Abstract
Adolescence is an important neurodevelopmental period marked by rapidly escalating rates of alcohol and drug use. Over the past decade, research has attempted to disentangle pre- and post-substance use effects on brain development by using sophisticated longitudinal designs. This review focuses on recent, prospective studies and addresses the following important questions: (1) what neuropsychological and neural features predate adolescent substance use, making youth more vulnerable to engage in heavy alcohol or drug use, and (2) how does heavy alcohol and drug use affect normal neural development and cognitive functioning? Findings suggest that pre-existing neural features that relate to increased substance use during adolescence include poorer neuropsychological functioning on tests of inhibition and working memory, smaller gray and white matter volume, changes in white matter integrity, and altered brain activation during inhibition, working memory, reward, and resting state. After substance use is initiated, alcohol and marijuana use are associated with poorer cognitive functioning on tests of verbal memory, visuospatial functioning, psychomotor speed, working memory, attention, cognitive control, and overall IQ. Heavy alcohol use during adolescence is related to accelerated decreases in gray matter and attenuated increases in white matter volume, as well as increased brain activation during tasks of inhibition and working memory, relative to controls. Larger longitudinal studies with more diverse samples are needed to better understand the interactive effects of alcohol, marijuana, and other substances, as well as the role of sex, co-occurring psychopathology, genetics, sleep, and age of initiation on substance use.
Keywords: Alcohol, Marijuana, Neural development, Substance use, Cognitive functioning, Neuropsychological testing, Magnetic resonance imaging (MRI), Functional MRI (fMRI)
Introduction
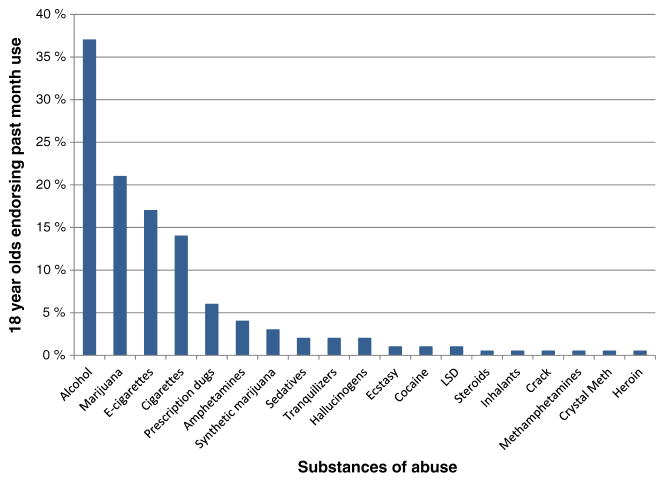
Historically, alcohol and other substance use disorders were perceived as conditions that emerged during adulthood. More recently, researchers are appreciating that substance use disorders are developmental problems that begin earlier in life, typically during adolescence [1, 2], with earlier initiation of substance use relating to poorer outcomes [3, 4]. Alcohol is by far the most commonly used substance among youth, with 37 % of 18-year olds endorsing alcohol use and 24 % reporting being drunk in the past month [5]. Marijuana is the second most used substance during adolescence, followed by e-cigarettes and cigarettes. Other drug use is relatively uncommon during this developmental period (see Fig. 1) [5]. Because of the comparatively high level of alcohol and marijuana use and low level of other drug use during adolescence, most studies have specifically focused on the effects of alcohol and marijuana on neural and cognitive development.
Fig. 1.
Alcohol and marijuana are the two most commonly used substances among adolescents. Data from Monitoring the Future [5]
Excessive alcohol and marijuana use during adolescence is concerning because this is a developmental stage characterized by significant neural development. While overall brain volume does not change during this time, there are substantial volume changes in gray and white matter regions of the brain. Gray matter is made up of neuronal cell bodies, dendrites, glial cells, synapses, and capillaries. During adolescence and into young adulthood, there is an overall decrease in gray matter, which is thought to be due to synaptic pruning (i.e., the elimination of underutilized or unnecessary neural connections), changes in the extracellular matrix, and white matter encroachment on gray matter [6–11]. Concurrent with gray matter decreases, white matter (i.e., myelinated axon tracts that connect gray matter regions) linearly increases during adolescence [11, 12]. This increase in white matter is thought to be due to the increased myelination of axons, which allow for more efficient communication between brain regions [13–15]. Decreases in gray matter and increases in white matter during adolescence are related to enhanced information processing, which is required for complex cognitive abilities [16]. In general, these neural changes begin primarily in posterior regions of the brain and progress to more anterior regions, with neural changes occurring well into the mid-to-late 20s [8]. Additionally, there is an imbalance in the way the brain develops, with mesolimbic and reward systems maturing before prefrontal and cognitive control areas [17–20]. Differences in the non-linear development of these two neural systems are believed to leave adolescents more vulnerable to engage in risk-taking behaviors like alcohol and other drug use [21]. During this crucial period of rapid neural development, the brain may be more vulnerable to the potentially persistent effects of neural insults, such as excessive alcohol and drug use [22–24].
Previous cross-sectional studies suggest that there is a relationship between adolescent substance use and brain development, but the direction of this relationship cannot be inferred given the cross-sectional design (i.e., it is unclear if the observed neural abnormalities in adolescent heavy drinkers are a pre-existing risk factor for initiation of substance use or a consequence of use). Over the past decade, researchers have attempted to delineate between pre-existing alterations and post-substance effects on brain development by using sophisticated prospective, longitudinal designs that assess youth before they have ever used any alcohol or drugs and continuing to assess them over time as a portion naturally transitions into substance use. This design allows for examination of normal developmental neural trajectories in youth who have never used alcohol or drugs during adolescence and compares their brain maturation to youth who transition into substance use. This review will mainly focus on these prospective longitudinal studies and will address the following important questions: (1) what neural features predate adolescent substance use and make youth more vulnerable to engage in alcohol or drug use, and (2) how does alcohol and drug use affect normal neural and cognitive development? This review covers the most recent, prospective longitudinal findings from the past four years. Cross-sectional studies or studies that do not include baseline pre-substance use data are not included.
Neural Features that Predate Adolescent Substance Use
Neuropsychological Precursors
Several cognitive and neural features may make youth more predisposed to engaging in heavy alcohol and marijuana use during adolescence. Inhibition, or impulse control, may be a key cognitive feature involved in regulating substance use [25]. Inhibition is a type of executive functioning that refers to the ability to withhold a pre-potent response in order to select a more appropriate, goal-directed response [26, 27]. One prospective study examined neurocognitive functioning in 175 substance-naive healthy 12- to 14-year-olds, and assessed participants’ substance use each year until age 18, by which 105 (60 %) transitioned into alcohol or marijuana use. Compromised inhibitory functioning during early adolescence, prior to the onset of substance use, was related to greater subsequent alcohol and marijuana use by age 18, even after controlling for common predictors of youth substance use including familial substance use disorders, externalizing behaviors, sex, pubertal development, academic achievement, and age [23]. Predictors accounted for 23 % of the total variance in substance use. This suggests poorer inhibitory functioning, in addition to demographic and genetic variables, predisposes youth to initiate substance use during adolescence. Importantly, this study was interested in cognitive predictors of substance use and not problem use. Other cognitive domains may be more predictive of problem use levels, as opposed to simple initiation of use, and should be examined in future studies. Similar to many of the existing studies on the effect of substance use on brain development, youth in this sample were, on average, from upper middle class families in affluent neighborhoods and had no co-occurring psychopathology. Findings could be more pronounced in youth with a greater number of environmental or genetic risk factors, including ADHD and depression. Despite these limitations, these findings are consistent with research showing pre-existing alterations in working memory and inhibitory functioning are related to escalation of drinking during early adolescence [28] and suggest that neuropsychological data could be used in preventative interventions to identify teens at risk for initiating problematic substance use.
Functional Brain Precursors
Aberrant brain structure and functioning may underlie observed premorbid differences in inhibitory functioning. By using functional magnetic resonance imaging (fMRI), a safe, non-invasive technique used to investigate brain activity, researchers have been able to study neural changes associated with adolescent substance use. fMRI studies on healthy, non-substance using youth have shown that neural circuitry underlying inhibitory control undergoes significant neurodevelopment during adolescence [29, 30]. Longitudinal fMRI studies of youth have shown that even in the presence of comparable performance, abnormal brain activation during inhibition tasks predicts alcohol use by mid-to-late adolescence [31–33], future substance use and dependence symptoms [33, 34], and significant alcohol-related consequences like blackouts (i.e., when a person is awake while drunk but does not remember pieces or large sections of time) [35]. Furthermore, less frontal and parietal brain activation on tasks of visual working memory in substance-naive youth have been found to be predictive of greater substance involvement by late adolescence [36].
Brain activation during reward processing has also been found to predict future adolescent substance use engagement. In a sample of 100 12– to 15-year - olds, reductions in resting-state cerebral blood flow (i.e., when participants were not performing a task) within reward and default mode networks were associated with greater alcohol consumption at a 3-year follow-up [37]. This study had a relatively small sample size, with a fifth of the sample having already initiated substance use at baseline and only a third transitioning into alcohol use by the follow-up. This sample also had higher rates of co-occurring externalizing behaviors in the drinking group that could have accounted for variations in blood flow. In a much larger multi-site European study of 692 youth [38], increased brain activity in superior frontal regions during reward processing at age 14 was predictive of initiation of alcohol use by age 16 [32]. In this sample, “drinking” was considered having at least three lifetime binge drinking episodes by age 16. It is unclear if this low level of alcohol engagement is clinically meaningful. Continued follow-up of this sample will help clarify how reward processing might predict transitioning into more problematic patterns of drinking during adolescence. Further, this study found that the most predictive variable of future drinking was cigarette use at baseline. This highlights the importance of having completely substance-naive youth at baseline to understand what underlying neural factors are associated with future use.
In regard to marijuana use, a Dutch study found that heavy marijuana-using young adults exhibited higher brain activation during reward processing than controls, which was predictive of greater marijuana use at a 6-month follow-up [39]. This was a relatively small sample (32 marijuana users, 41 controls); larger sample sizes with more information regarding co-occurring substance use are needed to understand how neural factors contribute to marijuana initiation and if these features confer risk to all substances or are specific to marijuana.
Overall, findings suggest that aberrations in brain activation during tasks of inhibition, working memory, and reward processing may be useful in predicting which youth will initiate alcohol and marijuana use during adolescence. It is also possible that prevention and intervention techniques targeting these cognitive domains could be helpful in staving off early adolescent substance use.
Structural Brain Precursors
Pre-existing structural brain differences may also predispose youth to engage in heavy substance use. In a prospective study of 121 youth, smaller orbitofrontal cortex volumes at age 12 predicted marijuana use by age 16 [40]. Findings have been replicated in other samples showing smaller frontal gray matter volume [32, 41, 42] and less cerebellar white matter volume [42] predict initiation of drinking by late adolescence, even after controlling for family history of substance use disorders [41]. Reward-related subcortical brain structures also appear to be involved in initiation of substance use. In a community sample of adolescents with no prior substance use, smaller left nucleus accumbens, a brain region involved in reward and reinforcement, predicted greater substance use at a 2-year follow-up [43]. Smaller volumes of the anterior cingulate, a region implicated in affective processes, self-control, and substance use, have also been found to predict later alcohol-related problems [44]. White matter integrity, as measured by diffusion tensor imaging, has also been related to future substance use. Lower white matter integrity in fronto-limbic regions at ages 16 to 19 predicted future alcohol and marijuana use and other delinquent behavior at an 18-month follow-up [45]. Overall, less volume in brain regions involved in impulsivity, reward sensitivity, and decision-making and altered white matter integrity appear to influence initiation of alcohol and marijuana use during adolescence.
Together, these findings suggest that pre-existing alterations, both in neurocognitive performance and neural response patterns during inhibition, working memory, and reward processing, as well as structural brain differences, could be useful markers of vulnerability to initiating substance use during adolescence. Larger studies including youth with more problematic substance use are needed to understand how these markers confer risk to future substance use and abuse, and if these neural and neuropsychological features are specific to substance use itself or to other risk-taking behaviors as well. Understanding neural markers that predispose youth to drinking or substance use may aid prevention and early intervention programs by identifying youth who are more likely to initiate substance use.
Neural Features that Follow Adolescent Substance Use
Longitudinal neuropsychological and neuroimaging studies have been useful in disentangling pre-existing neural features associated with adolescent substance use initiation from consequences directly related to substance use. Overall, adolescent substance use has been found to negatively affect behavior and brain structure and function; however, the level at which each substance separately affects brain functioning has been debated. Considering that it would be highly unethical to randomize youth to different substance-using groups, research is limited to natural observational studies. Alcohol and marijuana are the most commonly used substances during adolescence; therefore, most research has focused on these substances. When recruiting adolescents, it is relatively easy to recruit those who: (1) do not use any alcohol or drugs, (2) use only alcohol, or (3) use both alcohol and marijuana; however, it is very uncommon to recruit youth who only use marijuana, let alone other less frequently used substances. While some studies try to statistically control for alcohol and other drug use to parse the relative contribution of each substance on brain functioning, this method is imperfect given the high collinearity between alcohol and other drug use variables as well as potential interactive effects. Prospective studies with much larger sample sizes are currently underway and may help to answer this important question [38, 46]. Currently, studies typically combine heavy substance-using groups and compare them to non-using controls.
Substance-Related Changes in Neuropsychological Functioning
Collection of neuropsychological test data has enabled tracking cognitive skills over time to assess the effect of alcohol and marijuana on normal intellectual development. This is a particularly important area, as educational attainment is among the most critical developmental tasks of adolescence, and alcohol use and smoking behaviors at ages 12 to 14 predict lower educational achievement at later time points even after some confounding variables are taken into account [47]. In a sample of 234 healthy adolescents followed over 4 years, subdiagnostic alcohol and marijuana users showed worsening verbal memory, visuospatial functioning, and psychomotor speed after initiating intense or frequent alcohol and other substance use when compared to controls [48]. In a 10-year longitudinal study, heavy substance-using youth in treatment were assessed at age 16 and followed until early adulthood (~age 25). Youth who were heavy substance users showed poorer verbal learning and memory, visuospatial functioning, and working memory and attention at the 10-year follow-up [49, 50]. Alcohol use and drug withdrawal symptoms were related to worse verbal learning and memory, and stimulant use over the follow-up was related to worse visual learning and memory [50]. Heavier use patterns and greater hangover and withdrawal symptoms over time were related to worse cognitive functioning, suggesting a dose-dependent relationship between substance use and cognitive functioning [49] that has been replicated in other studies [48, 51]. Interestingly, youth who had met criteria for a substance use disorder at some point during the 10-year follow-up but remitted performed similarly to youth who had a persistent substance use disorder, suggesting heavy substance use during adolescence could have lasting effects into adulthood [50].
Given the recent legalization of medicinal and recreational marijuana in several states and the shifts in adolescents’ attitudes regarding marijuana use and increased rates of use [5], there has been elevated interest in understanding the deleterious effect of marijuana on cognitive development. A recent longitudinal study tracked heavy marijuana-using youth who also engaged in alcohol use from age 16 to 19. Heavy marijuana users showed worsening performance on several cognitive domains when compared to non-using youth, including worse performance on tests of complex attention, memory, processing speed, and visuospatial functioning. Earlier onset of marijuana use was associated with poorer processing speed and executive functioning by age 19, suggesting initiation of marijuana use during early adolescence (before age 16) may be more harmful to the developing brain than later initiation [52••]. There is some suggestion that cognitive domains are differentially impacted by marijuana use in adolescence, with attention, declarative memory, and cognitive control particularly affected (for review [53]). A large longitudinal birth cohort study (N = 1037) indicated that persistent adolescent-onset marijuana use is associated with neuropsychological decline broadly across domains of functioning, with more persistent use associated with greater decline (IQ decline of more than 5 points in the most persistent use group) and functioning not fully restored with cessation of use [54••]. These findings have been criticized as potentially representing a regression to the mean, given that the marijuana-using cohort had higher baseline IQ than the non-marijuana-using cohort. A recent longitudinal twin study found that IQ deficits observed in marijuana users may be attributable to confounding factors like familial and environmental influences rather than the direct neurotoxic effect of marijuana [55]. Of note, these studies examined a small number of IQ subtests and did not assess other measures of executive function or working memory that have been found to be affected by heavy marijuana use. Furthermore, the follow-up assessment period for this study ended in young adulthood, whereas the aforementioned study [54••] followed youth to age 38. Regular use over a prolonged period may result in more deleterious effects.
More studies are needed to understand if alcohol and marijuana-related effects are acute or are associated with long-term impairments. After a month of monitored abstinence, some cognitive deficits appear to persist for heavy substance using youth, with abstinence associated with improvements in verbal learning and memory but persistent deficits in attention [51, 56, 57]. A recent marijuana cessation study indicated that youth who attained abstinence during treatment demonstrated improved verbal memory and psychomotor task performance, compared to those who continued using marijuana [58]. Larger sample sizes with longer follow-ups are needed to understand the potential cognitive recovery related to alcohol and marijuana abstinence.
Substance-Related Changes in Brain Structure
Structural brain changes might help explain cognitive and behavioral differences between substance-using adolescents and non-users. In a recent prospective study, within-subject changes in brain volume were collected in the longest intervals and in the largest sample size of adolescents to date [59••]. Gray and white matter volume trajectories were compared between 75 youth who began drinking during adolescence and 59 continuously non-using controls over 4 years. The non-drinking adolescents studied over the same period served as a control group for estimating typical developmental trajectories over the same age range as the heavy drinkers. Heavy-drinking youth showed abnormal neurodevelopmental trajectories compared to continuously non-using controls, including accelerated decreases in gray matter volume (particularly in frontal and temporal regions) and attenuated increases in white matter volume over the follow-up, even after controlling for marijuana and other substance use [59••]. These findings replicated earlier longitudinal studies with smaller sample sizes showing heavy drinkers had accelerated decreases in gray matter over time compared to non-using controls [42, 60]. Potential interpretations of these findings include accelerated but non-beneficial pruning or, alternatively, premature cortical gray matter decline similar to volume declines related to accelerated aging in adult alcoholics [61] or even “normal” aging [62, 63]. Overall, heavy drinking appears to affect the normal developmental trajectories of gray and white matter maturation during adolescence. Existing studies tend to group youth by “drinkers” versus “controls”; future studies, with larger sample sizes, are needed to better understand the dose-dependent effect of alcohol and marijuana on neural development.
Normal adolescent brain development involves substantial cortical thinning, which has been linked to elimination of weak synaptic connections, changes in the extracellular matrix, and white matter encroachment [6–11]. Recent studies have examined how alcohol and marijuana affect normal cortical thinning during this developmental period. In regards to marijuana use, a 3-year prospective study showed that heavy marijuana users who also used alcohol showed greater cortical thickness, particularly in frontal and parietal lobes, at baseline and the 3-year follow-up. More cumulative marijuana use was associated with increased thickness estimates by the 3-year follow-up [64]. These findings appear to hold even after a month of monitored abstinence [65]. Of note, these findings are in contrast to the previously reported alcohol-focused study [59••] that showed alcohol use was related to accelerated gray matter declines. Importantly, both the heavy marijuana- and alcohol-using youth showed decreased gray matter over adolescence (as expected with normal development), but it was the alcohol-using group that showed the abnormal accelerated declines [59••]. This suggests a differential effect of alcohol and marijuana use on brain development. There are likely several factors contributing to these marijuana findings, including interactive effects of different substances [52••, 64], genetics [66–68], and the age of use initiation on brain development [52••, 69]. Again, larger studies are needed to parse these factors contributing to the observed deviations in normal neurodevelopment.
In contrast to gray matter, white matter (i.e., myelinated axon tracts that connect gray matter regions) linearly increases during adolescence [11, 12]. Several cross-sectional studies have shown abnormal white matter microstructure in adolescent substance users [70]. Recent longitudinal findings have helped disentangle pre-existing white matter differences from post-substance use. Adolescents with extensive marijuana-and alcohol-use histories showed worsening white matter integrity over an 18-month [71] and 3-year follow-up in association, projection, and interhemispheric white matter tracts when compared to non-using youth [72, 73]. In all three studies, substance-using youth showed consistently poorer white matter integrity compared to controls, as well as poorer performance on tests of neurocognitive functioning [71–73]. Marijuana-related changes in white matter microstructure may confer risk for co-occurring psychological disorders like schizophrenia [74].
Clearly, larger sample sizes over longer periods of time, including data on youth who have sustained long periods of abstinence, are needed to understand the nuanced cause and effect of alcohol and other drug use on neural development and to determine if aberrations are reversible. Future work will also need to examine interactions between differing use patterns of these substances to determine the contributions of each substance, as well as dose-dependent effects, on normal brain development.
Substance-Related Changes in Brain Functioning
fMRI studies have begun to elucidate neural contributions to the cognitive differences observed both pre- and post-substance use initiation. In a longitudinal study, 40 12- to 16-year-old adolescents were scanned before they ever used any alcohol or drugs and then were rescanned approximately 3 years later [36, 75]. Adolescents who transitioned into heavy drinking by late adolescence (approximately age 18) showed less brain activation during a visual working memory [36] and inhibition task [75] at baseline in frontal and parietal regions compared to demographically matched controls; this local brain response increased in youth who initiated drinking compared to those who remained abstinent over the follow-up, possibly suggesting that youth who initiated heavy drinking during adolescence required more cognitive energy to perform at the same level as controls. Less neural response during working memory and inhibition at baseline was related to greater rates of transitioning into substance use. Early maturation of neural features could be considered a vulnerability for youth, increasing the likelihood of engaging in sensation-seeking behaviors at an earlier age. Together, these studies suggest that neurodevelopmentally precocious youth may have a greater tendency to initiate and escalate risk-taking behaviors like substance use relative to peers [33, 76–78]. In a separate study, adolescent recent (past 90 day) binge drinkers were found to have reduced posterior cerebellar activity during reward processing above and beyond their baseline substance-naive neural functioning; more drinks per drinking day was related to less cerebellar activation. This study suggests binge drinking may affect the emotional component of reward processing, as damage to the posterior portion of the cerebellum has been associated with cognitive and emotional deficits [79, 80]. Observed changes in blood flow [81] and brain activation [82] between adolescent substance users and controls may remit after a month of abstinence. Taken together, these studies suggest that neural differences both predate and precede heavy drinking, mirroring the behavioral findings from neuropsychological and structural studies [42, 59••, 83], and sustained abstinence may be related to recovery in functioning. Of note, samples from these studies are small (≤20 drinkers in each study) and include mostly Caucasian participants from high socioeconomic status groups. Larger and more diverse sample sizes are needed, particularly to examine sex differences, which have been found in cross-sectional studies [83–86]. Additionally, these functional changes were not examined in relation to neuropsychological deficits; thus, it is not possible to infer whether changes in neural response were related to poorer cognitive outcomes. While several cross-sectional studies have shown fMRI brain activation differences between marijuana-using youth and controls [87–92], few longitudinal studies have been performed. In a sample of Dutch marijuana users, brain activation during a working memory task was not related to substance use over the 3-year follow-up [93]. More longitudinal fMRI studies focusing on marijuana-using adolescents are needed to better understand the specific effect of marijuana, above and beyond alcohol use, on neural functioning.
Summary and Perspective
Recent research has substantially advanced our understanding of the complicated relationship between adolescent brain development and substance use, with prospective, longitudinal designs parsing pre-existing vulnerabilities from alcohol and marijuana-related consequences. However, with the notable heterogeneity in patterns of substance use and co-use during this critical developmental period, more work is needed to characterize substance-specific concerns and to determine what developmental processes and cognitive domains may be most responsive to prevention and treatment efforts. The majority of existing studies include predominately Caucasian youth from upper middle class families and exclude youth who have co-occurring psychological disorders. These findings need to be replicated in more diverse samples to see if results generalize. Understanding the interactive effect of substance use on other disorders (e.g., ADHD, depression, anxiety) will be important, as adolescents with co-occurring psychological disorders are at the greatest risk of having long-term problems [68, 94]. Several other factors may contribute to the observed findings and need to be considered in future analyses, including sex [83–86], interactive effects of different substances [52••, 64], genetics [66–68], sleep habits [95], and the age at which use is initiated [52••, 69, 96]. Cigarette and e-cigarette usage are common among adolescents (see Fig. 1); however, most of the existing longitudinal studies were completed in cities that have low rates of adolescent smoking (e.g., San Diego; <1 % of the recruited sample), and therefore, the differential effect of these and other substances of abuse on the brain were not able to be examined. Because other substances have lower base rates of use (see Fig. 1), much larger sample sizes are needed to understand how drugs like opioids, amphetamines, cocaine, and hallucinogens might differentially affect neural development. Again, larger studies are needed to parse these factors contributing to the observed deviations in normal neurodevelopment, many of which are underway [38, 46] (http://addictionresearch.nih.gov/adolescent-brain-cognitive-development-study) and will help close the existing gaps in the current literature.
Of note, all of the existing studies relied on youth self-report of substance use. Incorporating real time measures (via smart phone technology) and biological markers of substance use would greatly improve the accuracy of reporting and would elucidate the more nuanced effects of substance use on behavior and cognition. Existing marijuana studies typically use crude measures of quantifying marijuana use (e.g., “days used marijuana in past month/year”). Collecting additional biomarker data on cannabis potency and content (e.g., THC/CBD ratios) will be important in more accurately quantifying the effect of marijuana on brain functioning. Further, it is necessary to use better quantity and frequency data when assessing substance use; some of the existing studies used ranges for self-report questionnaires, which weakens the ability to understand dose-dependent relationships. Universal definitions need to be adopted. For example, the IMAGEN study considered binge drinking as a “drinking episode that lead to drunkenness,”[38] while other studies typically defined binge drinking as having four or more drinks for women or five or more for men on one occasion. Similar to the need to be consistent across studies with substance use reporting, there needs to be reasonable consistency in measures used to assess cognitive functioning, in order to improve the ability to compare findings across studies. Having a common language among researchers will help when comparing studies and disseminating a clear message to the public.
At present, it is clear that assessment of neuropsychological, functional, and structural factors may help assess risk for problematic adolescent substance use, potentially informing targeted prevention efforts. Additionally, emerging work has helped characterize time-limited and potentially persisting effects of substance use on the developing brain, which may help guide treatment and rehabilitation efforts (see Table 1 for summary). Further research in this area has the potential to significantly impact public health, via better-informed prevention and intervention strategies to address adolescent-specific vulnerabilities.
Table 1.
Summary of pre- and post-substance use initiation on adolescent brain development
| Pre-existing neural features relating to ↑ substance use during adolescence | Effects of alcohol and marijuana on neural development | |
|---|---|---|
| Neuropsychological testing |
|
Alcohol:
|
| Brain structure |
|
Alcohol:
|
| Brain functioning |
|
Alcohol:
|
Acknowledgments
Funding Support The authors wish to acknowledge the funding sources for this work, including NIDA grants K12 DA031794 (Squeglia), U01 DA031779 (Gray), UG1 DA013727—CTN0053 (Gray), and R01 DA038700 (Gray). The funding source had no role other than financial support.
The authors would like to express gratitude to Jack McKee and Lindsay Meredith for their assistance with manuscript preparation.
Footnotes
Compliance with Ethical Standards
Conflict of Interest Lindsay M. Squeglia and Kevin M. Gray report grants from National Institute on Drug Abuse.
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
This article is part of the Topical Collection on Substance Use and Related Disorders
References
Papers of particular interest, published recently, have been highlighted as:
•• Of major importance
- 1.Squeglia LM, Boissoneault J, Van Skike CE, Nixon SJ, Matthews DB. Age-related effects of alcohol from adolescent, adult, and aged populations using human and animal models. Alcohol Clin Exp Res. 2014;38(10):2509–16. doi: 10.1111/acer.12531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown SA, McGue M, Maggs J, Schulenberg J, Hingson R, Swartzwelder S, et al. A developmental perspective on alcohol and youths 16 to 20 years of age. Pediatrics. 2008;121(Suppl 4):S290–310. doi: 10.1542/peds.2007-2243D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grant BF, Dawson DA. Age at onset of alcohol use and its association with DSM-IV alcohol abuse and dependence: Results from the National Longitudinal Alcohol Epidemiologic Survey. J Subst Abus. 1997;9:103–10. doi: 10.1016/s0899-3289(97)90009-2. [DOI] [PubMed] [Google Scholar]
- 4.Dawson DA, Goldstein RB, Chou SP, Ruan WJ, Grant BF. Age at first drink and the first incidence of adult-onset DSM-IV alcohol use disorders. Alcohol Clin Exp Res. 2008;32(12):2149–60. doi: 10.1111/j.1530-0277.2008.00806.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnston LD, O’Malley PM, Miech RA, Bachman JG, Schulenberg JE. Monitoring the Future national results on adolescent drug use: Overview of key findings, 2014. Ann Arbor: Michigan; 2015. [Google Scholar]
- 6.Paus T. Mapping brain maturation and cognitive development during adolescence. Trends Cogn Sci. 2005;9(2):60–8. doi: 10.1016/j.tics.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 7.Petanjek Z, Judas M, Simic G, Rasin MR, Uylings HB, Rakic P, et al. Extraordinary neoteny of synaptic spines in the human prefrontal cortex. Proc Natl Acad Sci U S A. 2011;108(32):13281–6. doi: 10.1073/pnas.1105108108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A. 2004;101(21):8174–9. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raznahan A, Shaw PW, Lerch JP, Clasen LS, Greenstein D, Berman R, et al. Longitudinal four-dimensional mapping of subcortical anatomy in human development. Proc Natl Acad Sci U S A. 2014;111(4):1592–7. doi: 10.1073/pnas.1316911111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sowell ER, Thompson PM, Holmes CJ, Jernigan TL, Toga AW. In vivo evidence for post-adolescent brain maturation in frontal and striatal regions. Nat Neurosci. 1999;2(10):859–61. doi: 10.1038/13154. [DOI] [PubMed] [Google Scholar]
- 11.Stiles J, Jernigan TL. The basics of brain development. Neuropsychol Rev. 2010;20(4):327–48. doi: 10.1007/s11065-010-9148-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bava S, Thayer R, Jacobus J, Ward M, Jernigan TL, Tapert SF. Longitudinal characterization of white matter maturation during adolescence. Brain Res. 2010;1327:38–46. doi: 10.1016/j.brainres.2010.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Asato MR, Terwilliger R, Woo J, Luna B. White matter development in adolescence: a DTI study. Cereb Cortex. 2010;20(9):2122–31. doi: 10.1093/cercor/bhp282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Benes FM. Myelination of cortical-hippocampal relays during late adolescence. Schizophr Bull. 1989;15(4):585–93. doi: 10.1093/schbul/15.4.585. [DOI] [PubMed] [Google Scholar]
- 15.Simmonds DJ, Hallquist MN, Asato M, Luna B. Developmental stages and sex differences of white matter and behavioral development through adolescence: a longitudinal diffusion tensor imaging (DTI) study. Neuroimage. 2014;92:356–68. doi: 10.1016/j.neuroimage.2013.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Squeglia LM, Jacobus J, Sorg SF, Jernigan TL, Tapert SF. Early adolescent cortical thinning is related to better neuropsychological performance. J Int Neuropsychol Soc. 2013;19(9):962–70. doi: 10.1017/S1355617713000878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Casey BJ, Getz S, Galvan A. The adolescent brain. Dev Rev. 2008;28(1):62–77. doi: 10.1016/j.dr.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ernst M, Nelson EE, Jazbec S, McClure EB, Monk CS, Leibenluft E, et al. Amygdala and nucleus accumbens in responses to receipt and omission of gains in adults and adolescents. Neuroimage. 2005;25(4):1279–91. doi: 10.1016/j.neuroimage.2004.12.038. [DOI] [PubMed] [Google Scholar]
- 19.Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35(1):217–38. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sturman DA, Moghaddam B. Reduced neuronal inhibition and coordination of adolescent prefrontal cortex during motivated behavior. J Neurosci. 2011;31(4):1471–8. doi: 10.1523/JNEUROSCI.4210-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Somerville LH, Jones RM, Casey BJ. A time of change: behavioral and neural correlates of adolescent sensitivity to appetitive and aversive environmental cues. Brain Cogn. 2010;72(1):124–33. doi: 10.1016/j.bandc.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Squeglia LM, Jacobus J, Tapert SF. The influence of substance use on adolescent brain development. Clin EEG Neurosci. 2009;40(1):31–8. doi: 10.1177/155005940904000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Squeglia LM, Jacobus J, Tapert SF. The effect of alcohol use on human adolescent brain structures and systems. Handb Clin Neurol. 2014;125:501–10. doi: 10.1016/B978-0-444-62619-6.00028-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jacobus J, Tapert SF. Neurotoxic effects of alcohol in adolescence. Annu Rev Clin Psychol. 2013;9:703–21. doi: 10.1146/annurev-clinpsy-050212-185610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.López-Caneda E, Rodríguez Holguín S, Cadaveira F, Corral M, Doallo S. Impact of alcohol use on inhibitory control (and vice versa) during adolescence and young adulthood: A review. Alcohol Alcohol. 2014;49(2):173–81. doi: 10.1093/alcalc/agt168. [DOI] [PubMed] [Google Scholar]
- 26.Luna B, Padmanabhan A, O’Hearn K. What has fMRI told us about the development of cognitive control through adolescence? Brain Cogn. 2010;72(1):101–13. doi: 10.1016/j.bandc.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stevens MC, Kiehl KA, Pearlson GD, Calhoun VD. Functional neural networks underlying response inhibition in adolescents and adults. Behav Brain Res. 2007;181(1):12–22. doi: 10.1016/j.bbr.2007.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khurana A, Romer D, Betancourt LM, Brodsky NL, Giannetta JM, Hurt H. Working memory ability predicts trajectories of early alcohol use in adolescents: The mediational role of impulsivity. Addiction. 2013;108(3):506–15. doi: 10.1111/add.12001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Durston S, Davidson MC, Tottenham N, Galvan A, Spicer J, Fossella JA, et al. A shift from diffuse to focal cortical activity with development. Dev Sci. 2006;9(1):1–8. doi: 10.1111/j.1467-7687.2005.00454.x. [DOI] [PubMed] [Google Scholar]
- 30.Velanova K, Wheeler ME, Luna B. Maturational changes in anterior cingulate and frontoparietal recruitment support the development of error processing and inhibitory control. Cereb Cortex. 2008;18(11):2505–22. doi: 10.1093/cercor/bhn012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Norman AL, Pulido C, Squeglia LM, Spadoni AD, Paulus MP, Tapert SF. Neural activation during inhibition predicts initiation of substance use in adolescence. Drug Alcohol Depend. 2011;119(3):216–23. doi: 10.1016/j.drugalcdep.2011.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Whelan R, Watts R, Orr CA, Althoff RR, Artiges E, Banaschewski T, et al. Neuropsychosocial profiles of current and future adolescent alcohol misusers. Nature. 2014;512(7513):185–9. doi: 10.1038/nature13402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heitzeg MM, Nigg JT, Hardee JE, Soules M, Steinberg D, Zubieta JK, et al. Left middle frontal gyrus response to inhibitory errors in children prospectively predicts early problem substance use. Drug Alcohol Depend. 2014;141:51–7. doi: 10.1016/j.drugalcdep.2014.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mahmood OM, Goldenberg D, Thayer R, Migliorini R, Simmons AN, Tapert SF. Adolescents’ fMRI activation to a response inhibition task predicts future substance use. Addict Behav. 2013;38(1):1435–41. doi: 10.1016/j.addbeh.2012.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wetherill RR, Castro N, Squeglia LM, Tapert SF. Atypical neural activity during inhibitory processing in substance-naïve youth who later experience alcohol-induced blackouts. Drug Alcohol Depend. 2013;128(3):243–9. doi: 10.1016/j.drugalcdep.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Squeglia LM, Pulido C, Wetherill RR, Jacobus J, Brown GG, Tapert SF. Brain response to working memory over three years of adolescence: Influence of initiating heavy drinking. J Stud Alcohol Drugs. 2012;73(5):749–60. doi: 10.15288/jsad.2012.73.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ramage AE, Lin AL, Olvera RL, Fox PT, Williamson DE. Resting-state regional cerebral blood flow during adolescence: Associations with initiation of substance use and prediction of future use disorders. Drug Alcohol Depend. 2015 doi: 10.1016/j.drugalcdep.2015.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schumann G, Loth E, Banaschewski T, Barbot A, Barker G, Büchel C, et al. IMAGEN consortium. The IMAGEN study: Reinforcement-related behaviour in normal brain function and psychopathology. Mol Psychiatry. 2010;15(12):1128–39. doi: 10.1038/mp.2010.4. [DOI] [PubMed] [Google Scholar]
- 39.Cousijn J, Wiers RW, Ridderinkhof KR, van den Brink W, Veltman DJ, Porrino LJ, et al. Individual differences in decision making and reward processing predict changes in cannabis use: A prospective functional magnetic resonance imaging study. Addict Biol. 2013;18(6):1013–23. doi: 10.1111/j.1369-1600.2012.00498.x. [DOI] [PubMed] [Google Scholar]
- 40.Cheetham A, Allen NB, Whittle S, Simmons JG, Yücel M, Lubman DI. Orbitofrontal volumes in early adolescence predict initiation of cannabis use: A 4-year longitudinal and prospective study. Biol Psychiatry. 2012;71(8):684–92. doi: 10.1016/j.biopsych.2011.10.029. [DOI] [PubMed] [Google Scholar]
- 41.Weiland BJ, Korycinski ST, Soules M, Zubieta JK, Zucker RA, Heitzeg MM. Substance abuse risk in emerging adults associated with smaller frontal gray matter volumes and higher externalizing behaviors. Drug Alcohol Depend. 2014;137:68–75. doi: 10.1016/j.drugalcdep.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Squeglia LM, Rinker DA, Bartsch H, Castro N, Chung Y, Dale AM, et al. Brain volume reductions in adolescent heavy drinkers. Dev Cogn Neurosci. 2014;9:117–25. doi: 10.1016/j.dcn.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Urošević S, Collins P, Muetzel R, Schissel A, Lim K, Luciana M. Effects of reward sensitivity and regional brain volumes on substance use initiation in adolescence. Soc Cogn Affect Neurosci. 2015;10(1):106–13. doi: 10.1093/scan/nsu022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cheetham A, Allen NB, Whittle S, Simmons J, Yücel M, Lubman DI. Volumetric differences in the anterior cingulate cortex prospectively predict alcohol-related problems in adolescence. Psychopharmacology. 2014;231(8):1731–42. doi: 10.1007/s00213-014-3483-8. [DOI] [PubMed] [Google Scholar]
- 45.Jacobus J, Thayer RE, Trim RS, Bava S, Frank LR, Tapert SF. White matter integrity, substance use, and risk taking in adolescence. Psychol Addict Behav. 2013;27(2):431–42. doi: 10.1037/a0028235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brown SA, Brumback T, Tomlinson K, Cummins K, Thompson WK, Nagel BJ, De Bellis MD, Hooper SR, Clark DB, Chung T, Hasler BP, Colrain IM, Baker FB, Prouty D, Pfefferbaum A, Sullivan EV, Pohl KM, Rohlfing T, Nichols BN, Chu W, Tapert SF. The National Consortium on Alcohol and NeuroDevelopment in Adolescence (NCANDA): A multi-site study of adolescent development and substance use. Journal of Studies on Alcohol and Drugs. doi: 10.15288/jsad.2015.76.895. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Latvala A, Rose RJ, Pulkkinen L, Dick DM, Korhonen T, Kaprio J. Drinking, smoking, and educational achievement: Cross-lagged associations from adolescence to adulthood. Drug Alcohol Depend. 2014;137:106–13. doi: 10.1016/j.drugalcdep.2014.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nguyen-Louie TT, Castro N, Matt GE, Squeglia LM, Brumback T, Tapert SF. Effects of emerging alcohol and marijuana use behaviors on adolescents’ neuropsychological functioning over four years. Journal of Studies on Alcohol and Drugs. doi: 10.15288/jsad.2015.76.738. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hanson KL, Cummins K, Tapert SF, Brown SA. Changes in neuropsychological functioning over 10 years following adolescent substance abuse treatment. Psychol Addict Behav. 2011;25(1):127–42. doi: 10.1037/a0022350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hanson KL, Medina KL, Padula CB, Tapert SF, Brown SA. Impact of adolescent alcohol and drug use on neuropsychological functioning in young adulthood: 10-year outcomes. J Child Adolesc Subst Abuse. 2011;20(2):135–54. doi: 10.1080/1067828X.2011.555272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Winward JL, Hanson KL, Tapert SF, Brown SA. Heavy alcohol use, marijuana use, and concomitant use by adolescents are associated with unique and shared cognitive decrements. J Int Neuropsychol Soc. 2014;20(8):784–95. doi: 10.1017/S1355617714000666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52••.Jacobus J, Squeglia LM, Infante MA, Castro N, Brumback T, Meruelo AD, Tapert SF. Neuropsychological performance in adolescent marijuana users with co-occurring alcohol use: A three-year longitudinal study. Neuropsychology. 2015;29(6):829–43. doi: 10.1037/neu0000203. This study examined neuropsychological functioning in a large sample of adolescent heavy marijuana and alcohol users over three years and found that substance use negatively affected domains of complex attention, memory, processing speed, and visuospatial functioning, with earlier age of marijuana use onset associated with poorer outcomes on tests of attention and executive functioning. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Randolph K, Turull P, Margolis A, Tau G. Cannabis and cognitive systems in adolescents. Adolesc Psychiatry. 2013;3(2):135–47. [Google Scholar]
- 54••.Meier MH, Caspi A, Ambler A, Harrington H, Houts R, Keefe RS, et al. Persistent cannabis users show neuropsychological decline from childhood to midlife. Proc Natl Acad Sci U S A. 2012;109:E2657–64. doi: 10.1073/pnas.1206820109. This study showed that, in a large longitudinal birth cohort from New Zealand, persistent adolescent-onset marijuana use was associated with neuropsychological decline broadly across domains of functioning. For the heaviest users, marijuana use was associated with an IQ decline of more than 5 points. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jackson NJ, Isen JD, Khoddam R, Irons D, Tuvblad C, Iacono WG, et al. Impact of adolescent marijuana use on intelligence: Results from two longitudinal twin studies. Proceedings of the National Academy of Sciences: PNAS. 2016 doi: 10.1073/pnas.1516648113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hanson KL, Winward JL, Schweinsburg AD, Medina KL, Brown SA, Tapert SF. Longitudinal study of cognition among adolescent marijuana users over three weeks of abstinence. Addict Behav. 2010;35(11):970–6. doi: 10.1016/j.addbeh.2010.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Winward JL, Hanson KL, Bekman NM, Tapert SF, Brown SA. Adolescent heavy episodic drinking: neurocognitive functioning during early abstinence. J Int Neuropsychol Soc. 2014;20(2):218–29. doi: 10.1017/S1355617713001410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Roten A, Baker NL, Gray KM. Cognitive performance in a placebo-controlled pharmacotherapy trial for youth with marijuana dependence. Addict Behav. 2015;45:119–23. doi: 10.1016/j.addbeh.2015.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59••.Squeglia LM, Tapert SF, Sullivan EV, Jacobus J, Meloy MJ, Rohlfing T, et al. Brain development in heavy-drinking adolescents. Am J Psychiatr. 2015;172(6):531–42. doi: 10.1176/appi.ajp.2015.14101249. This study used the largest neuroimaging sample size with the longest follow-up to date to examine neural volume trajectories in adolescent heavy drinkers compared to non-using youth. Findings suggest that heavy alcohol use during adolescence is associated with interruptions in both gray and white matter development. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Luciana M, Collins PF, Muetzel RL, Lim KO. Effects of alcohol use initiation on brain structure in typically developing adolescents. Am J Drug Alcohol Abuse. 2013;39(6):345–55. doi: 10.3109/00952990.2013.837057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pfefferbaum A, Lim KO, Zipursky RB, Mathalon DH, Rosenbloom MJ, Lane B, et al. Brain gray and white matter volume loss accelerates with aging in chronic alcoholics: A quantitative MRI study. Alcohol Clin Exp Res. 1992;16(6):1078–89. doi: 10.1111/j.1530-0277.1992.tb00702.x. [DOI] [PubMed] [Google Scholar]
- 62.Pfefferbaum A, Mathalon DH, Sullivan EV, Rawles JM, Zipursky RB, Lim KO. A quantitative magnetic resonance imaging study of changes in brain morphology from infancy to late adulthood. Arch Neurol. 1994;51(9):874–87. doi: 10.1001/archneur.1994.00540210046012. [DOI] [PubMed] [Google Scholar]
- 63.Pfefferbaum A, Rohlfing T, Rosenbloom MJ, Chu W, Colrain IM, Sullivan EV. Variation in longitudinal trajectories of regional brain volumes of healthy men and women (ages 10 to 85 years) measured with atlas-based parcellation of MRI. NeuroImage. 2013;65:176–93. doi: 10.1016/j.neuroimage.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jacobus J, Squeglia LM, Meruelo AD, Castro N, Brumback T, Giedd JN, Tapert SF. Cortical thickness in adolescent marijuana and alcohol users: A three-year prospective study from adolescence to young adulthood. Dev Cogn Neurosci. 2015;16:101–9. doi: 10.1016/j.dcn.2015.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jacobus J, Squeglia LM, Sorg SF, Nguyen-Louie TT, Tapert SF. Cortical thickness and neurocognition in adolescent marijuana and alcohol users following 28 days of monitored abstinence. J Stud Alcohol Drugs. 2014;75(5):729–43. doi: 10.15288/jsad.2014.75.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shollenbarger SG, Price J, Wieser J, Lisdahl K. Poorer frontolimbic white matter integrity is associated with chronic cannabis use, FAAH genotype, and increased depressive and apathy symptoms in adolescents and young adults. NeuroImage Clinical. 2015;8:117–1125. doi: 10.1016/j.nicl.2015.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wilson S, Malone SM, Thomas KM, Iacono WG. Adolescent drinking and brain morphometry: A co-twin control analysis. Dev Cogn Neurosci. 2015;16:130–8. doi: 10.1016/j.dcn.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Groenman AP, Greven CU, van Donkelaar MM, Schellekens A, van Hulzen K, Rommelse N, et al. Dopamine and serotonin genetic risk scores predicting substance and nicotine use in attention deficit/hyperactivity disorder. Addict Biol. 2015 doi: 10.1111/adb.12230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gruber SA, Dahlgren MK, Sagar KA, Gönenç A, Lukas SE. Worth the wait: Effects of age of onset of marijuana use on white matter and impulsivity. Psychopharmacology. 2014;231(8):1455–65. doi: 10.1007/s00213-013-3326-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Baker ST, Yücel M, Fornito A, Allen NB, Lubman DI. A systematic review of diffusion weighted MRI studies of white matter microstructure in adolescent substance users. Neurosci Biobehav Rev. 2013;37(8):1713–23. doi: 10.1016/j.neubiorev.2013.06.015. [DOI] [PubMed] [Google Scholar]
- 71.Bava S, Jacobus J, Thayer RE, Tapert SF. Longitudinal changes in white matter integrity among adolescent substance users. Alcohol Clin Exp Res. 2013;37(Suppl 1):E181–9. doi: 10.1111/j.1530-0277.2012.01920.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jacobus J, Squeglia LM, Bava S, Tapert SF. White matter characterization of adolescent binge drinking with and without co-occurring marijuana use: A 3-year investigation. Psychiatry Res. 2013;214(3):374–81. doi: 10.1016/j.pscychresns.2013.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jacobus J, Squeglia LM, Infante MA, Bava S, Tapert SF. White matter integrity pre- and post marijuana and alcohol initiation in adolescence. Brain Sci. 2013;3(1):396–414. doi: 10.3390/brainsci3010396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Epstein KA, Kumra S. White matter fractional anisotropy over two time points in early onset schizophrenia and adolescent cannabis use disorder: A naturalistic diffusion tensor imaging study. Psychiatry Res. 2015;232(1):34–41. doi: 10.1016/j.pscychresns.2014.10.010. [DOI] [PubMed] [Google Scholar]
- 75.Wetherill RR, Squeglia LM, Yang TT, Tapert SF. A longitudinal examination of adolescent response inhibition: neural differences before and after the initiation of heavy drinking. Psychopharmacology. 2013 doi: 10.1007/s00213-013-3198-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Berns GS, Moore S, Capra CM. Adolescent engagement in dangerous behaviors is associated with increased white matter maturity of frontal cortex. PLoS ONE. 2009;4(8):e6773. doi: 10.1371/journal.pone.0006773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li Q, Sun J, Guo L, Zang Y, Feng Z, Huang X, et al. Increased fractional anisotropy in white matter of the right frontal region in children with attention-deficit/hyperactivity disorder: A diffusion tensor imaging study. Neuroendocrinol Lett. 2010;31(6):747–53. [PubMed] [Google Scholar]
- 78.Sarkar S, Craig MC, Catani M, Dell’acqua F, Fahy T, Deeley Q, et al. Frontotemporal white-matter microstructural abnormalities in adolescents with conduct disorder: a diffusion tensor imaging study. Psychol Med. 2013;43(2):401–11. doi: 10.1017/S003329171200116X. [DOI] [PubMed] [Google Scholar]
- 79.Stoodley CJ, Schmahmann JD. Evidence for topographic organization in the cerebellum of motor control versus cognitive and affective processing. Cortex. 2010;46(7):831–44. doi: 10.1016/j.cortex.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cservenka A, Jones SA, Nagel BJ. Developmental Cognitive Neuroscience: Reduced cerebellar brain activity during reward processing in adolescent binge drinkers. 2015 doi: 10.1016/j.dcn.2015.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jacobus J, Goldenberg D, Wierenga CE, Tolentino NJ, Liu TT, Tapert SF. Altered cerebral blood flow and neurocognitive correlates in adolescent cannabis users. Psychopharmacology. 2012;222(4):675–84. doi: 10.1007/s00213-012-2674-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Brumback T, Squeglia LM, Jacobus J, Pulido C, Tapert SF, Brown SA. Adolescent heavy drinkers’ amplified brain responses to alcohol cues decrease over one month of abstinence. Addict Behav. 2015;46:45–52. doi: 10.1016/j.addbeh.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Squeglia LM, Spadoni AD, Infante MA, Myers MG, Tapert SF. Initiating moderate to heavy alcohol use predicts changes in neuropsychological functioning for adolescent girls and boys. Psychol Addict Behav. 2009;23(4):715–22. doi: 10.1037/a0016516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Squeglia LM, Schweinsburg AD, Pulido C, Tapert SF. Adolescent binge drinking linked to abnormal spatial working memory brain activation: differential gender effects. Alcohol Clin Exp Res. 2011;35(10):1831–41. doi: 10.1111/j.1530-0277.2011.01527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Squeglia LM, Sorg SF, Schweinsburg AD, Wetherill RR, Pulido C, Tapert SF. Binge drinking differentially affects adolescent male and female brain morphometry. Psychopharmacology. 2012;220(3):529–39. doi: 10.1007/s00213-011-2500-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Thatcher DL, Pajtek S, Chung T, Terwilliger RA, Clark DB. Gender differences in the relationship between white matter organization and adolescent substance use disorders. Drug Alcohol Depend. 2010;110(1–2):55–61. doi: 10.1016/j.drugalcdep.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Schweinsburg AD, Schweinsburg BC, Cheung EH, Brown GG, Brown SA, Tapert SF. fMRI response to spatial working memory in adolescents with comorbid marijuana and alcohol use disorders. Drug Alcohol Depend. 2005;79:201–10. doi: 10.1016/j.drugalcdep.2005.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Schweinsburg AD, Schweinsburg BC, Nagel BJ, Eyler LT, Tapert SF. Neural correlates of verbal learning in adolescent alcohol and marijuana users. Addiction. 2011;106(3):564–73. doi: 10.1111/j.1360-0443.2010.03197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.De Bellis MD, Wang L, Bergman SR, Yaxley RH, Hooper SR, Huettel SA. Neural mechanisms of risky decision-making and reward response in adolescent onset cannabis use disorder. Drug Alcohol Depend. 2013;133(1):134–45. doi: 10.1016/j.drugalcdep.2013.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Houck JM, Bryan AD, Feldstein Ewing SW. Functional connectivity and cannabis use in high-risk adolescents. Am J Drug Alcohol Abuse. 2013;39(6):414–23. doi: 10.3109/00952990.2013.837914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Orr C, Morioka R, Behan B, Datwani S, Doucet M, Ivanovic J, et al. Altered resting-state connectivity in adolescent cannabis users. Am J Drug Alcohol Abuse. 2013;39(6):372–81. doi: 10.3109/00952990.2013.848213. [DOI] [PubMed] [Google Scholar]
- 92.Jager G, Block RI, Luijten M, Ramsey NF. Cannabis use and memory brain function in adolescent boys: a cross-sectional multicenter functional magnetic resonance imaging study. J Am Acad Child Adolesc Psychiatry. 2010;49(6):561–72. doi: 10.1016/j.jaac.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cousijn J, Vingerhoets WA, Koenders L, de Haan L, van den Brink W, Wiers RW, et al. Relationship between working-memory network function and substance use: a 3-year longitudinal fMRI study in heavy cannabis users and controls. Addict Biol. 2014;19(2):282–93. doi: 10.1111/adb.12111. [DOI] [PubMed] [Google Scholar]
- 94.Chadwick B, Miller ML, Hurd YL. Cannabis use during adolescent development: susceptibility to psychiatric illness. Front Psychiatr. 2013;4:129. doi: 10.3389/fpsyt.2013.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hasler BP, Sitnick SL, Shaw DS, Forbes EE. An altered neural response to reward may contribute to alcohol problems among late adolescents with an evening chronotype. Psychiatry Res. 2013;214(3):357–64. doi: 10.1016/j.pscychresns.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lopez-Larson MP, Bogorodzki P, Rogowska J, McGlade E, King JB, Terry J, et al. Altered prefrontal and insular cortical thickness in adolescent marijuana users. Behav Brain Res. 2011;220(1):164–72. doi: 10.1016/j.bbr.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]