SUMMARY
Progressive multifocal leukoencephalopathy (PML) is a devastating and often fatal demyelinating disease of the central nervous system (CNS) for which effective therapies are lacking. It is caused by the replication of polyomavirus JC (JCV) in the oligodendrocytes and astrocytes leading to their cytolytic death and loss of myelin from the subcortical white matter. While the virus is very common in human populations worldwide, the incidence of the disease is very low and confined almost exclusively to individuals with some form of immunological dysfunction. However, the number of people who constitute the at-risk population is growing larger and includes individuals with HIV-1/AIDS and patients receiving immunomodulatory therapies such as multiple sclerosis patients treated with natalizumab. Further adding to the public health significance of this disease are the difficulties encountered in the diagnosis of PML and the lack of useful biomarkers for PML progression. In this review, we examine the diagnostic assays that are available for different aspects of the JCV life cycle, their usefulness and drawbacks, and the prospects for improvements.
Keywords: Progressive multifocal leukoencephalopathy, Diagnostic assays, Polyomavirus JC
1. INTRODUCTION
Progressive multifocal leukoencephalopathy (PML) is a severe demyelinating disease of the central nervous system (CNS) that is caused by the human polyomavirus JC (JCV)[1,2]. JCV replication occurs only in human glial cells, astrocytes and oligodendrocytes, and PML pathogenesis is attributable to the toxic and lytic effects of JCV on oligodendrocytes, which are responsible for the production of myelin in the CNS [3]. The polyomaviruses form a family of small, DNA tumor viruses, which have a circular, double-stranded DNA genome of about 5 kilobase pairs contained within in an icosahedral nonenveloped virion. They are common in vertebrates with each virus possessing a narrow host range that is usually limited to one species [4]. At least ten different species of polyomavirus are known to infect humans and each may or may not be associated with a pathological condition [5]. For example, the human polyomaviruses JC (JCV) and BK (BKV) are the etiological agents of progressive multifocal leukoencephalopathy (PML) and polyomavirus-associated nephropathy (PVAN) respectively [6,7]. On the other hand, other polyomaviruses have been isolated by large-scale molecular virus screening approaches to human diagnostic clinical samples and have not been associated with any known pathology, e.g., Karolinska Institute polyomavirus (KIV) was identified in nasopharyngeal aspirates [8] and Washington University polyomavirus (WUV), which was also identified using a high throughput DNA sequencing approach to a random library generated from a nasopharyngeal aspirate [9].
A feature that is common among the human polyomaviruses is that the incidence of virus-associated disease in the population is very low and yet a large percentage of people has antibodies to the virus indicating widespread infection. For example, most people become seropositive to JCV and BKV in childhood but very rarely virus can be detected in the blood (viremia) and usually only in patients with PML and PVAN respectively. Viruria (virus in the urine) can occur somewhat more often and tends to be episodic and at low levels in normal people. These observations underline that the immune system has a powerful role in the suppression of infections by human polyomaviruses. Viruria for JCV, BKV and the other human polyomaviruses can be increased by events associated with immunosuppression, e.g., viral shedding in the urine is increased during the third trimester pregnancy and in the elderly, for example, though in most cases it remains idiopathic [10,11]. Multiple sclerosis (MS) patients being treated with natalizumab have an increased risk of PML and increased JC viruria has been reported in natalizumab-treated MS patients without PML [12,13].
The life cycle of JCV is complex and many aspects are controversial, e.g., the site and mechanism of reactivation, the relationship between the archetypal transmitted form of the virus to the pathogenic neurovirulent form and the roles of different tissue compartments in the pathogenesis of PML: we have reviewed these issues recently [2,14]. Moreover, the diagnosis of PML is not always straightforward and there is a dearth of useful biomarkers for progression to PML. Taken together with the lack of an effective therapy to improve or reverse the course of the disease and the expanding size and diversity of the at-risk population, this emphasizes the need for careful and accurate quantification of the different aspects of the JCV life cycle. In this review, we will critically examine the clinical assays that are available for JCV and PML, their advantages and drawbacks, interpretation of results and opportunities for improvements. The life cycle of JCV and the assays available for the different stages that are discussed in this review are shown in Figure 1.
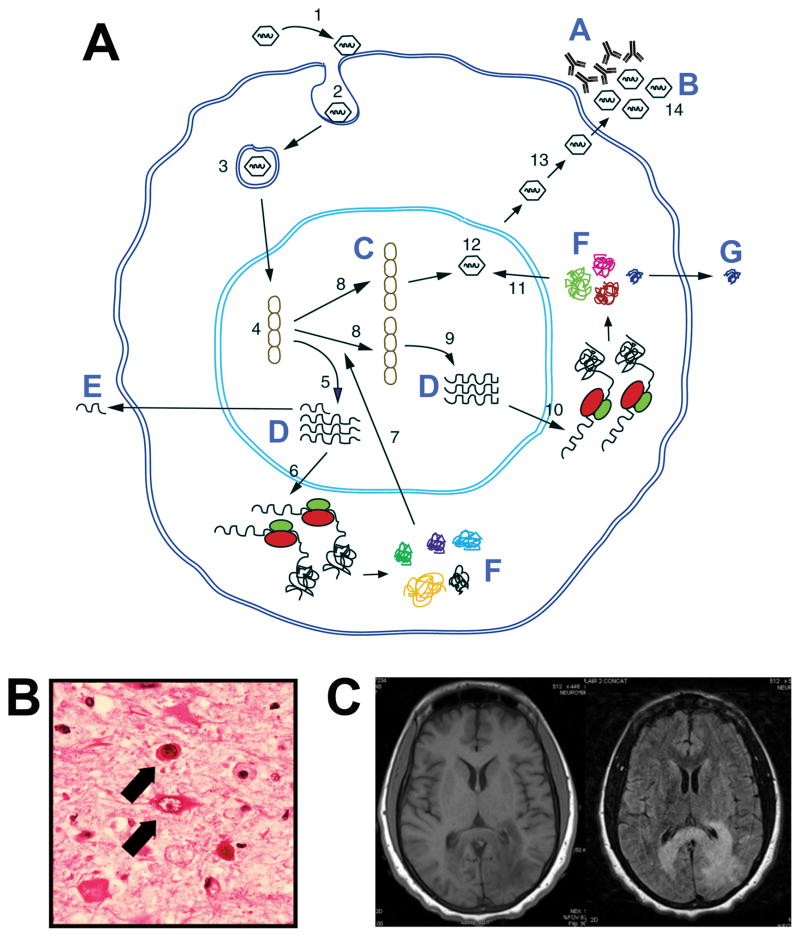
Figure 1. The Life Cycle of JCV and Diagnostic Assays for JCV and PML.
A. The following steps in the JCV life cycle are indicated with numbers: 1 — Binding of JC virions to cell surface receptor; 2 — endocytosis; 3 — nuclear import; 4 — uncoating; 5 — transcription of the early region; 6 — translation of the early proteins, T-antigen and t-antigen; 7 — viral DNA replication; 8 — transcription of the late region; 9 — translation of agnoprotein and the capsid proteins VP1, VP2 and VP3; 10 — virion assembly; 11 — nuclear export; 12 — virion release from the cell; 13—infectious viral particles. Assays for aspects of the JCV life cycle are indicated with letters: A — antibodies specific for JCV can be measured by hemagglutination inhibition or enzyme-linked immunoassay (Section 3.3); B — released virions can be measured by qPCR in serum, CSF or urine (Section 3.1); C — detection of JCV DNA in PML autopsy or biopsy material by in situ hybridization (Section 4.3); D — detection of JCV DNA in PML autopsy or biopsy material by RT-PCR (Section 3.2); E — detection of JCV miRNA by qRT-PCR (Section 3.2); F — detection of JCV proteins in PML autopsy or biopsy material by immunohistochemistry or Western blot (Section 4.2); G — detection of JCV agnoprotein released from infected cells (Section 3.5). Adapted from [96]. B. Hematoxylin and Eosin staining of a tissue section from a PML brain illustrating the presence of oligodendrocytes containing large nuclear inclusion bodies, indicated by the upper arrow. Also shown are bizarre astrocytes, indicated by the lower arrow [3]. C. MRI of PML [T1 weighted MRI left and Fluid attenuated T2 weighted image (FLAIR) right]. A large lesion is seen affecting the white matter of the left parieto-occipital lobe with extension across the splenium of the corpus callosum to the right hemisphere. The lesion is hypointense on T1WI and hyperintense on T2WI. The cortex is spared. The lesion exhibited no contrast enhancement.
2. DIAGNOSTIC CRITERIA FOR PROGRESSIVE MULTIFOCAL LEUKOENCEPHALOPATHY
The initial description of PML as a novel disorder was based on brain histopathology, which was characterized by a classic triad of features: demyelination, bizarre astrocytes, and oligodendrocytes containing nuclear inclusion bodies [15]. Demonstration of this triad in brain tissue in biopsy or autopsy material together with JCV DNA or protein detectable by in situ techniques permits a definitive diagnosis of PML as described in the recent consensus statement on PML diagnostic criteria from the American Academy of Neurology Neuroinfectious Disease Section [16]. Probable or CSF-confirmed PML is also known as laboratory-confirmed PML and has a lower degree of diagnostic certainty since it is based on JCV-specific PCR because of the inherent difficulties with sensitivity and specificity with these assays in different laboratories [17]. Thirdly, possible or presumptive PML has the lowest level of diagnostic certainty and is based on typical clinical and magnetic resonance imaging (MRI) findings consistent with PML diagnosis, where brain biopsy and lumbar puncture were either not performed or JCV DNA was not detected in CSF [18]. Thus, it is clear that no single criterion establishes a definitive diagnosis of PML and that multiple lines of evidence are required, which may be clinical, radiological and virological. These considerations are further explored in Sections 3.6 and 3.7. The three different forms of PML based on the diagnosis are summarized in Table 1.
TABLE 1.
The three different forms of PML based on the diagnosis
| Type | Diagnostic Criteria |
|---|---|
| Definitive | Classic triad of histopathogical features on biopsy:
|
| PLUS | |
| Detection of JCV DNA or protein | |
| Probable | Positive JCV-specific PCR in CSF |
| PLUS | |
| Typical clinical and MRI findings | |
| Possible | Typical clinical and MRI findings |
| WITH | |
| Brain biopsy and lumbar puncture not performed or JCV DNA not detected in CSF | |
| AND | |
| No other alternative diagnosis |
3. TESTS INVOLVING MINIMALLY INVASIVE ASSAYS
3.1 ASSAYS FOR JC VIRAL LOAD
It is likely that mixtures of multiple human polyomaviruses may be present in biological samples from clinical sources such as blood and urine and so assays for JC viral load must be highly specific. The development of quantitative polymerase chain reaction (qPCR) assays provides a specific, sensitive and quantitative method to measure JC viral load [19]. Pal et al [19] designed sets of primer/probes for BKV, JCV and SV40 specific for both the early and late coding regions of each virus. These were characterized and it was shown that they could detect between 1–10 copies of their cognate genomes while failing to react with as many as 108 copies of DNA from the other viruses [19]. Thus qPCR assays have the capacity to enumerate small numbers of viral genomes in the presence of large amounts of unrelated DNA. The reliability of any given set of primer/probes should be evaluated by generating standard curves at a broad range of dilutions and in the presence and absence of other viruses. It is important to note that the number of PCR cycles can affect both the sensitivity and specificity of the assay [19]. Another factor that needs to be considered is sequence divergence within the viral isolate. For example in the case of BKV, there is substantial diversity of the genomes of viral isolates [20,21]. Bayliss et al [22] reported that sequence analysis of the early region of JCV in four immunosuppressed patients revealed eight novel single nucleotide polymorphisms. However, Dumoulin and Hirsch [23] reevaluated and optimized JCV and BKV qPCR assays to detect rare sequence polymorphisms and updated the BKV assay for the occurrence of polymorphisms. They concluded that their original JCV and updated BKV qPCR assays were robust and able to detect rare variants routinely encountered in the clinic [23].
The noncoding regulatory region (NCCR) of JCV is variable and comes in two forms, the first of which is the archetypal form, which is shed in the urine of normal individuals, e.g. during pregnancy [10] and may be the transmissible form of the virus [24]. The second form of the JCV NCCR is the neurotropic form that is associated with PML pathogenesis and has multiple rearrangements, including mutations, deletions and duplications relative to the archetype and is typified by the Mad-1 strain [25]. It is possible that the neurotropic form is pathogenic because the rearranged JCV NCCR increases virus early gene expression and replication rate [26]. While, the relationship between the archetypal and neurotropic forms of the virus is still not fully elucidated [2], the association of the neurotropic form of the virus with PML makes the development of a qPCR assay that can differentiate between them an important goal. Ryschkewitsch et al [27] reported a multiplex qPCR assay for detection of JCV DNA claiming simultaneous identification of non-virulent from virulent variants. However, this assay simply measures the number of viral genomes with archetypal configuration using NCCR-specific primers relative to the total number of viral genomes measured using T-Ag-specific primers [27]. A true assay to measure the pathogenic neurotropic form of JCV has not yet been developed, presumably because it is more difficult owing to the multitude of different rearrangements that can occur to yield neurotropic virus [24]. The development of an assay for the neurotropic form of JCV would be an important advance in JCV/PML diagnostics.
The ability to quantify JC viral load is an important diagnostic tool. For example, in the presence of the PML histological triad, the detection of JCV in CSF allows a definitive diagnosis of confirmed PML [16]. JC viremia is very rare and is not predictive for the risk of PML [28–30]. JC viruria is more common and may be persistent or episodic in nature occurring at low levels in normal people when present. In a longitudinal study, Delbue and colleagues found that 61.9% shedding JCV did so persistently [13]. An increase in JC viruria has been reported in natalizumab-treated MS patients without PML [12,13] and JC viruria evaluation may be a useful way for early identification of those JCV-infected patients who have not already developed a humoral immune response to JCV [31]. Similarly, Berger et al [32] found that 37% of JCV seronegative patients receiving natalizumab in a single-center, retrospective cohort study had JCV viruria, i.e., JCV serostatus does not identify all patients infected with JCV and it is important not to conflate a negative JCV antibody result with an absence of JCV infection. [13]
3.2 ASSAYS FOR JC VIRAL RNA
Since JCV reactivation and onset of PML involves the activation of transcription of the viral genome [2], the measurement of viral RNA is, in principle, a possible assay for viral activity. While a few studies have looked at JC viral mRNAs in PML using reverse transcriptase PCR [33], most assays for JCV gene expression involve measurement of JC protein (described below). However, the discovery that micro-RNAs (miRNAs) are expressed by JCV [34] has made new assays possible. Seo et al [34] first reported that both JCV and BKV encode miRNAs and that the JCV miRNAs are readily detectable in brain tissues of PML patients. The function of these miRNAs appears to be to downregulate T-antigen expression late in infection, which may be involved in immune evasion by polyomaviruses [35]. In addition, the JCV miRNA downregulates the cellular stress-induced ligand ULBP3, which is recognized by the killer cell receptor NKG2D, and thus JCV miRNA reduces NKG2D-mediated killing of JCV-infected cells by natural killer cells [36]. Lagatie et al [37] investigated if JCV miRNA could be detected in plasma or urine by qRT-PCR in 50 healthy subjects and found that JCV miRNA was detected in 86% (12/14) and 57% (8/14) of plasma and urine samples, respectively for JCV-VP1 seronegative subjects. Thus, these data indicate that analysis of circulating viral miRNAs divulged the presence of latent JCV infection. Rocca et al [38] investigated the presence of JCV DNA and expression of JCV miRNA in clinical specimens from patients at risk for PML and found that JCV viral load was inversely correlated with the levels of JCV miRNA expression in blood and cerebrospinal fluid of patients at risk of PML indicating a potential clinical relevance for JCV miRNA in PML risk assessment. The field of viral miRNA is a fairly new one and it is probable that exciting new advances will be made in the future.
3.3 ASSAYS FOR SERUM ANTIBODIES TO JC VIRAL PROTEINS
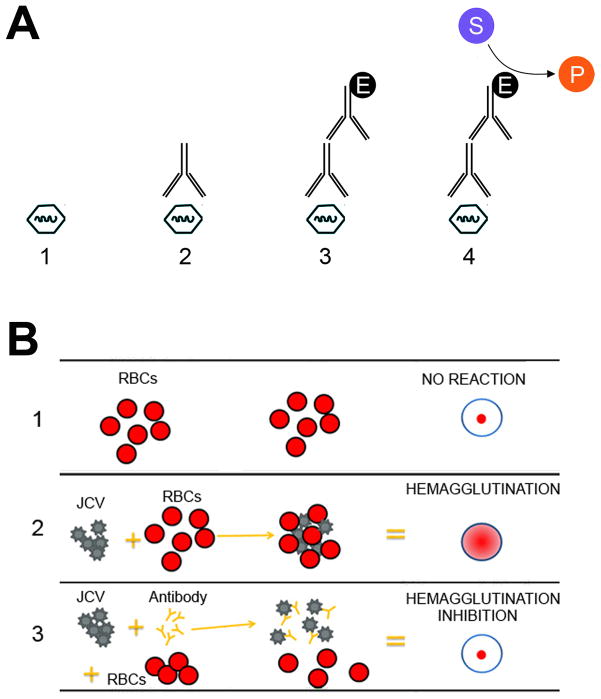
Infection by JCV is very common in populations worldwide and a review of the published seroepidemiological studies show that primary infection first occurs in childhood and the percentage of seropositivity measured in different studies showing estimated rates that vary between 33% and 91% [14]. There are a number of ways to measure serum antibodies to JCV. Hemagglutination inhibition (HI) was the first assay developed and it applies the process of hemagglutination, in which sialic acid receptors on the surface of red blood cells bind to JC virion glycoprotein and its inhibition by antibodies specific for the virus. More recent assays to measure antibodies to viral proteins utilize enzyme-linked immunoassay (ELISA) techniques since they have greater sensitivity and precision relative to HI. In a side-by-side comparison study, it was reported that antibody titers to JCV determined by ELISA were higher than those determined by HI [39]. Nonetheless, as ELISA titers increased so did HI titers, i.e., there is highly significant correlation between ELISA and HI titers. The ELISA for JCV can be further improved by using JCV VP1 virus-like particle (VLP)-based ELISAs normalized to a laboratory reference serum [40]. A schematic representation of the ELISA and HI assays for JCV are shown in Figure 2.
Figure 2. Assays for JCV-specific antibodies.
A. ELISA assay: (1) A microtiter plate is coated with antigen, e.g., JCV or JCV VP1 VLPs; (2) the sample is added, e.g. serum, and any antibody binds to the antigen; (3) an enzyme (E)-linked secondary antibody is added, e.g., rabbit anti-human-HRP and binds to antibody; (5) the substrate (S), e.g., luminol, is added, and is converted by enzyme to the detectable product (P), e.g., 3-aminophthalate. B. Hemagglutination inhibition assay: The HI test involves the interaction of red blood cells (RBCs), antibody and JC virus: Row 1 shows that in the absence of virus, RBCs in a solution will sink to the bottom of a microtiter plate well and look like a red dot; Row 2 shows that JC virus will bind to red blood cells when placed in the same solution, i.e., hemagglutination and is represented by the formation of the lattice structure, depicted in the center and right-hand columns of Row 2; Row 3 shows how antibodies that are antigenically similar to a virus being tested will recognize and bind to that influenza virus. This prevents the virus and RBCs from binding, and therefore, hemagglutination does not occur, i.e., hemagglutination inhibition occurs instead. Adapted from http://www.cdc.gov/flu/professionals/laboratory/antigenic.htm.
Interest in the serum titre for JCV antibody has been enkindled recently by the recognition that the risk for development of PML in MS patients taking natalizumab is linked to duration of therapy, the prior use of immunosuppressive agents and JC virus seropositivity [41,42]. This prompted the development and validation of a novel two-step ELISA assay to detect JCV antibodies in human serum, which has demonstrated potential clinical utility in identifying patients at increased risk of developing PML. The assay is robust and performs consistently and reproducibly in multiple laboratories [43–45]. More recently has seen the development and validation of a second-generation ELISA know as STRATIFY JCV™ DxSelect™ by Lee et al [46]. Focus Diagnostics, a subsidiary of Quest Diagnostics, provides STRATIFY JCV testing service to aid in risk stratification for PML in MS patients using technology licensed exclusively from Biogen. Many cohort studies of JCV antibodies have analyzed MS patients receiving natalizumab and seropositivity found to be 57.1% – 69.5% with no dependence on prior immunosuppressant use or duration of natalizumab treatment [47–50]. However, unlike these cross-sectional studies, a longitudinal study has reported a significant and substantial increase in anti-JCV index over time [51]. In another study, Warnke et al [52] reported that the onset of PML in five cases was accompanied by increasing ant-JCV antibodies in serum.
3.4 ASSAYS FOR CELL-MEDIATED IMMUNITY TO JCV
The occurrence of PML is restricted almost exclusively to persons who have some form of abnormality or impairment in the function of the immune system, especially JCV-specific cellular immunity [53,54]. The JCV-specific cellular immune response in patients with HIV-1/PML correlates with outcome [55] suggesting that JCV-specific cellular immunity is involved in restraining JCV infection and the development of PML. JCV-specific cytotoxic T cells (CTL) are associated with early control of PML [56,57] and the presence of JCV-specific CTL is associated with a trend toward longer survival times in patients with PML [58]. These data highlight the importance of JCV-specific CTL in containing viral replication and hence explain the role of immunosuppression in PML pathogenesis. The activity of the JCV-specific cellular immune response can be measured by two assays [59,60]. Firstly, JCV VP1-specific CTL can be measured by a functional lysis assay involving 51Cr release [61]. Secondly, binding to tetrameric HLA*0201/VP1 complex by specific CD8+ cells can be analyzed by flow cytometric analysis [59,60]. Since JCV-specific cellular immunity is inversely related to PML risk and disease outcome, the development of assays available for the clinic would be useful.
3.5 BIOMARKERS FOR JCV AND PML
The diagnosis of PML usually occurs at the time of presentation with neurological symptoms such as motor deficits, cognitive and behavioral changes, gait and limb ataxia, and visual symptoms [62]. However, the disorder may be detected before it has become clinically apparent in “at risk” patients, an entity referred to as “radiographically isolated PML” [41]. Therefore the development symptoms may be regarded as a late stage of disease progression. Few patients are at sufficiently high risk of PML, such as natalizumab treated patients, to warrant periodic screening MRIs. This highlights the urgent need to identify sensitive biomarkers that are detectable in the early phase of the disease. There are few potential biomarkers for early stage PML at this time. Recently L-selectin (CD62L) has emerged as a possible biomarker [63]. Schwab et al [64] examined 289 patients with MS, 224 of whom had been treated with natalizumab (18–80 months). The percentage of L-selectin-expressing CD4+ T cells was significantly lower in patients treated long-term with natalizumab (40.2%) when compared with patients not receiving natalizumab treatment (47.2%) or healthy controls (61.0%). An unusually low percentage (4.6%) was highly correlated with the risk of developing PML in the patient group with available pre-PML samples when compared with non-PML natalizumab-treated patients. Spadaro et al [65] confirmed that natalizumab specifically decreased CD62LCD4+ cells. Basnyat et al [66] reported a significant correlation between JCV serostatus and level of soluble L-selectin while Schwab et al [67] have confirmed that both anti-JCV antibodies and CD62L levels have merit for risk stratification and could be developed into a risk algorithm that could reduce PML incidence. There is also a report that CD62L might be a biomarker for HIV+ PML [68].
Another suggested biomarker for PML is C-reactive protein (CRP). Lanzillo et al [69] retrospectively analyzed natalizumab-treated MS patients who underwent the JCV STRATIFY antibody test to measure serum ultrasensitive C-reactive protein (usCRP) levels, and to perform blood and urine JCV PCR. The level of usCRP was higher in urinary JCV DNA-positive patients and correlated to the number of DNA copies in urine (P=0.028). They found that JC viruria was significantly correlated with a high JCV antibody index and high serum usCRP levels and suggested that usCRP might be useful as a marker of JCV reactivation.
Recently, it was found that the JCV late regulatory protein, agnoprotein, is able to be released from JCV-infected cells [70]. Since agnoprotein is only expressed by cells that are actively replicating the virus and is released into the extracellular matrix, it is possible that detection of agnoprotein in clinical samples could serve as a biomarker for JCV reactivation.
3.6 SPINAL TAP AND ANALYSIS OF CEREBROSPINAL FLUID (CSF) FOR JCV AND PML RISK ASSESSMENT
The main property of PCR is its extraordinary sensitivity and specificity allowing the detection of JCV at low copy number in CSF [71]. Biopsy and histopathology is the gold standard for diagnosis of PML and finding of the characteristic triad of histopathological features and JCV DNA allows a definitive diagnosis of PML [16]. Since biopsy of the brain is a hazardous procedure, there has been an increased reliance on MRI and CSF PCR. In the absence of biopsy, which is often not deemed necessary today, a widely employed approach to diagnosis relies upon: (i) the demonstration of JCV in the CSF by PCR; (ii) compatible clinical presentation; (iii) MRI finding consistent with PML (see next section); (iv) no other alternative diagnosis [62]. However, virus detection by PCR is not infallible and there are many reported cases of PML with false-negative CSF PCR despite progressive clinical course and radiological findings indicative of PML [72]. There are a number of factors that influence this. For example, in the age of combination antiretroviral therapy (cART), JCV titers in CSF tend to be lower and this is also especially the case for PML seen in the context of natalizumab therapy [16]. Also, virus is harder to detect in the presence of immune reconstitution inflammatory syndrome (IRIS) or in the early stages of PML [73,74]. Thus the absence of detection of JCV DNA by PCR in the CSF does not exclude a diagnosis of PML since the CSF compartment may not reflect the infection status of the brain parenchyma.
In a recent study of natalizumab treated MS patients with PML, decreased lipid-specific IgM bands in the CSF correlated with a higher risk of PML; whereas, increased IgM bands were associated with highly inflammatory MS and increased numbers of CSF B cells [75]. The investigators suggest that the risk of PML in individuals who are JCV seropositive and are negative for CSF IgM bands is high [75].
3.7 MAGNETIC RESONANCE IMAGING OF PML
MRI is much more sensitive than computed tomography for the detection of demyelinated lesions in patients with PML [62]. The occurrence of PML in MS patients receiving Natalizumab represents a diagnostic challenge in that PML demyelinated plaques must be differentiated from MS plaques. MRI scans of PML brain are described as bilateral, asymmetric, predominantly subcortical lesions appearing hyperintense on T2-weighted and FLAIR sequences and hypointense on T1-weighted sequences [76]. Mass effect is usually absent or mild, but not always and it is observed, it is generally indicative of IRIS. Large, confluent, and granular hyperintense T2-weighted lesions and deep gray matter lesions are more common in patients with PML than patients with MS and crescent-shaped lesions in the cerebellar hemispheres suggests PML. Periventricular white matter lesions and Dawson fingers are both common in MS but unexpected in PML [76]. The regular use of MRI imaging in MS patients may be able to detect the onset of PML at a very early stage before patients become clinically symptomatic [77,78]. MRI is the most powerful method to detect natalizumab-associated PML and generally but not invariably allowing differentiation between PML and MS lesions. Early, preferably asymptomatic, detection of PML may lead to more favorable outcomes with respect to survival and functional outcome [79].
4. TESTS INVOLVING ASSAYS ON BIOPSY OR AUTOPSY MATERIALS
4.1 HISTOPATHOLOGY
The recognition and characterization of PML the disease as a distinct clinical and neuropathological entity first occurred in 1958 by Åström and Richardson [15] who reported the clinical and histopathological aspects of the first three well-documented cases of PML, which was of unknown etiology at the time. The infectious nature of PML was later shown by electron microscopy studies performed by ZuRhein and Rubinstein, who showed viral particles in the nuclei of oligodendrocytes in PML [80]. Gross examination of the brain from PML patients reveals characteristic demyelinated plaques typically affecting the subcortical white matter. These lesions appear soft in consistency, discolored to a brownish-yellow and are irregular in shape with inconspicuous borders. Usually but not always, the lesions are multifocal and they may become confluent and coalescent in advanced cases leading to cavitation. In severe cases, demyelinated lesions may also be found in the cerebellum, basal ganglia and brainstem, always affecting white matter [81,82].
PML is uniquely characterized by three prominent histological features: plaques of demyelination, enlarged oligodendrocytes harboring intranuclear inclusion bodies and giant bizarre astrocytes. JCV-infected oligodendrocytes are about two to three times larger than normal oligodendrocytes and show intranuclear eosinophilic inclusion bodies. The inclusion bodies are the sites of active viral replication and characteristic icosahedral JC virion particles are visible by electron microscopy. Later stage demyelinated plaques contain foamy macrophages, which function in the phagocytosis of myelin breakdown products resulting from oligodendrocyte lysis. In larger demyelinated lesions, the enlarged oligodendrocytes with inclusion bodies are located mainly in the margins of the plaques. Bizarre astrocyte are present throughout the plaques and exhibit characteristic atypical, hyperchromatic nuclei and are often multinucleated with pleomorphic cytoplasm, which confers on them their atypical and bizarre appearance.
4.2 PROTEIN: IMMUNOHISTOCHEMISTRY AND WESTERN BLOTTING
By immunohistochemistry, viral proteins can be detected in both types of cells infected by JCV, enlarged oligodendrocytes and bizarre astrocytes. The early gene product T-Antigen is found in the nucleus. The capsid protein VP1, an indicator of the occurrence of active viral replication, is found in both the cytoplasm and the nuclei of infected cells, but predominantly the latter. The late accessory regulatory product agnoprotein is located almost exclusively in the cytoplasm of both cell types with a characteristic prominence in the perinuclear region and a small amount present in the nucleus [71,83,84]. Comparative studies show that there are no significant differences between AIDS and non-AIDS associated PML but perhaps a slight tendency towards more severe demyelination and a higher rate of infected cells in the AIDS-related cases reported [85].
Other histochemical features of PML offer interesting insights on cellular changes involved in the pathogenesis of the disease. For example, while expression of Rad51 is absent in normal astrocytes non-PML brain tissue, glial fibrillary acidic protein (GFAP)-positive bizarre astrocytes in PML show robust and abundant nuclear foci of the DNA repair protein Rad51 as do JCV-VP1-positive enlarged oligodendrocytes. This is indicative of the induction of the DNA damage response in these cells [86]. In another study, evaluation of HIV-1/PML clinical samples and non-PML controls for expression of TNF-α and its receptor TNFR1 showed an increase in overall expression in PML as measured by Western blot and specific induction in bizarre astrocytes and enlarged oligodendrocytes measured by immunohistochemistry as well as a redistribution of the transcription factors NF-κB and NFAT4 to preferential localization to the nucleus. This is consistent with the activation of this cytokine/transcription factor pathway in PML [87].
4.3 DNA: IN SITU HYBRIDIZATION
Finally, it is possible to detect JCV DNA in the oligodendrocytes and astrocytes in PML brain using in situ hybridization with a JCV-specific nucleic acid probe labeled radioactively or with biotin and this is as sensitive and specific as immunohistochemistry for diagnosis on formalin-fixed tissue [88–90]. The available diagnostic assays for JCV and PML are summarized in Table 2.
TABLE 2.
Available diagnostic assays for JCV and PML
| Assay | Outcome | Conclusion |
|---|---|---|
| Brain biopsy histopathology and/or Immunohistochemistry/In situ hybridization | Classic triad of features:
|
Gold standard for PML diagnosis |
| Brain biopsy Immunohistochemistry/In situ hybridization | Detection of JCV DNA or proteins | Definitive PML |
| qPCR for JCV DNA | Positive in blood | Viremia |
| Positive in urine | Viruria | |
| Positive in CSF | Probable/Possible PML | |
| qRT-PCR for JCV miRNA | Positive in plasma or urine | Latent JCV infection |
| HI or ELISA/STRATIFY | Antibodies to JCV protein | Latent JCV infection |
| JCV VP1-specific CTL (51Cr release or tetramer assays) | Cellular Immunity to JCV | Correlated to favorable outcome |
| L-selectin (CD62L) | Positive | Possible PML biomarker |
| C-reactive protein (CRP) | Positive | Possible PML biomarker |
| Agnoprotein | Positive | Possible PML biomarker |
| MRI | Findings consistent with PML | Probable/Possible PML |
5. CONCLUSIONS AND FUTURE DIRECTIONS
PML is a debilitating disease for which there is no convincingly effective therapy. The size of the at-risk population is growing larger and includes individuals with HIV-1/AIDS and patients receiving immunomodulatory therapies, e.g., MS patients being treated with natalizumab. The clinical manifestations of PML can be extremely diverse and, therefore, it should be considered in the differential diagnosis of any immunosuppressed person presenting with neurological disease. The most common symptoms are cognitive and behavioral disturbances and weakness [91]. Other common symptoms included headaches, gait disorders, visual problems, and sensory loss. The frequency with which abnormalities are detected by physical examination typically parallels the symptoms reported. Among the common signs are cognitive impairment, motor abnormalities, speech and language disorders, and visual disturbances including visual field defects due to retrochiasmal lesions and diplopia due to brainstem involvement [91]. Optic nerve disease has not been reported nor has clinically evident myelopathy, although spinal cord disease has been observed both at autopsy [92] and premortem by MRI [93]. Subtle differences in the frequency with which these symptoms and signs are observed may exist depending on the nature of the underlying disorder predisposing to PML as has been observed with HIV/AIDS-associated PML [94] and natalizumab-associated PML [95].
Adding to the problematic nature of this disease are the difficulties encountered in the diagnosis of PML and the lack of useful biomarkers for PML progression as we have discussed. Key areas for future research and development are building a better understanding of the virology and life cycle of JCV and the pathogenesis and immunopathology of PML. New and better assays and biomarkers for PML need to be developed and key areas are risk stratification and better understanding of the biology of the emergence and monitoring of the neurotropic rearranged form of JCV that is responsible for PML. New developments in these areas may pave the way for the development of new treatments for PML.
Acknowledgments
We wish to thank past and present members of the Department on Neuroscience and Center for Neurovirology for their continued support and insightful discussions. We also acknowledge the intellectual contributions of the Lewis Katz School of Medicine at Temple University Comprehensive Neuroaids Center (Basic Science Cores I and II). This work was supported, in part, by grants awarded by the NIH to MKW, IKS, JG and KK.
References
- 1.Safak M, Khalili K. An overview: Human polyomavirus JC virus and its associated disorders. J Neurovirol. 2003;9(Suppl 1):3–9. doi: 10.1080/13550280390195360. [DOI] [PubMed] [Google Scholar]
- 2.White MK, Khalili K. Pathogenesis of progressive multifocal leukoencephalopathy--revisited. J Infect Dis. 2011;203:578–586. doi: 10.1093/infdis/jiq097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gordon J, Gallia GL, Del Valle L, et al. Human polyomavirus JCV and expression of myelin genes. J Neurovirol. 2000;6(Suppl 2):S92–97. [PubMed] [Google Scholar]
- 4.DeCaprio JA, Imperiale MJ, Major EO. Polyomaviruses. In: Knipe DM, Howley PM, editors. Fields virology. 6. Philadelphia: Lippincott, Williams & Wilkins; 2013. pp. 1633–1661. [Google Scholar]
- 5.White MK, Gordon J, Khalili K. The rapidly expanding family of human polyomaviruses: recent developments in understanding their life cycle and role in human pathology. PLoS Pathog. 2013;9(3):e1003206. doi: 10.1371/journal.ppat.1003206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Padgett BL, Zu Rhein GM, Walker DL, et al. Cultivation of papova-like virus from human brain with progressive multifocal leukoencephalopathy. Lancet. 1971;i:1257–1260. doi: 10.1016/s0140-6736(71)91777-6. [DOI] [PubMed] [Google Scholar]
- 7.Gardner SD, Field AM, Coleman DV, Hulme B. New human papovavirus (B.K) isolated from urine after renal transplantation. Lancet. 1971;19:1253–1257. doi: 10.1016/s0140-6736(71)91776-4. [DOI] [PubMed] [Google Scholar]
- 8.Allander T, Andreasson K, Gupta S, et al. Identification of a third human polyomavirus. J Virol. 2007;81:4130–4136. doi: 10.1128/JVI.00028-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gaynor AM, Nissen MD, Whiley DM, et al. Identification of a novel polyomavirus from patients with acute respiratory tract infections. PLoS Pathog. 2007;3:e64. doi: 10.1371/journal.ppat.0030064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Markowitz RB, Eaton BA, Kubik MF, et al. BK virus and JC virus shed during pregnancy have predominantly archetypal regulatory regions. J Virol. 1991;65:4515–4519. doi: 10.1128/jvi.65.8.4515-4519.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Knowles WA, Pillay D, Johnson MA, et al. Prevalence of long-term BK and JC excretion in HIV-infected adults and lack of correlation with serological markers. J Med Virol. 1999;59:474–479. doi: 10.1002/(SICI)1096-9071(199912)59:4<474::AID-JMV9>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 12.Chen Y, Bord E, Tompkins T, et al. Asymptomatic reactivation of JC virus in patients treated with natalizumab. N Engl J Med. 2009;361:1067–1074. doi: 10.1056/NEJMoa0904267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Delbue S, Elia F, Carloni C, et al. JC virus urinary excretion and seroprevalence in natalizumab-treated multiple sclerosis patients. J Neurovirol. 2014 doi: 10.1007/s13365-014-0268-0. In Press. [DOI] [PubMed] [Google Scholar]
- 14.Wollebo HS, White MK, Gordon J, et al. Persistence and pathogenesis of the neurotropic polyomavirus JC. Ann Neurol. 2015;77:560–570. doi: 10.1002/ana.24371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Astrom KE, Mancall EL, Richardson EP. Progressive multifocal leuko-encephalopathy; a hitherto unrecognized complication of chronic lymphatic leukaemia and Hodgkin’s disease. Brain. 1958;81:93–111. doi: 10.1093/brain/81.1.93. [DOI] [PubMed] [Google Scholar]
- 16.Berger JR, Aksamit AJ, Clifford DB, et al. PML diagnostic criteria: consensus statement from the AAN Neuroinfectious Disease Section. Neurology. 2013;80:1430–1438. doi: 10.1212/WNL.0b013e31828c2fa1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tavazzi E, White MK, Khalili K. Progressive multifocal leukoencephalopathy: clinical and molecular aspects. Rev Med Virol. 2012;22:18–32. doi: 10.1002/rmv.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cinque P, Koralnik IJ, Gerevini S, et al. Progressive multifocal leukoencephalopathy in HIV-1 infection. Lancet Infect Dis. 2009;9:625–636. doi: 10.1016/S1473-3099(09)70226-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pal A, Sirota L, Maudru T, et al. Real-time, quantitative PCR assays for the detection of virus-specific DNA in samples with mixed populations of polyomaviruses. J Virol Methods. 2006;135:32–42. doi: 10.1016/j.jviromet.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 20.Chen Y, Sharp PM, Fowkes M, et al. Analysis of 15 novel full-length BK virus sequences from three individuals: evidence of a high intra-strain genetic diversity. J Gen Virol. 2004;85:2651–2663. doi: 10.1099/vir.0.79920-0. [DOI] [PubMed] [Google Scholar]
- 21.Moens U, Van Ghelue M. Polymorphism in the genome of non-passaged human polyomavirus BK: implications for cell tropism and the pathological role of the virus. Virology. 2005;331:209–231. doi: 10.1016/j.virol.2004.10.021. [DOI] [PubMed] [Google Scholar]
- 22.Bayliss J, Karasoulos T, McLean CA. Frequency and large T (LT) sequence of JC polyomavirus DNA in oligodendrocytes, astrocytes and granular cells in non-PML brain. Brain Pathol. 2012;22:329–336. doi: 10.1111/j.1750-3639.2011.00538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dumoulin A, Hirsch HH. Reevaluating and optimizing polyomavirus BK and JC real-time PCR assays to detect rare sequence polymorphisms. J Clin Microbiol. 2011;49:1382–1388. doi: 10.1128/JCM.02008-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yogo Y, Sugimoto C. The archetype concept and regulatory region rearrangement. In: Khalili K, Stoner GL, editors. Human polyomaviruses. Wiley-Liss; New York: 2001. pp. 127–148. [Google Scholar]
- 25.Frisque RJ, Bream GL, Cannella MT. Human polyomavirus JC virus genome. J Virol. 1984;51:458–469. doi: 10.1128/jvi.51.2.458-469.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gosert R, Kardas P, Major EO, Hirsch HH. Rearranged JC virus noncoding control regions found in progressive multifocal leukoencephalopathy patient samples increase virus early gene expression and replication rate. J Virol. 2010;84:10448–10456. doi: 10.1128/JVI.00614-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ryschkewitsch CF, Jensen PN, Major EO. Multiplex qPCR assay for ultra sensitive detection of JCV DNA with simultaneous identification of genotypes that discriminates non-virulent from virulent variants. J Clin Virol. 2013;57:243–248. doi: 10.1016/j.jcv.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Egli A, Infanti L, Dumoulin A, et al. Prevalence of polyomavirus BK and JC infection and replication in 400 healthy blood donors. J Infect Dis. 2009;199:837–846. doi: 10.1086/597126. [DOI] [PubMed] [Google Scholar]
- 29.Rudick RA, O’Connor PW, Polman CH, et al. Assessment of JC virus DNA in blood and urine from natalizumab-treated patients. Ann Neurol. 2010;68:304–310. doi: 10.1002/ana.22107. [DOI] [PubMed] [Google Scholar]
- 30.Viscidi RP, Khanna N, Tan CS, et al. JC virus antibody and viremia as predictors of progressive multifocal leukoencephalopathy in human immunodeficiency virus-1-infected individuals. Clin Infect Dis. 2011;53:711–715. doi: 10.1093/cid/cir507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pietropaolo V, Bellizzi A, Anzivino E, et al. Human polyomavirus JC replication and non-coding control region analysis in multiple sclerosis patients under natalizumab treatment. J Neurovirol. 2015 doi: 10.1007/s13365-015-0338-y. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berger JR, Houff SA, Gurwell J, et al. JC virus antibody status underestimates infection rates. Ann Neurol. 2013;74:84–90. doi: 10.1002/ana.23893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ishaq M, Stoner GL. Differential expression of mRNAs for JC virus large and small tumor antigens in brain tissues from progressive multifocal leukoencephalopathy patients with and without AIDS. Proc Natl Acad Sci USA. 1994;91:8283–8287. doi: 10.1073/pnas.91.17.8283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seo GJ, Fink LH, O’Hara B, et al. Evolutionarily conserved function of a viral microRNA. J Virol. 2008;82:9823–9828. doi: 10.1128/JVI.01144-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sullivan CS, Grundhoff AT, Tevethia S, et al. SV40-encoded microRNAs regulate viral gene expression and reduce susceptibility to cytotoxic T cells. Nature. 2005;435:682–686. doi: 10.1038/nature03576. [DOI] [PubMed] [Google Scholar]
- 36.Bauman Y, Nachmani D, Vitenshtein A, et al. An identical miRNA of the human JC and BK polyoma viruses targets the stress-induced ligand ULBP3 to escape immune elimination. Cell Host Microbe. 2011;9:93–102. doi: 10.1016/j.chom.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 37.Lagatie O, Van Loy T, Tritsmans L, Stuyver LJ. Viral miRNAs in plasma and urine divulge JC polyomavirus infection. Virol J. 2014;11:158. doi: 10.1186/1743-422X-11-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rocca A, Martelli F, Delbue S, et al. The JCPYV DNA load inversely correlates with the viral microrna expression in blood and cerebrospinal fluid of patients at risk of PML. J Clin Virol. 2015;70:1–6. doi: 10.1016/j.jcv.2015.06.104. [DOI] [PubMed] [Google Scholar]
- 39.Hamilton RS, Gravell M, Major EO. Comparison of antibody titers determined by hemagglutination inhibition and enzyme immunoassay for JC virus and BK virus. J Clin Microbiol. 2000;38:105–109. doi: 10.1128/jcm.38.1.105-109.2000. 0095-1137/00/$04.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kardas P, Leboeuf C, Hirsch HH. Optimizing JC and BK polyomavirus IgG testing for seroepidemiology and patient counseling. J Clin Virol. 2015;71:28–33. doi: 10.1016/j.jcv.2015.07.305. [DOI] [PubMed] [Google Scholar]
- 41.Chalkley JJ, Berger JR. Progressive multifocal leukoencephalopathy in multiple sclerosis. Curr Neurol Neurosci Rep. 2013;13:408. doi: 10.1007/s11910-013-0408-6. [DOI] [PubMed] [Google Scholar]
- 42.Chahin S, Berger JR. A risk classification for immunosuppressive treatment-associated progressive multifocal leukoencephalopathy. J Neurovirol. 2014 doi: 10.1007/s13365-014-0303-1. In Press. [DOI] [PubMed] [Google Scholar]
- 43.Gorelik L, Lerner M, Bixler S, et al. Anti-JC virus antibodies: implications for PML risk stratification. Ann Neurol. 2010;68:295–303. doi: 10.1002/ana.22128. [DOI] [PubMed] [Google Scholar]
- 44.Bloomgren G, Richman S, Hotermans C, et al. Risk of natalizumab-associated progressive multifocal leukoencephalopathy. N Engl J Med. 2012;366:1870–1880. doi: 10.1056/NEJMoa1107829. [DOI] [PubMed] [Google Scholar]
- 45.Plavina T, Subramanyam M, Bloomgren G, et al. Anti-JC virus antibody levels in serum or plasma further define risk of natalizumab-associated progressive multifocal leukoencephalopathy. Ann Neurol. 2014;76:802–812. doi: 10.1002/ana.24286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee P, Plavina T, Castro A, et al. A second-generation ELISA (STRATIFY JCV™ DxSelect™) for detection of JC virus antibodies in human serum and plasma to support progressive multifocal leukoencephalopathy risk stratification. J Clin Virol. 2013;57:141–146. doi: 10.1016/j.jcv.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 47.Bozic C, Subramanyam M, Richman S, et al. Anti-JC virus (JCV) antibody prevalence in the JCV Epidemiology in MS (JEMS) trial. Eur J Neurol. 2014;21:299–304. doi: 10.1111/ene.12304. [DOI] [PubMed] [Google Scholar]
- 48.da Silva AM, Santos ME Portuguese JEMS Study Investigators. JCV epidemiology in MS (JEMS)--epidemiology of anti-JCV antibody prevalence in multiple sclerosis patients--Portuguese data. J Neurol Sci. 2014;337:119–122. doi: 10.1016/j.jns.2013.11.031. [DOI] [PubMed] [Google Scholar]
- 49.Olsson T, Achiron A, Alfredsson L, et al. Anti-JC virus antibody prevalence in a multinational multiple sclerosis cohort. Mult Scler. 2013;19:1533–1538. doi: 10.1177/1352458513477925. [DOI] [PubMed] [Google Scholar]
- 50.Clausi V, Giannecchini S, Magnani E, et al. Markers of JC virus infection in patients with multiple sclerosis under natalizumab therapy. Neurol Neuroimmunol Neuroinflamm. 2013;2:e58. doi: 10.1212/NXI.0000000000000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Raffel J, Gafson AR, Malik O, Nicholas R. Anti-JC virus antibody titres increase over time with natalizumab treatment. Mult Scler. 2015 doi: 10.1177/1352458515599681. In Press. [DOI] [PubMed] [Google Scholar]
- 52.Warnke C, Ramanujam R, Plavina T. Changes to anti-JCV antibody levels in a Swedish national MS cohort. J Neurol Neurosurg Psychiatry. 2013;84:1199–1205. doi: 10.1136/jnnp-2012-304332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Koralnik IJ. Overview of the cellular immunity against JC virus in progressive multifocal leukoencephalopathy. J Neurovirol. 2002;8(Suppl 2):59–65. doi: 10.1080/13550280290167894. [DOI] [PubMed] [Google Scholar]
- 54.Beltrami S, Gordon J. Immune surveillance and response to JC virus infection and PML. J Neurovirol. 2014;20:137–149. doi: 10.1007/s13365-013-0222-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Koralnik IJ, Du Pasquier RA, Letvin NL. JC virus-specific cytotoxic T lymphocytes in individuals with progressive multifocal leukoencephalopathy. J Virol. 2001;75:3483–3487. doi: 10.1128/JVI.75.7.3483-3487.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Du Pasquier RA, Kuroda MJ, Zheng Y, et al. A prospective study demonstrates an association between JC virus-specific cytotoxic T lymphocytes and the early control of progressive multifocal leukoencephalopathy. Brain. 2004;127(Pt 9):1970–1978. doi: 10.1093/brain/awh215. [DOI] [PubMed] [Google Scholar]
- 57.Du Pasquier RA, Schmitz JE, Jean-Jacques J, et al. Detection of JC virus-specific cytotoxic T lymphocytes in healthy individuals. J Virol. 2004;78:10206–10210. doi: 10.1128/JVI.78.18.10206-10210.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Marzocchetti A, Tompkins T, Clifford DB, et al. Determinants of survival in progressive multifocal leukoencephalopathy. Neurology. 2009;73:1551–1558. doi: 10.1212/WNL.0b013e3181c0d4a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Koralnik IJ, Du Pasquier RA, Kuroda MJ, et al. Association of prolonged survival in HLA-A2+ progressive multifocal leukoencephalopathy patients with a CTL response specific for a commonly recognized JC virus epitope. J Immunol. 2002;168:499–504. doi: 10.4049/jimmunol.168.1.499. [DOI] [PubMed] [Google Scholar]
- 60.Du Pasquier RA, Kuroda MJ, Schmitz JE, et al. Low frequency of cytotoxic T lymphocytes against the novel HLA-A*0201-restricted JC virus epitope VP1(p36) in patients with proven or possible progressive multifocal leukoencephalopathy. J Virol. 2003;77:11918–11926. doi: 10.4049/jimmunol.168.1.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Grabstein K, Chen YU. In: Cell-mediated cytolytic responses in Selected Methods in Cellular Immunology. Mischell BB, Shiigi SM, editors. W.H. Freeman and Co; San Francisco, CA: 1980. p. 128. [Google Scholar]
- 62.Berger JR. The clinical features of PML. Cleve Clin J Med. 2011;78(Suppl 2):S8–12. doi: 10.3949/ccjm.78.s2.03. [DOI] [PubMed] [Google Scholar]
- 63.Antoniol C, Stankoff B. Immunological Markers for PML Prediction in MS Patients Treated with Natalizumab. Front Immunol. 2015;5:668. doi: 10.3389/fimmu.2014.00668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schwab N, Schneider-Hohendorf T, Posevitz V, et al. L-selectin is a possible biomarker for individual PML risk in natalizumab-treated MS patients. Neurology. 2013;81:865–871. doi: 10.1212/WNL.0b013e3182a351fb. [DOI] [PubMed] [Google Scholar]
- 65.Spadaro M, Caldano M, Marnetto F, et al. Natalizumab treatment reduces L-selectin (CD62L) in CD4+ T cells. J Neuroinflammation. 2015;12:146. doi: 10.1186/s12974-015-0365-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Basnyat P, Hagman S, Kolasa M, et al. Association between soluble L-selectin and anti-JCV antibodies in natalizumab-treated relapsing-remitting MS patients. Mult Scler Relat Disord. 2015;4:334–338. doi: 10.1016/j.msard.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 67.Schwab N, Schneider-Hohendorf T, Pignolet B, et al. PML risk stratification using anti-JCV antibody index and L-selectin. Mult Scler. 2015 Oct 2; doi: 10.1177/1352458515607651. pii: 1352458515607651. [DOI] [PubMed] [Google Scholar]
- 68.Schneider-Hohendorf T, Philipp K, Husstedt IW, et al. Specific loss of cellular L-selectin on CD4(+) T cells is associated with progressive multifocal leukoencephalopathy development during HIV infection. AIDS. 2014;28:793–795. doi: 10.1097/QAD.0000000000000201. [DOI] [PubMed] [Google Scholar]
- 69.Lanzillo R, Liuzzi R, Vallefuoco L, et al. JC virus antibody index in natalizumab-treated patients: correlations with John Cunningham virus DNA and C-reactive protein level. Ther Clin Risk Manag. 2014;10:807–814. doi: 10.2147/TCRM.S63295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Otlu O, De Simone FI, Otalora YL, et al. The agnoprotein of polyomavirus JC is released by infected cells: evidence for its cellular uptake by uninfected neighboring cells. Virology. 2014;468–470:88–95. doi: 10.1016/j.virol.2014.07.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cinque P, Scarpellini P, Vago L, et al. Diagnosis of central nervous system complications in HIV-infected patients: cerebrospinal fluid analysis by the polymerase chain reaction. AIDS. 1997;11:1–17. doi: 10.1097/00002030-199701000-00003. [DOI] [PubMed] [Google Scholar]
- 72.Babi MA, Pendlebury W, Braff S, Waheed W. JC Virus PCR Detection Is Not Infallible: A Fulminant Case of Progressive Multifocal Leukoencephalopathy with False-Negative Cerebrospinal Fluid Studies despite Progressive Clinical Course and Radiological Findings. Case Rep Neurol Med. 2015:643216. doi: 10.1155/2015/643216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Berger JR, Clifford DB. The manifold faces of PML and the challenge of diagnosis. Neurology. 2011;77:2006–2007. doi: 10.1212/WNL.0b013e31823b9c8f. [DOI] [PubMed] [Google Scholar]
- 74.Kuhle J, Gosert R, Bühler R, et al. Management and outcome of CSF-JC virus PCR-negative PML in a natalizumab-treated patient with MS. Neurology. 2011;77:2010–2016. doi: 10.1212/WNL.0b013e31823b9b27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Villar LM, Costa-Frossard L, Masterman T, et al. Lipid-specific immunoglobulin M bands in cerebrospinal fluid are associated with a reduced risk of developing progressive multifocal leukoencephalopathy during treatment with natalizumab. Ann Neurol. 2015;77:447–457. doi: 10.1002/ana.24345. [DOI] [PubMed] [Google Scholar]
- 76.Boster A, Hreha S, Berger JR, et al. Progressive multifocal leukoencephalopathy and relapsing-remitting multiple sclerosis: a comparative study. Arch Neurol. 2009;66:593–599. doi: 10.1001/archneurol.2009.31. [DOI] [PubMed] [Google Scholar]
- 77.Ayzenberg I, Lukas C, Trampe N, et al. Value of MRI as a surrogate marker for PML in natalizumab long-term therapy. J Neurol. 2012;259:1732–1733. doi: 10.1007/s00415-012-6426-5. [DOI] [PubMed] [Google Scholar]
- 78.Blair NF, Brew BJ, Halpern JP. Natalizumab-associated PML identified in the presymptomatic phase using MRI surveillance. Neurology. 2012;78:507–508. doi: 10.1212/WNL.0b013e318246d6d8. [DOI] [PubMed] [Google Scholar]
- 79.Wattjes MP, Barkhof F. Diagnosis of natalizumab-associated progressive multifocal leukoencephalopathy using MRI. Curr Opin Neurol. 2014;27:260–270. doi: 10.1097/WCO.0000000000000099. [DOI] [PubMed] [Google Scholar]
- 80.ZuRhein GM. Particles resembling papovavirions in human cerebral demyelinating disease. Science. 1965;148:1477–1479. doi: 10.1126/science.148.3676.1477. [DOI] [PubMed] [Google Scholar]
- 81.Del Valle L, Pina-Oviedo S. HIV disorders of the brain: pathology and pathogenesis. Front Biosci. 2006;11:718–732. doi: 10.2741/1830. [DOI] [PubMed] [Google Scholar]
- 82.Khalili K, Safak M, Del Valle L, White MK. JC virus molecular biology and the human demyelinating disease, progressive multifocal leukoencephalopathy. In: Shoshkes Reiss C, editor. Neurotropic virus infections. Cambridge University Press; Cambridge, UK: 2008. pp. 190–211. [Google Scholar]
- 83.Okada Y, Sawa H, Endo S, et al. Expression of JC virus agnoprotein in progressive multifocal leukoencephalopathy brain. Acta Neuropathol (Berl) 2002;104:130–136. doi: 10.1007/s00401-002-0526-8. [DOI] [PubMed] [Google Scholar]
- 84.Khalili K, White MK, Sawa H, et al. The agnoprotein of polyomaviruses: a multifunctional auxiliary protein. J Cell Physiol. 2005;204:1–7. doi: 10.1002/jcp.20266. [DOI] [PubMed] [Google Scholar]
- 85.Aksamit AJ, Gendelman HE, Orenstein JM, Pezeshkpour GH. AIDS-associated progressive multifocal leukoencephalopathy (PML): comparison to non-AIDS PML with in situ hybridization and immunohistochemistry. Neurology. 1990;40:1073–1078. doi: 10.1212/wnl.40.7.1073. [DOI] [PubMed] [Google Scholar]
- 86.Darbinyan A, White MK, Akan S, et al. Alterations of DNA damage repair pathways resulting from JCV infection. Virology. 2007;364:73–86. doi: 10.1016/j.virol.2007.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wollebo HS, Cotto B, Adiga R, et al. Expression of signaling molecules in progressive multifocal leukoencephalopathy. Curr HIV Res. 2016;14(1) doi: 10.2174/1570162x1401151102125319. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dörries K, Johnson RT, ter Meulen V. Detection of polyoma virus DNA in PML-brain tissue by (in situ) hybridization. J Gen Virol. 1979;42:49–57. doi: 10.1099/0022-1317-42-1-49. [DOI] [PubMed] [Google Scholar]
- 89.Aksamit AJ, Mourrain P, Sever JL, Major EO. Progressive multifocal leukoencephalopathy: investigation of three cases using in situ hybridization with JC virus biotinylated DNA probe. Ann Neurol. 1985;18:490–496. doi: 10.1002/ana.410180412. [DOI] [PubMed] [Google Scholar]
- 90.Aksamit AJ, Sever JL, Major EO. Progressive multifocal leukoencephalopathy: JC virus detection by in situ hybridization compared with immunohistochemistry. Neurology. 1986;36:499–504. doi: 10.1212/wnl.36.4.499. [DOI] [PubMed] [Google Scholar]
- 91.Berger JR. Progressive multifocal leukoencephalopathy. Handb Clin Neurol. 2014;123:357–376. doi: 10.1016/B978-0-444-53488-0.00017-1. [DOI] [PubMed] [Google Scholar]
- 92.Bernal-Cano F, Joseph JT, Koralnik IJ. Spinal cord lesions of progressive multifocal leukoencephalopathy in an acquired immunodeficiency syndrome patient. J Neurovirol. 2007;13:474–476. doi: 10.1080/13550280701469178. [DOI] [PubMed] [Google Scholar]
- 93.Murayi R, Schmitt J, Woo JH, Berger JR. Spinal cord progressive multifocal leukoencephalopathy detected premortem by MRI. J Neurovirol. 2015;21:688–690. doi: 10.1007/s13365-015-0342-2. [DOI] [PubMed] [Google Scholar]
- 94.Berger JR, Pall L, Lanska D, Whiteman M. Progressive multifocal leukoencephalopathy in patients with HIV infection. J Neurovirol. 1998;4:59–68. doi: 10.3109/13550289809113482. [DOI] [PubMed] [Google Scholar]
- 95.Clifford DB, De Luca A, Simpson DM, Arendt G, Giovannoni G, Nath A. Natalizumab-associated progressive multifocal leukoencephalopathy in patients with multiple sclerosis: lessons from 28 cases. Lancet Neurol. 2010;9:438–446. doi: 10.1016/S1474-4422(10)70028-4. [DOI] [PubMed] [Google Scholar]
- 96.Khalili K, Gordon J, White MK. The polyomavirus, JCV and its involvement in human disease. Adv Exp Med Biol. 2006;577:274–287. doi: 10.1007/0-387-32957-9_20. [DOI] [PubMed] [Google Scholar]