Abstract
Neurotrophins perform essential processes throughout neural development. They signal through Trk receptor proteins, typically in association with a “low affinity” p75NTR pan-neurotrophin co-receptor. Neurotrophins are synthesized as proproteins; the pro domains are removed proteolytically to yield the mature, presumably functional forms of the neurotrophins. Recent findings, however, have revealed a positive role for the proneurotrophins themselves. The proproteins bind with high affinity to the p75NTR pan-neurotrophin receptor in the absence of Trks to initiate a separate set of signaling cascades that actively oppose the effects of the mature growth factors. These experiments suggest that the balance between pro- and mature neurotrophin plays a critical role in tuning downstream signaling. This view changes the neurotrophin field substantially, and also points to the broader idea that the potential activities of precursor proteins deserve a closer look.
The neurotrophin family of growth factors, comprising nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), neurotrophin-3 (NT-3), and neurotrophin-4 (NT-4), regulate various processes during development of the central nervous system and peripheral nervous system, notably neuronal survival, but also synaptogenesis, axon and dendrite outgrowth, and activity-dependent plasticity (1). Neurotrophins are synthesized as larger proproteins that are cleaved to yield the mature growth factors and a short N-terminal propeptide. Neurotrophins bind to high-affinity receptors of the Trk (tropomyosin receptor kinase) family, stimulating the tyrosine kinase activity of the receptor to activate downstream signaling pathways, including mitogen-activated protein kinase, phosphoinositide 3-kinase, and phospholipase C– γ (2). Trks also enlist a co-receptor, called the pan-neurotrophin receptor or p75NTR, which can bind the neurotrophins on its own, albeit with rather lower affinity than do the Trks (3). p75NTR contains a “death domain” in its cytoplasmic portion, but the role of the p75NTR subunit in neurotrophin signaling has been enigmatic.
Two curious observations suggested that the old view of neurotrophins acting solely through Trk signaling required rethinking. First, p75NTR-null mice exhibited reduced, rather than increased, neuronal cell death in some contexts (4), and second, high concentrations of mature NGF caused cell death in p75NTR-expressing oligodendrocytes that lack Trk proteins (5). Building off these findings, Lee et al. made the seminal discovery that the uncleaved proNGF protein binds to the p75NTR receptor with high affinity to promote apoptosis (6). This was in stark contrast to mature NGF, which binds to the TrkA receptor to promote survival. These discoveries were followed quickly by new findings of proneurotrophins having roles in neuronal degeneration, pruning, axon injury, and long-term depression (LTD) of synaptic transmission (7, 8). The complementarity of these activities to the familiar effects of mature neurotrophins led Lu and colleagues to suggest a “yin and yang” model of neurotrophin action, whereby the pro- versus mature forms of neurotrophins were hypothesized to have opposing effects, perhaps arising from engagement of different receptors (8).
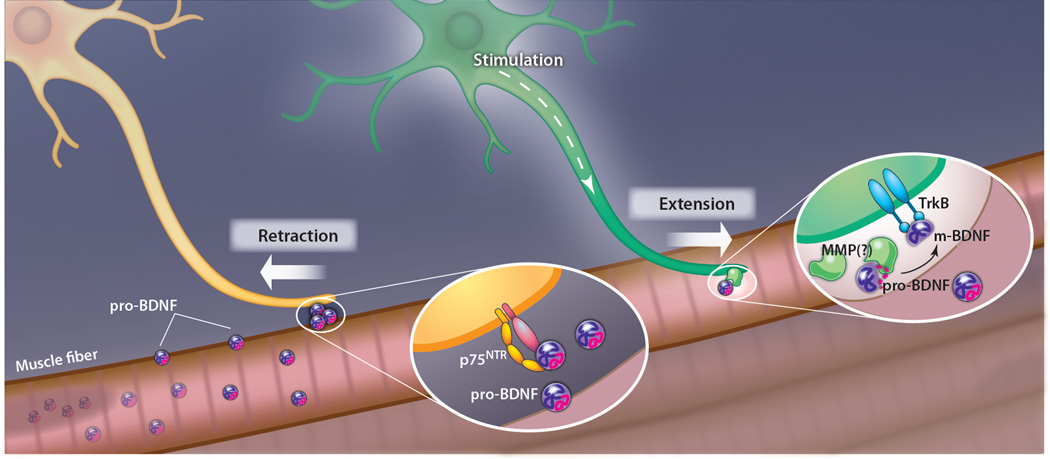
The interplay of pro- and mature neurotrophins has now been clearly demonstrated in “synaptic matching” at the Xenopus neuromuscular junction (NMJ). In vertebrate muscle development, several incoming motor axons initially form synapses to each muscle fiber. Over time, however, innervating axons are “matched” one-to-one to the available muscle fibers by strengthening a single synapse and eliminating the rest (9). In an elegant study, Je and colleagues show that the mature and precursor forms of BDNF provide, respectively, the “strengthening” and the “elimination” signals at the NMJ (10).
Je et al. prepared Xenopus nerve-muscle cocultures that included a mixture of neurons labeled with two different-color dyes, which identified axons coming from different neurons at a doubly innervated muscle fiber (Fig. 1). They then stimulated just one of the neurons by local photolysis of caged glutamate at the neuronal cell body. Time-lapse confocal imaging consistently showed stabilization or elongation of the stimulated axon, and simultaneous retraction of the unstimulated axon. The authors next modulated signaling in the cultures by adding either mature BDNF (m-BDNF) or pro-BDNF, and compared the effects each produced on the pattern of innervation. Upon decreasing m-BDNF action (by morpholino-oligonucleotide–mediated knockdown of its receptor, TrkB), both terminals retracted, whereas adding m-BDNF to the culture prevented retraction of both the unstimulated and the stimulated axons. In contrast, addition of pro-BDNF promoted withdrawal of inactive terminals, and blocking this signaling pathway with small interfering RNA against the p75NTR receptor led to reduced or no retraction of unstimulated terminals. This suggested a specific requirement for m-BDNF signaling to strengthen active terminals and for pro-BDNF signaling to eliminate inactive terminals.
Fig. 1.
Antagonistic effects of pro-BDNF and mature BDNF (m-BDNF) in synaptic competition. Muscle fiber (pink) releases pro-BDNF (purple and magenta circles), which binds p75NTR receptor complex on an innervating motor axon (yellow neuron) and causes it to retract. Electrical stimulation of a motoneuron (green neuron) causes it to release matrix metalloproteases (MMPs) that process pro-BDNF to mature BDNF (blue symbols), which binds to TrkB and promotes selective extension of that motor axon and stabilization of its synapse.
But how is the m-BDNF signal limited to the neighborhood of active terminals? Je and colleagues hypothesized that pro-BDNF processing was spatially localized. They constructed a bioprobe in which a pro-BDNF cleavage sequence lies between a fluorophore and a quencher, such that fluorescence emission is stimulated by proteolytic cleavage. Upon neuronal stimulation, increased fluorescence was seen at the muscle cell surface selectively along stimulated axons and not near unstimulated neurons or the rest of the muscle cell surface. Moreover, immunostaining showed that general electrical activation of the muscle (with K+ in the medium) caused the muscle to secrete pro-BDNF and that stimulation of innervating neurons (with glutamate) caused the secreted pro-BDNF near the axon to be transformed into m-BDNF, presumably by proteolytic cleavage. Consistent with this, application of inhibitors of matrix metalloproteases (MMPs) blocked the conversion of pro-BDNF to m-BDNF and resulted in retraction of both stimulated and unstimulated axons.
Therefore, the authors concluded that, at the NMJ, the postsynaptic muscle cell releases pro-BDNF that serves as a universal synapse elimination (“punishment”) signal to all innervating axons, but that an electrically active axon releases proteases—probably MMPs—that locally convert pro-BDNF to m-BDNF, thus creating a “reward” signal that stabilizes only that terminal. This is an elegant solution to the problem of ensuring mono-innervation of muscle fibers with 100% fidelity. Analog solutions, such as competition for a limiting trophic factor, would be expected to yield a distribution of innervation patterns, peaked at mono-innervation but also including di-, tri-, and noninnervated fibers. In contrast, the activity-dependent cleavage model results in a bistable switch, built around a single component with two mutually incompatible states, that allows the system to achieve mono-innervation with high fidelity.
One possible caveat to these results is that there is some disagreement as to whether “pro-BDNF–specific” antibodies recognize only the unprocessed proneurotrophin or whether, after processing, the antibody still labels the isolated N-terminal “propeptide” that has been cleaved off by the processing event, and which can be stabilized in vivo in some contexts (11). In Je et al., this concern is mitigated by the use of a reporter for processing activity and the modulatory effect of MMP inhibition, but additional experiments to rule out this critique would undoubtedly be worthwhile.
It is attractive to speculate that opposition between pro- and mature neurotrophins might be used in synaptic refinement elsewhere in the nervous system. For example, BDNF and NT-3 regulate dendrite growth in the developing cortex (12). Could their interaction be overlayed on regulation by the pro-proteins for these two neurotrophins? Similarly, given the opposing effects of pro- and mature neurotrophins on LTD versus LTP (long-term potentiation) (13, 14), it seems likely that the switch between these forms of plasticity is regulated in part by the processing state of the neurotrophins. From a different perspective, it may be that some of the conflicting data on effects of neurotrophins in vivo—discrepancies, for example, between overexpression, knockout phenotypes, and protein-blocking experiments—arise from the different degrees to which these various approaches alter the balance of pro- to mature neurotrophin. Reconsideration of these experiments may well be in order.
How might the forms of a single neurotrophin produce such diametrically opposing effects? Much is known about how mature neurotrophins promote axon growth and attraction by binding to Trk receptors. Far less is known about how proneurotrophins cause axon retraction. Deinhardt and colleagues began to redress this imbalance by defining signaling mechanisms downstream of pro-NGF that induce growth cone collapse in murine hippocampal neurons (15).
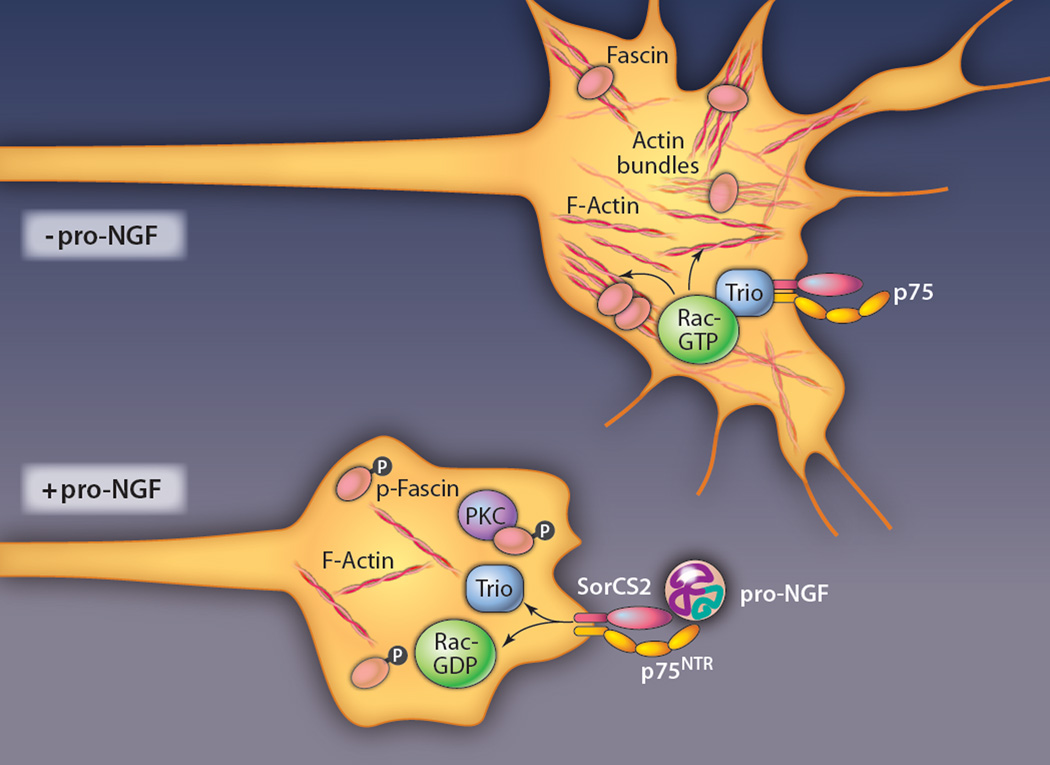
Upon adding pro-NGF to primary hippocampal cultures, Deinhardt et al. saw rapid cessation of growth cone movement and subsequent collapse of actin structures in a fraction of neurons in the culture (Fig. 2). Pro-NGF had previously been shown to signal through a complex of p75NTR together with a transmembrane protein called sortilin (16), and indeed, the pro-NGF–sensitive population of hippocampal cells were positive for p75NTR and a sortilin family member called SorCS2. The authors used coimmunoprecipitation to document a complex including pro-NGF, p75NTR, and SorCS2, and blocking SorCS2 with antibodies prevented growth cone collapse, showing that this complex mediates the response to pro-NGF.
Fig. 2.
Signaling mechanisms downstream of pro-NGF that induce growth cone collapse. Pro-NGF binds to a receptor complex containing p75NTR and SorCS2 on cultured hippocampal neurons. This causes Trio, a guanine exchange factor for Rac GTPase, to be released from the receptor complex. Consequent reduction of Rac activity, together with protein kinase C (PKC)–dependent phosphorylation and inactivation of the actin-bundling protein fascin, leads to actin disassembly and growth cone collapse.
The authors next used mass spectrometry to identify signaling proteins associated with the p75NTR and sortilin complex and identified Trio, a guanine nucleotide exchange factor (GEF) for the small guanosine triphosphatase (GTPase) Rac. Treatment with pro-NGF reduced the interaction of Trio with the receptor complex and caused loss of Trio from the tips of neurites, leading to the model that pro-NGF–dependent displacement of Trio from the p75NTR-sortilin complex, with consequent reduction of local Rac activity, was responsible for growth cone collapse. Indeed, the amount of active Rac was reduced in cultures exposed to pro-NGF, and blocking Rac activity pharmacologically caused growth cone collapse, much like pro-NGF treatment. Finally, to address how the effects of pro-NGF signaling are transmitted to the actin cytoskeleton, the authors showed that pro-NGF induces phosphorylation of the actin-bundling protein, fascin, by protein kinase C (PKC). This resulted in destabilization of actin bundles, with consequent collapse of the growth cone cortex. Whether PKC-dependent signaling to fascin is somehow linked to Trio and Rac or acts strictly in parallel is not yet clear.
Competition between opposing activities is a universal theme in biological regulation, and there are many ways in which neurotrophin action is switched between attractive and repulsive effects. For example, m-BDNF can repel sympathetic axons, acting through a complex of p75NTR with the receptor plexin A3, if m-BDNF is coadministered with the plexin ligand Sema3, or acting through a complex of p75NTR with the receptor EphB, if coadministered with ephrin B2 (17). In both of these cases, the repulsive activity is mediated through a pathway involving Rho GTPase and the kinase ROCK. Alternatively, the effect of NGF can be switched between attractive and repulsive by the interaction of 14-3-3 proteins and PKA (18). However, linking the switch in the sign of neurotrophin signaling to the irreversible proteolytic transformation of proprotein to mature protein provides unique opportunities for regulation.
Most transmembrane and secreted proteins undergo propeptide removal, so other pro-proteins usually thought of as silent precursors may also have unique functional properties that have been overlooked. For example, in Drosophila, the transforming growth factor–β (TGF-β) proteins Decapentaplegic (Dpp) and Screw (Scw) specify dorsal versus ventral cell identities in the embryo by forming a steep concentration gradient (19). Gradient formation, however, involves dorso-ventral transport of the unprocessed pro–TGF-βs, through their incorporation in a complex that includes the Drosophila Chordin ortholog Short Gastrulation (Sog) (20) and the Twisted Gastrulation protein (Tsg) (21). Transport of mature Dpp is quantitatively different, at least partly due to trapping by receptors on the embryo surface. In mammals, another TGF-β family member, Nodal, also has roles for both its processed and unprocessed forms. Although Nodal is cleaved by Furin and PACE4 to activate the growth of the mouse endoderm and other tissues in early development, uncleaved Nodal also binds to activin receptors to induce a feedback loop that increases Nodal expression and further cleavage in the extraembryonic ectoderm (22). It is evident that pro-forms of ligands may play a variety of signaling roles that have yet to be explored.
Acknowledgments
Funding: The authors were supported by the Basic Neuroscience Program in the Intramural Research Program of the National Institute of Neurological Disorders and Stroke, NIH (Z01-NS003013). L.K. was supported by a predoctoral fellowship from the NSF.
References
- 1.Huang EJ, Reichardt LF. Neurotrophins: Roles in neuronal development and function. Annu. Rev. Neurosci. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang EJ, Reichardt LF. Trk receptors: Roles in neuronal signal transduction. Annu. Rev. Biochem. 2003;72:609–642. doi: 10.1146/annurev.biochem.72.121801.161629. [DOI] [PubMed] [Google Scholar]
- 3.Bibel M, Hoppe E, Barde YA. Biochemical and functional interactions between the neurotrophin receptors trk and p75NTR. EMBO J. 1999;18:616–622. doi: 10.1093/emboj/18.3.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frade JM, Barde YA. Genetic evidence for cell death mediated by nerve growth factor and the neurotrophin receptor p75 in the developing mouse retina and spinal cord. Development. 1999;126:683–690. doi: 10.1242/dev.126.4.683. [DOI] [PubMed] [Google Scholar]
- 5.Casaccia-Bonnefil P, Carter BD, Dobrowsky RT, Chao MV. Death of oligodendrocytes mediated by the interaction of nerve growth factor with its receptor p75. Nature. 1996;383:716–719. doi: 10.1038/383716a0. [DOI] [PubMed] [Google Scholar]
- 6.Lee R, Kermani P, Teng KK, Hempstead BL. Regulation of cell survival by secreted proneurotrophins. Science. 2001;294:1945–1948. doi: 10.1126/science.1065057. [DOI] [PubMed] [Google Scholar]
- 7.Hempstead BL. Commentary: Regulating proNGF action: Multiple targets for therapeutic intervention. Neurotox. Res. 2009;16:255–260. doi: 10.1007/s12640-009-9054-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu B, Pang PT, Woo NH. The yin and yang of neurotrophin action. Nat. Rev. Neurosci. 2005;6:603–614. doi: 10.1038/nrn1726. [DOI] [PubMed] [Google Scholar]
- 9.Wyatt RM, Balice-Gordon RJ. Activity-dependent elimination of neuromuscular synapses. J. Neurocytol. 2003;32:777–794. doi: 10.1023/B:NEUR.0000020623.62043.33. [DOI] [PubMed] [Google Scholar]
- 10.Je HS, Yang F, Ji Y, Nagappan G, Hempstead BL, Lu B. Role of pro-brain-derived neurotrophic factor (proBDNF) to mature BDNF conversion in activity-dependent competition at developing neuromuscular synapses. Proc. Natl. Acad. Sci. U.S.A. 2012;109:15924–15929. doi: 10.1073/pnas.1207767109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dieni S, Matsumoto T, Dekkers M, Rauskolb S, Ionescu MS, Deogracias R, Gundelfinger ED, Kojima M, Nestel S, Frotscher M, Barde YA. BDNF and its pro-peptide are stored in presynaptic dense core vesicles in brain neurons. J. Cell Biol. 2012;196:775–788. doi: 10.1083/jcb.201201038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McAllister AK, Katz LC, Lo DC. Opposing roles for endogenous BDNF and NT-3 in regulating cortical dendritic growth. Neuron. 1997;18:767–778. doi: 10.1016/s0896-6273(00)80316-5. [DOI] [PubMed] [Google Scholar]
- 13.Schuman EM. Neurotrophin regulation of synaptic transmission. Curr. Opin. Neurobiol. 1999;9:105–109. doi: 10.1016/s0959-4388(99)80013-0. [DOI] [PubMed] [Google Scholar]
- 14.Yang F, Je HS, Ji Y, Nagappan G, Hempstead B, Lu B. Pro-BDNF-induced synaptic depression and retraction at developing neuromuscular synapses. J. Cell Biol. 2009;185:727–741. doi: 10.1083/jcb.200811147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deinhardt K, Kim T, Spellman DS, Mains RE, Eipper BA, Neubert TA, Chao MV, Hempstead BL. Neuronal growth cone retraction relies on proneurotrophin receptor signaling through Rac. Sci. Signal. 2011;4:ra82. doi: 10.1126/scisignal.2002060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nykjaer A, Lee R, Teng KK, Jansen P, Madsen P, Nielsen MS, Jacobsen C, Kliemannel M, Schwarz E, Willnow TE, Hempstead BL, Petersen CM. Sortilin is essential for proNGF-induced neuronal cell death. Nature. 2004;427:843–848. doi: 10.1038/nature02319. [DOI] [PubMed] [Google Scholar]
- 17.Naska S, Lin DC, Miller FD, Kaplan DR. p75NTR is an obligate signaling receptor required for cues that cause sympathetic neuron growth cone collapse. Mol. Cell. Neurosci. 2010;45:108–120. doi: 10.1016/j.mcn.2010.05.015. [DOI] [PubMed] [Google Scholar]
- 18.Kent CB, Shimada T, Ferraro GB, Ritter B, Yam PT, McPherson PS, Charron F, Kennedy TE, Fournier AE. 14-3-3 proteins regulate protein kinase a activity to modulate growth cone turning responses. J. Neurosci. 2010;30:14059–14067. doi: 10.1523/JNEUROSCI.3883-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shimmi O, O’Connor MB. Physical properties of Tld, Sog, Tsg and Dpp protein interactions are predicted to help create a sharp boundary in Bmp signals during dorsoventral patterning of the Drosophila embryo. Development. 2003;130:4673–4682. doi: 10.1242/dev.00684. [DOI] [PubMed] [Google Scholar]
- 20.Biehs B, François V, Bier E. The Drosophila short gastrulation gene prevents Dpp from autoactivating and suppressing neurogenesis in the neuroectoderm. Genes Dev. 1996;10:2922–2934. doi: 10.1101/gad.10.22.2922. [DOI] [PubMed] [Google Scholar]
- 21.Mason ED, Konrad KD, Webb CD, Marsh JL. Dorsal midline fate in Drosophila embryos requires twisted gastrulation, a gene encoding a secreted protein related to human connective tissue growth factor. Genes Dev. 1994;8:1489–1501. doi: 10.1101/gad.8.13.1489. [DOI] [PubMed] [Google Scholar]
- 22.Ben-Haim N, Lu C, Guzman-Ayala M, Pescatore L, Mesnard D, Bischofberger M, Naef F, Robertson EJ, Constam DB. The nodal precursor acting via activin receptors induces mesoderm by maintaining a source of its convertases and BMP4. Dev. Cell. 2006;11:313–323. doi: 10.1016/j.devcel.2006.07.005. [DOI] [PubMed] [Google Scholar]