Abstract
Diabetes is an independent risk factor of cardiovascular disease that can eventually cause cardiomyopathy and heart failure. Cardiac fibroblasts (CF) are the critical mediators of physiological and pathological cardiac remodeling, however, the effects of hyperglycemia on cardiac fibroblast function and differentiation is not well known. Here, we performed a comprehensive investigation on the effects of hyperglycemia on cardiac fibroblasts and show that hyperglycemia enhances cardiac fibroblast function and differentiation. We found that high glucose treatment increased collagen I, III and VI gene expression in rat adult cardiac fibroblasts. Interestingly, hyperglycemia increased CF migration and proliferation which is augmented by collagen I and III. Surprisingly, we found that short term hyperglycemia transiently inhibited ERK1/2 activation but increased AKT phosphorylation. Finally, high glucose treatment increased spontaneous differentiation of cardiac fibroblasts to myofibroblasts with increasing passage compared to low glucose. Taken together, these findings suggest that hyperglycemia induces cardiac fibrosis by modulating collagen expression, migration, proliferation and differentiation of cardiac fibroblasts.
Keywords: cardiac fibroblast, collagen, diabetes, high glucose, migration, myofibroblast, proliferation
INTRODUCTION
Diabetic patients are ten times more likely to develop cardiovascular disease compared to the general population (Deckert et al., 1978; Dorman et al., 1984; Orchard et al., 2006). After a myocardial infarction, diabetic patients are at a higher risk to develop heart failure and prone to developing diffuse cardiac fibrosis, specifically a drastic increase in fibrillar collagen (types I and III) (Aragno et al., 2008; Kelly et al., 2007; Loganathan et al., 2006; Mak et al., 1997; Shimizu et al., 1993; Tsutsui et al., 2007; Van Linthout et al., 2007). Cardiac fibroblasts (CF) are the major non-myocyte cell type in the heart and are responsible for extracellular matrix (ECM) deposition. The ECM operates as the glue of the myocardium and components including collagen types I and III provide a structural scaffold to the cells in the heart, but importantly they also alter the activity of cardiac fibroblasts via cell-matrix signaling. During cardiac remodeling in response to injury and insult, CFs are activated i.e. proliferate, migrate, and differentiate to the hypersecretory, wound healing myofibroblast phenotype. We have previously reported that collagen types I and III induce cardiac fibroblast proliferation whereas type VI promotes myofibroblast differentiation (Naugle et al., 2006).
Hyperglycemia alters proliferation and migration in other cell types; it promotes smooth muscle cell (SMC) proliferation in vitro (Natarajan et al., 1992; Watson et al., 2001; Yasunari et al., 1995). Interestingly, Peiro et al. (2001) and Zheng et al. (2007) reported contradictory results that high glucose inhibited SMC proliferation (Peiro et al., 2001; Zheng et al., 2007). Varma et al. (2005) reported that hyperglycemic concentrations of 20 mM and 40 mM glucose significantly decreased human umbilical vein endothelial cell (HUVEC) proliferation compared to 5 mM glucose media(Varma et al., 2005). High glucose also stimulates cardiac fibroblast proliferation in vitro and increased fibroblast collagen production (Asbun et al., 2005; Muona et al., 1993; Neumann et al., 2002; Tang et al., 2007). However, there are no comprehensive reports on the effects of hyperglycemia on specific types of collagen production and their influence on cardiac fibroblasts proliferation, migration and differentiation in the presence of high glucose. In the present study, we investigated the role of hyperglycemia and collagen matrices on adult rat CF proliferation, migration and differentiation and the underlying signaling mechanisms activated.
MATERIALS AND METHODS
Materials
Dulbecco’s Modified Eagle’s Medium (DMEM), penicillin/streptomycin, fungizone and fetal bovine serum (FBS) were all purchased from Invitrogen/GIBCO (Grand Island, NY). Anti-phospho-AKT and total AKT antibodies were obtained from Cell Signaling Technology (Beverly, MA). Anti-phospho-ERK 1/2 and anti-ERK 1/2 antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). α-SMA antibody, angiotensin II and epidermal growth factor were acquired from Sigma-Aldrich (St. Louis, MO). The non-radioactive cell proliferation assay was purchased from Promega (Madison, WI).
Isolation of Cardiac Fibroblasts
Cardiac fibroblasts were isolated from adult, male, Sprague-Dawley rats as described by our previous studies (Naugle et al., 2006; Olson et al., 2005; S et al., 2005). Rats were euthanized according to guidelines and approval of the Institutional Animal Care and Use Committee (IACUC) of the Northeast Ohio Medical University (NEOMED). Upon initial passing, cells were divided into three groups: low glucose, long-term high glucose, short-term high glucose. Low glucose cells were cultured in 5 mmol/l DMEM, which is comparable to the normal physiological level of blood glucose. Long-term high glucose cells were cultured in high glucose, 25 mmol/l, DMEM after initial plating and remained in high glucose until experimentation at passage 2. Short-term high glucose cells were cultured in low glucose DMEM until time of experimental stimulus when the cells were changed to high glucose DMEM. Passage 2 cells were used for all cardiac fibroblast experiments and cells were switched to serum-free DMEM, with the appropriate glucose concentration, 24 hours prior to experimentation.
Scratch-Wound Migration Assay
CFs were plated onto collagen I, III and tissue culture plastic. Cells were grown to 90-100% confluence, serum-starved for 24 hours, and scratched with a 200 μL pipet tip. Wounds were washed with 10% PBS and fresh 2% FBS DMEM media, low and high glucose depending upon the group was added. Images were taken at 0, 6, 12, and 24 hours.
ERK1/2 and AKT Phosphorylation
CFs from each group were plated onto 60 mm dishes and protein lysates were collected at 0 min, 5 min, 20 min, 60 min, 4 h, and 24 h following the addition of the high glucose stimulus for the short-term group, the other groups were treated with their original glucose levels. In some cases, cells were also pretreated with 20 nM ANG II and 20 nM EGF. Protein lysates were subjected to Western blot analysis and membranes were probed with phospho-specific and total ERK1/2 and AKT antibodies.
Measurement Cardiac Fibroblast Proliferation
Passage 2 fibroblasts from each group were plated onto uncoated or type I collagen and type III collagen coated 96 well tissue culture plates. At this step, high glucose media was added to the short term high glucose level cells. After 24 h, cells were incubated with MTT reagents and proliferation was measured via a colorimetric nonradioactive 96-well assay.
Cardiac fibroblasts differentiation to myofibroblasts
Cardiac fibroblasts were serum-starved for 24 h, followed by 24 h incubation in high glucose, and whole cell lysates were collected. Cell lysates were also collected from fibroblasts treated with low glucose or long-term high glucose at each passage up to passage 5. Cardiac fibroblast differentiation to myofibroblast was performed by assessing changes in α-SMA expression using Western blot analysis.
Western Blot Analysis
Cell lysates were collected as previously described by our lab (Olson et al., 2005; Olson et al., 2008; Shamhart et al., 2009). Protein levels were quantified with the BCA method (Pierce) and equal amount of protein were mixed with 2X sample buffer (100mM Tris base, 20% glycerol, 2% SDS, and 0.01% bromophenol blue) and boiled for 5 minutes. Then the proteins were separated by SDS-PAGE and transferred to a PVDF membrane and the membranes were blocked in 0.1% Tween-20-TBS containing either BSA for phospho-antibodies or milk for total antibodies for one hour at room temperature. The membranes were incubated overnight in primary antibody, washed three times in 0.1% Tween-20-TBS, and then incubated in secondary antibody for one hour at room temperature followed by five washes. The protein signals were detected using ECL supersignal (Pierce) and the band intensity was quantified by densitometric scanning using a Kodak 1D Digital Science Imaging System. Naphthol blue staining was used as a loading control for each Western blot.
Real-time quantitative PCR
The expression levels of collagen I, III and VI transcripts were determined with real-time qPCR. Total RNA was isolated from CFs cultured in low and high glucose media, cDNA was synthesized using reverse transcription and qPCR was performed using collagen I, III and VI pecific primers. GAPDH was used as a control and collagen levels were expressed relative to the GAPDH levels.
Statistical analysis
Statistical analysis was performed using GraphPad Prism 4.0 statistical software. Statistical significance (p<0.05) between groups was determined by one-way ANOVA.
RESULTS
High glucose increases collagen production in cardiac fibroblasts
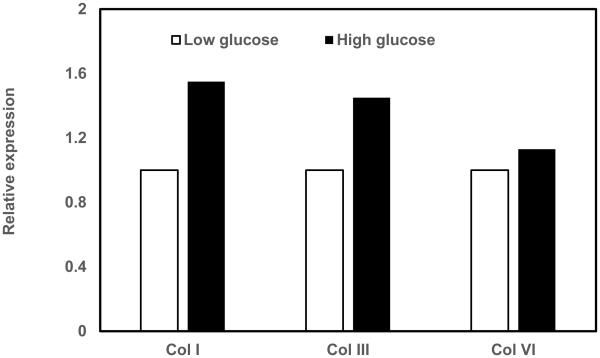
Since collagen production is one of the important functions of CF, we first investigated if high glucose induces expression of specific collagen types. We found that high glucose treatment increased gene expression of collagen I, collagen III and collagen VI (Fig.1).
Figure 1. RT-PCR analysis of collagens.
Cardiac fibroblasts were cultured in either low glucose (5 mM) or high glucose (25 mM) and total RNA was isolated and subjected cDNA was synthesized using reverse transcription. The expression of collagen I, III, VI and GAPDH transcripts were measured in real-time PCR, normalized for GAPDH and expressed relative to low glucose conditions.
High glucose augments collagen I and III induced cardiac fibroblast migration
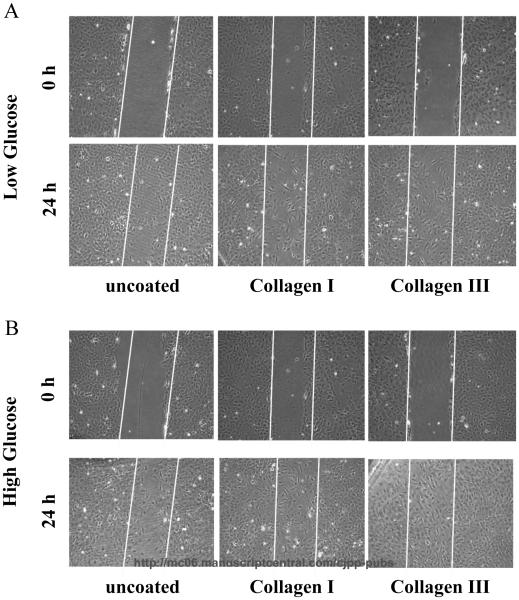
Next, we asked if specific collagen subtype influences (Naugle et al., 2006) fibroblast migration in the presence of low and high glucose. To achieve this, we measured CF migration using scratch-wound assay. We found that CF migration in low glucose was increased on type I and III collagens (20-25% more migration) compared to normal tissue culture plates after 24 h (Fig. 2A). We next determined the influence of high glucose on CF migration. Scratch-wound assay revealed that the high glucose treatment increased CF migration (10% more migration) on both collagen I, III and tissue culture plates compared to low glucose-treated cells (Fig. 2B).
Figure 2. Hyperglycemia and Collagen types I and III potently promote cardiac fibroblast migration.
Cardiac fibroblasts were plated onto collagen types I and III and tissue culture plates. Cells were grown to 90-100% confluence, serum-starved for 24 hours, and scratched with 200 μL pipet tip. The wounds were washed with PBS and fresh media with the appropriate glucose concentration was added to the cells. Panel A depicts increased fibroblast migration at 24 hours on both collagen types I and III in low glucose. Panel B reveals the compounded effects of hyperglycemia (high glucose) and collagen types I and III on cardiac fibroblast migration. Data are representative of triplicate wells from 4 separate CF preparations (n=4).
Hyperglycemia induces cardiac fibroblast proliferation
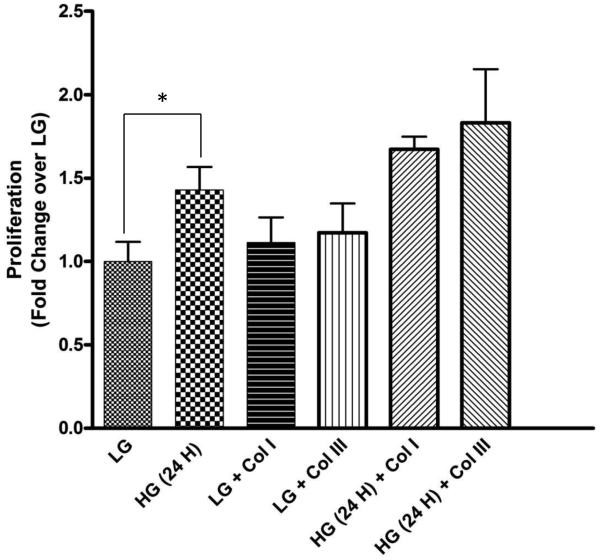
We next investigated if high glucose and/or collagen subtype influences CF proliferation. MTT assays revealed high glucose significantly increased CF proliferation compared to low glucose (1.43±0.11 fold; p<0.05) (Fig. 3). Collagen types I and III also induced proliferation of fibroblasts compared to low glucose treatment (1.11±0.15 and 1.17±0.17 fold, respectively) which was increased in presence of high glucose (1.17±0.07 and 1.28±0.32, respectively) (Fig. 3).
Figure 3. Hyperglycemia stimulates cardiac fibroblast proliferation.
Cardiac fibroblasts were cultured in 96 well culture plates uncoated or coated with collagen I and III in the presence of low glucose (LG) and high glucose (HG) for 24 h. High glucose significantly induced fibroblast proliferation. Data are representative of 5 separate wells from 3 separate CF preparations (n=5). Statistical significance (p<0.05) between groups was determined by one-way ANOVA.
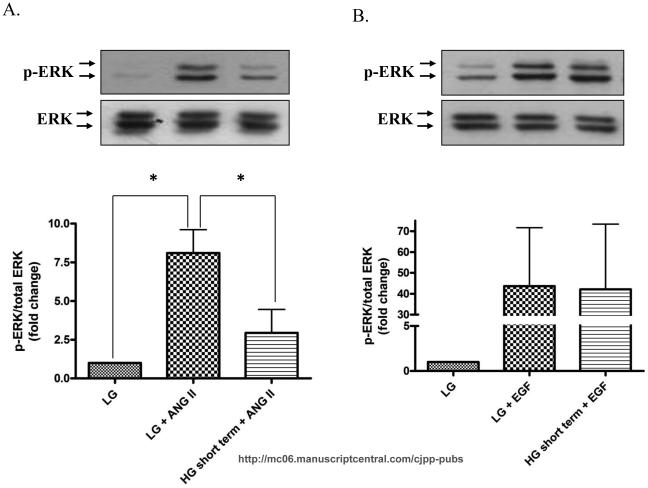
Short-term high glucose treatment transiently inhibits ERK 1/2 phosphorylation
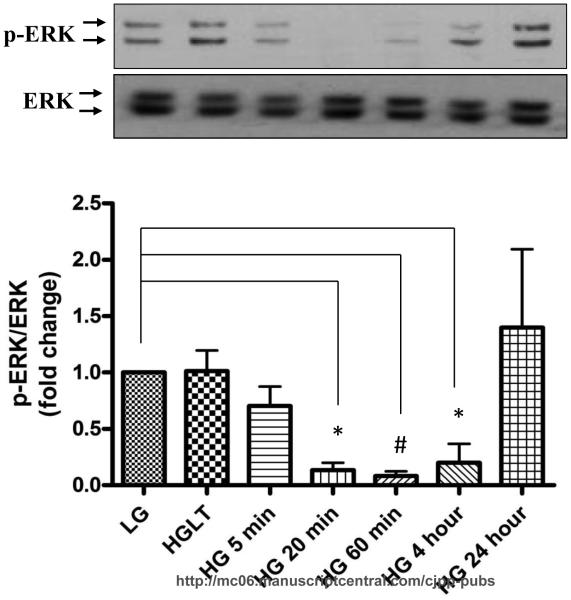
We next investigated the signaling mechanisms responsible for hyperglycemia induced CF proliferation. Initially we focused on the classical MAPK pathway because ERK 1/2 is a common mediator for high glucose stimulated proliferation in other cell types (Popov and Simionescu, 2006; Venkatachalam et al., 2008). Surprisingly, we found that short-term high glucose treatment significantly decreased basal ERK1/2 phosphorylation in a time-dependent manner from 5-60 min after which it started to increase at 4 h and reached to basal level at 24 h (Fig. 4).
Figure 4. High glucose transiently inhibits basal ERK 1/2 phosphorylation.
Cardiac fibroblasts were cultured in either low glucose (5 mM) or high glucose (25 mM) for the designated time and cell lysates were collected and subjected to Western blot analysis for phospho-ERK and total ERK. A) Basal ERK phosphorylation was lower in fibroblasts treated with high glucose for 20 min (0.13±0.06 fold, p<0.05), 60 min (0.08±0.42 fold, p<0.001), and 4 h (0.20±0.16 fold, p<0.05). Data are representative of duplicate wells from 4 separate CF preparations (n=4). Statistical significance (p<0.05) between groups was determined by one-way ANOVA.
Next, we asked if high glucose also influences agonist-induced ERK1/2 activation. We have previously reported that ANG II activates ERK 1/2 through concurrent calcium and PKCδ pathways (Olson et al., 2008). As expected, ANG II stimulated ERK 1/2 phosphorylation (p<0.05) (Fig. 5A), however, high glucose treatment significantly reduced the ANG II-induced ERK1/2 phosphorylation (Fig. 5A). Interestingly, hyperglycemic conditions did not inhibit EGF-induced ERK 1/2 phosphorylation (Fig. 5B).
Figure 5. High glucose inhibits ANG II-induced ERK 1/2 activation.
CF were either culture in low glucose or high glucose and then stimulated with ANG II or EGF for 5 minutes. Cell lysates were collected and subjected to Western blot analysis for phospho-ERK and total ERK. A) Representative Western blot and quantitative analysis depicting that high glucose pretreatment attenuates ANG II-induced ERK1/2 phosphorylation (p<0.05). B) In contrast, high glucose does not prevent EGF-induced ERK 1/2 activation. Data are representative of duplicate from 4 separate CF preparations (n=4).
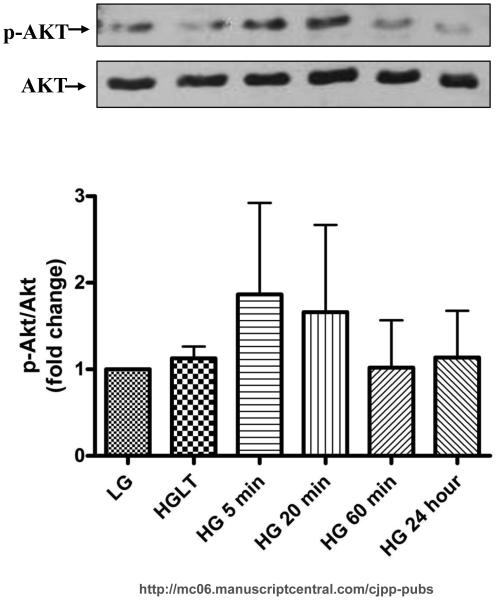
Previous studies in other cell types, including endothelial cells and mouse embryonic stem cells, revealed AKT activation mediates cell proliferation (Kim et al., 2006; Varma et al., 2005). Therefore, we next asked if high glucose mediates CF proliferation through activation of AKT. We found that high glucose induced AKT activation within 5 (1.86±0.19 fold) and 20 minute (1.661±1.000 fold) (Fig. 6).
Figure 6. Short-term hyperglycemia increases AKT phsphorylation.
CF were cultured in the indicated glucose concentrations for the time frame specified. The representative Western blot and quantitative analysis reveal that incubation with high glucose for 5 and 20 min increases AKT phosphorylation. Data are representative of duplicate wells from 4 separate CF preparations (n=4).
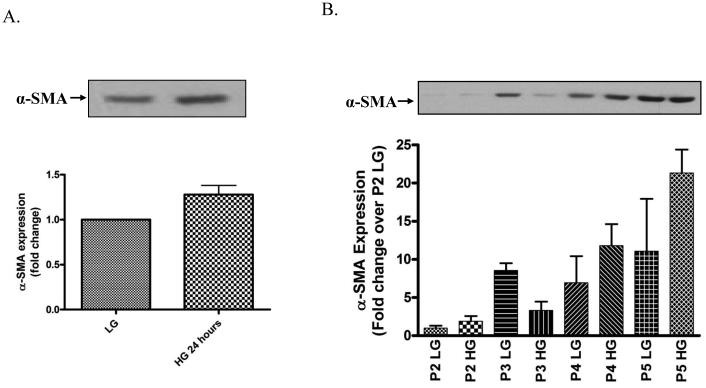
Hyperglycemia enhances cardiac fibroblast differentiation to myofibroblasts in vitro
Finally, we investigated the direct impact of short-term and long-term hyperglycemia on CF differentiation to myofibroblasts by measuring α-SMA levels. We found that short-term high glucose treatment for 24 h significantly increased the expression of α-SMA (1.28±0.10 fold) compared to CF cultured in low glucose medium (Fig. 7A). Further, we found that long-term high glucose treatment increased the spontaneous differentiation of CF to myofibroblasts as evidenced by increased α-SMA expression at each passage with maximum increase at passages 4 and 5 (Fig. 7B).
Figure 7. Hyperglycemia accelerates CFs differentiation to myofibroblasts.
Cardiac fibroblasts were cultured in either low glucose (5 mM) or high glucose (25 mM) for the designated time frame and cell lysates were collected and subjected to Western blot analysis for a-SMA expression. Representative Western blot and summary graph for α-SMA expression following a 24 h high glucose incubation (A) and at each cell culture passage (B). Each Western blot was normalized to bands from the napthol blue staining of the same membrane. Each blot and summary graph are mean fold change ± SEM and representative duplicate wells from 2 separate CF preparations (n=2).
DISCUSSION
In the present study, we demonstrate that hyperglycemia induced expression of collagen I, III and VI in cardiac fibroblasts and increased their migration, proliferation and differentiation. We also demonstrate that short-term high glucose transiently inhibits basal and agonist-induced ERK1/2 activation. Although there are few studies on high glucose effects on cardiac fibroblasts (Asbun et al., 2005; Tang et al., 2007; Venkatachalam et al., 2008; Yuen et al., 2010), we believe this work forms a comprehensive study on the effects of hyperglycemia on collagen expression, proliferation, migration and differentiation of cardiac fibroblasts.
Most of the past studies have centered on the influence of hyperglycemia on the cardiac myocytes, with few studies focusing on the cardiac fibroblasts. However, cardiac fibroblasts are the major matrix producing cells in the heart and are responsible for aberrant remodeling and fibrosis (Porter and Turner, 2009). Diabetes is an independent risk factor for cardiovascular disease and diabetic patients are predisposed to cardiovascular complications including fibrosis, therefore it is important to study the impact of hyperglycemia on fibroblast activation to determine specific targets that may potentially combat the progression of the fibrosis (Aragno et al., 2008; Kelly et al., 2007; Loganathan et al., 2006; Tsutsui et al., 2007; Van Linthout et al., 2007).
Since cardiac fibrosis is a critical event in diabetic cardiomyopathy and pathological remodeling of the heart, we first measured collagen expression by cardiac fibroblasts. Similar to previous studies, we also found that hyperglycemia increased collagen types I and III (Asbun et al., 2005; Tang et al., 2007). Interestingly, we found that hyperglycemia also induced collagen VI expression. We have previously shown that collagen I and III regulates CF proliferation whereas collagen VI induces CF differentiation. Therefore, we first investigated the effects of collagen I and III on CF migration in the presence and absence of high glucose. We found that adult cardiac fibroblasts stimulated with high glucose migrated faster than fibroblasts in low glucose. Collagen types I and III potently promoted CF migration, and plating on the collagen substrates had an equal additive effect on the hyperglycemia-induced CF migration. In contrast, neonatal fibroblasts plated on type I collagen and cultured in hyperglycemic media migrated slower than in normo-glycemic media (Zhang et al., 2007). This could be due to differences in the origin of fibroblasts and the type of migration assays i.e. migration of neonatal fibroblasts from aggregates in a three-dimensional collagen gel compared to scratch-wound assay with adult CFs in the present study.
We have previously reported that cardiac fibroblasts isolated directly from the diabetic myocardium were more proliferative than fibroblasts from the healthy, control myocardium (Shamhart et al., 2009). In fact, high glucose has been found to stimulate cardiac fibroblast proliferation in vitro but as described for migration, different labs utilized a unique cell culture system (Asbun and Villarreal, 2006; Neumann et al., 2002), therefore we sought to establish the impact of hyperglycemia on fibroblast proliferation in our system. Similar to previous studies, we found that high glucose significantly increased CF proliferation and collagen types I and III had an additive effect to the hyperglycemia-induced proliferation. However, in contrast to previous reports, we found that high glucose transiently inhibited ERK1/2 phosphorylation. ERK1/2 signaling is a common pathway for high glucose stimulated proliferation in CFs and other cell types (Popov and Simionescu, 2006; Venkatachalam et al., 2008). Tang et al. (2007) reported that high glucose exposure for 1 and 2 h increased ERK 1/2 activity in cardiac fibroblasts (Tang et al., 2007), but surprisingly we observed a significant decrease in ERK 1/2 phosphorylation at early time points (5- 60 min). The differing results may be from variations in the isolation or culture conditions or species or from the age of the animals. Importantly our data are from adult Sprauge-Dawley rat CF, whereas Tang et al. utilized CF from 1- to 3-day-old Wistar rats. Venkatachalam et al. (2009) reported that high glucose treatment for 1 h induced ERK1/2 activation in mouse CF, which was shown to be downstream of AKT activation (Venkatachalam et al., 2008). We have previously demonstrated that ANG II significantly induced ERK 1/2 phosphorylation (Olson et al., 2008) and CF differentiation, however, in the present study short-term high glucose treatment attenuated the ANG II-induced ERK 1/2 phosphorylation. Interestingly, it did not affect EGF-stimulated ERK1/2 activation. Our observation that hyperglycemia inhibits ERK 1/2 phosphorylation is quite surprising, therefore we explored if AKT is activated by hyperglycemia. AKT was shown to be activated by short-term hyperglycemic treatment in mouse cardiac fibroblasts (Venkatachalam et al., 2008), mouse embryonic stem cells and mesangial cells (Kim et al., 2006; Sheu et al., 2004). Moreover, AKT was shown to be upstream of ERK1/2 activation in mouse cardiac fibroblasts (Venkatachalam et al., 2008). Importantly, we found that high glucose induced an increase in AKT phosphorylation as early as 5 min which came back to basal levels by 24 h. These results suggest high glucose may differentially regulate ERK1/2 and AKT phosphorylation and that high glucose may mediate CF proliferation through the activation of AKT.
One of the critical events in cardiac remodeling is the differentiation of CFs to myofibroblasts. We found that incubation of CF in high glucose for 24 h increased α-SMA production. We also found that continuous culturing of CF in high glucose up to passage 5 accelerated α-SMA expression compared to CF cultured in low glucose at the respective passage. Zhang et al. (2007) demonstrated hyperglycemia treatment for 48 h promoted α-SMA expression in neonatal CF (Zhang et al., 2007). Yuen et al. have recently reported that modified collagen treated with methylglycoxal, a glucose metabolite, promoted myofibroblast differentiation (Yuen et al., 2010). These data collectively suggest hyperglycemia promotes myofibroblast differentiation in vitro. However, we have previously demonstrated that myofibroblast content is decreased in the heart of type1 diabetic rats(Shamhart et al., 2009). We believe this could be due to the short time effects of high glucose (days-2 weeks) compared to long term chronic effects of high glucose environment in diabetic hearts. This could also be due to a hyperglycemia-independent event occurring in type 1 diabetic myocardium. However, these findings indicate contrasting results between in vitro vs in vivo conditions. Indeed, we found that collagen VI promoted CF differentiation to myofibroblasts in vitro (Naugle et al., 2006), but myofibroblast content was unchanged in Collagen VI null mice (Luther et al., 2012).
In conclusion, our study demonstrates hyperglycemia increases collagen I, III and VI production, migration, proliferation and differentiation in cardiac fibroblasts. We also demonstrate that collagen I and III augments hyperglycemia-induced CF migration and proliferation. Further, we present evidence that hyperglycemia may mediate these effects through the activation of AKT rather than ERK1/2.
Acknowledgements
This work is supported by NIH-1R15HL106442-01 and Ohio Board of Regents (JGM and CKT).
REFERENCES
- Aragno M, Mastrocola R, Alloatti G, Vercellinatto I, Bardini P, Geuna S, Catalano MG, Danni O, Boccuzzi G. Oxidative stress triggers cardiac fibrosis in the heart of diabetic rats. Endocrinology. 2008;149:380–388. doi: 10.1210/en.2007-0877. [DOI] [PubMed] [Google Scholar]
- Asbun J, Manso AM, Villarreal FJ. Profibrotic influence of high glucose concentration on cardiac fibroblast functions: effects of losartan and vitamin E. Am J Physiol Heart Circ Physiol. 2005;288:H227–H234. doi: 10.1152/ajpheart.00340.2004. [DOI] [PubMed] [Google Scholar]
- Asbun J, Villarreal FJ. The Pathogenesis of Myocardial Fibrosis in the Setting of Diabetic Cardiomyopathy. Journal of American College of Cardiology. 2006;47:693–700. doi: 10.1016/j.jacc.2005.09.050. [DOI] [PubMed] [Google Scholar]
- Deckert T, Poulsen JE, Larsen M. Prognosis of diabetics with diabetes onset before the age of thirty-one. I. Survival, causes of death, and complications. Diabetologia. 1978;14:363–370. doi: 10.1007/BF01228130. [DOI] [PubMed] [Google Scholar]
- Dorman JS, Laporte RE, Kuller LH, Cruickshanks KJ, Orchard TJ, Wagener DK, Becker DJ, Cavender DE, Drash AL. The Pittsburgh insulin-dependent diabetes mellitus (IDDM) morbidity and mortality study. Mortality results. Diabetes. 1984;33:271–276. doi: 10.2337/diab.33.3.271. [DOI] [PubMed] [Google Scholar]
- Kelly DJ, Zhang Y, Connelly K, Cox AJ, Martin J, Krum H, Gilbert R. Tranilast attenuates diastolic dysfunction and structural injury in experimental diabetic cardiomyopathy. Am J Physiol Heart Circ Physiol. 2007;293:H2860–2869. doi: 10.1152/ajpheart.01167.2006. [DOI] [PubMed] [Google Scholar]
- Kim YH, Heo JS, Han HJ. High glucose increase cell cycle regulatory proteins level of mouse embryonic stem cells via PI3-K/Akt and MAPKs signal pathways. J Cell Physiol. 2006;209:94–102. doi: 10.1002/jcp.20706. [DOI] [PubMed] [Google Scholar]
- Loganathan R, Bilgen M, Al-Hafez B, Smirnova IV. Characterization of alterations in diabetic myocardial tissue using high resolution MRI. Int J Cardiovasc Imaging. 2006;22:81–90. doi: 10.1007/s10554-005-5386-6. [DOI] [PubMed] [Google Scholar]
- Luther DJ, Thodeti CK, Shamhart PE, Adapala RK, Hodnichak C, Weihrauch D, Bonaldo P, Chilian WM, Meszaros JG. Absence of type VI collagen paradoxically improves cardiac function, structure, and remodeling after myocardial infarction. Circulation Research. 2012;110:851–856. doi: 10.1161/CIRCRESAHA.111.252734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak KH, Moliterno DJ, Granger CB, Miller DP, White HD, Wilcox RG, Califf RM, Topol EJ. Influence of diabetes mellitus on clinical outcome in the thrombolytic era of acute myocardial infarction. GUSTO-I Investigators. Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries. J Am Coll Cardiol. 1997;30:171–179. doi: 10.1016/s0735-1097(97)00118-6. [DOI] [PubMed] [Google Scholar]
- Muona P, Jaakkola S, Zhang R-Z, Pan T-C, Pelliniemi L, Risteli L, Chu M-L, Uitto J, Peltonen J. Hyperglycemic Glucose Concentrations Up-Regulate the Expression of Type VI Collagen in Vitro. American Journal of Pathology. 1993;142:1586–1597. [PMC free article] [PubMed] [Google Scholar]
- Natarajan R, Gonzales N, Xu L, Nadler JL. Vascular smooth muscle cells exhibit increased growth in response to elevated glucose. Biochem Biophys Res Commun. 1992;187:552–560. doi: 10.1016/s0006-291x(05)81529-3. [DOI] [PubMed] [Google Scholar]
- Naugle JE, Olson ER, Zhang X, Mase SE, Pilati CF, Maron MB, G FH, Horne WI, Doane KJ, Meszaros JG. Type VI collagen induces cardiac myofibroblast differentiation: implications for postinfarction remodeling. Am J Physiol Heart Circ Physiol. 2006;290:H323–330. doi: 10.1152/ajpheart.00321.2005. [DOI] [PubMed] [Google Scholar]
- Neumann S, Huse K, Semrau R, Diegeler A, Gebhardt R, Buniatian GH, Scholz GH. Aldosterone and D-Glucose Stimulate the Proliferation of Human Cardiac Myofibroblasts In Vitro. Hypertension. 2002;39:756–760. doi: 10.1161/hy0302.105295. [DOI] [PubMed] [Google Scholar]
- Olson ER, Naugle JE, Zhang X, Bomser JA, Meszaros JG. Inihibition of cardiac fibroblast proliferation and myofibroblast differentiation by resveratrol. Am J Physiol Heart Circ Physiol. 2005;288:H1131–1138. doi: 10.1152/ajpheart.00763.2004. [DOI] [PubMed] [Google Scholar]
- Olson ER, Shamhart PE, Naugle JE, Meszaros JG. Angiotensin II-induced extracellular signal-regulated kinase 1/2 activation is mediated by protein kinase C delta and intracellular calcium in adult rat cardiac fibroblasts. Hypertension. 2008;51(3):704–11. doi: 10.1161/HYPERTENSIONAHA.107.098459. [DOI] [PubMed] [Google Scholar]
- Orchard TJ, Costacou T, Kretowski A, Nesto RW. Type 1 diabetes and coronary artery disease. Diabetes Care. 2006;29:2528–2538. doi: 10.2337/dc06-1161. [DOI] [PubMed] [Google Scholar]
- Peiro C, Lafuente N, Matesanz N, Cercas E, Llergo JL, Vallejo S, Rodriguez-Manas L, Sanchez-Ferrer CF. High glucose induces cell death of cultured human aortic smooth muscle cells through the formation of hydrogen peroxide. Br J Pharmacol. 2001;133:967–974. doi: 10.1038/sj.bjp.0704184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popov D, Simionescu M. Cellular mechanisms and signalling pathways activated by high glucose and AGE-albumin in the aortic endothelium. Arch Physiol Biochem. 2006;112:265–273. doi: 10.1080/13813450601094573. [DOI] [PubMed] [Google Scholar]
- Porter KE, Turner NA. Cardiac fibroblasts: at the heart of myocardial remodeling. Pharmacology & therapeutics. 2009;123:255–278. doi: 10.1016/j.pharmthera.2009.05.002. [DOI] [PubMed] [Google Scholar]
- S SJ, Roth DM, Olson ER, Naugle JE, Meszaros JG, Insel PA. Inhibition of cardiac myofibroblast formation and collagen synthesis by activation and overexpression of adenylyl cyclase. Proc Natl Acad Sci U S A. 2005;102:437–442. doi: 10.1073/pnas.0408704102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamhart PE, Luther DJ, Hodson BR, Koshy JC, Ohanyan V, Meszaros JG. Impact of type 1 diabetes on cardiac fibroblast activation: enhanced cell cycle progression and reduced myofibroblast content in the diabetic myocardium. Am J Physiol Endocrinol Metab. 2009;297(5):E1147–53. doi: 10.1152/ajpendo.00327.2009. [DOI] [PubMed] [Google Scholar]
- Sheu ML, Ho FM, Chao KF, Kuo ML, Liu SH. Activation of phosphoinositide 3-kinase in response to high glucose leads to regulation of reactive oxygen species-related nuclear factor-kappaB activation and cyclooxygenase-2 expression in mesangial cells. Mol Pharmacol. 2004;66:187–196. doi: 10.1124/mol.66.1.187. [DOI] [PubMed] [Google Scholar]
- Shimizu M, Umeda K, Sugihara N, Yoshio H, Ino H, Takeda R, Okada Y, Nakanishi I. Collagen remodelling in myocardia of patients with diabetes. J Clin Pathol. 1993;46:32–36. doi: 10.1136/jcp.46.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang M, Zhang W, Lin H, Jiang H, Dai H, Zhang Y. High glucose promotes the production of collagen types I and III by cardiac fibroblasts through a pathway dependent on extracellular-signal-regulated kinase 1/2. Mol Cell Biochem. 2007;301:109–114. doi: 10.1007/s11010-006-9401-6. [DOI] [PubMed] [Google Scholar]
- Tsutsui H, Matsushima S, Kinugawa S, Ide T, Inoue N, Ohta Y, Yokota T, Hamaguchi S, Sunagawa K. Angiotensin II type 1 receptor blocker attenuates myocardial remodeling and preserves diastolic function in diabetic hearts. Hypertens Res. 2007;30:439–449. doi: 10.1291/hypres.30.439. [DOI] [PubMed] [Google Scholar]
- Van Linthout S, Riad A, Dhayat N, Westermann D, Hilfiker-Kleiner D, Noutsias M, Laufs U, Schultheiss H-P, Tschope C. Anti-inflammatory effects of atorvastatin improve left ventricular function in experimental diabetic cardiomyopathy. Diabetologia. 2007;50:1977–1986. doi: 10.1007/s00125-007-0719-8. [DOI] [PubMed] [Google Scholar]
- Varma S, Lal B, Zheng RK, Breslin JW, Saito S, Pappas PJ, Hobson RWI, Duran WN. Hyperglycemia alters PI3k and Akt signaling and leads to endothelial cell proliferative dysfunction. Am J Physiol Heart Circ Physiol. 2005;289:H1744–1751. doi: 10.1152/ajpheart.01088.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatachalam K, Mummidi S, Cortez DM, Prabhu SD, Valente AJ, Chandrasekar B. Resveratrol inhibits high glucose-induced PI3K/Akt/ERK-dependent interleukin-17 expression in primary mouse cardiac fibroblasts. Am J Physiol Heart Circ Physiol. 2008;294:H2078–2087. doi: 10.1152/ajpheart.01363.2007. [DOI] [PubMed] [Google Scholar]
- Watson PA, Nesterova A, Burant CF, Klemm DJ, Reusch JE. Diabetes-related changes in cAMP response element-binding protein content enhance smooth muscle cell proliferation and migration. J Biol Chem. 2001;276:46142–46150. doi: 10.1074/jbc.M104770200. [DOI] [PubMed] [Google Scholar]
- Yasunari K, Kohno M, Kano H, Yokokawa K, Horio T, Yoshikawa J. Aldose reductase inhibitor prevents hyperproliferation and hypertrophy of cultured rat vascular smooth muscle cells induced by high glucose. Arterioscler Thromb Vasc Biol. 1995;15:2207–2212. doi: 10.1161/01.atv.15.12.2207. [DOI] [PubMed] [Google Scholar]
- Yuen A, Laschinger C, Talior I, Lee W, Chan M, Birek J, Young EW, Sivagurunathan K, Won E, Simmons CA, McCulloch CA. Methylglyoxal-modified collagen promotes myofibroblast differentiation. Matrix Biol. 2010;29(6):537–48. doi: 10.1016/j.matbio.2010.04.004. [DOI] [PubMed] [Google Scholar]
- Zhang X, Stewart JAJ, Kane ID, Massey EP, Cashatt DO, Carver WE. Effects of elevated glucose levels on interactions of cardiac fibroblasts with the extracellular matrix. In vitro cell.Dev.biol-Animal. 2007;43:297–305. doi: 10.1007/s11626-007-9052-2. [DOI] [PubMed] [Google Scholar]
- Zheng XL, Yuan SG, Peng DQ. Phenotype-specific inhibition of the vascular smooth muscle cell cycle by high glucose treatment. Diabetologia. 2007;50:881–890. doi: 10.1007/s00125-006-0543-6. [DOI] [PubMed] [Google Scholar]