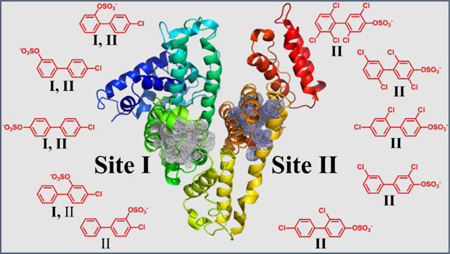
Abstract
The disposition of toxicants is often affected by their binding to serum proteins, of which the most abundant in humans is serum albumin (HSA). There is increasing interest in the toxicities of environmentally persistent polychlorinated biphenyls (PCBs) with lower numbers of chlorine atoms (LC-PCBs) due to their presence in both indoor and outdoor air. PCB sulfates derived from metabolic hydroxylation and sulfation of LC-PCBs have been implicated in endocrine disruption due to high affinity-binding to the thyroxine-carrying protein, transthyretin. Interactions of these sulfated metabolites of LC-PCBs with HSA, however, have not been previously explored. We have now determined the relative HSA-binding affinities for a group of LC-PCBs and their hydroxylated and sulfated derivatives by selective displacement of the fluorescent probes 5-dimethylamino-1-naphthalenesulfonamide and dansyl-L-proline from the two major drug-binding sites on HSA (previously designated as Site I and Site II). Values for half-maximal displacement of the probes indicated that the relative binding affinities were generally PCB sulfate ≥ OH-PCB > PCB, although this affinity was site- and congener-selective. Moreover, specificity for Site II increased as the numbers of chlorine atoms increased. Thus, hydroxylation and sulfation of LC-PCBs result in selective interactions with HSA which may affect their overall retention and toxicity.
Graphical Abstract
Introduction
The legacy of polychlorinated biphenyl (PCB) exposure and the resulting various toxicities have been well established in the scientific literature.1, 2 Once marketed as commercially available mixtures (e.g. Aroclors), PCBs were used worldwide throughout most of the 20th century in various industrial applications and in the production of consumer goods.3 The versatility of this class of compounds, i.e., a biphenyl core with up to 10 chlorine substituents, allowed for the production of mixtures with highly desirable qualities which include high thermal and electrical conductivity, high flash points and the resistance to degradation by chemical or biological means. These same physical and chemical properties that favored their use as flame retardants, lubricants, coolants, and insulating fluids also led to their ability to accumulate in the environment at virtually all trophic levels.4, 5 Their presence in the environment has been linked to various toxic effects in wildlife (e.g., birds and fish) as well as toxicity in laboratory animal models for disease6, 7 and in human populations.2, 8, 9 These toxicities encompass many human diseases, some of which include immunological disorders,10 hormonal dysfunction,11 carcinogenesis,12, 13 and neurotoxicity.8, 9
Although the worldwide levels of PCBs have been decreasing since their production and use was limited to distinct closed systems in the late 1970s, studies show that indoor and outdoor air samples in urban areas are high in lower chlorinated PCBs (LC-PCBs; ≤4 chlorine atoms per congener).14–17 Moreover, studies indicate that an additional current source of these toxic LC-PCBs may be their inadvertent production in the manufacture of certain dyes and pigments.18–20 When compared to higher chlorinated PCBs (>4 chlorine atoms per congener) also present in traditional Aroclor mixtures and in dyes and pigments, the LC-PCBs are more highly susceptible to metabolic transformation and exhibit a higher vapor pressure, thereby making them more easily volatilized.21 Exposure to these volatile PCBs via inhalation allows for their facile transfer into the blood via the lungs.22 As a point of reference, these lower chlorinated congeners may also be classified as non-dioxin-like PCBs, due to the fact that they are not agonists of the aryl hydrocarbon receptor.23
The metabolism and metabolites of PCBs have been widely studied in order to assess their toxic potential and to examine the possible use of metabolites as biomarkers for exposure or for determining body-burdens in human populations.24, 25 In fact, the analysis of oxidized metabolites such as hydroxylated PCBs (OH-PCBs) has been completed in biological samples from various species worldwide.5, 26, 27 Further oxidation of OH-PCBs to catechols, p-hydroquinones, and quinones has been linked to cytotoxic formation of reactive oxygen species28, 29 and the potential for carcinogenicity.30 Other PCB metabolites include PCB sulfates and glucuronides, as well as glutathione conjugates and their mercapturic acid derivatives.21
Of the aforementioned metabolites, PCB sulfates have recently garnered increased interest due to their potential activity as disruptors of endocrine signaling. While OH-PCBs have been shown to inhibit the sulfation of endogenous molecules including dehydroepiandrosterone (DHEA)31 and estradiol,32 many also serve as substrates for sulfate conjugation.33 The resulting PCB sulfates have been shown to bind to the thyroid hormone carrying protein transthyretin, where, in some cases, they bind with similar affinity to that observed with the native T4 ligand.34 The potential of OH-PCBs to serve as substrates for sulfation catalyzed by human and rat sulfotransferases has been published,33, 34 and a study in Sprague-Dawley rats has indicated that hydroxylation followed by sulfation accounts for more than half of the metabolic fate after treatment with the monochlorinated PCB 3.35 Furthermore, recent studies on 4-PCB 11 sulfate administered to rats indicate that PCB sulfates may be retained in vivo and undergo additional metabolism.36
The metabolic pathway that results in the sulfation of an exogenous compound has been traditionally thought of as a mode for its removal from the body. The sulfotransferase-mediated transfer of a sulfuryl group from 3’-phosphoadenosine 5’-phosphosulfate (PAPS) to an acceptor molecule often results in increased polarity, water solubility, and excretion of the sulfated product, however the potential for retention may exist in some cases. It has been previously shown that the formation of sulfated metabolites from either naphthol or para-nitrophenol increases their binding to human serum albumin (HSA) when compared both to the parent compounds and to their hydroxylated and glucuronidated counterparts.37 Similar to these sulfated aromatics, binding of PCB sulfates to HSA may, along with specific binding to lipids and proteins within tissues and organs, be substantial and may serve as a mode for their retention and distribution in vivo. The high concentration of this protein in blood and its ability to mediate the half-lives and distribution of many drugs and toxicants has led to it being referred to as the “silent receptor”.38 In addition to its other functions, HSA is largely responsible for maintaining colloidal osmotic pressure in the perivascular space, and it is also present in large amounts in extravascular compartments, being distributed to muscle, organs, and tissue. In fact, HSA in the extravascular space accounts for more than half of its total body distribution.39
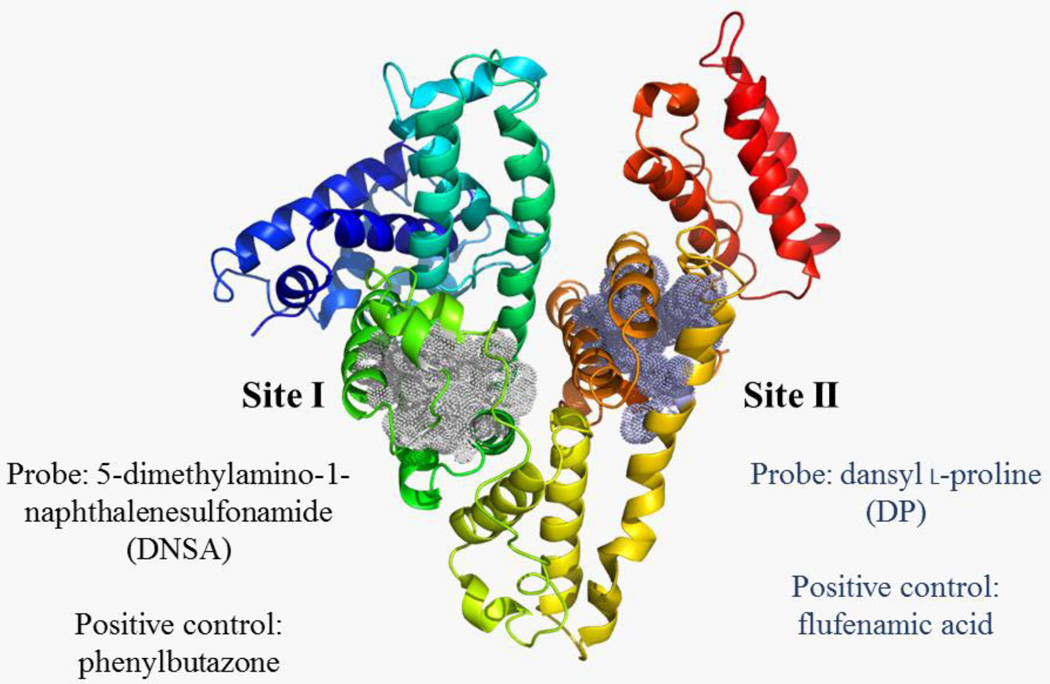
The work of Sudlow, et. al.,40 has elucidated the presence of two major drug binding sites within HSA by use of a small library of drug molecules and fluorescent probes (mostly dansylated amino acids). Site I is situated within subdomain IIa and is characteristic of binding bulky hydrophobic molecules with negative charges on opposing ends. This includes dicarboxylic acids and xenobiotics including warfarin, phenylbutazone, and a dansylated amine (5-dimethylamino-1-naphthalenesulfonamide, DNSA). Site II is situated within subdomain IIIa and typically binds compounds with a negative charge at one end of a molecule with a hydrophobic core. This includes diazepam, flufenamic acid, and dansylated proline. Whereas the larger area and flexibility of Site I imparts on it a more promiscuous binding, the smaller dimensions and less flexibility of Site II result in more stringent binding constraints. Although there might be a potential for lower affinity interactions at other sites, this model using the two major drug-binding sites in HSA has served as a good predictor of many high-affinity xenobiotic-HSA interactions.41 Figure 1 illustrates the residues that form the hydrophobic core of both sites I and II, as well as representative ligands for these binding sites.42We hypothesized that, similar to the studies on naphthol and para-nitrophenol described above, the sulfated congeners of LC-PCBs would exhibit increased HSA-binding when compared to their PCB or OH-PCB counterparts, and that this binding would display selectivity between the two major drug-binding sites in HSA. Since sulfation is a significant metabolic reaction for PCBs with lower numbers of chlorine atoms, our objective was to determine the potential selectivity of interactions between such PCB sulfates and human serum albumin. The method employed for the current study involves the displacement of site-selective fluorescent probes from these two major drug binding sites. Although this approach does not allow for the direct determination of an affinity constant (Ka), it gives information on selectivity for these two drug-binding sites, and, when reported alongside positive control compounds, gives a relative determination of binding affinity.
Figure 1.
HSA crystal structure indicating residues that form Sites I and II as well as site-selective fluorescent probes and positive control ligands. The HSA crystal structure (PDB ID: 1UOR) was modified to highlight the binding sites using the PyMOL Molecular Graphics System, Version 1.7.4 Schrödinger, LLC. Residues that surround sites I and II42 have been highlighted in dotted spheres, and the appropriate fluorescent probes and positive control ligands for each site have been annotated.
Materials and Methods
Materials
Human serum albumin (fatty acid and globulin free, ≥99% pure), phenylbutazone, and 5-dimethylamino-1-naphthalenesulfonamide (dansylamide; DNSA) were purchased from Sigma-Aldrich (St. Louis, MO). Flufenamic acid was purchased from Fluka Analytical (Buchs, Switzerland), and dansyl-L-proline (DP) was purchased from TCI (Cambridge, MA). The synthesis and characterization of 4 OH PCB 52 (2,2’,5,5’-tetrachlorobiphenyl-4-ol) and 4 PCB 52 sulfate (sulfuric acid mono-(2,2’,5,5’-tetrachlorobiphenyl-4-yl) ester, ammonium salt) are reported in the Supporting Information. All other PCB metabolites were prepared and characterized in the synthesis core of the Iowa Superfund Research Program as previously described.43–45
Human Serum Albumin (HSA) binding assay
The displacement of site-selective fluorescent probes from HSA has been used to identify the relative binding affinity and selectivity of a large number of compounds. We used this approach for LC-PCBs and their metabolites by employing a modification of a method that was first described by Sudlow et. al.40, 46 Briefly, a 96-well plate format was used for analysis of solutions containing 10 µM HSA in 0.1 M potassium phosphate buffer, pH 7.4, a fluorescent probe (20µM for Site I-selective DNSA and 5 µM for Site II-selective DP), and increasing concentrations of ligand (i.e., PCB, OH-PCB, PCB sulfate, or positive control ligand). The experiments were performed using a quartz 96-well microplate purchased from Molecular Devices (Sunnyvale, CA). Stock solutions of ligands were made in DMSO, and the final assay concentration of DMSO was less than 0.8% v/v. This concentration of DMSO was shown to have no significant effect on the fluorescence of either HSA-DP or HSA-DNSA. After mixing using a multipipettor, the fluorescence intensity was obtained after 5 minutes at 25°C (DNSA Excitation: 350nm; Emission: 460nm) (DP Excitation: 375nm; Emission: 460nm) in a Spectramax M5 fluorimeter (Molecular Devices, Sunnyvale, CA). Each ligand was assayed in triplicate and each entry was an average of six fluorescence readings and reported as a percent of control signal. Controls contained the same amount of DMSO solution, but without any ligand. Values for 50% decrease in the fluorescence intensity of the probe (EC50) were determined by plotting the percentage of control fluorescence intensity versus the log of the concentration of the test ligand and then utilizing a sigmoidal dose response ligand-binding algorithm (SigmaPlot v.11.0, Systat Software, Chicago, IL). The EC50 binding parameters for all ligands were calculated by a best fit to the equation: ; where min and max are the minima and maxima of the sigmoidal curve, y and x are the percent of control fluorescence and ligand concentration, respectively; Hillslope characterizes the slope of the curve at its midpoint, and the EC50 parameter is the ligand concentration where half of the total fluorescent probe displacement occurs. A further requirement for these studies was that at least 50% of the total probe must have been displaced within the range of concentrations examined. It is important to note that, in the case of high affinity binding to both sites, the overall binding to HSA will be somewhat underestimated by this method due to the fact that only one site is observed at any given time, regardless of whether or not the other site is occupied by the ligand. Moreover, due to limitations of the specific assay conditions requited, the lowest EC50 values that can be reliably quantitated are 5.0 µM and 2.5 µM for sites I and II, respectively.
HPLC analysis of recovery and reversibility in the binding of PCB sulfates to HSA
A solution of HSA (50 µM) and PCB sulfate (50 µM) was prepared in 0.1 M potassium phosphate buffer, pH 7.4, and 100 µL aliquots were sampled and extracted after incubation at 25°C for 0, 30, 90, 180, and 270 minutes. Each aliquot was extracted with 100µL acetonitrile after the addition of 15 mg NaCl, and the samples were subjected to vortex-mixing followed by centrifugation at 5000 rpm for 5 minutes. The acetonitrile layer was analyzed by HPLC using a Shimadzu Model LC-20-AT liquid chromatograph equipped with an SPD-20-AT UV/VIS detector and a C18 AQ 5µm (4.6 × 250 mm) column (Grace, Deerfield, IL). The HPLC separation was achieved by using linear gradients formed between 0.04% TEA acetate (pH 7.4) and the indicated concentration of acetonitrile (MeCN): 0–1 min at 15% MeCN, 1–10 min at 15–100% MeCN, 10–14 min at 100% MeCN, and 14–15 min at 15% MeCN. Analysis of the eluate was carried out by absorbance at 254 nm. Concentrations of PCB sulfates were determined by relating the peak area to a standard curve obtained using synthetic standards.
Results and Discussion
Serum albumin is the most abundant protein in human plasma, with a concentration of approximately 700µM in a healthy adult.47 Ligand-bound crystal structures for the 66.5kDa protein show the various drug-, fatty acid-, ion-, and heme-binding sites of HSA,48 and many methods have been employed to determine binding parameters.41, 49–51 In our present studies, we have measured the displacement of site-selective fluorescent probes from the two major drug-binding sites on HSA (i.e., Site I and Site II) as described by Sudlow et al.40 In this examination of LC-PCBs and their hydroxylated and sulfated metabolites, the displacement of: 5-dimethylamino-1-naphthalenesulfonamide (DNSA) for Site I and dansyl L-proline (DP) for Site II, shown in Figure 1, was used.40 The displacement of these probes from their respective binding sites has been used to determine site-selectivity for ligands as well as to provide relative estimates of their binding affinities.52 It is noted here, however, that a lack of site-selective binding observed for these two high affinity drug-binding sites does not preclude HSA-binding at other sites on the protein. Indeed, due to high concentrations of serum albumin some sites where these molecules bind with lower affinity might become important. They may, however, be competing with higher affinity endogenous ligands. While such interactions have been noted for higher-chlorinated PCBs and OH-PCBs53–55, the current study is, to our knowledge, the first to examine high affinity interactions with lower-chlorinated PCBs and their metabolites.
The LC-PCBs and their metabolites chosen for this study represent a variety of chlorine atom-substitution patterns and, in several cases, they represent LC-PCBs that are commonly found in samples taken from indoor and outdoor air (e.g., PCBs 3, 8, 11, and 52).14, 56 The data are presented as EC50 values for displacement of the appropriate site-selective probe from HSA. A lower value of EC50 indicates greater affinity for that site and the EC50 values for site-selective positive controls (i.e., phenylbutazone for Site I and flufenamic acid for Site II) are included for reference. The Ka values for these reference ligands have been previously published (phenylbutazone Ka = 4.22 × 105 M−1; flufenamic acid Ka = 1.56 × 105 M−1).57
Displacement of probes from HSA by PCBs, OH-PCBs, and PCB Sulfates
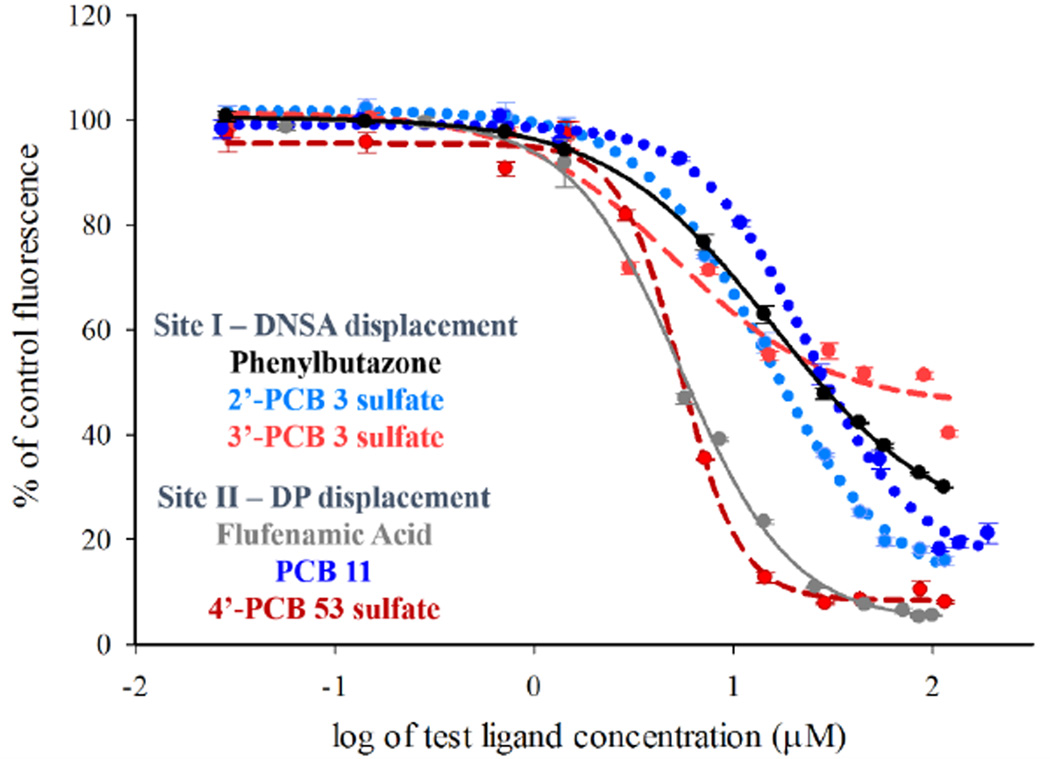
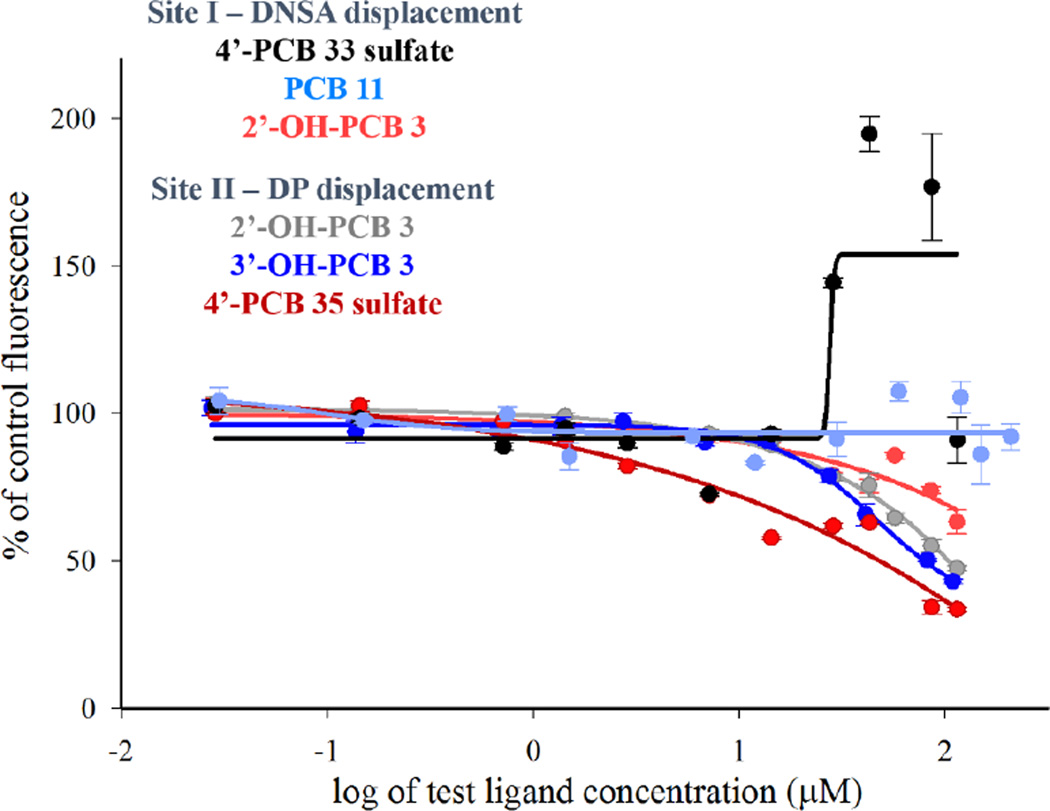
After incubation of the HSA with a site-selective probe, changes in fluorescence intensity of the probe were determined upon addition of a potential ligand. Representative graphs of the change in fluorescence intensity (% change) vs. log of ligand concentration are provided in Figures 2 and 3. In order to confidently ascribe an EC50 value to a ligand based on its displacement of a probe, we used the criterion that it must displace at least 50% of the probe within the range of ligand-concentrations tested. Those ligands that did not significantly interact with the HSA at concentrations less than 50µM are simply reported as having an EC50 greater than 50µM. It was noted, however, that in a few cases (e.g., 4’-PCB 33 sulfate and 4’-PCB 35 sulfate in Figure 3), the fluorescence of the site I probe, DNSA, increased at higher concentrations of ligand. This effect may be due to the high flexibility of HSA, as well as the potential binding of the ligands to any of its multiple binding sites. These include the seven fatty acid binding sites as well as other undefined or low-affinity sites found throughout the protein.
Figure 2.
Binding curves for compounds that exhibited binding to Site I and Site II as determined by site-selective fluorescent probe displacement. Plots are of percent of control fluorescence vs. increasing ligand concentration. Each experiment consists of 10µM HSA and 20µM DNSA or 5µM DP. Data was fit to sigmoidal dose response ligand-binding algorithms (SigmaPlot v.11.0, Systat Software, Chicago, IL) and EC50 values are reported in Figures 4 through 6. Mean ± SE, n=3.
Figure 3.
Binding curves for compounds that did not exhibit binding to either Site I or Site II as determined by site-selective fluorescent probe displacement. Plots are of percent of control fluorescence vs. increasing ligand concentration. Each experiment consists of 10µM HSA and 20µM DNSA or 5µM DP. Data was fit to sigmoidal dose response ligand-binding algorithms (SigmaPlot v.11.0, Systat Software, Chicago, IL) and EC50 values are reported in Figures 4 through 6. Mean ± SE, n=3.
Recovery of PCB sulfates following incubation with HSA
Since HSA has been reported to catalyze hydrolysis of some carboxylic acid esters,58, 59 we sought to determine if a PCB sulfate could serve as a substrate for hydrolysis catalyzed by the protein. Moreover, it was also important to establish that the PCB sulfate concentration was not changing throughout the course of the experiment, with full recovery of the ligand. Analyses that were conducted by acetonitrile extraction and HPLC showed that during incubations up to 270 min, the PCB sulfates were stable. These results, shown in Supporting Information Fig. S12, indicated that no hydrolysis of the PCB sulfate to the corresponding phenol was occurring during the experiment, and the quantitative extraction at each time point further indicated that no chemical changes were occurring to the ligand in question.
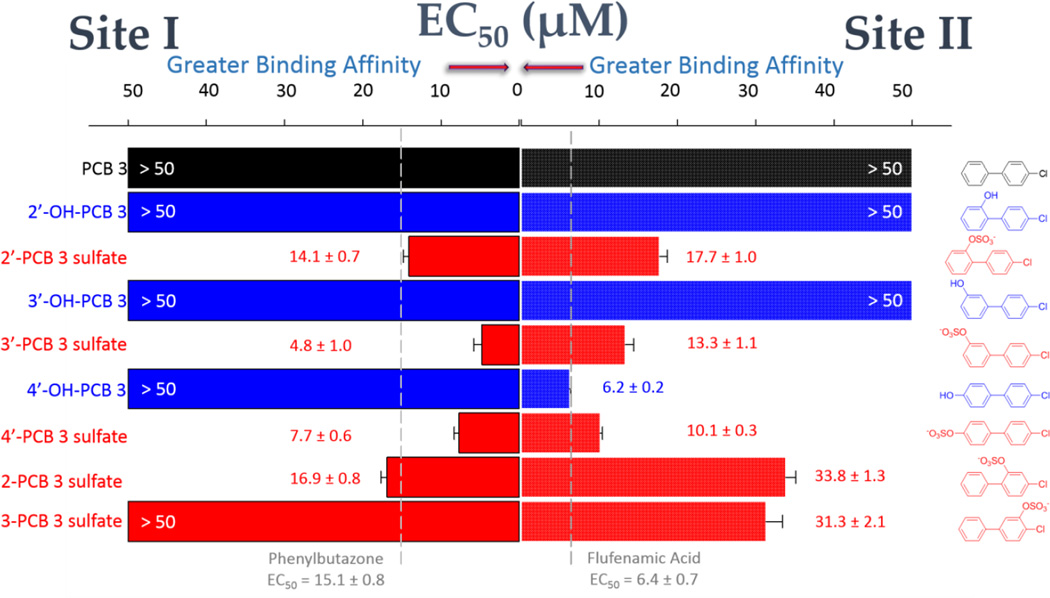
Binding of the monochlorinated PCB 3 and its hydroxylated and sulfated metabolites to HSA
The data shown in Figure 4 illustrate the binding characteristics of the monochlorinated PCB 3 and its hydroxylated and sulfated metabolites. In vivo studies with Sprague-Dawley rats have shown that treatment with PCB 3 (via intraperitoneal injection or inhalation) is largely metabolized to hydroxylated (3’-OH-PCB 3, and 4’-OH-PCB 3) and sulfated (3-PCB 3 sulfate, 2’-PCB 3 sulfate, 3’-PCB 3 sulfate, and 4’-PCB 3 sulfate) derivatives, where the 4’-PCB 3 sulfate was the primary component.35, 60, 61 In the current work, neither PCB 3 nor its 2’-OH and 3’-OH metabolites bound with significant affinity to either of the two drug-binding sites on HSA. In comparison, their sulfated counterparts showed moderate to high affinity for both sites. In the case of the 4’ metabolites, where both the chlorine and sulfate functional groups are para to the biphenyl linkage, there was significant binding to Site I of both the OH-PCB and PCB sulfate, while the latter retained its high affinity for Site II. A sulfate group at the 2-position of the same aromatic ring as the 4-chloro, however, resulted in some selectivity in binding at HSA Site I in preference to Site II. The results obtained for PCB 3 and its hydroxylated and sulfated derivatives highlight a general trend in the binding of the LC-PCBs and metabolites in this study, namely that HSA-binding affinity follows the trend: PCB sulfate ≥ OH-PCB > PCB.
Figure 4.
HSA-binding of the monochlorinated PCB 3 and its hydroxylated and sulfated metabolites. Site I binding (DNSA displacement) is shown at the left of the graph and Site II binding (DP displacement) is shown at the right. Color coding is used to differentiate the PCBs (black), OH-PCBs (blue), and PCB sulfates (red). Compound names appear on the left of the graph and the corresponding structure is at the right. EC50 values were obtained as described in Materials and Methods. The vertical dashed lines indicate the EC50 values for the positive control ligands at each site.
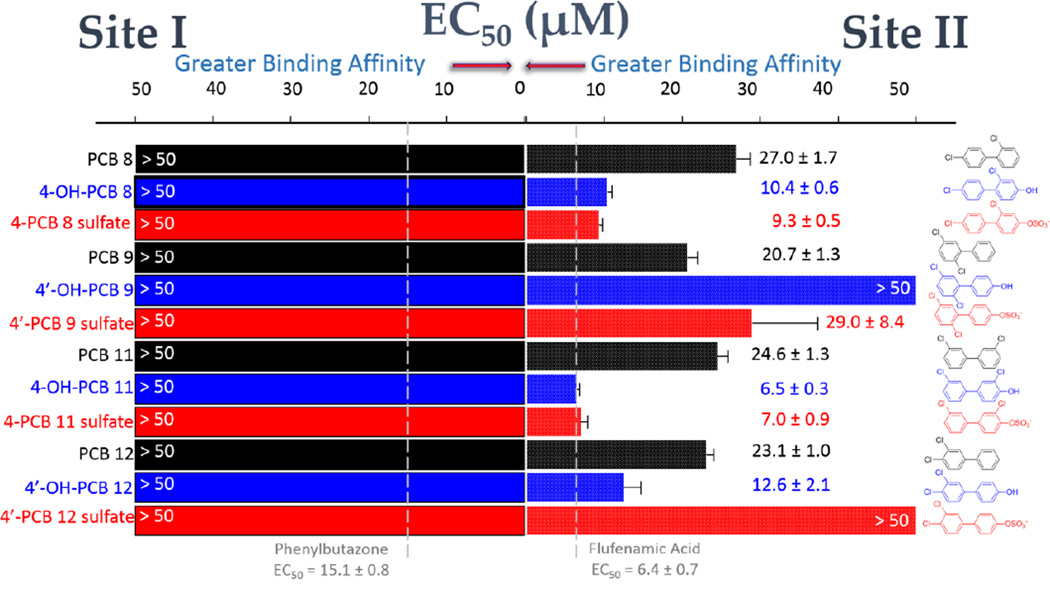
Binding of dichlorinated PCB congeners and their metabolites to HSA
As seen in Figure 5, the binding of dichlorinated PCB congeners and their metabolites displayed significant selectivity for HSA Site II. Unlike the results seen for the monochlorinated PCB sulfates, there was no significant binding to Site I observed for any of the dichlorinated PCB metabolites. Binding affinity to Site II, however, was increased for the dichlorinated PCBs and OH-PCBs in comparison to their monochlorinated counterparts. It is interesting to note that in this set of compounds, one of the PCB sulfates (4’-PCB 12 sulfate) and one of the OH-PCBs (4’-OH-PCB 9) showed no significant binding to either site. We have also noted a similar lack of interaction with either binding site on HSA for the trichlorinated 4’-PCB 35 sulfate. A common feature of these two PCB sulfates is the presence of a 3,4-dichloro substitution pattern in the aromatic ring that does not bear the 4’-sulfate. Further studies will be needed to ascertain the basis for this structural specificity for Site II binding.
Figure 5.
HSA-binding of dichlorinated PCB congeners and their hydroxylated and sulfated metabolites. Site I binding (DNSA displacement) is shown at the left of the graph and Site II binding (DP displacement) is shown at the right. Color coding is used to differentiate the PCBs (black), OH-PCBs (blue), and PCB sulfates (red). Compound names appear on the left of the graph and the corresponding structure is at the right. EC50 values were obtained as described in Materials and Methods. The vertical dashed lines indicate the EC50 values for the positive control ligands at each site.
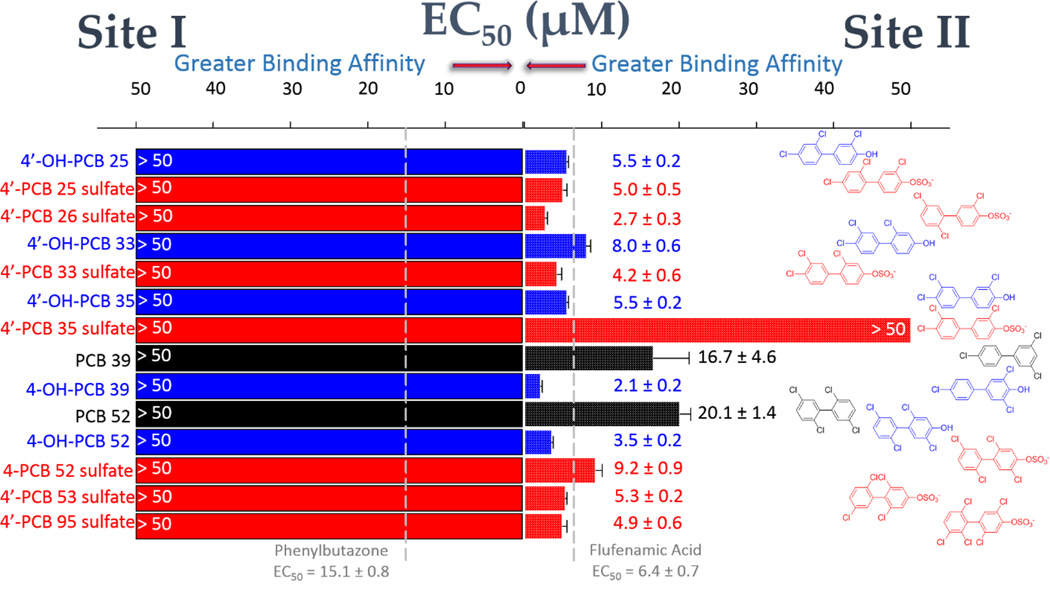
Binding of tri-, tetra-, and penta- PCB congeners and their metabolites to HSA
The interactions of selected tri- and tetra-chlorinated PCBs and their metabolites are shown in Figure 6. These results were consistent with the interpretation that HSA Site II was the major binding site for most of the dichlorinated PCB derivatives studied. With the exception of the tri-chlorinated 4’-PCB 35 sulfate mentioned above, there was a positive association between the degree of chlorination and binding affinity for the hydroxylated and sulfated PCBs (i.e., when comparing to monochlorinated and dichlorinated congeners). Furthermore, while most of the tri-, tetra-, and penta-chlorinated PCB derivatives generally appeared to have greater affinity for Site II than those of the dichlorinated congeners, it was consistently observed that there was no binding of these compounds to Site I.
Figure 6.
HSA-binding of tri-, tetra-, and penta- PCB congeners and their hydroxylated and sulfated metabolites. Site I binding (DNSA displacement) is shown at the left of the graph and Site II binding (DP displacement) is shown at the right. Color coding is used to differentiate the PCBs (black), OH-PCBs (blue), and PCB sulfates (red). Compound names appear on the left of the graph and the corresponding structure is at the right. EC50 values were obtained as described in Materials and Methods. The vertical dashed lines indicate EC50 values for the positive control ligands at each site.
The data presented here indicate that LC-PCB sulfates generally bind to HSA with higher affinity than their corresponding PCB or OH-PCB counterparts. Furthermore, LC-PCBs (those with 2+ chlorine atoms) and their hydroxylated and sulfated metabolites preferentially bind to Site II of the major drug binding sites in HSA. Interestingly, however, the monochlorinated metabolites bound with high affinity to both Sites I and II. Previous studies on the binding of individual higher chlorinated PCB congeners to HSA found that binding occurred at Site II.49, 50 The binding of PCB 153 and the PCB 180 to HSA was determined using a combination of intrinsic fluorescence, probe displacement and molecular modelling. It was determined that both compounds bound to a single site of HSA, Sudlow Site II, and that binding was dominated by hydrophobic interactions.49, 50 Interestingly, a separate study of PCB 118, 126, and 153 found that these compounds bound with high affinity to Sudlow Site I, with no binding to Site II.51 These seemingly contradictory conclusions may stem from the differences in the design of the studies.51 Although the approach reported here does not assess total protein binding, the high affinity binding of hydroxylated and sulfated LC-PCBs to HSA measured in this study underscores the importance that binding to human serum albumin may play in their subsequent distribution and effective half-lives in the body.
Environmental Relevance
The work presented here highlights the critical role that albumin may play in the binding, transport, and/or disposition of environmental toxic species such as PCBs and their metabolites. The presence and shared physiological roles of serum albumin in all vertebrates62 makes the understanding of this interaction helpful in determining the fates of these potential toxicants and their distribution to sensitive tissues in humans and other animals. This includes the HSA-mediated transfer of drugs and other xenobiotics transplacentally to the developing fetus63–66, which may implicate OH-PCBs and PCB sulfates in the neurodevelopmental effects associated with PCB exposure. This potential for retention and redistribution of PCB sulfates supports the idea that this has been a largely overlooked component of the overall picture of PCB exposure and toxicity. Finally, the selectivity of specific PCB metabolites for the major drug/toxicant binding sites on HSA may also be a factor that needs to be considered in the overall assessment of the contribution to toxic responses made by individual PCB congeners derived from exposure to PCB mixtures. This may be particularly important for airborne exposures to LC-PCBs where the PCB itself may be metabolized and not be detected in standard assays, yet OH-PCBs and PCB sulfates may be retained and either have toxic effects of their own or be further converted to other metabolites with adverse effects.
Supplementary Material
Acknowledgments
The authors thank Drs. Xianran He, Yang Song, Sanjay Telu and Sandhya Vyas from the Synthesis Core of the Iowa Superfund Research Program for the synthesis and characterization of the various PCB derivatives. This study was supported by a research grant P42 ES013661 from the National Institute of Environmental Health Sciences, NIH. We also acknowledge programmatic support through the University of Iowa Environmental Health Sciences Research Center (NIEHS/NIH P30 ES05605).
Footnotes
Associated Content
Supporting Information
- Synthesis and characterization of 2,2’,5,5’-tetrachloro-4-methoxybiphenyl, 2,2’,5,5’-tetrachlorobiphenyl-4-ol (4 OH PCB52), 2,2’,5,5’-tetrachlorobiphenyl-4-yl 2,2,2-trichloroethyl ester, and sulfuric acid mono-(2,2’,5,5’-tetrachlorobiphenyl-4-yl) ester, ammonium salt (4 PCB52 sulfate); Site I binding curves of LC-PCBs, OH-LC-PCBs, and LC-PCB sulfates to HSA; Site II binding curves of LC-PCBs, OH-LC-PCBs, and LC-PCB sulfates to HSA; HPLC analysis of the recovery and reversibility of HSA-binding for representative PCB sulfates.
References
- 1.Crinnion WJ. Polychlorinated biphenyls: persistent pollutants with immunological, neurological, and endocrinological consequences. Altern. Med. Rev. 2011;16(1):5–13. [PubMed] [Google Scholar]
- 2.ATSDR. US Department of Health and Human Services, Public Health Service Agency for Toxic Substances and Disease Registry. Atlanta, GA: 2000. Toxicological profile for polychlorinated biphenyls (PCBs) [PubMed] [Google Scholar]
- 3.Erickson MD, Kaley RG., 2nd Applications of polychlorinated biphenyls. Environ. Sci. Pollut. Res. 2011;18(2):135–151. doi: 10.1007/s11356-010-0392-1. [DOI] [PubMed] [Google Scholar]
- 4.Hope B, Scatolini S, Titus E, Cotter J. Distribution patterns of polychlorinated biphenyl congeners in water, sediment and biota from Midway Atoll (North Pacific Ocean) Mar. Pollut. Bull. 1997;34(7):548–563. [Google Scholar]
- 5.Gutleb AC, Cenijn P, Velzen M, Lie E, Ropstad E, Skaare JU, Malmberg T, Bergman A, Gabrielsen GW, Legler J. In vitro assay shows that PCB metabolites completely saturate thyroid hormone transport capacity in blood of wild polar bears (Ursus maritimus) Environ. Sci. Technol. 2010;44(8):3149–3154. doi: 10.1021/es903029j. [DOI] [PubMed] [Google Scholar]
- 6.Fonnum F, Mariussen E. Mechanisms involved in the neurotoxic effects of environmental toxicants such as polychlorinated biphenyls and brominated flame retardants. J. Neurochem. 2009;111(6):1327–1347. doi: 10.1111/j.1471-4159.2009.06427.x. [DOI] [PubMed] [Google Scholar]
- 7.de Cock M, van de Bor M. Obesogenic effects of endocrine disruptors, what do we know from animal and human studies? Environ. Int. 2014;70:15–24. doi: 10.1016/j.envint.2014.04.022. [DOI] [PubMed] [Google Scholar]
- 8.Schantz SL, Widholm JJ, Rice DC. Effects of PCB exposure on neuropsychological function in children. Environ. Health Perspect. 2003;111(3):357–576. doi: 10.1289/ehp.5461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Verner MA, Hart JE, Sagiv SK, Bellinger DC, Altshul LM, Korrick SA. Measured prenatal and estimated postnatal levels of polychlorinated biphenyls (PCBs) and ADHD-related behaviors in 8-year-old children. Environ. Health Perspect. 2015;123(9):888–894. doi: 10.1289/ehp.1408084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Serdar B, LeBlanc WG, Norris JM, Dickinson LM. Potential effects of polychlorinated biphenyls (PCBs) and selected organochlorine pesticides (OCPs) on immune cells and blood biochemistry measures: a cross-sectional assessment of the NHANES 2003–2004 data. Environ. Health. 2014;13:114. doi: 10.1186/1476-069X-13-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bell MR. Endocrine-disrupting actions of PCBs on brain development and social and reproductive behaviors. Curr. Opin. Pharmacol. 2014;19:134–144. doi: 10.1016/j.coph.2014.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robertson LW, Ludewig G. Polychlorinated Biphenyl (PCB) carcinogenicity with special emphasis on airborne PCBs. Gefahrstoffe - Reinhaltung der Luft. 2011;71(1–2):25–32. [PMC free article] [PubMed] [Google Scholar]
- 13.Lauby-Secretan B, Loomis D, Grosse Y, El Ghissassi F, Bouvard V, Benbrahim-Tallaa L, Guha N, Baan R, Mattock H, Straif K International Agency for Research on Cancer Monograph Working Group, IARC, Lyon France. Carcinogenicity of polychlorinated biphenyls and polybrominated biphenyls. Lancet Oncol. 2013;14(4):287–288. doi: 10.1016/S1470-2045(13)70104-9. [DOI] [PubMed] [Google Scholar]
- 14.Hu D, Lehmler HJ, Martinez A, Wang K, Hornbuckle KC. Atmospheric PCB congeners across Chicago. Atmos. Environ. 2010;44(12):1550–1557. doi: 10.1016/j.atmosenv.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liebl B, Schettgen T, Kerscher G, Broding HC, Otto A, Angerer J, Drexler H. Evidence for increased internal exposure to lower chlorinated polychlorinated biphenyls (PCB) in pupils attending a contaminated school. Int. J. Hyg. Environ. Health. 2004;207(4):315–324. doi: 10.1078/1438-4639-00296. [DOI] [PubMed] [Google Scholar]
- 16.Pedersen EB, Ebbehoj NE, Goen T, Meyer HW, Jacobsen P. Exposure to 27 polychlorinated biphenyls in the indoor environment of a workplace: a controlled bio-monitoring study. Int Arch Occup Environ Health. 2016;89(1):43–47. doi: 10.1007/s00420-015-1050-1. [DOI] [PubMed] [Google Scholar]
- 17.Carpenter DO. Exposure to and health effects of volatile PCBs. Rev. Environ. Health. 2015;30(2):81–92. doi: 10.1515/reveh-2014-0074. [DOI] [PubMed] [Google Scholar]
- 18.Hu D, Hornbuckle KC. Inadvertent polychlorinated biphenyls in commercial paint pigments. Environ. Sci. Technol. 2010;44(8):2822–2827. doi: 10.1021/es902413k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rodenburg LA, Guo J, Du S, Cavallo GJ. Evidence for unique and ubiquitous environmental sources of 3,3'-dichlorobiphenyl (PCB 11) Environ Sci Technol. 2010;44(8):2816–2821. doi: 10.1021/es901155h. [DOI] [PubMed] [Google Scholar]
- 20.Shang H, Li Y, Wang T, Wang P, Zhang H, Zhang Q, Jiang G. The presence of polychlorinated biphenyls in yellow pigment products in China with emphasis on 3,3'-dichlorobiphenyl (PCB 11) Chemosphere. 2014;98:44–50. doi: 10.1016/j.chemosphere.2013.09.075. [DOI] [PubMed] [Google Scholar]
- 21.Grimm FA, Hu D, Kania-Korwel I, Lehmler HJ, Ludewig G, Hornbuckle KC, Duffel MW, Bergman A, Robertson LW. Metabolism and metabolites of polychlorinated biphenyls. Crit. Rev. Toxicol. 2015;45(3):245–272. doi: 10.3109/10408444.2014.999365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu X, Adamcakova-Dodd A, Thorne PS. The fate of inhaled (14)C-labeled PCB11 and its metabolites in vivo. Environ. Int. 2014;63:92–100. doi: 10.1016/j.envint.2013.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van den Berg M, Birnbaum LS, Denison M, De Vito M, Farland W, Feeley M, Fiedler H, Hakansson H, Hanberg A, Haws L, Rose M, Safe S, Schrenk D, Tohyama C, Tritscher A, Tuomisto J, Tysklind M, Walker N, Peterson RE. The 2005 World Health Organization reevaluation of human and Mammalian toxic equivalency factors for dioxins and dioxin-like compounds. Toxicol. Sci. 2006;93(2):223–241. doi: 10.1093/toxsci/kfl055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Quinete N, Schettgen T, Bertram J, Kraus T. Occurrence and distribution of PCB metabolites in blood and their potential health effects in humans: a review. Environ. Sci. Pollut. Res. Int. 2014;21(20):11951–11972. doi: 10.1007/s11356-014-3136-9. [DOI] [PubMed] [Google Scholar]
- 25.Quinete N, Schettgen T, Bertram J, Kraus T. Analytical approaches for the determination of PCB metabolites in blood: a review. Anal. Bioanal. Chem. 2014;406(25):6151–6164. doi: 10.1007/s00216-014-7922-5. [DOI] [PubMed] [Google Scholar]
- 26.Marek RF, Thorne PS, DeWall J, Hornbuckle KC. Variability in PCB and OH-PCB serum levels in children and their mothers in urban and rural U.S. communities. Environ. Sci. Technol. 2014;48(22):13459–13467. doi: 10.1021/es502490w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schafer P, Muller M, Kruger A, Steinberg CE, Menzel R. Cytochrome P450-dependent metabolism of PCB52 in the nematode Caenorhabditis elegans. Arch. Biochem. Biophys. 2009;488(1):60–68. doi: 10.1016/j.abb.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 28.Spencer WA, Lehmler HJ, Robertson LW, Gupta RC. Oxidative DNA adducts after Cu(2+)-mediated activation of dihydroxy PCBs: role of reactive oxygen species. Free Radic. Biol. Med. 2009;46(10):1346–1352. doi: 10.1016/j.freeradbiomed.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu D, Li L, Liu L, Dong H, Deng Q, Yang X, Song E, Song Y. Polychlorinated biphenyl quinone induces mitochondrial-mediated and caspase-dependent apoptosis in HepG2 cells. Environ. Toxicol. 2015;30(9):1063–1072. doi: 10.1002/tox.21979. [DOI] [PubMed] [Google Scholar]
- 30.Flor S, Ludewig G. Polyploidy-induction by dihydroxylated monochlorobiphenyls: structure-activity-relationships. Environ. Int. 2010;36(8):962–969. doi: 10.1016/j.envint.2010.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ekuase EJ, Liu Y, Lehmler HJ, Robertson LW, Duffel MW. Structure-activity relationships for hydroxylated polychlorinated biphenyls as inhibitors of the sulfation of dehydroepiandrosterone catalyzed by human hydroxysteroid sulfotransferase SULT2A1. Chem Res Toxicol. 2011;24(10):1720–1728. doi: 10.1021/tx200260h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kester MH, Bulduk S, van Toor H, Tibboel D, Meinl W, Glatt H, Falany CN, Coughtrie MW, Schuur AG, Brouwer A, Visser TJ. Potent inhibition of estrogen sulfotransferase by hydroxylated metabolites of polyhalogenated aromatic hydrocarbons reveals alternative mechanism for estrogenic activity of endocrine disrupters. J. Clin. Endocrinol. Metab. 2002;87(3):1142–1150. doi: 10.1210/jcem.87.3.8311. [DOI] [PubMed] [Google Scholar]
- 33.Liu Y, Apak TI, Lehmler HJ, Robertson LW, Duffel MW. Hydroxylated polychlorinated biphenyls are substrates and inhibitors of human hydroxysteroid sulfotransferase SULT2A1. Chem. Res. Toxicol. 2006;19(11):1420–1425. doi: 10.1021/tx060160+. [DOI] [PubMed] [Google Scholar]
- 34.Grimm FA, Lehmler HJ, He X, Robertson LW, Duffel MW. Sulfated metabolites of polychlorinated biphenyls are high-affinity ligands for the thyroid hormone transport protein transthyretin. Environ. Health Perspect. 2013;121(6):657–662. doi: 10.1289/ehp.1206198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dhakal K, He X, Lehmler HJ, Teesch LM, Duffel MW, Robertson LW. Identification of sulfated metabolites of 4-chlorobiphenyl (PCB3) in the serum and urine of male rats. Chem Res Toxicol. 2012;25(12):2796–2804. doi: 10.1021/tx300416v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grimm FA, He X, Teesch LM, Lehmler HJ, Robertson LW, Duffel MW. Tissue Distribution, Metabolism, and Excretion of 3,3'-Dichloro-4'-sulfooxy-biphenyl in the Rat. Environ. Sci. Technol. 2015;49(13):8087–8095. doi: 10.1021/acs.est.5b01499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mizuma T, Komori M, Ueno M, Horikoshi I. Sulphate conjugation enhances reversible binding of drug to human serum albumin. J. Pharm. Pharmacol. 1991;43(6):446–448. doi: 10.1111/j.2042-7158.1991.tb03509.x. [DOI] [PubMed] [Google Scholar]
- 38.Muller WE, Wollert U. Human serum albumin as a 'silent receptor' for drugs and endogenous substances. Pharmacology. 1979;19(2):59–67. doi: 10.1159/000137289. [DOI] [PubMed] [Google Scholar]
- 39.Peters TJ. Biochemistry, Genetics, and Medical Applications. San Diego: Academic Press; 1996. All About Albumin. [Google Scholar]
- 40.Sudlow G, Birkett DJ, Wade DN. The characterization of two specific drug binding sites on human serum albumin. Mol. Pharmacol. 1975;11(6):824–832. [PubMed] [Google Scholar]
- 41.Kragh-Hansen U, Chuang VT, Otagiri M. Practical aspects of the ligand-binding and enzymatic properties of human serum albumin. Biol. Pharm. Bull. 2002;25(6):695–704. doi: 10.1248/bpb.25.695. [DOI] [PubMed] [Google Scholar]
- 42.He XM, Carter DC. Atomic structure and chemistry of human serum albumin. Nature. 1992;358(6383):209–215. doi: 10.1038/358209a0. [DOI] [PubMed] [Google Scholar]
- 43.Li X, Parkin S, Duffel MW, Robertson LW, Lehmler HJ. An efficient approach to sulfate metabolites of polychlorinated biphenyls. Environ. Int. 2010;36(8):843–848. doi: 10.1016/j.envint.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lehmler HJ, Robertson LW. Synthesis of hydroxylated PCB metabolites with the Suzuki-coupling. Chemosphere. 2001;45(8):1119–1127. doi: 10.1016/s0045-6535(01)00052-2. [DOI] [PubMed] [Google Scholar]
- 45.Lehmler HJ, He X, Li X, Duffel MW, Parkin S. Effective synthesis of sulfate metabolites of chlorinated phenols. Chemosphere. 2013;93(9):1965–1971. doi: 10.1016/j.chemosphere.2013.06.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sudlow G, Birkett DJ, Wade DN. Spectroscopic techniques in the study of protein binding: the use of 1-anilino-8-naphthalenesulphonate as a fluorescent probe for the study of the binding of iophenoxic and iopanoic acids to human serum albumin. Mol. Pharmacol. 1973;9(5):649–657. [PubMed] [Google Scholar]
- 47.Fasano M, Curry S, Terreno E, Galliano M, Fanali G, Narciso P, Notari S, Ascenzi P. The extraordinary ligand binding properties of human serum albumin. IUBMB Life. 2005;57(12):787–796. doi: 10.1080/15216540500404093. [DOI] [PubMed] [Google Scholar]
- 48.Ghuman J, Zunszain PA, Petitpas I, Bhattacharya AA, Otagiri M, Curry S. Structural basis of the drug-binding specificity of human serum albumin. J. Mol. Biol. 2005;353(1):38–52. doi: 10.1016/j.jmb.2005.07.075. [DOI] [PubMed] [Google Scholar]
- 49.Fang S, Li H, Liu T, Xuan H, Li X, Zhao C. Molecular interaction of PCB180 to human serum albumin: insights from spectroscopic and molecular modelling studies. J. Mol. Model. 2014;20(4):2098. doi: 10.1007/s00894-014-2098-7. [DOI] [PubMed] [Google Scholar]
- 50.Han C, Fang S, Cao H, Lu Y, Ma Y, Wei D, Xie X, Liu X, Li X, Fei D, Zhao C. Molecular interaction of PCB153 to human serum albumin: insights from spectroscopic and molecular modeling studies. J. Hazard. Mater. 2013;248–249:313–321. doi: 10.1016/j.jhazmat.2012.12.056. [DOI] [PubMed] [Google Scholar]
- 51.Rownicka-Zubik J, Sulkowski L, Toborek M. Interactions of PCBs with human serum albumin: in vitro spectroscopic study. Spectrochim. Acta. A Mol. Biomol. Spectrosc. 2014;124:632–637. doi: 10.1016/j.saa.2014.01.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen YM, Guo LH. Fluorescence study on site-specific binding of perfluoroalkyl acids to human serum albumin. Arch. Toxicol. 2009;83(3):255–261. doi: 10.1007/s00204-008-0359-x. [DOI] [PubMed] [Google Scholar]
- 53.Mohammed A, Eklund A, Ostlund-Lindqvist AM, Slanina P. Distribution of toxaphene, DDT, and PCB among lipoprotein fractions in rat and human plasma. Arch Toxicol. 1990;64(7):567–571. doi: 10.1007/BF01971836. [DOI] [PubMed] [Google Scholar]
- 54.Guo YL, Emmett EA, Pellizzari ED, Rohde CA. Influence of serum cholesterol and albumin on partitioning of PCB congeners between human serum and adipose tissue. Toxicology and applied pharmacology. 1987;87(1):48–56. doi: 10.1016/0041-008x(87)90083-4. [DOI] [PubMed] [Google Scholar]
- 55.Borlakoglu JT, Welch VA, Wilkins JP, Dils RR. Transport and cellular uptake of polychlorinated biphenyls (PCBs)--I. Association of individual PCB isomers and congeners with plasma lipoproteins and proteins in the pigeon. Biochem Pharmacol. 1990;40(2):265–272. doi: 10.1016/0006-2952(90)90687-g. [DOI] [PubMed] [Google Scholar]
- 56.Persoon C, Peters TM, Kumar N, Hornbuckle KC. Spatial distribution of airborne polychlorinated biphenyls in Cleveland, Ohio and Chicago, Illinois. Environ. Sci. Technol. 2010;44(8):2797–2802. doi: 10.1021/es901691s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Aki H, Yamamoto M. Thermodynamic characterization of drug binding to human serum albumin by isothermal titration microcalorimetry. J. Pharm. Sci. 1994;83(12):1712–1716. doi: 10.1002/jps.2600831213. [DOI] [PubMed] [Google Scholar]
- 58.Baraka-Vidot J, Planesse C, Meilhac O, Militello V, van den Elsen J, Bourdon E, Rondeau P. Glycation alters ligand binding, enzymatic, and pharmacological properties of human albumin. Biochemistry. 2015;54(19):3051–3062. doi: 10.1021/acs.biochem.5b00273. [DOI] [PubMed] [Google Scholar]
- 59.Kragh-Hansen U. Molecular and practical aspects of the enzymatic properties of human serum albumin and of albumin–ligand complexes. Biochimica et Biophysica Acta (BBA) - General Subjects. 2013;1830(12):5535–5544. doi: 10.1016/j.bbagen.2013.03.015. [DOI] [PubMed] [Google Scholar]
- 60.Dhakal K, Uwimana E, Adamcakova-Dodd A, Thorne PS, Lehmler HJ, Robertson LW. Disposition of phenolic and sulfated metabolites after inhalation exposure to 4-chlorobiphenyl (PCB3) in female rats. Chem. Res. Toxicol. 2014;27(8):1411–1420. doi: 10.1021/tx500150h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dhakal K, Adamcakova-Dodd A, Lehmler HJ, Thorne PS, Robertson LW. Sulfate conjugates are urinary markers of inhalation exposure to 4-chlorobiphenyl (PCB3) Chem. Res. Toxicol. 2013;26(6):853–855. doi: 10.1021/tx4001539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Doolittle RF. The Evolution of the Vertebrate Plasma-Proteins. Biol. Bull. 1987;172(3):269–283. [Google Scholar]
- 63.Mathiesen L, Rytting E, Mose T, Knudsen LE. Transport of benzo[alpha]pyrene in the dually perfused human placenta perfusion model: effect of albumin in the perfusion medium. Basic Clin. Pharmacol. Toxicol. 2009;105(3):181–187. doi: 10.1111/j.1742-7843.2009.00431.x. [DOI] [PubMed] [Google Scholar]
- 64.Earhart AD, Patrikeeva S, Wang X, Abdelrahman DR, Hankins GD, Ahmed MS, Nanovskaya T. Transplacental transfer and metabolism of bupropion. J. Matern. Fetal Neonatal Med. 2010;23(5):409–416. doi: 10.1080/14767050903168424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nanovskaya TN, Patrikeeva S, Hemauer S, Fokina V, Mattison D, Hankins GD, Ahmed MS. Effect of albumin on transplacental transfer and distribution of rosiglitazone and glyburide. J. Matern. Fetal Neonatal Med. 2008;21(3):197–207. doi: 10.1080/14767050801929901. [DOI] [PubMed] [Google Scholar]
- 66.Nanovskaya TN, Bowen RS, Patrikeeva SL, Hankins GD, Ahmed MS. Effect of plasma proteins on buprenorphine transfer across dually perfused placental lobule. J. Matern. Fetal Neonatal Med. 2009;22(8):646–653. doi: 10.1080/14767050802610328. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.