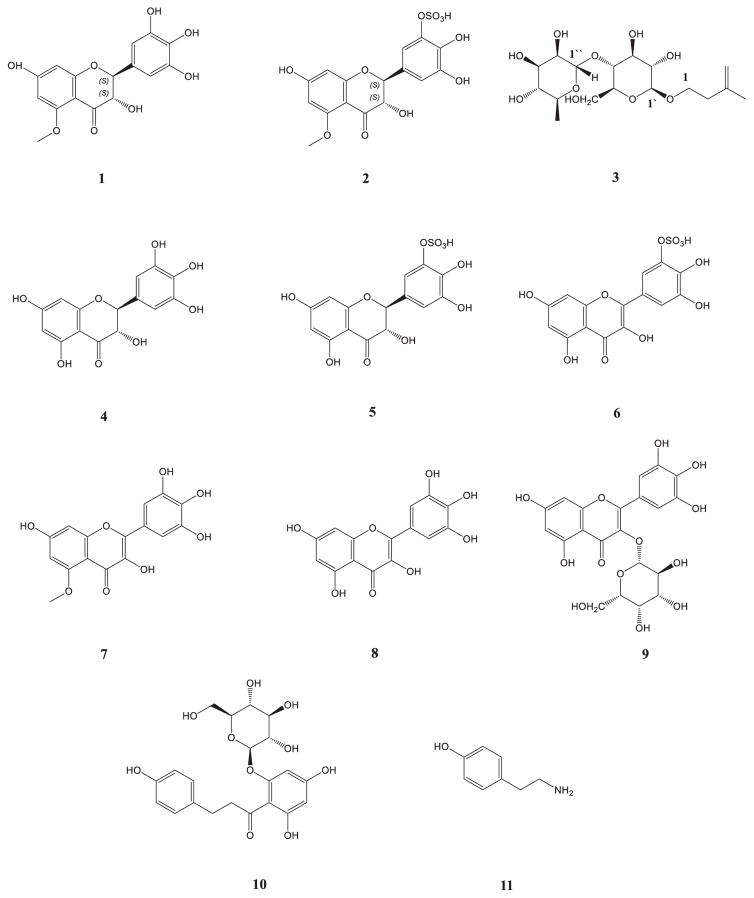
Abstract
Three new compounds, (2S,3S)-5-methyldihydromyricetin (1), (2S,3S)-5-methyldihydromyricetin-3′-O-sulfate (2) and β-D-glucopyranoside, 3-methyl, but-3-en-1-yl 4-O-α-L-rhamnopyranosyl (3) have been isolated from the Limonium caspium, together with dihydromyricetin (4), dihydromyricetin-3′-O-sulfate (5), myricetin-3′-O-sulfate (6), 5-methylmyricetin (7), myricetin (8), myricetin-3-O-β-glucoside (9), as well as phloridzin (10), and tyramine (11). Compounds 5 and 6 were isolated for the first time as acids. This is the first report of all these compounds from this plant. Their structures were established by extensive NMR studies (1H NMR, 13C NMR, DEPT, 1H–1H COSY, HSQC, HMBC) as well as HRESIMS. All isolated compounds were evaluated for their antibacterial, antifungal, antimalarial and antileishmanial activities. Compounds 7, 8 and 9 exhibited good antifungal activity against Candida glabrata with IC50 values of 6.79, 15.37 and 8.53 μg/mL, respectively. Compound 8 displayed significant antimalarial activity against resistant and sensitive strains of Plasmodium falciparum with IC50 values of 1.82 and 1.51 μg/mL, respectively. Compounds 1, 4, 6, 8 and 9 showed excellent activity against Trypanosoma brucei with IC50 values of 6.93, 9.65, 8.52, 7.67 and 6.31 μg/mL, respectively. To date, this is the first report on the phytochemical and biological activity of secondary metabolites from L. caspium.
Keywords: Limonium caspium, Sulfated flavonoids, Myricetin, Dihydromyricetin, Antimalarial, Antifungal, Antileishmanial
1. Introduction
Ethnopharmacology, the study of plants traditionally used for medical purposes with the aim of discovering their pharmacological properties, is a promising area for the discovery of novel medicinal compounds [1]. Salt tolerant plants, known as halophytes, for example, have traditionally been used to treat a wide variety of medical conditions, including viruses and other diseases and symptoms of aging, and continue to be used in rural areas for these therapeutic purposes [2]. Among the more than 2500 known salt-tolerant halophytes there are several that could be useful as cash crops. Cakile maritima has various potential medical uses, including antiscorbutic, diuretic, purgative and digestive properties [3]. The Limonium genus of salt-tolerant halophytes has a number of potentially useful species. As examples, Limonium brasiliense has been reported to possess anti-inflammatory and anti-bacterial qualities [4], Limonium wrightii is reported to treat arthritis and fever [5], Limonium tetragonum and Limonium sinense have been reported to possess antiviral properties [6], and Limonium axillare and Limonium californicum have been shown to possess antibacterial and cytotoxic effects [7].
As a part of our search for novel, plant-derived biological agents, Limonium caspium was studied. Previous phytochemical studies of Limonium revealed the presence of several kinds of active chemical components such as polysaccharides, tannins, alkaloids, flavonoids, terpenes, aliphatic compounds, amino acids, and minerals [8]. The present study was aimed to investigate the constituent of L. caspium aerial parts. The fractionation, isolation and structural elucidation yielded a total of 11 compounds, which are shown in Fig. 1. All isolated compounds were evaluated for their antimalarial, antileishmanial, antifungal and antimicrobial activities.
Fig. 1.
Compounds isolated from Limonium caspium.
2. Results and discussion
In this study the ethanolic extract derived from the aerial parts of L. caspium was subjected to multiple chromatographic fractionations over silica gel yielding a series of myricetin derivatives including two new dihydromyricetins: (2S,3S)-5-methyldihydromyricetin (1), and (2S,3S)-5-methyldihydromyricetin-3′-O-sulfate (2), six previously reported myricetins; dihydromyricetin (4) [9], dihydromyricetin-3′-O-sulfate (5) [11], myricetin-3′-O-sulfate (6) [11], 5-methylmyricetin (7), myricetin (8) [10], myricetin-3-β-glucoside (9) [12], and a new β-D-glucopyranoside, 3-methyl, but-3-en-1-yl 4-O-α-L-rhamnopyranosyl (3), phloridzin (10) [13], and tyramine (11) [14] (Fig. 1).
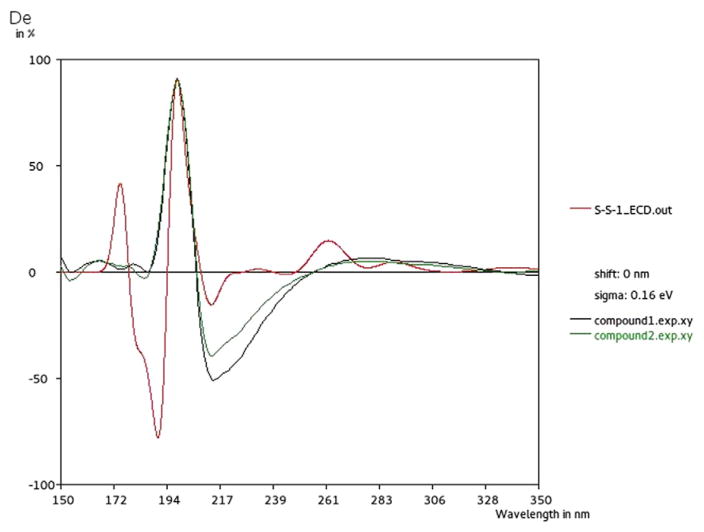
Compound 1 was isolated as a yellowish amorphous solid. The 1H NMR showed a three proton singlet signal at δH 3.75, suggesting one methoxy group, and two protons resonating at δH 6.38 (2H, d, J = 2.16 Hz) related to the B-ring (H-2′ and H-6′). A dihydromyricetin skeleton was evident from the coupling pattern of C-3 and C-2, which resonate at δH 4.16 (1H, d, J = 10.6), and δH 4.81 (1H, d, J = 10.45). Analysis of the 13C NMR spectrum and DEPT experiments, confirmed a dihydromyricetin moiety with the presence of a ketone carbonyl signal at δC 189.8, and a typical non-substituted A-ring with signals at δC 93.3 and 95.5 for C-8 and C-6, respectively. The 1H NMR and 13C NMR spectroscopic data of 1 were found to be similar to those of dihydromyricetin (4) [9] except for the presence of a methoxy group (Table 1). The nature and identity of this dihydromyricetin were deduced from the NMR experiments (COSY, NOESY, TOCSY, and HSQC). HMBC correlations allowed the complete assignments: the proton at δH 4.81 (1H, d, J = 10.45 Hz, H-2) showed strong correlation with the carbon at δC 106.9 (C-2′) and a weak one with δC 127.5 (C-1′), as well as, the correlation between the signal at δH 6.38 (2H, s) and the signals at δC 82.7 (C-2), δC 145.8 (C-3′), and δC 133.4 (C-4′) (Table 1). The position of the methoxy group assigned through the strong correlation of the signal at δH 3.75 with the carbon C-5 resonating at δC 162.2, which together with published data [9], identified this compound (1) as 4H-1-benzopyran-4-one, 2,3-dihydro-3,5-dihydroxy-5-methoxy-2-(3,4,5-trihydroxyphenyl). Compound 1 had [M + H]+ and [M − H]− ions in the HRESIMS at m/z 335.0750 and 333.0393 respectively, which is consistent with the molecular formula C16H14O8. To determine the absolute configuration of the stereogenic center at C-2 and C-3, its ECD spectrum was measured and compared to calculated values for the 2R,3R and 2S,3S enantiomers. While both 2R,3S and 2S,3R enantiomers were eliminated based on the coupling constant of the signals for H-2 and H-3. The two proton signals of 1 were double and double peaks: 4.81 (1H, J = 10.45 Hz, 2-H), 4.16 (1H, J = 10.6 Hz, 3-H), whereas in its 2R,3S isomer they were both single peaks, which indicated that 2-H and 3-H are either 2R,3R or 2S,3S enantiomers [27]. The experimental ECD spectrum showed a positive Cotton effect at 197 nm and a negative Cotton effect at 217 nm (Fig. 2). The B3LYP/6-31G simulated ECD spectrum [28] for 2S,3S enantiomer, generated from 40 excited states using Gaussian band shapes for the peaks, had peaks at 197 and 217 nm. The calculated ECD of the 2S,3S enantiomer showed excellent agreement with the experimental data. The compound 1 is a newly reported dihydromyricetin-5-methyl ether, and is named (2S,3S)-5-methylampelopsin.
Table 1.
1H and 13C NMR data for 1 and 4 (in DMSO).
| No. | 1
|
δcb | 4
|
δcb |
|---|---|---|---|---|
| δH (mult; J in Hz)a | δH (mult; J in Hz) a | |||
| 2 | 4.81 (d, 10.45) | 82.7 | 4.91 (d, 10.8) | 83.7 |
| 3 | 4.16 (d, 10.6) | 72.9 | 4.42 (d, 10.72) | 72.1 |
| 4 | 189.8 | 198.0 | ||
| 5 | 162.2 | 162.9 | ||
| 6 | 5.93 (s) | 95.5 | 5.91 (d, 1.8) | 96.4 |
| 7 | 164.8 | 167.2 | ||
| 8 | 6.08 (s) | 93.3 | 5.86 (d, 1.76) | 95.4 |
| 9 | 163.7 | 163.8 | ||
| 10 | 102.6 | 100.9 | ||
| 1′ | 127.5 | 127.7 | ||
| 2′ | 6.38 (s) | 106.9 | 6.40 (s) | 107.2 |
| 3′ | 145.8 | 146.1 | ||
| 4′ | 133.4 | 133.8 | ||
| 5′ | 145.8 | 146.1 | ||
| 6′ | 6.38 (s) | 106.9 | 6.38 (s) | 107.2 |
| —CH3 | 3.75 (s) | 55.8 |
1H NMR measured at 400 MHz.
13C NMR measured at 100 MHz [9].
Fig. 2.
Experimental (black for 1 and green for 2) spectrum compared to the calculated (red) ECD spectrum of 2S,3S enantiomer.
Compound 2 was obtained as a pale yellow, amorphous powder. Treating compound 2 with 10% H2SO4 and heating on TLC gave a yellowish pink color. The 1H NMR spectrum (Table 2) of 2 showed a meta-coupled two doublet proton at δH 6.10 (J = 1.96 Hz) and 5.98 (J = 1.64 Hz) corresponding to the C-6 and C-8 protons of a flavonoid skeleton. Protons at δH 7.05 (1H, d, J = 1.8 Hz, H-1′) and 6.87 (1H, d, J = 1.8 Hz, H-6′) showed meta-coupled doublets related to a flavonoid B-ring, which suggests that this ring possesses an unsymmetrical 3′,4′,5′-trisubstituted pattern. The 13C NMR spectrum of 2 revealed 15 carbon signals, including δc 82.9 (C-2), 72.9 (C-3), and 191.4 (C-4), which are characteristic features of a flavanonol moiety. Dihydromyricetin (4) showed good agreement with the carbon chemical shifts of 2 (Table 2), except those of the B-ring [δc 128.0 (C-1′), 113.3 (C-2′), 146.3 (C-3′), 138.0 (C-4′), 140.2 (C-5′), and 112.0 (C-6′)] and the C-5 of the A-ring. Compound 2 has [M + H]− ion in the HRESIMS at m/z 414.02053 consistent with the molecular formula C16H14O11S. The structure of 2 was suggested to be 3′-O-sulfate-methyldihydromyricetin. The effect of O-sulfate as an electron-withdrawing group was observed in the 13C NMR spectrum [15]. The carbon atoms ortho to the sulfate group show a down-field shift of 5.1–6.9 ppm, which is the result of decreased electron density. This contrasts with the up-field shift of 3.7 ppm shown by the carbon atom bound to the sulfate group, which results from an increased electron density. These are in line with the chemical shifts calculated in the past for quercetin-3′-sulfate and phloroglucinol sulfates [16]. The experimental ECD spectrum showed a negative Cotton effect peak at 217 nm and a positive Cotton effect peak at 197 nm (Fig. 2) which is similar to that of 1. Based on these results, (2S,3S)-3′-O-sulfate-5-O-methyldihyd-romyricetin was assigned as the structure of 2. Two other sulfate flavonoids, 5 and 6, were isolated in acidic form for the first time and were identified by spectroscopic analysis and comparison of the resulting data with the literature [11]. Although sulfated compounds occur ubiquitously in higher plants, it is unclear what physiological significance these compounds have in plants. It is possible that they play a role in neutralizing reactive hydroxyl groups. In addition, because these compounds accumulate in plants which grow in saline conditions, it is possible that they play a role in sequestering sulfate ions [17]. The presence of many sulfated-form flavonoids is well-known in plants [15]. Lignan, (+)-isoloarisiresinol-3α-O-sulfate [18], and the coumarin sulfates [19], both containing sulfated alcohol groups, are examples of other types of phenolic sulfates.
Table 2.
1H and 13C NMR data for 2, 5, and 6 (in CD3OD).
| No. | 6
|
δcb | 5
|
δcb | 2
|
δcb |
|---|---|---|---|---|---|---|
| δH (mult; J in Hz)a | δH (mult; J in Hz)a | δH (mult; J in Hz)a | ||||
| 2 | 147.1 | 4.91 (d, 11.56) | 83.5 | 4.86 (d, 11.68) | 82.9 | |
| 3 | 137.6 | 4.53 (d, 11.64) | 72.3 | 4.38 (d, 11.72) | 72.9 | |
| 4 | 177.3 | 196.9 | 191.4 | |||
| 5 | 162.3 | 163.9 | 164.6 | |||
| 6 | 6.19 (s) | 99.3 | 5.93 (d, 2.12) | 96.0 | 6.10 (d, 1.96) | 95.7 |
| 7 | 165.6 | 167.3 | 165.9 | |||
| 8 | 6.42 (s) | 94.5 | 5.90 (d, 2.2) | 94.9 | 5.98 (d, 1.64) | 93.1 |
| 9 | 158.1 | 163.0 | 162.7 | |||
| 10 | 104.5 | 100.5 | 102.3 | |||
| 1′ | 123.3 | 127.8 | 128.0 | |||
| 2′ | 7.80 (s) | 115.3 | 7.04 (d, 2.16) | 113.3 | 7.05 (d, 1.8) | 113.3 |
| 3′ | 147.6 | 146.3 | 146.3 | |||
| 4′ | 141.1 | 138.0 | 138.0 | |||
| 5′ | 141.6 | 140.2 | 140.2 | |||
| 6′ | 7.68 (s) | 113.5 | 6.86 (d, 2.16) | 112.1 | 6.87 (d, 1.56) | 112.0 |
| —CH3 | 3.83 (s) | 55.0 |
1H NMR measured at 400 MHz.
13C NMR measured at 100 MHz [9].
Compound 3 gave [M + Na]+ and [M + Cl]− ions in the HRESIMS at m/z 417.1737 and 429.1544 respectively, consistent with the molecular formula C17H30O10. The 1H NMR spectrum displayed two anomeric proton signals at δH 5.19 (1H, s br) and 4.36 (1H, d, J = 7.6 Hz), corresponding to rhamnose and glucose anomeric protons, respectively. The 13C NMR displayed signals at 16.6 (C-6″; q), 68.3 (C-5″; d), 72.6 (C-4″; d) 70.4 (C-3″; d), 70.9 (C-2″; d) and 100.8 (C-1″; d) attributable to a terminal α-L-rhamnose, while the δC 61.3 (C-6′; t), 76.3 (C-5′; d), 77.9 (C-4′; d), 77.7 (C-3′; d), 70.8 (C-2′; d) and 101.6 (C-1′; d) illustrated the inner β-D-glucose (Table 3). The glycosylation shift of the C-4′ [20,21] suggested that the terminal rhamnose unit is connected to the C-4′ of the glucose moiety. In addition to the signals for the sugars, the 1H NMR showed signals representative of one methyl at δH 1.75 (3H, s), one aliphatic methylene at δH 2.36 (2H, t, J = 7.6 Hz), one hydroxyl methylene at δH 4.0 (1H, m) and 3.6 (1H, m) and an exomethylene at δH 1.75 (2H, d, J = 6.9 Hz). Their 13C NMR signals appeared at δC 21.5 (C-5; q), 37.3 (C-2; t) 67.8 (C-1; t), 110.7 (C-4; t) and 142.3 (C-3; s), respectively, suggesting the presence of CH2==C(CH3)CH2CH2O-. In the HMBC experiment H-1′ showed a clear correlation with C-1, and H-1″ with C-3′ and C-2′. In the HMBC experiment the proton at δH 1.75 (3H, s) showed strong correlations with the carbon at δC 142.3 (C-3), 110.7 (C-4) and 37.3 (C-2), as well as, the correlations between the signal at δH 2.36 (1H, t, J = 7.6) and the signals at δC 142.3 (C-3), 110.7 (C-4) and 67.8 (C-1). The assignments were based on COSY, HMBC and HMQC correlations. The above evidence was used to propose the structure of compound 3 which is β-D-glucopyranoside, 3-methyl, but-3-en-1-yl 4-O-α-L-rhamnopyranosyl (Fig. 1).
Table 3.
1H and 13C NMR data for 3 (in CD3OD).
| No. | 3
|
δcb |
|---|---|---|
| δH (mult; J in Hz)a | ||
| 1 | 4.0 (m) | 67.8 |
| 3.6 (m) | ||
| 2 | 2.36 (t, 7.6) | 37.3 |
| 3 | 142.3 | |
| 4 | 1.75 (d, 6.9). | 110.7 |
| 5 | 1.75 (s) | 21.5 |
| 1′ | 4.36 (d, 7.6) | 101.6 |
| 2′ | 3.93 | 70.8 |
| 3′ | 3.40 | 77.7 |
| 4′ | 3.49 | 77.9 |
| 5′ | 3.29 | 76.3 |
| 6′ | 3.87 | 61.3 |
| 1 ″ | 5.19 (s br) | 100.8 |
| 2 ″ | 3.97 | 70.9 |
| 3 ″ | 3.27 | 70.4 |
| 4 ″ | 3.41 | 72.6 |
| 5 ″ | 3.67 | 68.3 |
| 6 ″ | 1.23 (d, 6.1) | 16.6 |
1H NMR measured at 400 MHz.
13C NMR measured at 100 MHz.
The structures of the known compounds were confirmed by comparison of their spectroscopic properties with published data. To date, this is the first phytochemical and biological evaluation of L. caspium. The isolated compounds 1–11 (Fig. 1) were evaluated for their antibacterial, antifungal, antimalarial and antileishmanial activities. The antibacterial activities were evaluated using Staphylococcus aureus, Methicillin-resistant S. aureus (MRSA), Escherichia coli, Pseudomonas aeruginosa, and Mycobacterium intracellulare. None of these compounds showed in vitro antibacterial activity. The antifungal activities were evaluated against a panel of pathogenic fungi (Candida albicans, Candida glabrata, Candida krusei, Cryptococcus neoformans, and Aspergillus fumigatus) associated with opportunistic infections. Compounds 7, 8 and 9 exhibited good antifungal activity against C. glabrata with an IC50 values of 6.79, 15.37 and 8.53 μg/mL, respectively. Compound 8 showed potent antimalarial activity against both resistant and sensitive strains of Plasmodium falciparum with IC50 values of 1.82 and 1.51 μg/mL, respectively. Compounds 1, 4, 6, 8 and 9 showed significant activity against Trypanosoma brucei with IC50 values of 6.93, 9.65, 8.52, 7.67 and 6.31 μg/mL, respectively. Compounds 1–11 were evaluated at a concentration of 10 μM for their affinity to bind with cannabinoid (CB1 and CB2) and opioid (mu, kappa and delta) receptors. None of these compounds showed affinity for these receptors.
3. Experimental
3.1. Plant material and extraction
The plant, L. caspium (Willd), was collected during its flowering stage on July, 29th 2004 in the piedmont steppe of the Ulytau Mountains and identified by Botanical Garden, Institute of Botany and Phytointroduction, Almaty, Kazakhstan.
The aerial parts (0.24 kg) were air-dried followed by grinding in a Willey-Mill plant grinder. Ground plant material was extracted at room temperature using two different solvents, methylene chloride (1.7 L) and ethanol (2.2 L) provided 7.4 g and 11.3 g respectively of the crude extracts after evaporation of the solvents. Lastly, extraction with water (2.5 L) provided 17.8 g of extract after lyophilization to remove the water.
3.2. Isolation
The ethanol extract of L. caspium (11.3 g) was fractionated using column chromatography with reversed phase C18 silica gel stepwise from water to methanol to yield seven fractions (water; 4:1 H2O–MeOH; 3:2 H2O–MeOH; 1:1 H2O–MeOH; 2:3 H2O–MeOH; 1:4 H2O–MeOH and MeOH). The fraction eluted with water was subsequently chromatographed on an open column using reversed phase C18 silica gel starting with water and followed with a stepwise gradient elution to MeOH, yielding nine subfractions (water (1); 95:5 H2O–MeOH (2); 90:10 H2O–MeOH (3); 80:20 H2O–MeOH (4); 70:30 H2O–MeOH (5); 60:40 H2O–MeOH (6); 40:60 H2O–MeOH (7); 20:80 H2O–MeOH (8) and MeOH (9)). The subfraction (2), eluted with 5% MeOH in water, was rechromatographed using Sephadex LH-20 eluted with methanol to afford compounds 2 (22 mg) and 5 (12 mg). Subfraction (3), eluted with 10% MeOH in water, was rechromatographed using Sephadex LH-20 eluted with methanol to yield compounds 1 (34 mg), 7 (33 mg) and 4 (15 mg). Subfraction (4), eluted with 20% MeOH in water, was rechromatographed using Sephadex LH-20 eluted with MeOH yielding eighteen subfractions (A–R) (50 mL each). Subfraction P and Q afforded as compound 8 (50 mg). Subfraction E was purified on Sephadex LH-20 to furnish compound 11 (6 mg). Compound 10 (25 mg) was purified with Sephadex LH-20 eluted with MeOH from subfraction (7), eluted with 60% MeOH in water. The subfraction (5), eluted with 30% MeOH in water, was rechromatographed on Sephadex LH-20 eluted with methanol to yield compounds 3 (15 mg), 6 (12 mg), and 9 (21 mg).
3.3. General experimental procedures
1H and 13C NMR spectra were obtained on a Bruker model AMX 500 NMR spectrometer with standard pulse sequences, operating at 500 MHz in 1H and 125 MHz in 13C. The chemical shift values were reported in parts per million units (ppm) and trimethylsilane (TMS) or known solvent shifts, used as internal chemical shift references. Coupling constants were recorded in Hertz (Hz). Standard pulse sequences were used for COSY, HMQC, HMBC, TOCSY, NOESY and DEPT. High-resolution mass spectra (HRMS) were measured on a Micromass Q-T of Micro mass spectrometer with a lock spray source. Column chromatography was carried out on silica gel (70–230 mesh, Merck) and Sephadex LH-20 (Mitsubishi Kagaku, Tokyo, Japan). TLC (silica gel 60 F254) was used to monitor fractions from column chromatography. Visualization of the TLC plates was achieved with a UV lamp (l = 254 and 365 nm) and anisaldehyde/acid spray reagent (MeOH:acetic acid:anisaldehyde:sulfuric acid, 85:9:1:5). All chemicals used were purchased from Sigma-Aldrich (St. Louis, MO) with the following exceptions: for the binding experiments [3H]-CP-55,940 (174.8 Ci/mmol), [3H]-DAMGO (53.4 Ci/mmol), [3H]-U-69,593 (42.7 Ci/mmol), and [3H]-Enkephalin (45 Ci/mmol) were purchased from Perkin-Elmer Life Sciences Inc. (Boston, MA, U.S.A.). CP-55,940, DAMGO, DPDPE, nor-Binaltorphimine and Win 55,212-2 were purchased from Tocris Bioscience (Ellisville, MO, U.S.A.). CD spectra were measured on a JASCO J-715 spectrometer.
3.4. In vitro antimicrobial assay
Compounds 1–11 were tested for antimicrobial activity against a panel of microorganism obtained from the American Type Culture Collection (Manassas, VA) and included C. albicans ATCC 90028, C. glabrata ATCC 90030, C. krusei ATCC 6258, C. neoformans ATCC 90113, and A. fumigatus ATCC 204305, S. aureus ATCC 29213, methicillin-resistant S. aureus ATCC 33591 (MRSA), E. coli ATCC 35218, P. aeruginosa ATCC 27853, and Mycobacterium intracellulare ATCC 23068. The bioassays were performed as previously described [22,24].
3.5. In vitro antimalarial and antileishmanial assays
Antimalarial activity was determined in vitro against chloroquine sensitive (D6, Sierra Leone) and resistant (W2, Indo China) strains of P. falciparum by measuring plasmodial LDH activity, as described earlier [24]. The antileishmanial activity of the compounds was tested in vitro against a culture of Leishmania donovani promastigotes Pentamidine and Amphotericin B were used as positive controls [22,23].
3.6. Radioligand displacement for cannabinoid receptor subtypes
Compounds evaluated in the assay were run in competition experiments using both cannabinoid receptor subtypes, CB1 and CB2 [25,26]. Cannabinoid receptor binding assays were performed under the following conditions: 10 μM of each compound was incubated with 0.5 nM [3H]-CP 55,940, a potent cannabinoid agonist with affinity to both receptor subtypes, and 10 μg CB1 or CB2 membrane [26] for 90 min in a 96-well plate. The reaction was terminated via rapid vacuum filtration through GF/C filters presoaked with 0.3% BSA using a Perkin Elmer 96-well Unifilter (Perkin Elmer Life Sciences Inc., Boston, Mass. U.S.A.) followed by 10 washes with 50 mM Tris–EDTA buffer containing 0.2% BSA. Plates were read using a Perkin Elmer Topcount (Perkin Elmer Life Sciences Inc., Boston, Mass. U.S.A.). Total binding was defined as binding in the presence of 1.0% DMSO. Nonspecific binding was the binding observed in the presence of 1.0 μM CP-55,940. Specific binding was defined as the difference between total and nonspecific binding. Percent binding was calculated using the following formula: .
Ki and IC50 values were calculated using Graph-Pad Prism 5.
3.7. Radioligand displacement for opioid receptor subtypes
All compounds evaluated in the assay were run in competition binding against the opioid receptor subtypes (δ, κ, μ), as well as the cannabinoid receptor subtypes mentioned above [26]. Saturation experiments were performed after each batch of membrane was scraped for each of the three opioid cell lines (δ, κ, μ) to determine the optimal tritium and membrane concentration to be used in the assay. Saturation experiments determine the receptor number and radioligand affinity for the membrane. Opioid binding assays were performed under the following conditions: 10 μM of each compound was incubated with [3H]-DAMGO (μ), [3H]-U-69,593 (κ), or [3H]-enkephalin (δ) for 60 min in a 96-well plate. The reaction was terminated via rapid vacuum filtration through GF/B filters presoaked with 0.3% BSA using a Perkin Elmer 96-well Unifilter followed by 10 washes with 50 mM Tris–HCl. Plates were read using a Perkin Elmer Topcount. Total binding was defined as binding in the presence of 1.0% DMSO. Nonspecific binding was the binding observed in the presence of 10 μM DAMGO (μ), nor-binaltorphimine (κ), or DPDPE (δ). Specific binding was defined as the difference between total and nonspecific binding. Percent binding was calculated using the following formula: .
Ki and IC50 values were calculated using Graph-Pad Prism 5.
3.8. Computational
Conformational analysis of compounds 1 and 2 were performed with Schrödinger Macromodel 9.9 (Schrödinger, LLC, New York) employing the OPLS2005 (optimized potential for liquid simulations) force field in MeOH [29]. Four conformers within a 2 kcal/mol energy window from the global minimum were selected, based on Boltzmann distribution calculated from Schrödinger software [29]. The output files for these conformers were prepared by Avogadro software for Gaussian 09 calculation, geometrical optimization and energy calculation applied on these conformers at B3LYP/6-31G [30]. Vibrational evaluation was done at the same level to confirm minimal excitation energy (denoted by wavelength in nm), rotatory strength dipole, and dipole length that were calculated in MeOH by TD-DFT/B3LYP/6-31G performed by the Gaussian 09 software package [30,31]. ECD curves were obtained in the SpecDis 1.62 program [32].
Acknowledgments
We are grateful to the Government of Kazakhstan, National Center for Natural Products Research (NCNPR), University of Mississippi, USA, and the International Science and Technology Center (ISTC) project K1896 for financial support. Also we are grateful to Dr. Melissa Jacob, Dr. Babu Tekwani, and Dr. Shabana Khan for preforming antibacterial, antifungal, antimalarial and antileishmanial activities, and to Sara Pettaway and Janet Lambert for performing cannabinoid and opioid receptor binding studies. This work was supported by the National Institute of General Medical Sciences (NIGMS) of the National Institute of Health (NIH), grant number P20GM104932. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIGMS or the NIH.
References
- 1.Cos P, Maes L, Vanden Berghe D, Hermans N, Pieters L, Vlietinck A. Plant substances as anti-HIV agents selected according to their putative mechanism of action. J Nat Prod. 2004;67(2):284–293. doi: 10.1021/np034016p. [DOI] [PubMed] [Google Scholar]
- 2.Ksouri R, Megdiche-Ksouri W, Jallali I, Debez A, Magné C, Hiroko I, Abdelly C. Medicinal halophytes: potent source of health promoting biomolecules with medical, nutraceutical and food applications. Crit Rev Biotechnol. 2011:1–38. doi: 10.3109/07388551.2011.630647. [DOI] [PubMed] [Google Scholar]
- 3.Davy AJ, Scott R, Cordazzo CV. Biological flora of the British Isles: Cakile maritima. Scop. J Ecol. 2006;94(3):695–711. [Google Scholar]
- 4.Murray AP, Rodriguez S, Frontera MA, Tomas MA, Mulet MC. Antioxidant metabolites from Limonium brasiliense (Boiss.) Kuntze. Z Naturforsch. 2004;59:477–480. doi: 10.1515/znc-2004-7-804. [DOI] [PubMed] [Google Scholar]
- 5.Aniya Y, Miyagi C, Nakandakari A, Kamiya S, Imaizumi N, Ichiba T. Free radical scavenging action of the medicinal herb Limonium wrightii from the Okinawa islands. Phytomedicine. 2002;9:239–244. doi: 10.1078/0944-7113-00112. [DOI] [PubMed] [Google Scholar]
- 6.Yuh-Chi K, Lie-Chwen L, Wei-Jern T, Cheng-Jen C, Szu-Hao K, Yen-Hui H. Samarangenin B from Limonium sinense suppresses herpes simplex virus type 1 replication in vero cells by regulation of viral macromolecular synthesis. Antimicrob Agents Chemother. 2002;46(9):2854–2864. doi: 10.1128/AAC.46.9.2854-2864.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kandil FE, Ahmed KM, Hussieny HA, Soliman AM. A new flavonoid from Limonium axillare. Arch Pharm J Pharmacol Med Chem. 2000;333:275–277. doi: 10.1002/1521-4184(20008)333:8<275::aid-ardp275>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 8.Medini F, Fellah H, Ksouri R, Abdelly C. Total phenolic, flavonoid and tannin contents and antioxidant and antimicrobial activities of organic extracts of shoots of the plant Limonium delicatulum. J Taibah Univ Sci. 2014;8(3):216–224. [Google Scholar]
- 9.Jeon SH, Chun W, Choi YJ, Kwon YS. Cytotoxic constituents from the bark of Salix hulteni. Arch Pharm Res. 2008;31(8):978–982. doi: 10.1007/s12272-001-1255-9. [DOI] [PubMed] [Google Scholar]
- 10.Movsumov IS. Flavonoids of the roots of Limonium caspium. Chem Nat Compd. 1996;32(6):922–922. [Google Scholar]
- 11.Kim HH, Oh MH, Park KJ, Heo JH, Lee MW. Anti-inflammatory activity of sulfate-containing phenolic compounds isolated from the leaves of Myrica rubra. Fitoterapia. 2014;92:188–193. doi: 10.1016/j.fitote.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 12.Scharbert S, Holzmann N, Hofmann T. Identification of the astringent taste compounds in black tea infusions by combining instrumental analysis and human bioresponse. J Agric Food Chem. 2004;52(11):3498–3508. doi: 10.1021/jf049802u. [DOI] [PubMed] [Google Scholar]
- 13.Shao X, Bai N, He K, Ho CT, Yang CS, Sang S. Apple polyphenols, phloretin and phloridzin: new trapping agents of reactive dicarbonyl species. Chem Res Toxicol. 2008;21(10):2042–2050. doi: 10.1021/tx800227v. [DOI] [PubMed] [Google Scholar]
- 14.García-Egido E, Paz J, Iglesias B, Muñoz L. Synthesis of cyanoformamides from primary amines and carbon dioxide under mild conditions. Synthesis of ceratinamine. Org Biomol Chem. 2009;7(19):3991–3999. doi: 10.1039/b912043b. [DOI] [PubMed] [Google Scholar]
- 15.Barron D, Varin L, Ibrahim RK, Harborne JB, Williams CA. Sulphated flavonoids — an update. Phytochemistry. 1988;27(8):2375–2395. [Google Scholar]
- 16.Op de Beck P, Dijoux MG, Cartier G, Mariotte AM. Quercitrin 3′-sulphate from leaves of Leea guinensis. Phytochemistry. 1998;47(6):1171–1173. [Google Scholar]
- 17.Harborne JB. Flavonoid sulphates: a new class of sulphur compounds in higher plants. Phytochemistry. 1975;14(5):1147–1155. [Google Scholar]
- 18.Zhong XN, Ide T, Otsuka H, Hirata E, Takeda Y. (+)-Isolarisiresinol 3a-O-sulphate from leaves of Myrsine seguinii. Phytochemistry. 1998;49(6):1777–1778. doi: 10.1016/s0031-9422(98)00256-8. [DOI] [PubMed] [Google Scholar]
- 19.Lemmich J, Shabana M. Coumarin sulphates of Seseli libanotis. Phytochemistry. 1984;23(4):863–865. [Google Scholar]
- 20.Shinozaki Y, Tobita T, Mizutani M, Matsuzaki AT. Isolation and identification of two new diterpene glycosides from Nicotiana tabacum. Biosci Biotechnol Biochem. 1996;60(5):903–905. doi: 10.1271/bbb.60.903. [DOI] [PubMed] [Google Scholar]
- 21.Sousa EAD, Da Silva AA, Cavalheiro AJ, Lago JHG, Chaves MH. A new flavonoid derivative from leaves of Oxandra Sessiliflora RE Fries. J Braz Chem Soc. 2014;25(4):704–708. [Google Scholar]
- 22.Ma G, Khan SI, Jacob MR, Tekwani BL, Li Z, Pasco DS, Walker LA, Khan IA. Antimicrobial and antileishmanial activities of hypocrellins A and B. Antimicrob Agents Chemother. 2004;48:4450–4452. doi: 10.1128/AAC.48.11.4450-4452.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hamid R, Rotshteyn Y, Rabadi L, Parikh R, Bullock P. Comparison of alamar blue and MTT assays for high through-put screening. Toxicol in Vitro. 2004;18:703–710. doi: 10.1016/j.tiv.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 24.Bharate SB, Khan SI, Yunus NA, Chauthe SK, Jacob MR, Tekwani BL, Khan IA, Singh IP. Antiprotozoal and antimicrobial activities of O-alkylated and formylated acylphloroglucinols. Bioorg Med Chem. 2007;15:87–96. doi: 10.1016/j.bmc.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 25.Ross RA, Gibson TM, Stevenson LA, Saha B, Crocker P, Razdan RK, Pertwee RG. Structural determinants of the partial agonist-inverse agonist properties of 6′-azidohex-2′-yne-Δ8-tetrahydrocannabinol at cannabinoid receptors. Br J Pharmacol. 1999;128(3):735–743. doi: 10.1038/sj.bjp.0702836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.León F, Gao J, Dale OR, Wu Y, Habib E, Husni AS, Hill RA, Cutler SJ. Secondary metabolites from Eupenicillium parvum and their in vitro binding affinity for human opioid and cannabinoid receptors. Planta Med. 2013;79(18):1756–1761. doi: 10.1055/s-0033-1351099. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Y, Que S, Yang X, Wang B, Qiao L, Zhao Y. Isolation and identification of metabolites from dihydromyricetin. Magn Reson Chem. 2007;45(11):909–916. doi: 10.1002/mrc.2051. [DOI] [PubMed] [Google Scholar]
- 28.Sang YM, Yan LK, Wang JP, Su ZM. TDDFT studies on the electronic structures and chiroptical properties of mono-tin-substituted Wells–Dawson polyoxotungstates. J Phys Chem A. 2012;116(16):4152–4158. doi: 10.1021/jp211262b. [DOI] [PubMed] [Google Scholar]
- 29.Macro Model 9.9. Schrodinger LLC; 2012. [accessed May 10, 2015]. ( http://www.schrodinger.com/productpage/14/11/) [Google Scholar]
- 30.Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA, Peralta JE, Jr, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ. Gaussian 09 (Revision B.01) Gaussian Inc; Wallingford, CT (USA): 2010. [Google Scholar]
- 31.Open-source molecular builder and visualization tool. Version1.1.1, http://avogadro.openmolecules.net/
- 32.Bruhn T, Schaumlöffel A, Hemberger Y, Bringmann G. SpecDis version 1.62. University of Wuerzburg; Germany: 2014. [Google Scholar]