Abstract
The expression of kidney injury molecule-1 (KIM-1), a very promising sensitive and specific urinary biomarker for acute renal injury, is markedly upregulated in injured and regenerating renal proximal tubular epithelial cells following ischemic or toxic insults, suggesting a possible role for this molecule in renal repair process. In the present study we report that expression of KIM-1 facilitates renal tubular epithelial cell repair by promoting cell migration and proliferation. KIM-1 expression also enhances ERK MAPK activation, and the modulatory effect of KIM-1 on cellular repair process is likely mediated via ERK MAPK signaling pathway.
Keywords: Kidney injury molecule-1 (KIM-1), cellular repair, cell migration, cell proliferation, ERK MAPK
INTRODUCTION
Acute kidney injury is a common medical disease associated with very high overall mortality [1]. It is unlikely that this high mortality and associated cost will be reduced until we have a better understanding of the cellular and molecular mechanisms for renal cell injury and repair. Epithelial cell injury is fundamental to the pathophysiology of acute kidney injury, and the S3 segment of the proximal tubule is particularly susceptible to ischemic and toxic injury [2]. Unlike the brain and heart, where ischemically injured tissue cannot recover after injury, the kidney with its complex architecture has the ability to recover both morphologically as well as functionally. However, the underlying mechanism regulated renal epithelial cell repair processes remains largely unknown.
Kidney injury molecule-1 (KIM-1), also known as HAVCR (hepatitis A virus cellular receptor) [3] and TIM-1 (T-cell immunoglobulin and mucin protein) [4], is a type 1 transmembrane glycoprotein markedly upregulated in the injured and regenerating renal proximal tubule epithelial cells of the human and rodent kidney following ischemic [5] and toxic [6] injury to the kidney. KIM-1 protein is localized primarily to the apical membrane of the cells and the ectodomain of KIM-1 undergoes membrane–proximal cleavage via a highly regulated process, which leads to the release of soluble KIM-1 into the urine [7, 8]. Urinary KIM-1 has been shown to be a very promising novel biomarker in human and rodents and outperforms traditional biomarker of kidney injury in preclinical biomarker qualification studies [9–12].
Recently KIM-1 has been shown to be a phosphatidylserine receptor that confers a phagocytic phenotype on epithelial cells [13]. The expression of this molecule in renal tubules mediates epithelial cell phagocytosis of apoptotic cells, which protects the kidney after acute injury by downregulating innate immunity and inflammation [14]. KIM-1 also physically interacts with and inhibits cellular Ga12 activation after inflammatory stimuli by blocking GTP binding to Ga12, which protects mice against renal ischemia-reperfusion injury [15]. On the other hand, renal tubule repair process is generally characterized by migration and proliferation of the intrinsic dedifferentiated epithelia across the denuded tubule basement membrane [16], but the mechanisms that regulate these processes remain poorly understood. Since renal epithelial cells that express KIM-1 are typically dedifferentiated, characterized by a loss of the brush border membrane and expression of mesenchymal proteins such as the intermediate filament protein vimentin [5], the present study was therefore designed to examine the role of KIM-1 in renal tubular epithelial cell repair focusing on the migration and proliferation processes.
MATERIALS AND METHODS
Materials
Dulbecco’s modified Eagle’s medium (DMEM), Dulbecco’s modified Eagle’s medium/Han’s F12 (DMEM/F12), hygromycin, and G418 were obtained from Mediatech (VA, USA). Fetal bovine serum (FBS), doxycycline, MTT (Methylthiazolyldiphenyl-tetrazolium bromide), and U0126 were obtained from Sigma (MO, USA). Epidermal growth factor (EGF) and matrigel were purchased from BD Biosciences (NY, USA). Antibodies against human KIM-1 were generated as described [7] and have been used in our previous study [8].
Cell culture and transfections
LLC-PK1 cells were grown in DMEM supplemented with 10% FBS and maintained at 37°C in 5% CO2. These cells were transfected with eukaryotic expression vector pcDNA3-neo containing the cDNAs encoding for wild type human KIM-1 or pcDNA3-neo alone using Superfect reagent (Qiagen, CA, USA). Stable clones were selected and maintained in DMEM containing G418 (400 μg/ml). KIM1-tet-off MDCKII cells (referred as MDCK-KIM-1 here) were generous gift from Dr. Benjamin Humphreys (Harvard medical school). They were generated as described in previous study [13] and cultured in DMEM/F-12 supplemented with 10% FBS, 250 μg/ml hygromycin, and 100 μg/ml of G418. Cells were routinely maintained in 100 ng/ml doxycycline to prevent KIM-1 transcription until induction was initiated by its withdrawal.
Epithelium wound healing assay
Cellular repair was assessed by an in vitro model of epithelium wound healing. Confluent cell monolayers grown in glass coverslips were wounded with a sterile surgical blade and remained in culture for various times. At the end of experiment, the monolayers were washed with ice-cold PBS, fixed with 100% methanol, and stained with 0.1% crystal violet, and photographed. In some experiments, the “wounded area” (cell free region) was assessed using Image J program (NIH). The “wound-healing” at different time points were calculated and expressed as a percentage of the wounded area at baseline (0 h).
Cell migration
To assess the complex migration processes of epithelial monolayers in cell culture without available time-lapse video microscopy, we have developed a test system that reproducibly measures the ability of cells to cover a cell culture surface coated with selected components of extracellular matrix. For this assay, a small glass cover slip covered with monolayer of confluent cells was placed upside-down on cell culture plate coated with or without matrigel (2.5 μg/ml) and cultured in complete media for various times. Cell migration from the edge of cover slip was then observed under a phase contrast microscope and photographed. To distinguish cell migration from proliferation, mitomycin C (Sigma, MO, USA) at 0.01mg/ml was added to culture media to inhibit cell proliferation.
Cell proliferation
After overnight serum deprivation, cells were harvested with trypsin/EDTA (inactivated by trypsin inhibitor, not serum containing media), seeded in 96-well cell culture plate, and cultured in the presence or absence of 20% FBS for various times. Cell proliferation was then assessed by MTT staining and expressed as percentage of cell proliferation according to the following formula: Cell survival (%) = OD (optical density) at various times/OD at baseline (0 h) × 100.
Activation of ERK MAPK
Confluent cells were serum deprived overnight and then incubated with various concentrations of FBS or EGF for various times. Activation of ERK MAPK was then assessed by western blot analysis using phospho-specific antibody against ERK MAPK.
Western blot analysis
The cell monolayers were lysed on ice in lysis buffer supplemented with protease inhibitors. Aliquots of cell lysates were boiled in SDS-PAGE sample buffer, fractionated on 10% SDS-PAGE gel, and transferred to PVDF membrane. After blocking with 5% nonfat dry milk in TBS/0.05% Tween, the blots were then incubated with the respective primary antibodies overnight at 4°C, followed by peroxidase-conjugated secondary antibody and ECL detection.
Statistical Analyses
Continuous variables were reported as mean ± standard deviation. The differences between groups were analyzed by the Student t test or one-way analysis of variance (ANOVA). A p value of less than 0.05 was considered statistically significant.
RESULTS
Heterologous expression of KIM-1 facilitates renal tubular epithelial cell repair
LLC-PK1 cells, a porcine renal tubular epithelial cell line, were chosen for the current study because they do not express detectable endogenous KIM-1. Overexpression of human KIM-1 in these cells was achieved via stable transfection. As shown in Figure 1A, the expression pattern of KIM-1 in KIM-1-LLC cells was similar to that of 769-P cells, a human renal cell adenocarcinoma cell line expressing high levels of endogenous KIM-1 [7, 8]. The 100 kDa band is the actual cell surface glycosylated protein (marked with*) and the 60 kDa band most likely corresponds to KIM-1 transiting through the Golgi [7]. By contrast, there is no KIM-1 expression at all in control (pcDNA3-LLC) cells. In the current study, we tested the effect of KIM-1 overexpression in an in vitro model of epithelium wound healing. As shown in Figure 1B, a mechanical wound was completely healed in less than 16 hours in KIM-1 expressing LLC-PK1 cells; while in control pcDNA3-LLC cells, it remained unhealed even after 24 hours, indicating that upregulation of KIM-1 facilitates renal tubular epithelial cell repair.
Figure 1. Stable expression of KIM-1 enhances renal tubular epithelial cell repair.
A. Overexpression of KIM-1 in LLC-PK1 cells. LLC-PK1 cells were stably transfected with eukaryotic expression vector pcDNA3-neo encoding cDNA of human KIM-1 (KIM-1-LLC) or empty vector alone (pcDNA3-LLC). Levels of human KIM-1 expression were determined by western blot analysis in cell lysates (upper panel). The mature, fully glycosylated KIM-1 protein is marked with an (*). Detection of ERK-2 in the same blot was included to assess sample loading (lower panel). B. KIM-1 expression facilitates “epithelium wound healing”. Stably transfected LLC-PK1 cells were grown on a glass cover slip. The cell monolayers were then wounded via mechanical scratch, and the wounded areas were recorded at various times.
KIM-1 expression promotes migration and proliferation of renal tubular epithelial cells
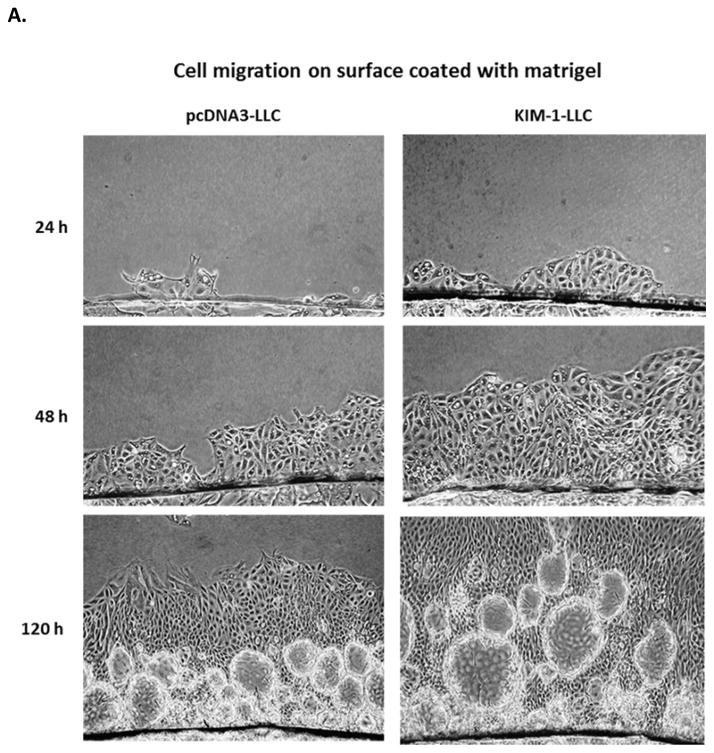
Cell migration and proliferation are two most important cellular events during the repair processes. We next examined whether the cellular repair conferred by KIM-1 overexpression are related to these cellular functions. Since a time-lapse video microscopy is not easily accessible, we have developed a novel assay to assess the migration of cell monolayer under physiologic condition. As shown in Figure 2A, cell migration over a surface coated with matrigel, an extract of the EHS sarcoma that is enriched in basement membrane matrix, is significantly faster in KIM-1 expressing LLC-PK1 cells compared to their controls (pcNDA3-LLC), indicating KIM-1 overexpression promotes renal epithelial cell migration. On the other hand, migration over an uncoated surface is slow in both cells without notable differences between them (Figure 2B), suggesting that the enhanced cell migration conferred by KIM-1 expression is not just a general feature but is specific to interaction with basement membrane components, similar to a wound healing process.
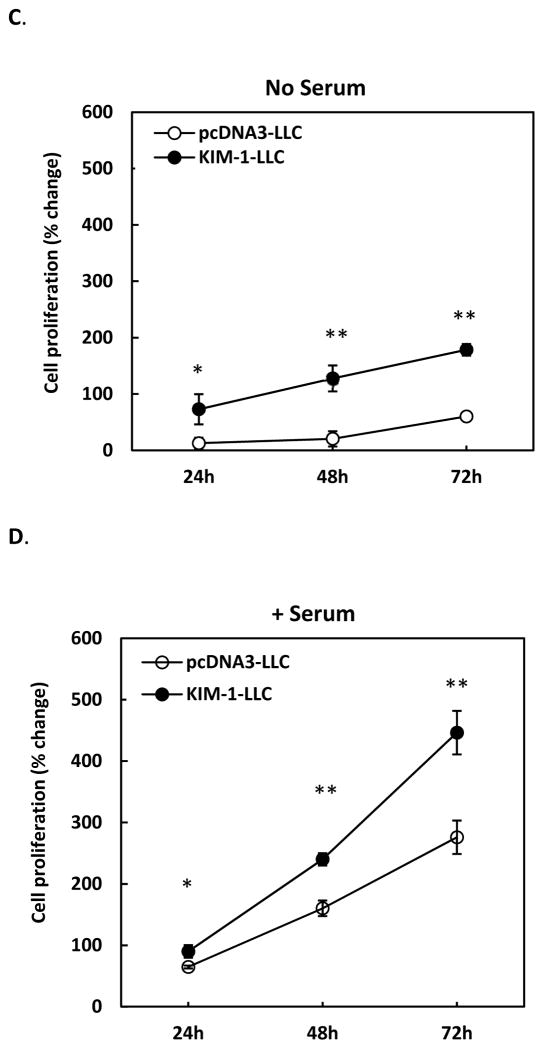
Figure 2. Overexpression of KIM-1 enhances renal tubular epithelial cell migration and proliferation.
LLC-PK1 cells were stably transfected with human KIM-1 (KIM-1-LLC) or empty vector alone (pcDNA3-LLC). To assess cell migration, confluent cell monolayer grown on a small glass cover slip was placed upside-down on cell culture plate coated with (A) or without matrigel (B) and cultured for various times. Cell migration from the edge of cover slip was then recorded. For cell proliferation, cells were seeded in a 96-well cell culture plate in the absence (C) or presence (D) of serum (10% FBS) and incubated for various times. Proliferation of these cells was then measured by MTT staining and expressed as percentage of growth. Results are mean +/− standard deviation from 3 different experiments performed in triplicate for each treatment. ** p < 0.01 compared with pcDNA3-LLC.
We have also evaluated the effect of KIM-1 overexpression on cell proliferation. As shown in Figure 2C&D, KIM-1 expressing LLC-PK1 cells grew significantly faster than the control pcDNA3-LLC cells in the absence or presence of serum. More interestingly, in response to serum-derived growth factor, KIM-1 expressing cells grew exponentially, as compared to a nearly linear pattern of growth in controls cells (Figure 2D), suggesting KIM-1 expression may enhance cellular response to growth signaling and promote cell proliferation.
Regulated expression of KIM-1 enhances renal tubular epithelial cell repair
Stable transfection may alter the phenotypic characteristics of cells over time and results from those experiments may not reflect the situation of endogenous KIM-1 expression under physiologic conditions. We have therefore established a clone of MDCK cells (MDCK-KIM-1) baring a doxycycline sensitive promoter, which has the ability to control when and how much of KIM-1 is expressed. MDCK is a canine renal tubular epithelial cell line expressing only modest amount of endogenous KIM-1. As shown in Figure 3A, withdrawal of doxycycline in the culture media resulted in a time-dependent expression of KIM-1 in MDCK-KIM-1 cells as early as less than 12 hours, achieving a significant level of expression in these cells after 48 hours of doxycycline withdrawal. Thus, to allow sufficient KIM-1 expression, doxycycline was withdrawn from culture media for at least 48 hours prior to experiment. In a similar model of epithelium wound healing, the wounded area was significantly smaller in cells expressing KIM-1 (with doxycycline withdrawn) compared with control cells that were cultured in the presence of doxycycline to prevent KIM-1 transcription (Figure 3B&C). These results were consistent with that performed with stable transfection in different cell line, indicating our findings were common features in renal epithelial cells.
Figure 3. Regulated expression of KIM-1 enhances renal tubular epithelial cell repair.
MDCK-KIM-1 cells, a clone of MDCK cells in which the expression of human KIM-1 can be regulated under the control of a doxycycline sensitive promoter, were routinely cultured in media containing doxycycline to suppress KIM-1 expression. The expression of KIM-1 in these cells was then induced by withdrawal of doxycycline from culture media for various times and the levels of human KIM-1 expression were determined in cell lysates by western blot analysis (A). The mature, fully glycosylated KIM-1 protein is marked with an (*), and detection of ERK-2 in the same blot was included to assess sample loading (lower panel). To evaluate the effect of regulated expression of KIM-1 on epithelial cell repair, MDCK-KIM-1 cells were grown on a glass cover slip in the presence or absence of doxycycline for at least 48 hours to allow sufficient expression of KIM-1 prior to experiment. The cell monolayers were then wounded via mechanical scratch, and the wounded areas were recorded (B) and quantitated (C) after 6 hours. * p < 0.01 compared with MDCK-KIM-1 cells cultured with doxycycline.
KIM-1 expression enhances ERK MAPK activation
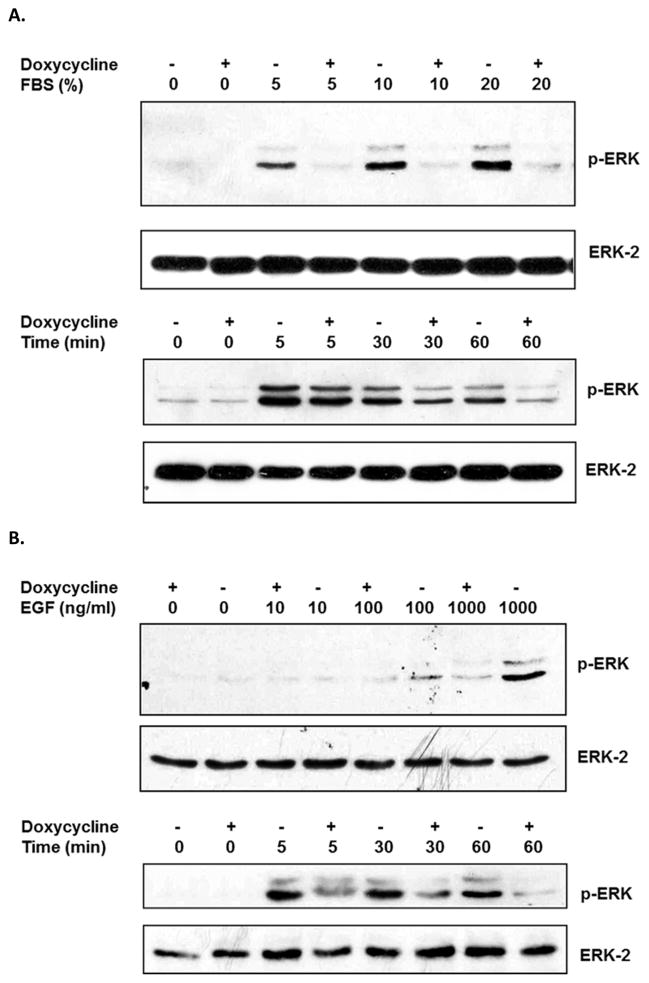
Activation of ERK MAPK plays important role in epithelial wound healing process [17]. To evaluate whether overexpression of KIM-1 influences ERK MAPK activities, serum-deprived MDCK-KIM-1 cells were exposed to various concentration of FBS and ERK activation was monitored. In the presence of doxycycline, lack of KIM-1 expression in these cells resulted in a weak activation of ERK MAPK in response to high concentration of FBS (20%, Figure 4A, upper panel). On the other hand, induction of KIM-1 expression in the absence of doxycycline led to a very strong response to FBS even at a concentration as low as 5% (Figure 4A, upper panel). Time course study also indicated that the activation of ERK MAPK was more sustained in KIM-1 expressing cells (Figure 4A, lower panel). Similarly, KIM-1 expression induced a more potent and sustained ERK MAPK activation in response to EGF (Figure 4B), one of the most important growth factors involved in epithelial wound healing process [18].
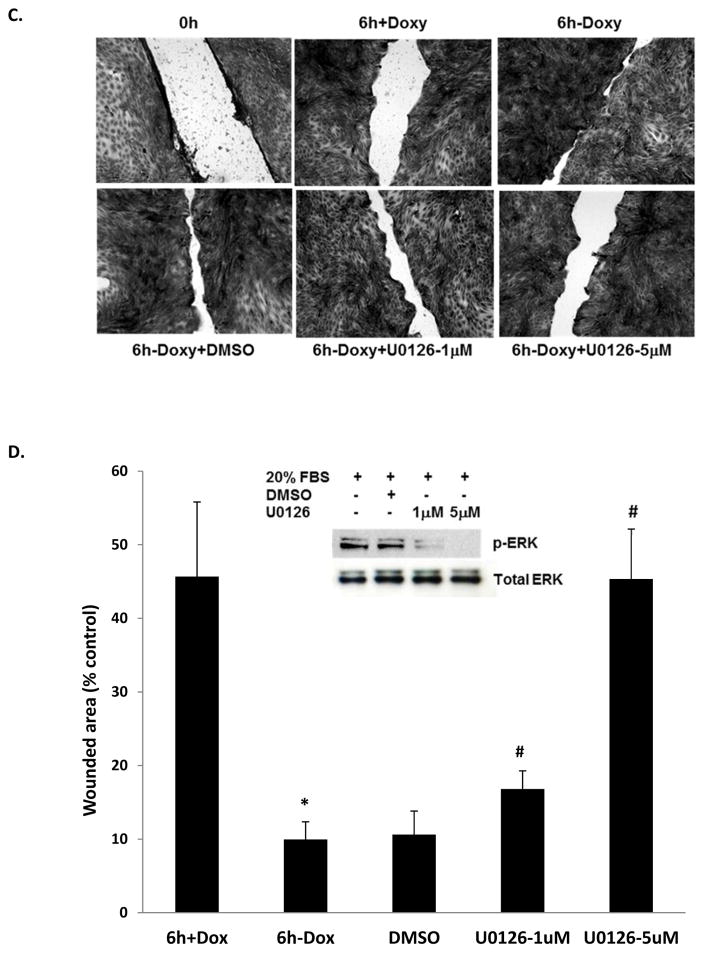
Figure 4. KIM-1-modulated renal tubular epithelial cell repair is mediated by ERK MAPK signaling pathway.
MDCK-KIM-1 cells were cultured in the presence or absence of doxycycline for at least 48 hours to allow sufficient expression of KIM-1 prior to experiment. A. Activation of ERK MAPK in response to FBS. Cells were exposed to various dilution of FBS for 1 hour (upper panel) or exposed to 20% FBS for various times (lower panel) after overnight serum deprivation, The levels of ERK MAPK activation were determined in cell lysates by western blot analysis using an antibody against phosphorylated ERK, and detection of ERK-2 in the same blot was included to assess sample loading (lower lane of each panel). B. Activation of ERK MAPK in response to EGF. Cells were exposed to various concentrations of EGF for 1 hour (upper panel) or exposed to 1000 ng/ml of EGF for various times (lower panel) after overnight serum deprivation. The levels of ERK MAPK activation were determined in cell lysates by western blot analysis using an antibody against phosphorylated ERK, and detection of ERK-2 in the same blot was included to assess sample loading (lower lane of each panel). C-D. Effect of ERK MAPK inhibition on epithelium wound healing. The cell monolayers were wounded via mechanical scratch and cultured in the presence of various concentrations of U0126 or its vehicle DMSO. The wounded areas were then recorded (C) and quantitated (D) after 6 hours. The inhibition of ERK MAPK activation by U0126 was also confirmed in these cells (D). * p < 0.01 compared with MDCK-KIM-1 cells cultured with doxycycline, # p < 0.01 compared with MDCK-KIM-1 cells cultured without doxycycline.
The modulatory effect of KIM-1 on cellular repair is mediated via ERK MAPK signaling pathway
Since KIM-1 expression enhances ERK MAPK activation, the modulatory effect of KIM-1 on renal epithelial cell repair could be mediated by ERK MAPK signaling pathway. To test this hypothesis, we examined whether the enhanced wound healing process modulated via overexpression of KIM-1 will be altered by the inhibition of ERK MAPK activities. As shown in Figure 4C&D, induction of KIM-1 expression in the absence of doxycycline resulted in a significant decrease in the wounded area (10.06 ± 2.29% vs 45.68 ± 10.14 %, p < 0.01). Incubation with vehicle DMSO did not affect the wound healing process (wounded area 10.62 ± 1.30 % vs 10.06 ± 2.29%). By contrast, incubation with U0126, a specific inhibitor of ERK MAPK, resulted in a significantly dose-dependent inhibition of wound healing (Figure 4C&D), which is associated with its ability to inhibit ERK MAPK activation (Figure 4D, top panel). The enhanced wound healing conferred by KIM-1 expression was affected in the presence of U0126 even at a concentration as low as 1 μM (wound area 16.81 ± 2.47% vs 10.06 ± 2.29%, p < 0.01, Figure 4D), and was further compromised in the presence of 5 μM of U0126 (45.34 ± 6.83% vs 10.06 ± 2.29%, p < 0.01, Figure 4D) that is similar to baseline without KIM-1 expression (45.68 ± 10.14% in the presence of doxycycline, Figure 4D), suggesting the modulatory effect of KIM-1 on cell repair is likely mediated via ERK MAPK signaling pathway.
DISCUSSION
In the present study, we found that KIM-1 expression facilitates renal tubular epithelial cell repair process by promoting cell migration and proliferation, which is likely mediated via ERK MAPK signaling pathway. The complexity of renal tubular structures is lined with epithelial cells that are frequently exposed to various ischemic and toxic insults. Epithelial cell injury is therefore fundamental to the pathophysiology of acute kidney injury [2]. To restore epithelial integrity and function following injury, several cellular events are engaged sequentially, including cell migration, proliferation, and differentiation [19]. Cell migration plays a central role in a wide variety of biological phenomena [20]. Movement of the regenerating cells to cover the denuded basement membranes is one of the first mechanisms of epithelial repair. Indeed, migration of renal epithelial cells specific on basement membrane matrix matrigel (not observed on plastic surface) is significantly enhanced by overexpression of KIM-1 in our study, suggesting a potential role for KIM-1 in regulating this event to initiate the renal tubular epithelial cell repair process.
Proliferation of epithelial cells forms the cornerstone of wound healing. There had been a debate about the origin of cells that repair renal tubular epithelia after injury. Recent studies have challenged the traditional belief that those cells are come from endogenous surviving tubules [21]. They suggested that bone marrow-derived cells, including hematopoietic stem cells (HSCs) and mesenchymal stem cells, could possibly replenish the epithelial cells that have been lost [22, 23]. Additional study [24], however, indicated that bone marrow-derived cells do not directly replace the tubule epithelial cells that are lost with injury, but exert paracrine effects that facilitate repair potentially by reducing inflammation, which may be mediated by microvesicles that transfer membrane receptors, proteins, mRNAs, microRNAs, and organelles [25–28]. The most definite evidence came from genetic fate-mapping techniques employed in transgenic mice, and the results demonstrated that intratubular progenitor cells proliferate and this accounts for replenishment of the tubular epithelium after ischemia [16]. Since we observed that proliferation of renal tubular epithelial cells, either in the presence or absence of growth factors is significantly increased via upregulation of KIM-1 expression, a possible role for KIM-1 in propagating the renal tubular epithelial cell repair process by stimulation of endogenous progenitor cell proliferation could also be speculated.
In response to injury, renal epithelial cells initiate several signaling pathways to act as intracellular communication lines that regulate the tubular structural and functional restoration. Mitogen-activated protein kinases (MAPKs) are among the most commonly studied signaling pathways involved in these processes [29]. Extracellular signal-regulated kinases (ERKs; ERK1 and ERK2), the classic and best characterized MAPKs identified in the context of growth factor-related signaling, have been implicated in a variety of kidney diseases [30, 31]. Our study indicated that overexpression of KIM-1 induces not only more robust but also more sustained activation of ERK MAPK in response to serum derived growth factors or more specifically, EGF. The activation of ERK MAPK in response to growth factor is typically biphasic, a rapid phase appearing at 5–10 min followed by a late and sustained phase lasting for hours [32]. The second phase is usually more important since it is correlated well with the mitogenic potential and is an obligatory event for growth factor-induced cellular functions [32]. The booster effect of KIM-1 on the sustained phase of ERK MAPK activation is likely critical, since the modulatory effect of KIM-1 on renal epithelial cell repair disappears after inhibition of ERK MAPK pathway.
In summary, we demonstrate that KIM-1 enhances cell repair, likely by promoting cell migration and proliferation in renal tubular epithelial cells. KIM-1 also induces a more robust and sustained activation of ERK MAPK signaling pathway, which could mediate its cellular functions. Modulation of KIM-1 expression in renal tubular epithelial cells could represent a novel therapeutic approach to facilitate renal recovery after acute kidney injury.
Acknowledgments
This study was supported by Individual National Research Service Award 1F32DK10126 from National Institute of Health to Z. Zhang, and Institutional Research Fund from VA Loma Linda healthcare system and Loma Linda Veterans Association for Research for Research & Education. We are grateful to Amy Parker and Andrew Franklin for excellent technical assistances.
References
- 1.Waikar SS, Liu KD, Chertow GM. Diagnosis, epidemiology and outcomes of acute kidney injury. Clin J Am Soc Nephrol. 2008;3:844–61. doi: 10.2215/CJN.05191107. [DOI] [PubMed] [Google Scholar]
- 2.Thadhani R, Pascual M, Bonventre JV. Acute renal failure. N Engl J Med. 1996;334:1448–60. doi: 10.1056/NEJM199605303342207. [DOI] [PubMed] [Google Scholar]
- 3.Feigelstock D, Thompson P, Mattoo P, Zhang Y, Kaplan GG. The human homolog of HAVcr-1 codes for a hepatitis A virus cellular receptor. J Virol. 1998;72:6621–8. doi: 10.1128/jvi.72.8.6621-6628.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McIntire JJ, Umetsu SE, Akbari O, Potter M, Kuchroo VK, Barsh GS, Freeman GJ, Umetsu DT, DeKruyff RH. Identification of Tapr (an airway hyperreactivity regulatory locus) and the linked Tim gene family. Nat Immunol. 2001;2:1109–16. doi: 10.1038/ni739. [DOI] [PubMed] [Google Scholar]
- 5.Ichimura T, Bonventre JV, Bailly V, Wei H, Hession CA, Cate RL, Sanicola M. Kidney injury molecule-1 (KIM-1), a putative epithelial cell adhesion molecule containing a novel immunoglobulin domain, is up-regulated in renal cells after injury. J Biol Chem. 1998;273:4135–42. doi: 10.1074/jbc.273.7.4135. [DOI] [PubMed] [Google Scholar]
- 6.Ichimura T, Hung CC, Yang SA, Stevens JL, Bonventre JV. Kidney injury molecule-1: a tissue and urinary biomarker for nephrotoxicant-induced renal injury. Am J Physiol Renal Physiol. 2004;286:F552–63. doi: 10.1152/ajprenal.00285.2002. [DOI] [PubMed] [Google Scholar]
- 7.Bailly V, Zhang Z, Meier W, Cate R, Sanicola M, Bonventre JV. Shedding of kidney injury molecule-1, a putative adhesion protein involved in renal regeneration. J Biol Chem. 2002;277:39739–48. doi: 10.1074/jbc.M200562200. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Z, Humphreys BD, Bonventre JV. Shedding of the urinary biomarker kidney injury molecule-1 (KIM-1) is regulated by MAP kinases and juxtamembrane region. J Am Soc Nephrol. 2007;18:2704–14. doi: 10.1681/ASN.2007030325. [DOI] [PubMed] [Google Scholar]
- 9.Han WK, Waikar SS, Johnson A, Betensky RA, Dent CL, Devarajan P, Bonventre JV. Urinary biomarkers in the early diagnosis of acute kidney injury. Kidney Int. 2007 doi: 10.1038/sj.ki.5002715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Han WK, Bailly V, Abichandani R, Thadhani R, Bonventre JV. Kidney Injury Molecule-1 (KIM-1): a novel biomarker for human renal proximal tubule injury. Kidney Int. 2002;62:237–44. doi: 10.1046/j.1523-1755.2002.00433.x. [DOI] [PubMed] [Google Scholar]
- 11.Liangos O, Perianayagam MC, Vaidya VS, Han WK, Wald R, Tighiouart H, MacKinnon RW, Li L, Balakrishnan VS, Pereira BJ, Bonventre JV, Jaber BL. Urinary N-acetyl-beta-(D)-glucosaminidase activity and kidney injury molecule-1 level are associated with adverse outcomes in acute renal failure. J Am Soc Nephrol. 2007;18:904–12. doi: 10.1681/ASN.2006030221. [DOI] [PubMed] [Google Scholar]
- 12.Vaidya VS, Ozer JS, Dieterle F, Collings FB, Ramirez V, Troth S, Muniappa N, Thudium D, Gerhold D, Holder DJ, Bobadilla NA, Marrer E, Perentes E, Cordier A, Vonderscher J, Maurer G, Goering PL, Sistare FD, Bonventre JV. Kidney injury molecule-1 outperforms traditional biomarkers of kidney injury in preclinical biomarker qualification studies. Nat Biotechnol. 2010;28:478–85. doi: 10.1038/nbt.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ichimura T, Asseldonk EJ, Humphreys BD, Gunaratnam L, Duffield JS, Bonventre JV. Kidney injury molecule-1 is a phosphatidylserine receptor that confers a phagocytic phenotype on epithelial cells. J Clin Invest. 2008;118:1657–68. doi: 10.1172/JCI34487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang L, Brooks CR, Xiao S, Sabbisetti V, Yeung MY, Hsiao LL, Ichimura T, Kuchroo V, Bonventre JV. KIM-1-mediated phagocytosis reduces acute injury to the kidney. J Clin Invest. 2015;125:1620–36. doi: 10.1172/jci75417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ismail OZ, Zhang X, Wei J, Haig A, Denker BM, Suri RS, Sener A, Gunaratnam L. Kidney Injury Molecule-1 Protects against Galpha12 Activation and Tissue Damage in Renal Ischemia-Reperfusion Injury. Am J Pathol. 2015;185:1207–15. doi: 10.1016/j.ajpath.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Humphreys BD, Valerius MT, Kobayashi A, Mugford JW, Soeung S, Duffield JS, McMahon AP, Bonventre JV. Intrinsic epithelial cells repair the kidney after injury. Cell Stem Cell. 2008;2:284–91. doi: 10.1016/j.stem.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 17.Matsubayashi Y, Ebisuya M, Honjoh S, Nishida E. ERK activation propagates in epithelial cell sheets and regulates their migration during wound healing. Curr Biol. 2004;14:731–5. doi: 10.1016/j.cub.2004.03.060. [DOI] [PubMed] [Google Scholar]
- 18.Moulin V. Growth factors in skin wound healing. Eur J Cell Biol. 1995;68:1–7. [PubMed] [Google Scholar]
- 19.Bonventre JV, Yang L. Cellular pathophysiology of ischemic acute kidney injury. J Clin Invest. 2011;121:4210–21. doi: 10.1172/jci45161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lauffenburger DA, Horwitz AF. Cell migration: a physically integrated molecular process. Cell. 1996;84:359–69. doi: 10.1016/s0092-8674(00)81280-5. [DOI] [PubMed] [Google Scholar]
- 21.Bonventre JV. Dedifferentiation and proliferation of surviving epithelial cells in acute renal failure. J Am Soc Nephrol. 2003;14(Suppl 1):S55–61. doi: 10.1097/01.asn.0000067652.51441.21. [DOI] [PubMed] [Google Scholar]
- 22.Kale S, Karihaloo A, Clark PR, Kashgarian M, Krause DS, Cantley LG. Bone marrow stem cells contribute to repair of the ischemically injured renal tubule. J Clin Invest. 2003;112:42–9. doi: 10.1172/jci17856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin F, Cordes K, Li L, Hood L, Couser WG, Shankland SJ, Igarashi P. Hematopoietic stem cells contribute to the regeneration of renal tubules after renal ischemia-reperfusion injury in mice. J Am Soc Nephrol. 2003;14:1188–99. doi: 10.1097/01.asn.0000061595.28546.a0. [DOI] [PubMed] [Google Scholar]
- 24.Duffield JS, Park KM, Hsiao LL, Kelley VR, Scadden DT, Ichimura T, Bonventre JV. Restoration of tubular epithelial cells during repair of the postischemic kidney occurs independently of bone marrow-derived stem cells. J Clin Invest. 2005;115:1743–55. doi: 10.1172/jci22593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lange C, Togel F, Ittrich H, Clayton F, Nolte-Ernsting C, Zander AR, Westenfelder C. Administered mesenchymal stem cells enhance recovery from ischemia/reperfusion-induced acute renal failure in rats. Kidney Int. 2005;68:1613–7. doi: 10.1111/j.1523-1755.2005.00573.x. [DOI] [PubMed] [Google Scholar]
- 26.Togel F, Hu Z, Weiss K, Isaac J, Lange C, Westenfelder C. Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. Am J Physiol Renal Physiol. 2005;289:F31–42. doi: 10.1152/ajprenal.00007.2005. [DOI] [PubMed] [Google Scholar]
- 27.Duffield JS, Bonventre JV. Kidney tubular epithelium is restored without replacement with bone marrow-derived cells during repair after ischemic injury. Kidney Int. 2005;68:1956–61. doi: 10.1111/j.1523-1755.2005.00629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Camussi G, Deregibus MC, Bruno S, Cantaluppi V, Biancone L. Exosomes/microvesicles as a mechanism of cell-to-cell communication. Kidney Int. 2010;78:838–48. doi: 10.1038/ki.2010.278. [DOI] [PubMed] [Google Scholar]
- 29.Tian W, Zhang Z, Cohen DM. MAPK signaling and the kidney. Am J Physiol Renal Physiol. 2000;279:F593–604. doi: 10.1152/ajprenal.2000.279.4.F593. [DOI] [PubMed] [Google Scholar]
- 30.Zhang W, Liu HT. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res. 2002;12:9–18. doi: 10.1038/sj.cr.7290105. [DOI] [PubMed] [Google Scholar]
- 31.Feliers D, Kasinath BS. Erk in kidney diseases. J Signal Transduct. 2011;2011:768512. doi: 10.1155/2011/768512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meloche S, Seuwen K, Pages G, Pouyssegur J. Biphasic and synergistic activation of p44mapk (ERK1) by growth factors: correlation between late phase activation and mitogenicity. Mol Endocrinol. 1992;6:845–54. doi: 10.1210/mend.6.5.1603090. [DOI] [PubMed] [Google Scholar]