rac1/Total‐Rac1 protein. *represents P < 0.05 versus the corresponding non‐si‐Rac1 group, #represents P < 0.05 versus the Control group, & represents P < 0.05 versus the OGD group. (E and F) Immunofluorescence staining of TJ and AJ proteins. Nucleus (blue) was labelled with Hoechst.
Discussion
In this study, we displayed that EGF administration attenuated neurofunctional deficits by preventing BSCB disruption in rats after SCI. EGF treatment induced phosphorylation and activation of Akt, and Rac1 (GTP‐Rac1). Ultimately, EGF treatment led to the increasing of TJ (Occludin, Claudin‐5) and AJ (P120‐Catenin, β‐Catenin) at 24 hrs after SCI. However, co‐administration of PI3K inhibitor LY294002 and EGF reversed the protective effect of EGF on BSCB, implying that the observed protection of BSCB was mediated by EGF‐induced activation of the PI3K/Akt/Rac1 signalling pathway. Here, we present the mechanism of EGF that affects the BSCB integrity after injury.
Blood–spinal cord barrier integrity plays a pivotal role in maintaining the normal functions of spinal cord. Disruption of BSCB integrity happens owing to numerous pathological conditions such as amyotrophic lateral sclerosis and SCI 3, which leads to an increasing of vascular permeability. It has been demonstrated that grow factors like NGF, FGF‐2 improve functional recovery after SCI 25, 27. However, the study of grow factors on BSCB is little done. Epidermal growth factor is a key growth factor involved in a wide range of physiological and pathologic processes including angiogenesis and wound healing 28. It plays an essential role in migration, proliferation and differentiation of endothelial cells. In the present study, we demonstrated the protective effect and molecular mechanism of EGF on BSCB in vivo and in vitro. Previous report shows that activation of EGFR and PI3K/Akt increases transepithelial resistance and improves epithelial paracellular permeability in canine kidney II cells 29. Epidermal growth factor administration promotes intestinal proliferation and improves mucosal barrier by decreasing gut permeability, recruiting TJ proteins and modulating inflammation responses after ischaemia/reperfusion injury in rats 30. In addition, EGF increases the expression of claudin‐3 protein mediated by the ERK1/2 and PI3K‐Akt pathways in HT‐29 cells 31. It improves epithelial paracellular permeability by regulating claudin‐2 and ‐4 expression through Src and STAT3 in MDCK cells 32. However, few studies report concerning the role of EGF in the regulation of BSCB permeability. A previous study demonstrates that EGF promotes neurovascular protection by targeting early BBB disruption after ischaemia–reperfusion in rats 33. As shown in this study, EGF attenuated the disruption of TJ and AJ, thereby reduced the permeability of BSCB both in vitro and in vivo.
However, the underlying mechanism between EGF and permeability barrier function is still not fully understood. As is known to all, PI3K/Akt pathway can be triggered by EGF or other trophic factors, such as bFGF and NGF 34, 35, 36. The previous study shows that bFGF improves recovery from SCI by activating PI3K/Akt pathway 37. Meanwhile PI3K/Akt is a major signalling pathway in regulating angiogenesis in vivo and in vitro 38, 39. It has been reported that PI3K/Akt signalling pathway promotes the VEGF expression as well as makes an increase in the development of choroidal neovascularization 40. Moreover, the PI3K/Akt signalling pathway regulates paracellular claudin‐5 expression in mouse brain endothelial cells and improves intestinal epithelial permeability by regulating Occludin and Claudin‐1 in Caco‐2 cells 16, 41. FGFs preserve BBB integrity after experimental intracerebral haemorrhage in mice involves PI3K/Akt pathway 42. Activation of PI3K/Akt pathway attenuates BBB disruption following neonatal hypoxia‐ischaemia in rats 43. Above research indicate that PI3K/Akt pathway is essential for mediating barrier permeability under a variety of circumstances. Compared to these findings, our results revealed that EGF elevated the level of Akt activity in vivo and in vitro. Furthermore, the specific chemical inhibitor (LY294002) for this protein causes the reduction in TJ and AJ, thereby accelerating the disruption of the permeability of BSCB. Therefore, it may be reasonable to speculate that EGF enhances the expression of TJ and AJ proteins mediated through PI3K/Akt signalling pathway.
Rac1, a member of small GTPases in the Rho family, regulates intercellular junctions and the cytoskeleton. It is a major signalling component that is known to regulate various pathways downstream of the EGFR 44. Rac1 activation promotes angiogenesis of various types of vascular endothelial cell, meanwhile it is also known to regulate the formation and function of AJ and TJ. For instance, Rac1 signalling protects AJ (E‐cadherin and β‐Catenin) from disassembly during ATP depletion in MDCK cells 13. In pulmonary endothelial barrier, Rac1 activation is necessary for VE‐cadherin redistribution 45. Therefore, we explored the role of Rac1 in BSCB. As is known to all, Rac1 appears as a vital effector to stabilize endothelial E‐cadherin and β‐Catenin, and promote AJ formation through stimulating lamellipodia formation 46, 47. Inhibition of Rac1 activation disrupts AJ, leading to increase in endothelial permeability in mesenteric microvessels and cultured cells 48, 49. The above results of study reveal that Rac1 activation is essential for mediating barrier permeability. In the present study, it was found that EGF‐induced Rac1 activation in vivo and in vitro. While preventing Rac1 activity by Rac1 siRNA increased the TJ and AJ reduction under OGD, reversed the protection of EGF on BSCB in HBMECs, suggesting that Rac1 activity was responsible for regulating the expression of TJ and AJ proteins. Previous study shows that Rac1 acts as the downstream of PI3K/Akt 50, 51, 52. Rac1 activation is stimulated by hypoxia in breast cancer cells via PI3K/Akt signalling 53. PI3K is involved in Rac1 activation in cardiomyocytes during LPS stimulation 54. Moreover, NGF accelerates cutaneous wound healing by promoting the migration of dermal fibroblasts via the PI3K/Akt‐Rac1‐JNK and ERK pathways 55. In this study, we examined that PI3K inhibitor (LY294002) caused the reduction of EGF‐induced Rac1 activity in vitro and in vivo, thus reversed the protection of EGF on BSCB integrity. Meanwhile, silencing the expression of Rac1 by Rac1 siRNA also prevented the protective effect of EGF on BSCB integrity. Therefore, it may be reasonable to believe that EGF‐induced activation of Rac1 and enhanced the expression of TJ and AJ proteins mediated through PI3K/Akt signalling pathway.
In conclusion, this study examines the protective effect of EGF on BSCB integrity after SCI. Our study shows that EGF attenuates BSCB disruption and improves functional recovery after SCI via the PI3K/Akt/Rac1 signalling pathway. These results indicate that the PI3K/Akt/Rac1 signalling pathway is an important signalling cascade involved in BSCB integrity. Our results suggest that EGF may provide potential therapeutic interventions for preventing BSCB disruption after SCI.
Conflicts of interest
The authors confirm that the content of this article has no conflicts of interest.
Supporting information
Figure S1 LY294002 doesn't have a toxic and devastating effect by itself in the in vivo and in the in vitro treatments. (A) Representative whole spinal cords show that Evan's Blue dye permeabilized into injury spinal cord at 1 day (n = 4/group). (B) Quantification of the amount of Evan's Blue at 1 day (μg/g). (C) Protein expressions of p120‐Catenin, β‐Catenin, Occludin, Claudin‐5 in the spinal cord segment at the contusion epicentre. (D and E) The optical density analysis of p120‐Catenin, β‐Catenin, Occludin and Claudin‐5 protein. (F) Protein expressions of p120‐Catenin, β‐Catenin, Occludin, Claudin‐5 in endothelial cells. (G and H) The optical density analysis of p120‐Catenin, β‐Catenin, Occludin and Claudin‐5 protein. *represents P > 0.05 versus the Sham group, #represents P > 0.05 versus the SCI group.
Acknowledgements
This study was partly supported by research grants from the Zhejiang Provincial Natural Science Funding (LY14H170002, LY14H150010, LY14H090013), National Natural Science Funding of China (81472165, 81372112, 81200958, 81302775), Zhejiang Provincial Program for the Cultivation of High‐level Innovative Health Talents (to J.X.), Zhejiang Provincial Program of Medical and Health Science (2014KYA131), Zhejiang Undergraduate Talent Project (2015R413079), Wenzhou Program of Science and Technology (Y20140003) and State Key Basic Research Development Program (2012CB518105).
References
- 1. Whetstone WD, Hsu JY, Eisenberg M, et al Blood‐spinal cord barrier after spinal cord injury: relation to revascularization and wound healing. J Neurosci Res. 2003; 74: 227–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Muresanu DF, Sharma A, Lafuente JV, et al Nanowired delivery of growth hormone attenuates pathophysiology of spinal cord injury and enhances insulin‐like growth factor‐1 concentration in the plasma and the spinal cord. Mol Neurobiol. 2015; 52: 837–45. [DOI] [PubMed] [Google Scholar]
- 3. Bartanusz V, Jezova D, Alajajian B, et al The blood‐spinal cord barrier: morphology and clinical implications. Ann Neurol. 2011; 70: 194–206. [DOI] [PubMed] [Google Scholar]
- 4. Hawkins BT, Davis TP. The blood‐brain barrier/neurovascular unit in health and disease. Pharmacol Rev. 2005; 57: 173–85. [DOI] [PubMed] [Google Scholar]
- 5. Zlokovic BV. The blood‐brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008; 57: 178–201. [DOI] [PubMed] [Google Scholar]
- 6. Winkler EA, Sengillo JD, Sagare AP, et al Blood‐spinal cord barrier disruption contributes to early motor‐neuron degeneration in ALS‐model mice. Proc Natl Acad Sci USA. 2014; 111: E1035–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hakoshima T, Shimizu T, Maesaki R. Structural basis of the Rho GTPase signaling. J Biochem. 2003; 134: 327–31. [DOI] [PubMed] [Google Scholar]
- 8. McDonald P, Veluthakal R, Kaur H, et al Biologically active lipids promote trafficking and membrane association of Rac1 in insulin‐secreting INS 832/13 cells. Am J Physiol Cell Physiol. 2007; 292: C1216–20. [DOI] [PubMed] [Google Scholar]
- 9. Barros P, Jordan P, Matos P. Rac1 signaling modulates BCL‐6‐mediated repression of gene transcription. Mol Cell Biol. 2009; 29: 4156–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yamada S, Nelson WJ. Localized zones of Rho and Rac activities drive initiation and expansion of epithelial cell‐cell adhesion. J Cell Biol. 2007; 178: 517–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jou TS, Schneeberger EE, Nelson WJ. Structural and functional regulation of tight junctions by RhoA and Rac1 small GTPases. J Cell Biol. 1998; 142: 101–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ebnet K, Suzuki A, Horikoshi Y, et al The cell polarity protein ASIP/PAR‐3 directly associates with junctional adhesion molecule (JAM). EMBO J. 2001; 20: 3738–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gopalakrishnan S, Dunn KW, Marrs JA. Rac1, but not RhoA, signaling protects epithelial adherens junction assembly during ATP depletion. Am J Physiol Cell Physiol. 2002; 283: C261–72. [DOI] [PubMed] [Google Scholar]
- 14. Terakado M, Gon Y, Sekiyama A, et al The Rac1/JNK pathway is critical for EGFR‐dependent barrier formation in human airway epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2011; 300: L56–63. [DOI] [PubMed] [Google Scholar]
- 15. Daniels BP, Holman DW, Cruz Orengo L, et al Viral pathogen‐associated molecular patterns regulate blood‐brain barrier integrity via competing innate cytokine signals. MBio. 2014; 5: e01476–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang L, Shen J, Cheng J, et al MicroRNA‐21 regulates intestinal epithelial tight junction permeability. Cell Biochem Funct. 2015; 33: 235–40. [DOI] [PubMed] [Google Scholar]
- 17. Liu Y, Leo LF, McGregor C, et al Pigment epithelium‐derived factor (PEDF) peptide eye drops reduce inflammation, cell death and vascular leakage in diabetic retinopathy in Ins2(Akita) mice. Mol Med. 2012; 18: 1387–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gunduz D, Thom J, Hussain I, et al Insulin stabilizes microvascular endothelial barrier function via phosphatidylinositol 3‐kinase/Akt‐mediated Rac1 activation. Arterioscler Thromb Vasc Biol. 2010; 30: 1237–45. [DOI] [PubMed] [Google Scholar]
- 19. Iwakura Y, Piao YS, Mizuno M, et al Influences of dopaminergic lesion on epidermal growth factor‐ErbB signals in Parkinson's disease and its model: neurotrophic implication in nigrostriatal neurons. J Neurochem. 2005; 93: 974–83. [DOI] [PubMed] [Google Scholar]
- 20. Birecree E, King LE Jr, Nanney LB. Epidermal growth factor and its receptor in the developing human nervous system. Brain Res Dev Brain Res. 1991; 60: 145–54. [DOI] [PubMed] [Google Scholar]
- 21. Abe K, Saito H. Epidermal growth factor selectively enhances NMDA receptor‐mediated increase of intracellular Ca2+ concentration in rat hippocampal neurons. Brain Res. 1992; 587: 102–8. [DOI] [PubMed] [Google Scholar]
- 22. Kojima A, Tator CH. Intrathecal administration of epidermal growth factor and fibroblast growth factor 2 promotes ependymal proliferation and functional recovery after spinal cord injury in adult rats. J Neurotrauma. 2002; 19: 223–38. [DOI] [PubMed] [Google Scholar]
- 23. Jimenez Hamann MC, Tator CH, Shoichet MS. Injectable intrathecal delivery system for localized administration of EGF and FGF‐2 to the injured rat spinal cord. Exp Neurol. 2005; 194: 106–19. [DOI] [PubMed] [Google Scholar]
- 24. Karimi Abdolrezaee S, Schut D, Wang J, et al Chondroitinase and growth factors enhance activation and oligodendrocyte differentiation of endogenous neural precursor cells after spinal cord injury. PLoS ONE. 2012; 7: e37589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhang HY, Wang ZG, Wu FZ, et al Regulation of autophagy and ubiquitinated protein accumulation by bFGF promotes functional recovery and neural protection in a rat model of spinal cord injury. Mol Neurobiol. 2013; 48: 452–64. [DOI] [PubMed] [Google Scholar]
- 26. Chen X, Lan X, Roche I, et al Caffeine protects against MPTP‐induced blood‐brain barrier dysfunction in mouse striatum. J Neurochem. 2008; 107: 1147–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhu SP, Wang ZG, Zhao YZ, et al Gelatin Nanostructured Lipid Carriers Incorporating Nerve Growth Factor Inhibit Endoplasmic Reticulum Stress‐Induced Apoptosis and Improve Recovery in Spinal Cord Injury. Mol Neurobiol. 2015; DOI:10.1007/s12035‐015‐9372‐2. [DOI] [PubMed] [Google Scholar]
- 28. Jin E, Kim JM, Kim SW. Priming of mononuclear cells with a combination of growth factors enhances wound healing via high angiogenic and engraftment capabilities. J Cell Mol Med. 2013; 17: 1644–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Singh AB, Sugimoto K, Dhawan P, et al Juxtacrine activation of EGFR regulates claudin expression and increases transepithelial resistance. Am J Physiol Cell Physiol. 2007; 293: C1660–8. [DOI] [PubMed] [Google Scholar]
- 30. Geng Y, Li J, Wang F, et al Epidermal growth factor promotes proliferation and improves restoration after intestinal ischemia‐reperfusion injury in rats. Inflammation. 2013; 36: 670–9. [DOI] [PubMed] [Google Scholar]
- 31. De Souza WF, Fortunato Miranda N, Robbs BK, et al Claudin‐3 overexpression increases the malignant potential of colorectal cancer cells: roles of ERK1/2 and PI3K‐Akt as modulators of EGFR signaling. PLoS ONE. 2013; 8: e74994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Garcia Hernandez V, Flores Maldonado C, Rincon Heredia R, et al EGF regulates claudin‐2 and ‐4 expression through Src and STAT3 in MDCK cells. J Cell Physiol. 2015; 230: 105–15. [DOI] [PubMed] [Google Scholar]
- 33. Pillai DR, Shanbhag NC, Dittmar MS, et al Neurovascular protection by targeting early blood‐brain barrier disruption with neurotrophic factors after ischemia‐reperfusion in rats*. J Cereb Blood Flow Metab. 2013; 33: 557–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Reichardt LF, Mobley WC. Going the distance, or not, with neurotrophin signals. Cell. 2004; 118: 141–3. [DOI] [PubMed] [Google Scholar]
- 35. Itoh N, Ornitz DM. Fibroblast growth factors: from molecular evolution to roles in development, metabolism and disease. J Biochem. 2011; 149: 121–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cao L, Zhang L, Chen S, et al BDNF‐mediated migration of cardiac microvascular endothelial cells is impaired during ageing. J Cell Mol Med. 2012; 16: 3105–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhang HY, Zhang X, Wang ZG, et al Exogenous basic fibroblast growth factor inhibits ER stress‐induced apoptosis and improves recovery from spinal cord injury. CNS Neurosci Ther. 2013; 19: 20–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chung BH, Kim JD, Kim CK, et al Icariin stimulates angiogenesis by activating the MEK/ERK‐ and PI3K/Akt/eNOS‐dependent signal pathways in human endothelial cells. Biochem Biophys Res Commun. 2008; 376: 404–8. [DOI] [PubMed] [Google Scholar]
- 39. Chung BH, Cho YL, Kim JD, et al Promotion of direct angiogenesis in vitro and in vivo by Puerariae flos extract via activation of MEK/ERK‐, PI3K/Akt/eNOS‐, and Src/FAK‐dependent pathways. Phytother Res. 2010; 24: 934–40. [DOI] [PubMed] [Google Scholar]
- 40. Yang XM, Wang YS, Zhang J, et al Role of PI3K/Akt and MEK/ERK in mediating hypoxia‐induced expression of HIF‐1alpha and VEGF in laser‐induced rat choroidal neovascularization. Invest Ophthalmol Vis Sci. 2009; 50: 1873–9. [DOI] [PubMed] [Google Scholar]
- 41. Camire RB, Beaulac HJ, Brule SA, et al Biphasic modulation of paracellular claudin‐5 expression in mouse brain endothelial cells is mediated through the phosphoinositide‐3‐kinase/AKT pathway. J Pharmacol Exp Ther. 2014; 351: 654–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Huang B, Krafft PR, Ma Q, et al Fibroblast growth factors preserve blood‐brain barrier integrity through RhoA inhibition after intracerebral hemorrhage in mice. Neurobiol Dis. 2012; 46: 204–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Li L, McBride DW, Doycheva D, et al G‐CSF attenuates neuroinflammation and stabilizes the blood‐brain barrier via the PI3K/Akt/GSK‐3beta signaling pathway following neonatal hypoxia‐ischemia in rats. Exp Neurol. 2015; 272: 135–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wu R, Coniglio SJ, Chan A, et al Up‐regulation of Rac1 by epidermal growth factor mediates COX‐2 expression in recurrent respiratory papillomas. Mol Med. 2007; 13: 143–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wang L, Bittman R, Garcia JG, et al Junctional complex and focal adhesion rearrangement mediates pulmonary endothelial barrier enhancement by FTY720 S‐phosphonate. Microvasc Res. 2015; 99: 102–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mehta D, Konstantoulaki M, Ahmmed GU, et al Sphingosine 1‐phosphate‐induced mobilization of intracellular Ca2+ mediates rac activation and adherens junction assembly in endothelial cells. J Biol Chem. 2005; 280: 17320–8. [DOI] [PubMed] [Google Scholar]
- 47. Komarova Y, Malik AB. Regulation of endothelial permeability via paracellular and transcellular transport pathways. Annu Rev Physiol. 2010; 72: 463–93. [DOI] [PubMed] [Google Scholar]
- 48. Wojciak Stothard B, Potempa S, Eichholtz T, et al Rho and Rac but not Cdc42 regulate endothelial cell permeability. J Cell Sci. 2001; 114: 1343–55. [DOI] [PubMed] [Google Scholar]
- 49. Waschke J, Baumgartner W, Adamson RH, et al Requirement of Rac activity for maintenance of capillary endothelial barrier properties. Am J Physiol Heart Circ Physiol. 2004; 286: H394–401. [DOI] [PubMed] [Google Scholar]
- 50. Petpiroon N, Suktap C, Pongsamart S, et al Kaempferol‐3‐O‐rutinoside from Afgekia mahidoliae promotes keratinocyte migration through FAK and Rac1 activation. J Nat Med. 2015; 69: 340–8. [DOI] [PubMed] [Google Scholar]
- 51. Zhu G, Fan Z, Ding M, et al An EGFR/PI3K/AKT axis promotes accumulation of the Rac1‐GEF Tiam1 that is critical in EGFR‐driven tumorigenesis. Oncogene. 2015; 34: 5971–82. [DOI] [PubMed] [Google Scholar]
- 52. Madhyastha HK, Radha KS, Nakajima Y, et al uPA dependent and independent mechanisms of wound healing by C‐phycocyanin. J Cell Mol Med. 2008; 12: 2691–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Du J, Xu R, Hu Z, et al PI3K and ERK‐induced Rac1 activation mediates hypoxia‐induced HIF‐1alpha expression in MCF‐7 breast cancer cells. PLoS ONE. 2011; 6: e25213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Zhang T, Lu X, Beier F, et al Rac1 activation induces tumour necrosis factor‐alpha expression and cardiac dysfunction in endotoxemia. J Cell Mol Med. 2011; 15: 1109–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Chen JC, Lin BB, Hu HW, et al NGF accelerates cutaneous wound healing by promoting the migration of dermal fibroblasts via the PI3K/Akt‐Rac1‐JNK and ERK pathways. Biomed Res Int. 2014; 2014: 547187. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 LY294002 doesn't have a toxic and devastating effect by itself in the in vivo and in the in vitro treatments. (A) Representative whole spinal cords show that Evan's Blue dye permeabilized into injury spinal cord at 1 day (n = 4/group). (B) Quantification of the amount of Evan's Blue at 1 day (μg/g). (C) Protein expressions of p120‐Catenin, β‐Catenin, Occludin, Claudin‐5 in the spinal cord segment at the contusion epicentre. (D and E) The optical density analysis of p120‐Catenin, β‐Catenin, Occludin and Claudin‐5 protein. (F) Protein expressions of p120‐Catenin, β‐Catenin, Occludin, Claudin‐5 in endothelial cells. (G and H) The optical density analysis of p120‐Catenin, β‐Catenin, Occludin and Claudin‐5 protein. *represents P > 0.05 versus the Sham group, #represents P > 0.05 versus the SCI group.


