Abstract
Objective
Bevacizumab, the first FDA-approved anti-angiogenesis agent, has been used for metastatic colorectal cancer since 2004. This study evaluated the utilization of bevacizumab among elderly metastatic colorectal cancer patients in the United States.
Methods
Using Surveillance and Epidemiology and End Results (SEER)-Medicare data, this retrospective cohort study consisted of individuals aged 65 years or older with a colorectal cancer diagnosis between 2005 and 2009, who received chemotherapy any time through 2010. This included patients with newly diagnosed metastatic colorectal cancer and patients who progressed from initially diagnosed earlier-stage disease. We ascertained comorbid conditions using ICD-9 codes and conducted logistic regression to identify patients’ characteristics associated with bevacizumab use.
Results
A total of 8645 patients were identified (mean age 74 years; 52% male); 57% of patients received bevacizumab with initially diagnosed metastatic colorectal cancer and 44% of patients with treated progressive or recurrent disease. After adjusting for other covariates, we found that patients aged ≥80 years were less likely to receive bevacizumab compared with those aged 65–69 years (odds ratio (OR), 0.64 (95% confidence interval (CI): 0.57–0.73)), or if they had evidence of comorbid cardiomyopathy/congestive heart failure (OR, 0.82 (CI: 0.70–0.95)) or arrhythmic disorder (OR, 0.85 (CI: 0.75–0.96)). Adoption of bevacizumab into practice was rapid following its approval, and the use increased from 36% to 40% from 2005 to 2010 (p = 0.013). There were significant regional variations in bevacizumab use.
Conclusions
Despite rapid uptake since its original approval, there appears to be low use of bevacizumab in elderly metastatic colorectal cancer patients in the United States. Regional variations and the strong effects of age and comorbidity suggest lack of consensus among oncologists regarding benefits and risks of bevacizumab in elderly patients.
Keywords: Bevacizumab utilization, colorectal cancer, elderly, SEER-Medicare
Introduction
Colorectal cancer (CRC) is the third-most common cancer and the third leading cause of cancer death for both men and women in the United States, with 142,820 new cases and 50,830 deaths projected for year 2013.1 Among newly diagnosed CRC patients, 15–30% have metastatic colorectal cancer (mCRC) at diagnosis, whereas disease recurrence and development of distant metastases can occur in up to 50% of patients who were initially diagnosed at earlier stages.2
In the past two decades, improvements in the survival of patients with mCRC have been made with efficacious systemic therapies, including cytotoxic drugs and targeted monoclonal antibody treatments. Bevacizumab (Bev, Avastin®), targeting the vascular endothelial growth factor, received approval from the US Food and Drug Administration in 2004 as the first anti-angiogenesis drug with fluorouracil-based chemotherapy as first-line treatment for mCRC. Clinical trials show that Bev improves median overall survival by 1–5 months depending on combined use with different chemotherapy regimens.3–5 Clinical trials also report that Bev use is associated with the following severe adverse events: gastrointestinal (GI) perforations, surgery and wound-healing complications, hemorrhage, non-GI fistula formations, arterial thromboembolism, and hypertension.4,6 However, clinical trial results may not generalize well to older patients and those with comorbidities, who are frequently underrepresented in clinical trials and often undertreated with standard therapy. For example, the two pivotal trials of Bev included patients with a mean age of 60 years,3,5 whereas more than 40% of newly diagnosed mCRC patients in the United States are aged 75 years and older. Studies have shown that older patients are less likely to receive treatment for every stage of CRC than younger patients, including chemotherapy,10,11 and they are more likely to receive single-agent rather than multiple-agent treatment with their chemotherapy.11
Bev has been associated with an increased risk of GI perforation and arterial thromboembolic events among mCRC patients aged 65 years or above in large observational cohorts.12–16 Given the potential for elevated risks of serious adverse events in older patients, and the small gain in survival shown in randomized trials that may not generalize to this subgroup, it is possible that Bev may not yet be widely adopted in US oncology practice. Using the most recently updated linked Surveillance and Epidemiology and End Results (SEER) registry-Medicare claims database,17 we investigated the trends, variations, and utilization patterns of Bev in mCRC patients aged 65 years or older.
Methods
Source data
The SEER data are from a comprehensive population-based US cancer registry program funded by the National Cancer Institute, which collects cancer incidence and survival data from 17 population-based cancer registries covering approximately 26% of the US population. Medicare is a US federal health insurance program serving 97% of people aged 65 years or older. Approximately 94% of SEER patients aged 65 years or above have been linked with their Medicare claims.18 The linked SEER-Medicare data, one of the largest cancer registry-claims data in the United States, is unique in its generalizability for US community practice and is used extensively in cancer-related health services research. The most recent update was available in December 2012, providing data including all Medicare eligible persons diagnosed with cancer in the SEER through year 2009 and their Medicare claims through 2010.17
From the SEER registries, we obtained information on population characteristics, year of diagnosis, clinical stage, and geographic region of all incident CRC patients. We used Medicare claims to ascertain information regarding chemotherapy and other medication utilization, as well as comorbidity information from inpatient hospital records, institutional outpatient providers, physician claims, and durable medical equipment files.
Sample selection and variable extraction
Our study cohort included patients aged 65 years or above with a diagnosis of CRC as their first or primary cancer from 1 January 2005 through 31 December 2009. The follow-up medical claims of these patients were through December 2010. For this study’s purposes, we looked at patients who received chemotherapy any time from diagnosis to the end of their follow-up (death or end of 2010). This included patients who were initially diagnosed with mCRC (stage IV) and patients who were initially diagnosed with earlier stages and later experienced a progression or recurrence of CRC. We defined this progression as receiving more than one standard chemotherapy agent (5-fluorouracil, irinotecan, oxaliplatin) at least 8 months after their initial diagnosis of non-stage-IV CRC. We defined the index date as the first service date associated with chemotherapy use. In order to avoid bias owing to prior treatment for any other cancers, we excluded patients who had been diagnosed with a prior cancer other than CRC. We also excluded those who were enrolled in Health Maintenance Organizations (HMOs) or were not enrolled in both Medicare A and B programs because these patients do not have complete longitudinal claims histories in Medicare.
We extracted chemotherapy use and Bev use using Healthcare Common Procedure Coding System (HCPCS) codes present in Part B Medicare claims. We began with year 2005 because there was not a specific HCPCS code in use to accurately identify Bev during 2004, although Bev was first approved by the FDA in February of 2004. Patient characteristics derived from SEER data included demographic covariates (age, sex, race/ethnicity, location by registry site), ecological socio-economic status (marital status, income, education), and clinical variables (cancer stage, comorbidities). Census-tract level median household income and percentage of adults with less than high school education were created in SEER-Medicare by linking patient’s census tract at the time of diagnosis with data collected by the US Census Bureau.19 Income and education variables were categorized by quartiles. Clinical variables included the tumor, node, metastasis stage at initial diagnosis20 and co-morbid conditions before index date. We identified co-morbid conditions using ICD-9 codes within 1 year prior to the index date from inpatient, outpatient, and physician claims. Based on Bev-associated risks reported in the literature,6,13,14 we included GI perforation, arterial thromboembolism, cardiomyopathy or congestive heart failure (CM/CHF), arrhythmic disorders, and other cardiac conditions (including hypertension, pericardial disorder, aortic wall disorders, and cardiopulmonary arrest). As a measure of other comorbid conditions, we calculated a modified Charlson index by excluding cancer and the previously mentioned conditions from the original Charlson index.21 The details of coding that we used to capture treatment regimens are provided in the appendix (supplementary material online).
Statistical analysis
We summarized patient characteristics by the use of Bev. We reported continuous variables as means ± standard deviations (SDs) and summarized categorical variables as percentages. The bivariate analyses were conducted comparing these variables between patients who did or did not use Bev. Chi-square tests assessed the statistical significance of percentage differences in categorical variables and t-tests tested for mean differences in continuous variables. Wilcoxon rank sum tests tested median differences in continuous variables. The Cochran-Armitage trend test tested the time trend in the adoption of Bev. For explanatory analysis, we constructed a multivariate logistic regression model to estimate likelihoods of Bev use as a function of all measured patient characteristics, including demographic information, socioeconomic status, and clinical covariates. The regression model included interaction terms between age, race, and comorbidities to test the potential modifying effects between these characteristics. Data analyses were carried out using SAS 9.2 (Cary, NC). All p-values were two-sided, and a p-value less than 0.05 was considered statistically significant.
Results
A total of 8645 patients with mCRC met our eligibility criteria for inclusion. Patient characteristics are shown in Table 1. The study sample had a mean (±SD) age of 74 (±6.3) years. There were 4502 (52%) patients who received Bev with 14 days as the median time from index date (initial chemotherapy) to Bev use. The average number of Bev cycles was 12.2 (median = 9), and median duration of Bev use was 176 days from the first to the last Bev use. Fifty-nine percent of 6529 patients who received oxaliplatin also used Bev, whereas 80% used Bev among 3385 patients who received irinotecan. Among 2777 patients who received both oxaliplatin and irinotecan, 15% used Bev. Overall, Bev was used in 57% of patients with an initial mCRC diagnosis and 44% of patients with treated progressive or recurrent disease, with median time from index date to Bev use 1 versus 76 days, respectively (p< 0.0001). There were no differences between these two subgroups with respect to the median cycles of Bev or the median duration of Bev use.
Table 1.
Characteristics of CRC patients who were 65 years or older and treated with chemotherapy with versus without bevacizumab (Bev) use.a
| n | Total 8645 |
Bev 4502 |
No Bev 4143 |
p |
|---|---|---|---|---|
| Age at first chemotherapy (years) | ||||
| 65–69 | 29.5% | 31.3% | 27.5% | <0.0001 |
| 70–74 | 27.5% | 27.9% | 27.1% | |
| 75–79 | 22.5% | 23.2% | 21.8% | |
| 80 or above | 20.5% | 17.7% | 23.6% | |
| Sex | ||||
| Male | 52.1% | 52.5% | 51.8% | 0.53 |
| Female | 47.9% | 47.5% | 48.2% | |
| Race/ethnicity | ||||
| White | 82.4% | 83.3% | 81.5% | 0.062 |
| Black | 9.1% | 9.0% | 9.2% | |
| Hispanic/Latino | 1.7% | 1.4% | 1.9% | |
| Asian | 3.8% | 3.6% | 4.0% | |
| Other | 3.0% | 2.6% | 3.4% | |
| Marital status | ||||
| Married | 57.0% | 58.6% | 55.1% | 0.001 |
| Other | 43.0% | 41.4% | 44.9% | |
| Median household income (group-level) | ||||
| Q1 | 24.1% | 23.6% | 24.6% | 0.66 |
| Q2 | 24.3% | 24.7% | 23.9% | |
| Q3 | 25.0% | 25.1% | 25.0% | |
| Q4 | 26.2% | 26.1% | 26.3% | |
| Education | ||||
| Q1 | 25.4% | 25.0% | 25.9% | 0.20 |
| Q2 | 25.3% | 25.0% | 25.7% | |
| Q3 | 24.0% | 23.8% | 24.3% | |
| Q4 | 24.9% | 25.8% | 23.8% | |
| CRC stage at diagnosis | ||||
| I | 3.1% | 2.8% | 3.4% | <0.0001 |
| II | 10.3% | 9.2% | 11.4% | |
| III | 25.8% | 21.2% | 30.7% | |
| IV | 60.9% | 66.8% | 54.5% | |
| Co-morbid conditions | ||||
| GI perforation | 2.5% | 2.2% | 2.8% | 0.062 |
| Arterial thromboembolism | 4.6% | 3.9% | 5.3% | 0.001 |
| CM/CHF | 10.2% | 8.6% | 12.0% | <0.0001 |
| Arrhythmic disorder | 17.2% | 15.2% | 19.3% | <0.0001 |
| Other cardiac condition | 66.7% | 64.3% | 69.4% | <0.0001 |
| Modified Charlson index (mean±SD) | 0.626 ±0.916 | 0.582 ±0.876 | 0.675 ±0.956 | <0.0001 |
Q1–Q4: quartile 1–quartile 4 from lowest to highest; CRC: colorectal cancer; CM/CHF: cardiomyopathy or congestive heart failure; GI: gastrointestinal; SD: standard deviation.
Modified Charlson index was calculated excluding all other listed conditions.
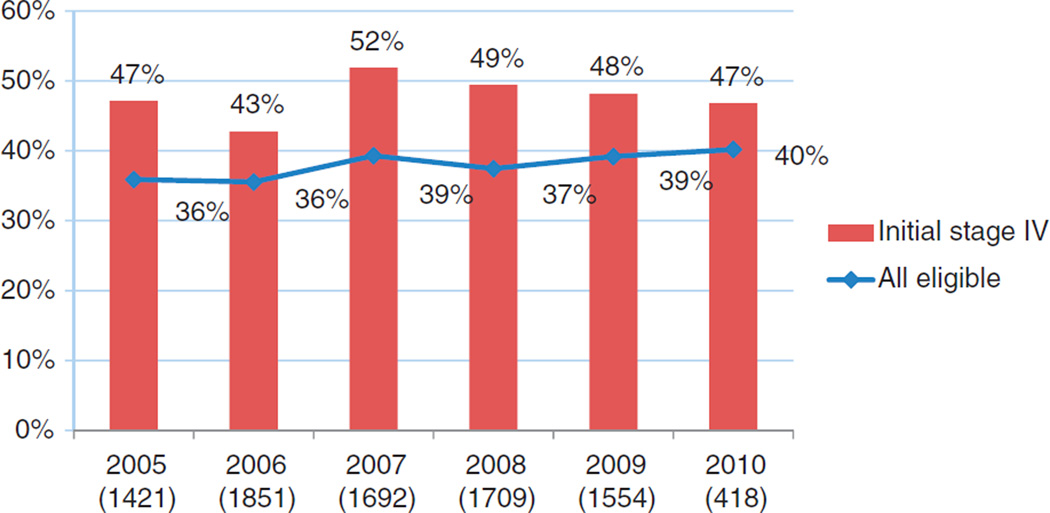
We observed that the adoption of Bev into practice was quite rapid, with an initial rate of 36% in its first full year after FDA approval in 2004. There is a modest yet significant increase in Bev use from 2005 to 2010, with annual percentage of use ranging from 36% to 40% (p = 0.013 for trend analysis) (Figure 1). The percentage of annual Bev use among patients diagnosed with stage IV CRC ranged between 43% and 52%, but this trend was not statistically significant.
Figure 1.
Bevacizumab use by calendar year (all eligible versus initially diagnosed stage IV colorectal cancer patients; number of all patients in parentheses).
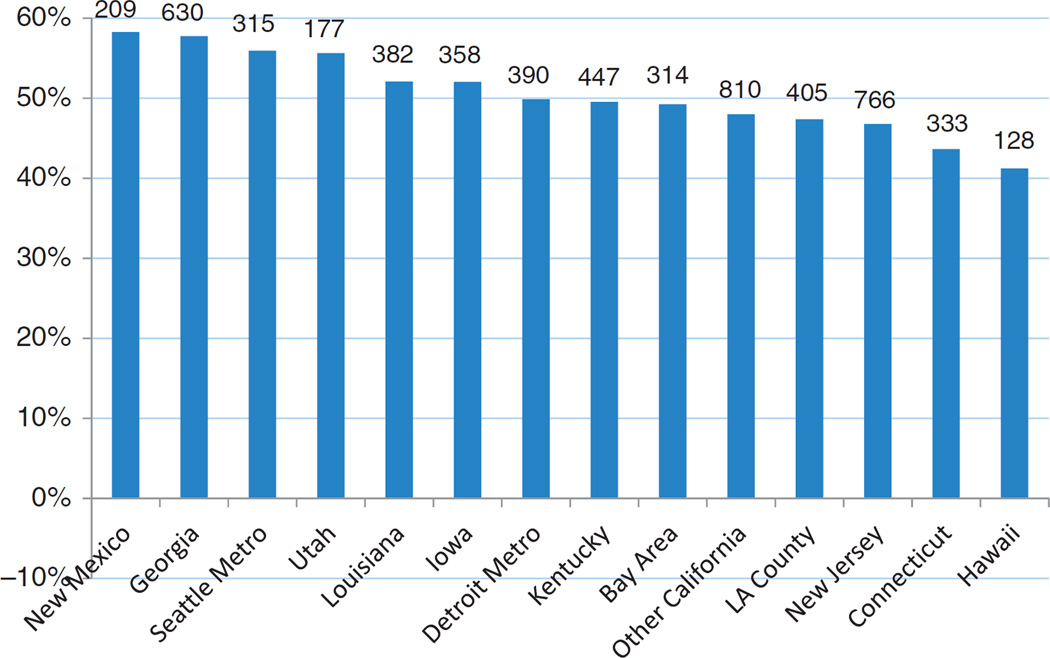
In bivariate analysis (Table 1), we found that patients who used Bev were younger (p<0.0001) and were more likely to be married (p = 0.001) than those who did not use Bev. There were significant variations in Bev use by region, ranging from 41% in Hawaii to 58% in New Mexico (Figure 2). In terms of clinical factors, 61% of patients in our study who received any chemotherapy were initially diagnosed with mCRC. The remainder were initially diagnosed with stages I–III disease and by our definition, received multiple chemotherapy agents for progressive or recurrent CRC. Patients who received Bev were more likely to have an initial stage IV diagnosis (67% versus 55%, p < 0.0001) and less likely to have an initial diagnosis of earlier stages than those who did not receive Bev. Patients who received Bev were less likely to have co-morbid arterial thromboembolism, CM/CHF, arrhythmic disorder, other cardiac conditions, and non-cardiac comorbid conditions (measured as a modified Charlson index) prior to their index date.
Figure 2.
Bevacizumab use by geographic area (number of all patients on top of bar).
Table 2 shows only those factors that are significantly associated with Bev use after adjustment for all other covariates that are shown in Table 1 using a multivariate logistic regression. We did not identify any significant effects from interactions between age, race, and comorbidities, so we dropped these interaction terms from the final model. Patients were less likely to receive Bev if they were aged>80 years compared with those aged 65–69 years (odds ratio (OR) = 0.64 (95% confidence interval (CI): 0.57–0.73); p < 0.0001) after adjusting for all other covariates in the model. There was a significant overall geographic effect (p < 0.0001, not shown in Table 2) for Bev use, with higher use in New Mexico, Georgia, Seattle metro area, Louisiana, and Detroit metro area compared with other areas. Married patients were more likely to use Bev (OR = 1.11 (95% CI: 1.01–1.21); p = 0.033). Patients in the highest quartile of household income were more likely to use Bev compared with patients in the lowest quartile of household income (OR = 1.20 (95% CI: 1.03–1.40); p = 0.021). Patients initially diagnosed with mCRC were more likely to use Bev than those with treated progressive or recurrent disease (OR = 1.78 (95% CI: 1.63–1.95); p<0.0001). Additionally, patients who had CM/CHF, arrhythmic disorder, and more non-cardiac comorbid conditions prior to chemotherapy initiation were less likely to use Bev after adjustment for all other factors.
Table 2.
Logistic regression of likelihood of bevacizumab (Bev) use among CRC patients who were 65 years or older and treated with chemotherapy.a
| Odds ratio |
95% Confidence interval |
p | ||
|---|---|---|---|---|
| Age groups | ||||
| 65–69 years (reference) | ||||
| 80 years or above | 0.64 | 0.57 | 0.73 | <0.0001 |
| Cancer registry site | ||||
| New Mexico | 1.60 | 1.16 | 2.19 | 0.004 |
| Georgia | 1.55 | 1.30 | 1.86 | <0.0001 |
| Seattle metro area | 1.42 | 1.12 | 1.79 | 0.003 |
| Louisiana | 1.27 | 1.03 | 1.57 | 0.029 |
| Detroit metro area | 1.23 | 1.01 | 1.50 | 0.041 |
| New Jersey (reference) | ||||
| Marital status | ||||
| Married | 1.11 | 1.01 | 1.21 | 0.033 |
| Other (reference) | ||||
| Income | ||||
| Q1 (reference) | ||||
| Q4 | 1.20 | 1.03 | 1.40 | 0.021 |
| CRC stage at diagnosis | ||||
| I–III (reference) | ||||
| IV | 1.78 | 1.63 | 1.95 | <0.0001 |
| Co-morbid conditions | ||||
| CM/CHF | 0.82 | 0.70 | 0.95 | 0.009 |
| Arrhythmic disorder | 0.85 | 0.75 | 0.96 | 0.009 |
| Modified Charlson index | 0.93 | 0.89 | 0.98 | 0.007 |
Q1–Q4: quartile 1–quartile 4 from lowest to highest; CRC: colorectal cancer; CM/CHF: cardiomyopathy or congestive heart failure.
Variables with significant results are listed in the table. The model also adjusted for the effects of sex, race/ethnicity, other registry sites, education, other co-morbid conditions including gastrointestinal perforation, arterial thromboembolism, and other cardiac conditions.
Discussion
Bev is the most widely used anti-angiogenic monoclonal antibody for treatment of several tumors, particularly as first-line therapy for mCRC.4 Using a large US population-based database, we observed that approximately half of eligible patients aged 65 years or older used Bev. To our knowledge, this is the first report on patterns of Bev use through 2010 among this sub-population using SEER-Medicare data.
A previous study analyzed the electronic medical records of 1655 adult patients with mCRC from 91 oncology practices in the United States.22 This study reported that Bev was the most frequently administered biologic therapy, with 69% of patients receiving it sometime during the study period (January 2004 to January 2008).22 However, this study did not specifically assess older patients, nor did it examine factors associated with Bev use. Our finding that 52% of eligible patients aged 65 years and older used Bev is lower than the previously published 69% in all patients. However, this is not surprising, since physicians may be often reluctant to aggressively treat older patients due to lack of sufficient clinical evidence and concerns with toxicity.23–25 The propensity toward more conservative use of Bev is consistent with results from the BRiTE observational cohort study evaluating the safety and effectiveness of Bev-based first-line mCRC therapy for elderly patients26 and another study analyzing the Cancer Care Outcomes Research Surveillance Consortium (CanCORS) data.27 Both indicated that elderly patients were less likely to receive Bev than younger patients. Our study found that age is a significant factor contributing to lower Bev use, even among patients aged 65 year and older, consistent with an earlier study through 2007 using the SEER-Medicare data.15
In addition to age, we observed that patients’ history of CM/CHF, arrhythmic disorder, and non-cardiac comorbid conditions significantly contributes to lower Bev utilization. Data from clinical trials demonstrates the association between Bev and several cardiotoxicities;4,6,28 hence, physicians may carefully prescribe Bev to patients, avoiding those either with a history of or with risk factors for cardiac conditions. This finding is consistent with prior studies using SEER-Medicare data.13–15
Our results are consistent with another report that found that chemotherapy use may also be related to patients’ preference to undergo therapy and physicians’ recommendations.29 Physicians in particular may be partly hampered by lack of comparative outcomes data that pertain to this patient subgroup, which is not typically well represented in prospective randomized trials of Bev. Our results are consistent with treatment patterns for older patients with other cancers,30–32 who generally receive less aggressive cancer therapies than their younger counterparts due likely to greater uncertainty regarding the benefit–risk tradeoffs of such therapies.
The rapid early uptake and then small increasing trend in Bev use we observed demonstrates the diffusion of an important medical advance in GI oncology within this patient population. Additionally, we found that patients with higher household income are more likely to use Bev, suggesting a possible association between higher socio-economic status and Bev use. The drug cost of Bev is approximately US$4000–5000 per month.33 Though presumably most of the cost is covered by the Medicare program for this patient population, the out-of-pocket financial burden may not be trivial.34 Thus, future research could focus on the economic impact of Bev use on patients’ financial well-being. Our finding of an association between marital status and Bev use is consistent with other cancer care literature, which has often found that being currently married is positively associated with better outcomes relative to being single, widowed, or divorced.35,36
There is substantial geographic variation in Bev use even after adjusting for all patient characteristics. We observed patients in the Northeast region were less likely to use Bev compared with patients in the West or South regions. This variation underscores uncertainty and professional disagreement37 in the adoption of this drug. This phenomenon may also reflect variations in insurance guidelines and/or institutional policies in local regions. To better understand the reasons for geographic variations and physician practice patterns, future studies should assess the attitudes and practices of physicians and health plan regarding the adoption of Bev and other high-cost cancer medications.
The present study has several strengths. We included a large cohort of approximately 9000 subjects aged 65 years or above, a subgroup typically underrepresented in randomized clinical trials. Our results are thus generalizable to older patients with mCRC within US oncological practice. Additionally, we used the most recently updated SEER-Medicare linked data to provide the most updated report as possible on practice.
As with all analyses based on observational data, the study findings should be viewed in light of some limitations. First, we restricted our sample to patients newly diagnosed with CRC without a history of other cancers. This maintained the internal validity of the study but limited our generalizability to patients with CRC as their second or higher cancer diagnosis. Second, we defined patients who were initially diagnosed at earlier stages who later experienced progressive or recurrent disease as those receiving more than one standard chemotherapy agent (5-fluorouracil, irinotecan, oxaliplatin) at least 8 months after initial diagnosis. However, some patients, especially those at an older age or with comorbidities, may decide not to receive aggressive treatments but only receive one agent or none for the control of their metastatic disease. Due to the exclusion of these patients, the denominator of all eligible patients may be larger; hence, the true utilization rate is probably lower than what we observed. Third, the comorbidities identified in this study were based on ICD-9 codes within claims data rather than medical charts. For example, the ascertainment of CHF using claims has high specificity but moderate sensitivity,38 since a diagnosis of CHF may often appear as a diagnosis of CM. We included CHF with CM to minimize this potential misclassification error. It would also be possible that certain comorbidities were not claimed within the baseline year, which led to underestimate of comorbid conditions. Fourth, this study included only older patients with Medicare health insurance. Those remained employed after the age of 65 and who maintain their private health insurance plans, or who are in HMO plans, are not included.
Conclusion
Our study showed that approximately half of Bev-indicated patients aged 65 years or older in the United States receive Bev. Patients’ age and history of cardiac conditions significantly contributed to lower Bev utilization. These results from an older patient population in community practice demonstrate the rapid uptake of Bev since its original approval, the regional variations in Bev adoption, and several other patient factors in addition to age that are strongly associated with Bev use. Our study complements the current literature on clinical efficacy, safety, and effectiveness in US oncology practice by describing existing practices for older patients with this disease.
Acknowledgments
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Funding
The work was supported in part by award number P30CA051008 from the National Cancer Institute.
Footnotes
Conflict of interest
Dr Marshall serves as a consultant for Genentech Inc. All other authors have no conflicts of interest.
Contributor Information
Alex Z Fu, Cancer Prevention and Control Program, Georgetown University Medical Center, Washington, DC, USA.
Huei-Ting Tsai, Cancer Prevention and Control Program, Georgetown University Medical Center, Washington, DC, USA.
John L Marshall, Lombardi Comprehensive Cancer Center, Georgetown University Medical Center, Washington, DC, USA.
Andrew N Freedman, Division of Cancer Control and Population Sciences, National Cancer Institute, Bethesda, MD, USA.
Arnold L Potosky, Cancer Prevention and Control Program, Georgetown University Medical Center, Washington, DC, USA.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Kindler HL, Shulman KL. Metastatic colorectal cancer. Curr Treat Options Oncol. 2001;2(6):459–471. doi: 10.1007/s11864-001-0068-7. [DOI] [PubMed] [Google Scholar]
- 3.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350(23):2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 4.Galfrascoli E, Piva S, Cinquini M, et al. Risk/benefit profile of bevacizumab in metastatic colon cancer: a systematic review and meta-analysis. Dig Liver Dis. 2011;43(4):286–294. doi: 10.1016/j.dld.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 5.Saltz LB, Clarke S, Diaz-Rubio E, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol. 2008;26(12):2013–2019. doi: 10.1200/JCO.2007.14.9930. [DOI] [PubMed] [Google Scholar]
- 6.Roche Bevacizumab US prescribing information. 2010 [Google Scholar]
- 7.National Cancer Institute. Fast stats, surveillance epidemiology and end results. http://seer.cancer.gov/faststats/index.php (2012, accessed 30 April 2012).
- 8.Taylor M, Chu L, Sugrue M, et al. 2008 Gastrointestinal cancers symposium. Orlando, FL: 2008. Jan, Median survival in newly diagnosed metastatic colorectal cancer patients and its variability by age and comorbidity; pp. 25–28. abstract 397. [Google Scholar]
- 9.Edwards BK, Howe HL, Ries LA, et al. Annual report to the nation on the status of cancer, 1973–1999, featuring implications of age, aging on U.S cancer burden. Cancer. 2002;94(10):2766–2792. doi: 10.1002/cncr.10593. [DOI] [PubMed] [Google Scholar]
- 10.Schrag D, Cramer LD, Bach PB, et al. Age and adjuvant chemotherapy use after surgery for stage III colon cancer. J Natl Cancer Inst. 2001;93(11):850–857. doi: 10.1093/jnci/93.11.850. [DOI] [PubMed] [Google Scholar]
- 11.Hardiman KM, Cone M, Sheppard BC, et al. Disparities in the treatment of colon cancer in octogenarians. Am J Surg. 2009;197(5):624–628. doi: 10.1016/j.amjsurg.2008.12.018. [DOI] [PubMed] [Google Scholar]
- 12.Ranpura V, Hapani S, Chuang J, et al. Risk of cardiac ischemia and arterial thromboembolic events with the angiogenesis inhibitor bevacizumab in cancer patients: a meta-analysis of randomized controlled trials. Acta Oncol. 2010;49(3):287–297. doi: 10.3109/02841860903524396. [DOI] [PubMed] [Google Scholar]
- 13.Meyerhardt JA, Li L, Sanoff HK, et al. Effectiveness of bevacizumab with first-line combination chemotherapy for Medicare patients with stage IV colorectal cancer. J Clin Oncol. 2012;30(6):608–615. doi: 10.1200/JCO.2011.38.9650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsai HK, Marshall JL, Smith SW, et al. Bevacizumab use and risk of cardiovascular adverse events among elderly patients with colorectal cancer receiving chemotherapy: a population-based study. Ann Oncol. 2013;24(6):1574–1579. doi: 10.1093/annonc/mdt019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shankaran V, Mummy D, Koepl L, et al. Adverse events associated with bevacizumab and chemotherapy in older patients with metastatic colorectal cancer. Clin Colorectal Cancer. 2013;12(3):204–213. doi: 10.1016/j.clcc.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 16.Hapani S, Chu D, Wu S. Risk of gastrointestinal perforation in patients with cancer treated with bevacizumab: a meta-analysis. Lancet Oncol. 2009;10(6):559–568. doi: 10.1016/S1470-2045(09)70112-3. [DOI] [PubMed] [Google Scholar]
- 17.National Cancer Institute. SEER-Medicare: about the data files. http://appliedresearch.cancer.gov/seermedicare/aboutdata/ (2013, accessed 24 September 2013).
- 18.Warren JL, Klabunde CN, Schrag D, et al. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40(8 Suppl):IV–3–18. doi: 10.1097/01.MLR.0000020942.47004.03. [DOI] [PubMed] [Google Scholar]
- 19.Krieger N. Overcoming the absence of socioeconomic data in medical records: validation and application of a census-based methodology. Am J Public Health. 1992;82(5):703–710. doi: 10.2105/ajph.82.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Edge SB, Byrd DR, Compton CC, et al., editors. AJCC cancer staging manual. 7th ed. New York: Springer; 2010. [Google Scholar]
- 21.Klabunde CN, Potosky AL, Legler JM, et al. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53(12):1258–1267. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 22.Hess GP, Wang PF, Quach D, et al. Systemic therapy for metastatic colorectal cancer: patterns of chemotherapy and biologic therapy use in US medical oncology practice. J Oncol Pract. 2010;6(6):301–307. doi: 10.1200/JOP.2010.000072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.El SM, Scarfe A, Yasui Y, et al. Reasons physicians do not recommend and patients refuse adjuvant chemotherapy for stage III colon cancer: a population based chart review. BMC Res Notes. 2012;5:269. doi: 10.1186/1756-0500-5-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keating NL, Landrum MB, Klabunde CN, et al. Adjuvant chemotherapy for stage III colon cancer: do physicians agree about the importance of patient age and comorbidity? J Clin Oncol. 2008;26(15):2532–2537. doi: 10.1200/JCO.2007.15.9434. [DOI] [PubMed] [Google Scholar]
- 25.Francois E, Guerin O, Follana P, et al. Use of bevacizumab in elderly patients with metastatic colorectal cancer. J Geriatr Oncol. 2011;2(1):64–71. [Google Scholar]
- 26.Kozloff MF, Berlin J, Flynn PJ, et al. Clinical outcomes in elderly patients with metastatic colorectal cancer receiving bevacizumab and chemotherapy: results from the BRiTE observational cohort study. Oncology. 2010;78(5–6):329–339. doi: 10.1159/000320222. [DOI] [PubMed] [Google Scholar]
- 27.Zafar SY, Malin JL, Grambow SC, et al. Early dissemination of bevacizumab for advanced colorectal cancer: a prospective cohort study. BMC Cancer. 2011;11:354. doi: 10.1186/1471-2407-11-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shamloo BK, Chhabra P, Freedman AN, et al. Novel adverse events of bevacizumab in the US FDA adverse event reporting system database: a disproportionality analysis. Drug Saf. 2012;35(6):507–518. doi: 10.2165/11597600-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 29.Zafar SY, Malin JL, Grambow SC, et al. Chemotherapy use and patient treatment preferences in advanced colorectal cancer: a prospective cohort study. Cancer. 2013;119(4):854–862. doi: 10.1002/cncr.27815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pang A, Ho S, Lee SC. Cancer physicians’ attitude towards treatment of the elderly cancer patient in a developed Asian country. BMC Geriatr. 2013;13(1):35. doi: 10.1186/1471-2318-13-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guth U, Myrick ME, Kandler C, et al. The use of adjuvant endocrine breast cancer therapy in the oldest old. Breast. 2013;22(5):863–868. doi: 10.1016/j.breast.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 32.Zauderer MG, Sima CS, Korc-Grodzicki B, et al. Toxicity of initial chemotherapy in older patients with lung cancers. J Geriatr Oncol. 2013;4(1):64–70. doi: 10.1016/j.jgo.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mayer RJ. Two steps forward in the treatment of colorectal cancer. N Engl J Med. 2004;350(23):2406–2408. doi: 10.1056/NEJMe048098. [DOI] [PubMed] [Google Scholar]
- 34.Davidoff AJ, Erten M, Shaffer T, et al. Out-of-pocket health care expenditure burden for Medicare beneficiaries with cancer. Cancer. 2013;119(6):1257–1265. doi: 10.1002/cncr.27848. [DOI] [PubMed] [Google Scholar]
- 35.Rendall MS, Weden MM, Favreault MM, et al. The protective effect of marriage for survival: a review and update. Demography. 2011;48(2):481–506. doi: 10.1007/s13524-011-0032-5. [DOI] [PubMed] [Google Scholar]
- 36.Kravdal H, Syse A. Changes over time in the effect of marital status on cancer survival. BMC Public Health. 2011;11:804. doi: 10.1186/1471-2458-11-804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wennberg JE, Copper MM, editors. The Dartmouth atlas of health care. Chicago, IL: American Hospital Publishing; 1996. [PubMed] [Google Scholar]
- 38.Birman-Deych E, Waterman AD, Yan Y, et al. Accuracy of ICD-9-CM codes for identifying cardiovascular and stroke risk factors. Med Care. 2005;43(5):480–485. doi: 10.1097/01.mlr.0000160417.39497.a9. [DOI] [PubMed] [Google Scholar]