Abstract
Background:
Over the last 400 years, cocoa and chocolate have been described as having potential medicinal value, being consumed as a beverage or eaten as food. Concentration–dependant, antiproliferation, and cytotoxic effects of some of their polyphenolic constituents have been demonstrated against various cancers. Such an effect remains to be demonstrated in ovarian cancer
Objective:
To investigate the effect of cocoa procyanidins against ovarian cancer in vitro using OAW42 and OVCAR3 cell lines.
Materials and Methods:
Cocoa procyanidins were extracted and enriched from non alkalized cocoa powder. The polyphenolic content and antioxidant activity were determined. Effect on cell viability was determined after the treatment with ≤1000 μg/mL cocoa procyanidin-rich extract on OAW42 and OVCAR3 and normal human dermal fibroblasts. Similarly, chemosensitization effect was determined by pretreating cancer cell lines with extract followed by doxorubicin hydrochloride treatment. The effect of treatment on cell cycle and P-glycoprotein (P-gp) expression was determined using flow cytometry.
Results:
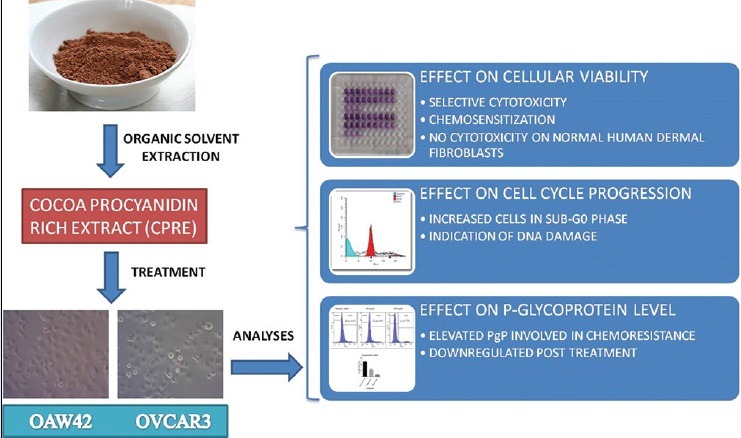
The cocoa extract showed high polyphenolic content and antioxidant activity. Treatment with extract caused cytotoxicity and chemosensitization in OAW42 and OVCAR3 cell lines. Normal dermal fibroblasts showed an increase in cell viability post treatment with extract. Treatment with extract affected the cell cycle and an increasing percentage of cells in hypodiploid sub-G1/G0 phase was observed. Treatment of OVCAR3 with the extract caused reduction of P-gp expression.
Conclusion:
Cocoa procyanidins were found to be selectively cytotoxic against epithelial ovarian cancer, interfered with the normal cell cycle and sensitized cells to subsequent chemotherapeutic treatment. Chemosensitization was found to be associated with P-gp reduction in OVCAR3 cells.
SUMMARY
Among the naturally occurring flavonoids, procyanidins have been shown to be effective against cancers
Non alkalized cocoa powder is one of the richest sources of procyanidins
Cocoa procyanidin-rich extract (CPRE) caused cytotoxicity and chemosensitization in ovarian carcinoma cell lines OAW42 and OVCAR3
CPRE affected normal cell cycle progression
CPRE also downregulated P-glycoprotein, which mediates chemoresistance in multidrug-resistant OVCAR3 cell line.
Abbreviations used: P-gp: P-glycoprotein, CPRE: Cocoa procyanidin rich extract, DMAC: 4-dimethylaminocinnamaldehyde, DPPH: Diphenylpicrylhydrazyl, ABTS: 2,2’;-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid), PI: Propidium iodide, FITC: Fluorescein isothiocyanate, MTT: 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, TLC: Thin layer chromatography, HPTLC: High-performance thin layer chromatography.
Keywords: Chemosensitization, cocoa procyanidins, epithelial ovarian cancer, P-glycoprotein
INTRODUCTION
Ovarian cancer is a common and highly aggressive gynecological malignancy in women associated with a high mortality[1,2] and low 5 years survival rates, with epithelial ovarian cancer being the leading cause.[3] Current treatment strategies include a combination of surgical removal and chemotherapy using platinum-based drugs and taxanes. However, the disease has a poor prognosis with a high chance of relapse,[4] mainly attributed to the development of acquired resistance to chemotherapeutic drugs.[5] P-glycoprotein (P-gp), a membrane protein belonging to the ABC transporter family, is an efflux pump involved in the removal of chemotherapeutic drugs out of the cell and is implicated to be the main cause of resistance to the drugs. P-gp mediated drug resistance is common among a variety of cancers and the downregulation of P-gp is being targeted as an approach to counteract the acquired resistance in cancer cells. As conventional chemotherapeutics not only pose the problem of acquired resistance but also nonselective cytotoxicity toward normal cells, use of naturally occurring compounds such as polyphenols in plants is being explored as an alternative approach in cancer treatment.[6]
Polyphenols, especially flavonoids, are a widely distributed class of secondary metabolites in plants with varied health benefits, specifically as anticancer agents.[7,8,9] Among flavonoids, procyanidins have garnered attention for their anticancer, antiageing, antihypertensive, and cardioprotective effects, owing to their high antioxidant and pro-oxidant activity.[10,11] They have also been proven to be potent P-gp inhibitors in immortalized cell lines and the blood-brain barrier.[6] Rich sources of procyanidins include many regularly consumed foods such as apples, cocoa and cocoa products, berries, and grapes.[12] Among these, cocoa (Theobroma cacao L., Sterculiaceae) and its products, such as non alkalized cocoa powder, are considered to be one of the richest sources of catechins and procyanidins.[13,14] Cocoa, native to South America, was cultivated more than 1500 years ago by the Mayas.[15] Consumption of cocoa-derived products has been shown to offer a wide range of health benefits such as cardioprotection, reduction of chronic inflammation, antihypertensive effect, and cancer prevention as evident through various human intervention and cohort studies.[16,17,18,19]
In this study, non alkalized cocoa powder was used to extract and enrich procyanidins using solvent extraction. The cocoa procyanidin-rich extract (CPRE) was screened for phytochemicals, polyphenolic content, and antioxidant activity. The effect on cell viability was evaluated in vitro using OAW42[20] and multidrug refractory OVCAR3[21] epithelial ovarian cancer cell lines. The effect of extract cotreatment with doxorubicin was determined in order to establish chemo sensitizing effect. Effect of the extract on the cell cycle was evaluated. Furthermore, the involvement of P-gp expression in the development of acquired drug resistance was assessed using flow cytometry.
MATERIALS AND METHODS
Source material
Non alkalized cocoa powder was purchased from Morde Foods Pvt. Ltd, Mumbai, India. As per the Certificate of Analysis (No. FRM/QC/026) provided by the manufacturer, the cocoa powder met the required standards of appearance, flavor, aroma, total fat (11.2%), and moisture content (2.14%), particle size (200 mesh), total (7.78%), and acid insoluble ash content (0.22%). The powder was stored in vacuum packaged polyethylene pouches at room temperature until use. All experiments were performed using only one batch of obtained material.
Chemicals and reagents
4-dimethylaminocinnamaldehyde (DMAC), diphenylpicrylhydrazyl (DPPH), 2,2’-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS), and propidium iodide (PI) were purchased from Sigma Aldrich, USA. 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was purchased from HiMedia Laboratories Pvt. Ltd., France. Gallic acid was purchased from Loba Chemie Pvt. Ltd., India, (+) catechin from Natural Remedies Pvt. Ltd, India, and procyanidin B2 from Santa Cruz Biotechnology, USA. Doxorubicin hydrochloride API was a generous gift from Veeda Clinical Research Pvt. Ltd., India. Cell culture media and reagents were purchased from Genetix Biotech Asia Pvt. Ltd., India. P-gp monoclonal antibody (clone UIC2) and IgG2a isotype control, conjugated with fluorescein isothiocyanate (FITC), were purchased from Abcam, UK. All the other analytical chemicals and solvents were purchased from Fisher Scientific, USA.
Extraction of polyphenols and enrichment of procyanidins from non alkalized cocoa powder
Non alkalized cocoa powder (25 g) was defatted in n-hexane (cocoa powder: n-hexane, 1:4, w/v) on a shaker for 1 h. The solvent was decanted, and the powder was allowed to dry overnight. Defatted material was extracted with 70% aqueous acetone using ultrasonication (3 cycles × 250 mL, 30 min per ultrasonication cycle).[22] The extracts were pooled and filtered through Whatman paper No. 1. An equal volume of ethyl acetate was added to the extract; the mixture was transferred to a separating funnel, shaken vigorously, and allowed to stand to enable separation. The lower aqueous layer was re-extracted with an equal volume of ethyl acetate. Liquid-liquid extraction with ethyl acetate[23] was repeated one more time. The upper organic layer fractions were pooled and dried to give pale brown dry powder expected to contain a high quantity of procyanidins, and the extraction yield was calculated. This powder was labeled as CPRE and stored at room temperature for further analysis.
Phytochemical screening
Qualitative phytochemical screening of CPRE was performed using standard methods.[24] The preliminary tests were carried out for the detection of carbohydrates (Molisch test), proteins (Biuret test), flavonoids (NaOH test and lead acetate test), tannins, and phenols (Ferric chloride test) and alkaloids (Dragendorrf's test, Mayer's test, and Wagner's test).
Determination of total phenol and total procyanidin content
Total phenol content of CPRE was determined using a modified folin-Ciocalteau method.[25] Gallic acid (20–100 μg/mL) in methanol was used as standard. CPRE (1 mg/mL, 1:5 v/v diluted) was used as the test sample. The final volume was kept at 1 mL. A blank containing 1 mL of methanol was maintained. About 5 mL folin-ciocalteau reagent (1:10 v/v in distilled water) was added to each tube. After 5 min, 4 mL of 7.5% sodium carbonate was added and allowed to react for 15 min at room temperature. The absorbance was measured at 760 nm using ultraviolet (UV) visible spectrophotometer (Lambda 25, Perkin Elmer, Inc., USA). The results were expressed as mg gallic acid equivalents or mg GAE/g of extract.
Total procyanidins in CPRE were determined by colorimetric reaction with DMAC.[26] Procyanidin B2 (10–60 μg/mL) in methanol was used as a standard. CPRE (1 mg/mL) was diluted 1:5 with methanol. To set up a reaction mixture, 20 μl each of various standard dilutions and extract were diluted with 2.38 mL of methanol. About 2.4 mL of methanol was taken in the blank tube. To each of the tubes, 100 μl of 2% DMAC (prepared in chilled 1:1 6N H2SO4:methanol, v/v) was added and incubated at room temperature for 15 min. The absorbance was measured at 640 nm. The results were expressed as mg procyanidin B2 equivalents or mg PB2E/g of extract.
Evaluation of antioxidant activity
The original method of Blois[27] was slightly modified and used for the determination of scavenging activity of DPPH free radical. CPRE was dissolved and diluted in methanol, ranging from 15 to 40 μg/mL. 400 μl of 0.9 mM DPPH prepared in methanol was added to each tube. A control tube containing methanol was maintained. The reaction mixture was incubated in dark at room temperature for 30 min. The absorbance was measured at 516 nm.
Radical scavenging activity of CPRE was also evaluated using the ABTS assay. The monocation of ABTS•+ is generated by oxidation of ABTS with potassium persulfate.[28] The ABTS•+ was produced by allowing 7 mM ABTS stock solution to react with 2.45 mM potassium persulfate (final concentration) in the dark for 14–16 h before use, at room temperature. The ABTS•+ solution was diluted with phosphate buffered saline (PBS, pH 7.4) to an absorbance value of 0.70 (±0.02) at 734 nm. To 20 μl of CPRE or standard diluted in methanol, 1 mL of this solution was added. Concentrations of the extract ranged from 100 to 500 μg/mL. Absorbance was measured at 734 nm.
The radical scavenging activity was calculated as follows:
% Scavenging activity = ([Absorbancecontrol − Absorbancetest)/Absorbancecontrol] × 100. Results were represented as IC50 in μg/mL.
Antioxidant activity of CPRE was determined by ferric reducing power assay, modified from the original method.[29] Various concentrations of the extract (20–100 μg/mL) in methanol were mixed with 0.2M sodium phosphate buffer and 1% potassium ferricyanide (2.5 mL each). This mixture was kept at 50°C for 20 min. About 2.5 mL of 10% trichloroacetic acid was added and centrifuged at 3000 rpm for 10 min. The upper layer of solution was mixed with distilled water, and 0.5 mL of freshly prepared 0.1% ferric chloride solution was added to it. The absorbance was measured at 700 nm. Control was prepared in similar manner wherein extract/standard was replaced with methanol. The increase in reducing power is indicated by an increase in absorbance value. The reducing power was represented as EC0.5 (effective concentration having 0.5 absorbance value) L-ascorbic acid was used as a standard for all the anti-oxidant activity assays.
Thin layer chromatography
Thin layer chromatography (TLC) was carried out on precoated silica gel F254 plates (0.2 mm, Merck, Darmstadt, Germany) as stationary phase and ethyl acetate: Glacial acetic acid: Formic acid: Methanol (7.5:0.2:0.3:1, v/v) as mobile phase. Plates were derivatized using 1% DMAC in 3M hydrochloric acid[30] that is specific for procyanidins (+) catechin was used as standard. Antioxidant bioautography[31] using 0.1% DPPH as spray reagent was carried out. High-performance TLC (HPTLC) study was carried out for confirming the presence of (+) catechin and procyanidin B2 reported to be present in cocoa powder.[13] HPTLC fingerprinting was performed on precoated silica gel F254 plates at room temperature. Solutions of standards and sample were applied to the plates using the Camag (Muttenz, Switzerland) linomat V sample applicator equipped with a 100 μl Hamilton (USA) syringe. Ascending development to a distance of 80 mm was performed using the same mobile phase as that used in aforementioned TLC, in a Camag glass twin trough chamber saturated with mobile phase vapor for 25 min. After development, the plates were dried and then scanned using a Camag TLC scanner with WINCAT software (Camag, Switzerland).
Cell culture and conditions
OAW42 and OVCAR3 cell lines were a generous gift from Dr. Sharmila Bapat from National Centre for Cell Sciences, Pune, India. OAW42 and OVCAR3 were maintained and propagated in Dulbecco's Modified Eagle's Medium and Roswell Park medium Institute 1640 and streptomycin (100 μg/mL medium) and incubated at 37°C in a humidified atmosphere containing 5% CO2.
Assessment of in vitro effect of cocoa procyanidin-rich extract on cell viability and chemosensitization
Effect of CPRE on cell viability of ovarian cancer cell lines was evaluated by MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay. OAW42 and OVCAR3 were seeded in 96 well plates at 3 × 103 cells/well and 1.5 × 104 cells/well, respectively, and incubated for 24 h. Cells were treated with 400, 600, 800, and 1000 μg/mL of CPRE in serum free media. OAW42 was treated for 24 h and OVCAR3 for 72 h. During post treatment, medium in each well was discarded and wells were washed with DPBS (Dulbecco's PBS). About 100 μl serum-free medium containing MTT (0.5 mg/mL) was added to each well. The plate was incubated at 37°C for 4 h. Media was removed, and the purple formazan product was dissolved by addition of 150 μl dimethylsulfoxide. Absorbance was recorded after 15 min at 570 nm against a reference wavelength of 655 nm.
The cell viability was calculated as follows:
% Cell viability = (absorbance of treated cells/absorbance of untreated cells) *100. IC50, i.e., the concentration toxic to 50% of the cell population was calculated. The effect of CPRE on the viability of human dermal fibroblasts was also evaluated similarly.
For evaluating chemosensitization, the cells were pretreated with suboptimal IC50 concentrations of CPRE for OAW42 and OVCAR3 for their respective effective period, followed by doxorubicin hydrochloride (1 μg/mL) treatment for 24 h. Controls were maintained for doxorubicin or extract treatment alone. Results were reported as percentage cell viability.
Cell cycle analysis
Effect of CPRE on the cell cycle was evaluated by staining of cells with PI followed by flow cytometry.[32] OAW42 and OVCAR3 were seeded in 6 well plates at a seeding density of 0.3 × 106 cells/well and 0.5 × 106 cells/well, respectively, and incubated for 24 h. Cells were treated with 600, 800, and 1000 μg/mL of CPRE for 24 h in serum free media. Doxorubicin hydrochloride was used as a positive control. Post treatment, the cells were trypsinized and fixed with cold 70% ethanol. Cells were centrifuged washed with DPBS twice to remove traces of ethanol. About 0.8 μl of DNAse-free RNAse was added to each tube and incubated at 37°C for 10 min. Staining was carried out by adding 500 μl of PI (50 μg/mL) to each tube. Flow cytometry data was acquired for 10,000 cells using BD FACSAria system and BD FACSDiva software (BD Biosciences, USA). The data were analyzed offline using ModFit LT 4.1 software, Verity Software House, USA.
Analysis of P-glycoprotein expression
For evaluating P-gp expression, OVCAR3 cells were seeded onto 6 well plates, at a density of 0.5 × 106 cells/well and incubated for 24 h. The cells were treated with 600, 800, and 1000 μg/mL of CPRE for 24 h in respective serum free media. Doxorubicin hydrochloride was used as a positive control. After incubation, the cells were washed with DPBS, trypsinized, and centrifuged at 1200 rpm for 5 min. The cells were the resuspended in DPBS containing 10% heat-inactivated FBS, and P-gp antibody (UIC2) conjugated with FITC (diluted as per manufacturer's instructions). Mouse IgG2a-FITC was used as an isotype control. After incubation with antibody for 30 min at 37°C in the dark, the cells were washed twice with DPBS containing 10% FBS, suspended in ice-cold PBS buffer, and kept on ice until analysis. Flow cytometry data was acquired for 10,000 cells using BD FACSAria system and BD FACSDiva software (BD Biosciences, USA). Nonlabeled cells were analyzed in order to detect autofluorescence. FITC fluorescence indicated cells bound to the labeled P-gp antibody and therefore, positive for P-gp expression.
Statistical analysis
All quantitative experiments were carried out in triplicate (n = 3). Results were expressed as mean ± standard deviation. Regression analysis to calculate IC50 and EC0.5 values was done using Microsoft Excel Version 2007 (Microsoft Corporation, USA). Statistical analysis was carried out using GraphPad Prism 5.0 software (GraphPad Software, Inc., USA) using one-way and two-way ANOVA. Results at P < 0.05 were considered significant.
RESULTS
Cocoa procyanidin-rich extract was found to be rich in polyphenols and possessed high anti-oxidant activity
The qualitative phytochemical screening of CPRE indicated the presence of flavonoids, such as tannins and phenols. Alkaloids were found to be absent. The percentage yield was 3.53%. The total polyphenolic content was found to be 143.04 ± 3.41 mg GAE/g of extract, and the total procyanidin content was 65.99 ± 9.12 mg PB2E/g of extract. TLC showed the presence of flavonoids characterized by quenching of short UV radiation at 254 nm [Figure 1a]. The ethyl acetate fraction showed the enriched presence of procyanidin group of compounds on derivatization with 1% DMAC reagent as highly specific green colored bands [Figure 1b] that are characteristic to procyanidin group of compounds.[26] HPTLC fingerprinting of the extract confirmed the presence of (+) catechin (Rf value −0.68) and procyanidin B2 (Rf value − 0.62) by comparison of the spectral scan of extract with those of the two standards [Figure 1c].
Figure 1.
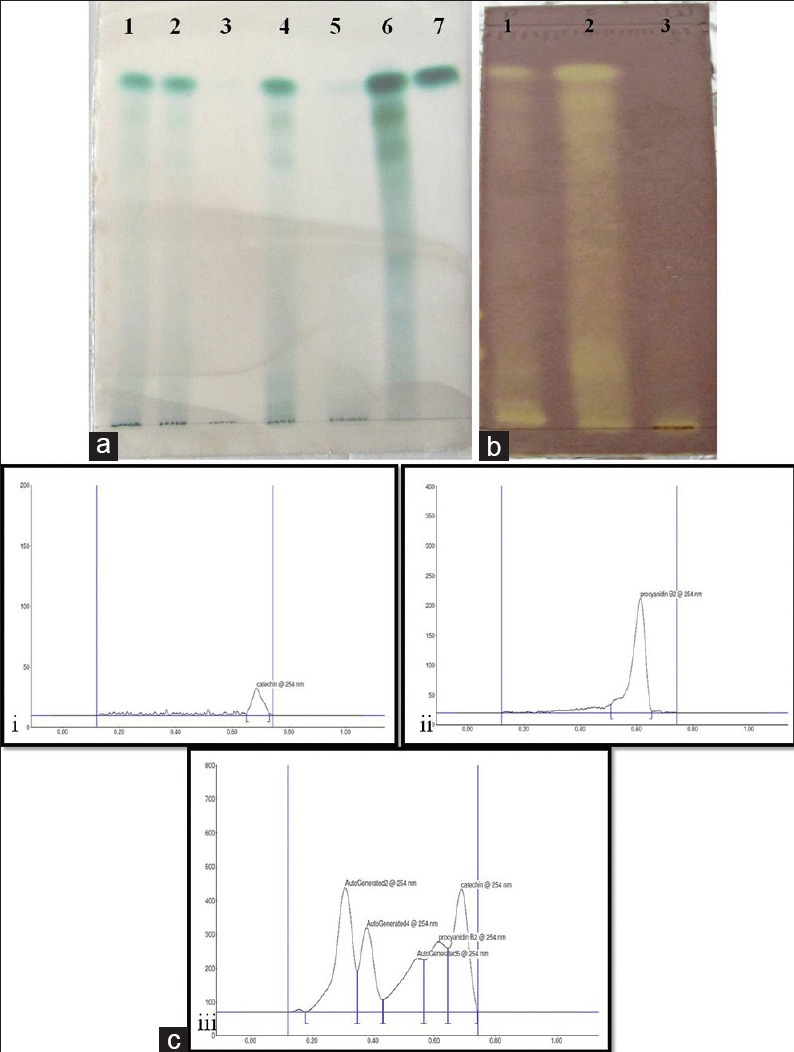
Thin layer chromatography of extract at various extraction stages. (a) Derivatized with acidified 1% 4-dimethylaminocinnamaldehyde. Lane 1: Extraction cycle 1, Lane 2: Extraction cycle 2, Lane 3: Extraction cycle 3, Lane 4: Pooled crude extract, Lane 5: Aqueous fraction, Lane 6: Ethyl acetate fraction and Lane 7: (+)-catechin. (b) Thin layer chromatography bioautography of cocoa extracts with 0.1% diphenylpicrylhydrazyl. Lane 1: Pooled extract, Lane 2: Ethyl acetate extract, Lane 3: Water fraction of extract. (c) Ultraviolet spectra analysis of standards and extracts after high-performance thin layer chromatography. (i) (+)-catechin, (ii) procyanidin B2 and (iii) ethyl acetate extract of cocoa powder
TLC autography using 0.1% DPPH showed maximum antioxidant activity in the ethyl acetate fraction as compared to the aqueous and crude extract, with antioxidant constituents appearing as yellow bands against a purple background [Figure 1b].[33] Thus, the radical scavenging activity of this fraction was evaluated using DPPH and ABTS assays, and the IC50 values were 31.75 ± 0.94 μg/mL and 294.15 ± 38.4 μg/mL, respectively. The fluorescence recovery after photobleaching EC0.5 value for the extract was 266.72 ± 14.85 μg/mL. The difference in values might be due to the different mechanisms and reaction characteristics of each assay.[34]
Cocoa procyanidin-rich extract was selectively cytotoxic to cancer cells and sensitized them to doxorubicin treatment
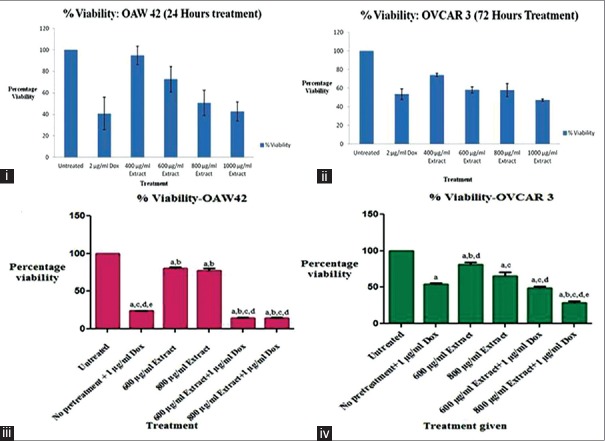
CPRE exerted cytotoxicity on OAW42 [Figure 2.i] and OVCAR3 [Figure 2.ii] posttreatment of 24 and 72 h, respectively. The IC50 obtained for OAW42 was 863.84 ± 115.04 μg/mL and for OVCAR3 was 896.84 ± 70.01 μg/mL. No cytotoxicity was observed on normal human dermal fibroblasts. On the contrary, there was an increase in cell viability, indicated by increased formation of formazan product in treated cells as compared to untreated cells (data not shown). Successive treatment of cells with CPRE followed by doxorubicin hydrochloride caused significantly higher cytotoxicity in both OAW42 and OVCAR3 as compared to treatment with either of them alone [Figure 2.iii and 2.iv], thus demonstrating chemosensitization.
Figure 2.
Percentage viability of treated (i) OAW42 and (ii) OVCAR3 cells by MTT assay. Chemosensitization assay for (iii) OAW42 and (iv) OVCAR3 represented as percentage viability versus treatment given to the cells. Statistical analysis was carried out using one-way ANOVA followed by Bonferroni's posttest. Results were considered to be significant at P< 0.05. The significance amongst treatment groups is denoted as lower case alphabets placed on top of the error bars, representing the following comparisons: (a) Treated groups versus untreated group; (b) only doxorubicin versus other treatment groups; (c) 600 μg/mL versus other treatment groups; (d) 800 μg/mL versus other treatment groups (e) 600 μg/mL versus 800 μg/mL treatment group
Appearance of hypodiploid sub-G1/G0 phase in treated cells indicated possible DNA damage and cell death
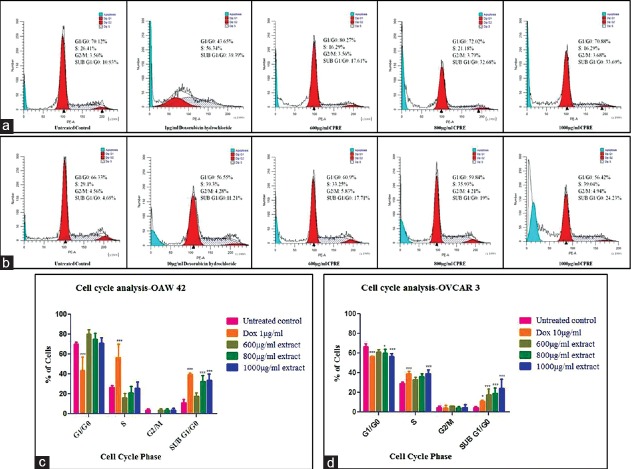
CPRE was found to interfere with normal cell cycle progression in both cell lines. Treatment of OAW42 and OVCAR3 with various concentrations of CPRE showed a significant percentage of cells in sub-G1/G0 (hypodiploid) phase, which increased with increasing concentration. Significant accumulation of cells in the S phase was seen in OVCAR3 cells treated with 1000 μg/mL of CPRE. Doxorubicin hydrochloride, used as positive control, showed a significant percentage of cells arrested in S-phase and in sub-G1/G0 phase [Figure 3].
Figure 3.
Cell cycle distribution represented as histograms for (a) OAW42 (b) OVCAR3. (c and d) graphical representation of statistical significance within each phase for OAW42 and OVCAR3, respectively. Statistical analysis was carried out using two-way ANOVA followed by Bonferroni's posttest at significance *P < 0.05 for treated cells as compared to untreated control within each phase
Reduced P-glycoprotein expression was observed posttreatment with cocoa procyanidin-rich extract
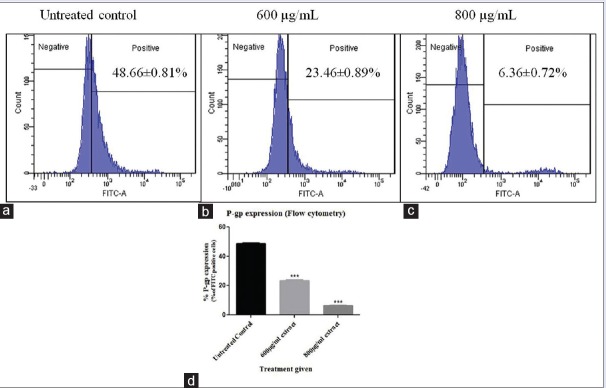
Effect of CPRE on P-gp expression was evaluated in OVCAR3 cells only, as OAW42 cells did not show initial P-gp expression (data not shown). P-gp expression was found to decrease post treatment with CPRE, as compared to untreated control and the results were statistically significant [Figure 4]. No nonspecific binding of the isotype control was observed (data not shown), indicating that the labeled P-gp antibody bound specifically to P-gp present on the cell membrane.
Figure 4.
P-glycoprotein expressing (fluorescein isothiocyanate positive) OVCAR3 cells represented as histograms for (a) untreated cells (b) 600 μg/mL extract treatment (c) 800 μg/mL extract treatment and (d) graphical representation of statistical significance. Statistical analysis was carried out using one-way ANOVA followed by Dunnett's posttest, and results were significant at P < 0.05 as compared to the untreated control
DISCUSSION
Cocoa and its products have been consumed since the Mesoamerican civilization, later spreading to Spain and Christian European Countries.[35] Over the last 400 years, many medicinal uses have been revealed for cocoa or chocolate.[36] Cocoa polyphenols are associated with many medicinal properties such as antiangiogenic,[37] cardioprotective,[38] cosmetic,[39] anti-inflammatory,[40] and anticancer.[41] Dutching or alkalization of naturally obtained cocoa powder leads to a loss in the polyphenol content.[42] Therefore, non alkalized cocoa powder was used as source material to successfully extract and enrich procyanidin group of compounds, which are polymeric condensation products of catechins (flavan-3-ols). The resultant extract possessed high polyphenol content, enriched with (+)-catechin, procyanidin B2, and other related compounds. It also possessed efficient antioxidant activity. Several factors such as the choice of solvent, method of extraction influence the extraction yield, and polyphenol content.[43] Moreover, cocoa powder from different sources may differ in polyphenol content due to cultivar, conditions of growth, sampling, and analytical procedures.[14,44]
The effect of CPRE on cell viability was evaluated in vitro on OAW42 and OVCAR3 cell lines using the MTT assay. Reduced cellular viability was observed in both cell lines and was measured in terms of IC50 value. CPRE was found to be differentially cytotoxic to OAW42 (IC50 = 863.84 ± 115.04 μg/mL, 24 h treatment) and OVCAR3 (IC50 = 896.84 ± 70.01 μg/mL, 72 h treatment). OVCAR3 not only has a higher doubling time than OAW42 but is also drug resistant.[45,46] This difference in duration of treatment could be attributed to the fact that OAW42 divides more rapidly than OVCAR3. Previously, procyanidins from cranberry have been shown to be more cytotoxic to rapidly dividing cells.[47] CPRE treatment was found to be nontoxic to normal human dermal fibroblasts. Conversely, it caused an increase in viability of human dermal fibroblasts by virtue of the antioxidant properties of polyphenolic constituents.[48] Treatment with CPRE demonstrated selective cytotoxicity of the extract toward cancer cells, leaving the normal cells unharmed.
Targeting the cell cycle is one of the approaches in cancer therapy.[49] CPRE treatment on both, OAW42 and OVCAR3, interfered with the normal cell cycle progression. An increasing trend was observed in the appearance of the hypodiploid sub-G1/G0 phase, with increasing CPRE concentration, indicative of DNA damage and cell death.[32]
Pretreatment of OAW42 and OVCAR3 with CPRE augmented the cytotoxic effect of subsequent doxorubicin hydrochloride treatment. A previous report has similarly demonstrated chemosensitizing effect of procyanidins on another ovarian cancer cell line, SKOV3.[50] OVCAR3 is a multidrug-resistant cell line having acquired resistance to clinically relevant concentrations of doxorubicin, cisplatin, and melphalan.[21] Acquired multidrug-resistance, though governed by a variety of cellular mechanisms,[51] is largely linked to the over-expression of P-gp. OVCAR3 cells treated with extract showed reduced P-gp levels as compared to those of untreated cells, indicative of lowered resistance and subsequent chemosensitization of cancer cells.[6]
CONCLUSION
The study demonstrates cytotoxic effect of procyanidin group of compounds enriched from non alkalized or natural cocoa powder on ovarian cancer cell lines OAW42 and OVCAR3. This is the first report of its kind, to the best of our knowledge. Procyanidins were successfully extracted and enriched from natural cocoa powder. The CPRE or CPRE demonstrated good anti-oxidant activity, which is one of the factors that contributes to the toxicity of polyphenols against cancer.[52] The extract was found to be selectively cytotoxic to ovarian cancer cells and sensitized them to doxorubicin. Chemosensitization of the cells was accompanied by decrease in P-gp levels in OVCAR3 cells treated with the extract. Both OAW42 and OVCAR3 cells showed a dose-dependant increase of cell population in the hypodiploid sub-G0 phase on treatment with CPRE, indicating irreversible DNA damage which may have contributed to its cytotoxic effect. CPRE has shown therapeutic potential against ovarian cancer in vitro, by interfering with the regular cell cycle progression and overcoming chemoresistance conferred by P-gp. Efforts to elucidate the mechanism by which CPRE exerts toxicity and to identify of the molecular components involved are currently underway.
Financial support and sponsorship
Ms. Shruti Taparia is a recipient of the Senior Research Fellowship from the Council of Scientific and Industrial Research (CSIR), India under the Grant sanction no. 09/1083(0001)-2012-EMR-I.
Conflicts of interest
There are no conflicts of interest.
ABOUT AUTHOR

Aparna Khanna
Aparna Khanna obtained her Ph.D. degree in 1995 from Jawaharlal Nehru University, New Delhi, while working at the National Institute of Immunology. Dr. Khanna was a Wellcome Trust Postdoctoral fellow (1995–1996) at the University of Glasgow, UK. She currently holds the position of Dean at the Sunandan Divatia School of Science, NMIMS (deemed to be) University, Mumbai, India. Dr. Khanna has been working in the area of stem cells since the year 2003, with expertise in establishment and characterization of cell cultures in vitro. Currently, she is involved in projects related to stem cell imaging and differentiation as well as evaluation of phytoextracts for hepatoprotective and anticancer activity, using cell lines as a model system.
Acknowledgments
All flow cytometry analyses were performed at Sophisticated Analytical Instrument Facility, Indian Institute of Technology, Mumbai, India.
REFERENCES
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–86. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 3.Chen SS, Michael A, Butler-Manuel SA. Advances in the treatment of ovarian cancer: A potential role of antiinflammatory phytochemicals. Discov Med. 2012;13:7–17. [PubMed] [Google Scholar]
- 4.Agarwal R, Kaye SB. Prognostic factors in ovarian cancer: How close are we to a complete picture? Ann Oncol. 2005;16:4–6. doi: 10.1093/annonc/mdi104. [DOI] [PubMed] [Google Scholar]
- 5.Fehrmann RS, Li XY, van der Zee AG, de Jong S, Te Meerman GJ, de Vries EG, et al. Profiling studies in ovarian cancer: A review. Oncologist. 2007;12:960–6. doi: 10.1634/theoncologist.12-8-960. [DOI] [PubMed] [Google Scholar]
- 6.Abdallah HM, Al-Abd AM, El-Dine RS, El-Halawany AM. P-glycoprotein inhibitors of natural origin as potential tumor chemo-sensitizers: A review. J Adv Res. 2015;6:45–62. doi: 10.1016/j.jare.2014.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lewandowska U, Gorlach S, Owczarek K, Hrabec E, Szewczyk K. Synergistic interactions between anticancer chemotherapeutics and phenolic compounds and anticancer synergy between polyphenols. Postepy Hig Med Dosw (Online) 2014;68:528–40. doi: 10.5604/17322693.1102278. [DOI] [PubMed] [Google Scholar]
- 8.Peng L, Khan N, Afaq F, Ye C, Mukhtar H. In vitro and in vivo effects of water extract of white cocoa tea (Camellia ptilophylla) against human prostate cancer. Pharm Res. 2010;27:1128–37. doi: 10.1007/s11095-010-0052-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Somasagara RR, Hegde M, Chiruvella KK, Musini A, Choudhary B, Raghavan SC. Extracts of strawberry fruits induce intrinsic pathway of apoptosis in breast cancer cells and inhibits tumor progression in mice. PLoS One. 2012;7:e47021. doi: 10.1371/journal.pone.0047021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beecher GR. Proanthocyanidins: Biological activities associated with human health. Pharm Biol. 2004;42 Suppl 1:2–20. [Google Scholar]
- 11.Dai J, Mumper RJ. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules. 2010;15:7313–52. doi: 10.3390/molecules15107313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gu L, Kelm MA, Hammerstone JF, Beecher G, Holden J, Haytowitz D, et al. Concentrations of proanthocyanidins in common foods and estimations of normal consumption. J Nutr. 2004;134:613–7. doi: 10.1093/jn/134.3.613. [DOI] [PubMed] [Google Scholar]
- 13.Gu L, House SE, Wu X, Ou B, Prior RL. Procyanidin and catechin contents and antioxidant capacity of cocoa and chocolate products. J Agric Food Chem. 2006;54:4057–61. doi: 10.1021/jf060360r. [DOI] [PubMed] [Google Scholar]
- 14.Oracz J, Zyzelewicz D, Nebesny E. The content of polyphenolic compounds in cocoa beans (Theobroma cacao L.), depending on variety, growing region, and processing operations: A review. Crit Rev Food Sci Nutr. 2015;55:1176–92. doi: 10.1080/10408398.2012.686934. [DOI] [PubMed] [Google Scholar]
- 15.Motamayor JC, Risterucci AM, Lopez PA, Ortiz CF, Moreno A, Lanaud C. Cacao domestication I: The origin of the cacao cultivated by the Mayas. Heredity (Edinb) 2002;89:380–6. doi: 10.1038/sj.hdy.6800156. [DOI] [PubMed] [Google Scholar]
- 16.Andújar I, Recio MC, Giner RM, Ríos JL. Cocoa polyphenols and their potential benefits for human health. Oxid Med Cell Longev. 2012;2012:906252. doi: 10.1155/2012/906252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bayard V, Chamorro F, Motta J, Hollenberg NK. Does flavanol intake influence mortality from nitric oxide-dependent processes. Ischemic heart disease, stroke, diabetes mellitus, and cancer in Panama? Int J Med Sci. 2007;4:53–8. doi: 10.7150/ijms.4.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maskarinec G. Cancer protective properties of cocoa: A review of the epidemiologic evidence. Nutr Cancer. 2009;61:573–9. doi: 10.1080/01635580902825662. [DOI] [PubMed] [Google Scholar]
- 19.Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic Biol Med. 2010;49:1603–16. doi: 10.1016/j.freeradbiomed.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilson AP. Characterization of a cell line derived from the ascites of a patient with papillary serous cystadenocarcinoma of the ovary. J Natl Cancer Inst. 1984;72:513–21. [PubMed] [Google Scholar]
- 21.Hamilton TC, Young RC, McKoy WM, Grotzinger KR, Green JA, Chu EW, et al. Characterization of a human ovarian carcinoma cell line (NIH:OVCAR-3) with androgen and estrogen receptors. Cancer Res. 1983;43:5379–89. [PubMed] [Google Scholar]
- 22.Vladimir-Kneževic S, Blažekovic B, Štefan MB, Alegro A, Koszegi T, Petrik J. Antioxidant activities and polyphenolic contents of three selected Micromeria species from Croatia. Molecules. 2011;16:1454–70. doi: 10.3390/molecules16021454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lorrain B, Ky I, Pechamat L, Teissedre PL. Evolution of analysis of polyhenols from grapes, wines, and extracts. Molecules. 2013;18:1076–100. doi: 10.3390/molecules18011076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khandelwal KR. Practical Pharmacognosy. 12th ed. Pune: Nirali Prakashan; 2004. [Google Scholar]
- 25.Singleton VL, Rossi JA. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic. 1965;16:144–58. [Google Scholar]
- 26.Wallace TC, Giusti MM. Evaluation of parameters that affect the 4-dimethylaminocinnamaldehyde assay for flavanols and proanthocyanidins. J Food Sci. 2010;75:C619–25. doi: 10.1111/j.1750-3841.2010.01734.x. [DOI] [PubMed] [Google Scholar]
- 27.Blois MS. Antioxidant determinations by the use of a stable free radical. Nature. 1958;181:1199–200. [Google Scholar]
- 28.Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999;26:1231–7. doi: 10.1016/s0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 29.Oyaizu M. Studies on products of browning reactions: Antioxidative activities of products of browning reaction prepared from glucosamine. Jpn J Nutr. 1986;44:307–15. [Google Scholar]
- 30.Glavnik V, Simonovska B, Vovk I. Densitometric determination of (+)-catechin and (-)-epicatechin by 4-dimethylaminocinnamaldehyde reagent. J Chromatogr A. 2009;1216:4485–91. doi: 10.1016/j.chroma.2009.03.026. [DOI] [PubMed] [Google Scholar]
- 31.Marston A. Thin-layer chromatography with biological detection in phytochemistry. J Chromatogr A. 2011;1218:2676–83. doi: 10.1016/j.chroma.2010.12.068. [DOI] [PubMed] [Google Scholar]
- 32.Riccardi C, Nicoletti I. Analysis of apoptosis by propidium iodide staining and flow cytometry. Nat Protoc. 2006;1:1458–61. doi: 10.1038/nprot.2006.238. [DOI] [PubMed] [Google Scholar]
- 33.Wang J, Yue YD, Tang F, Sun J. TLC screening for antioxidant activity of extracts from fifteen bamboo species and identification of antioxidant flavone glycosides from leaves of Bambusa. textilis McClure. Molecules. 2012;17:12297–311. doi: 10.3390/molecules171012297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grace-Lynn C, Darah I, Chen Y, Latha LY, Jothy SL, Sasidharan S. In vitro antioxidant activity potential of lantadene A, a pentacyclic triterpenoid of Lantana plants. Molecules. 2012;17:11185–98. doi: 10.3390/molecules170911185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lippi D. Chocolate in history: Food, medicine, medi-food. Nutrients. 2013;5:1573–84. doi: 10.3390/nu5051573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rusconi M, Conti A. Theobroma cacao L. the food of the gods: A scientific approach beyond myths and claims. Pharmacol Res. 2010;61:5–13. doi: 10.1016/j.phrs.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 37.Li WW, Li VW, Hutnik M, Chiou AS. Tumor angiogenesis as a target for dietary cancer prevention. J Oncol. 2012;2012:879623. doi: 10.1155/2012/879623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mehrinfar R, Frishman WH. Flavanol-rich cocoa: A cardioprotective nutraceutical. Cardiol Rev. 2008;16:109–15. doi: 10.1097/CRD.0b013e31815d95e2. [DOI] [PubMed] [Google Scholar]
- 39.Karim AA, Azlan A, Ismail A, Hashim P, Abd Gani SS, Zainudin BH, et al. Phenolic composition, antioxidant, anti-wrinkles and tyrosinase inhibitory activities of cocoa pod extract. BMC Complement Altern Med. 2014;14:381. doi: 10.1186/1472-6882-14-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Selmi C, Cocchi CA, Lanfredini M, Keen CL, Gershwin ME. Chocolate at heart: The anti-inflammatory impact of cocoa flavanols. Mol Nutr Food Res. 2008;52:1340–8. doi: 10.1002/mnfr.200700435. [DOI] [PubMed] [Google Scholar]
- 41.Krstic M, Stojadinovic M, Smiljanic K, Stanic-Vucinic D, Cirkovic Velickovic T. The anti-cancer activity of green tea, coffee and cocoa extracts on human cervical adenocarcinoma HeLa cells depends on both pro-oxidant and anti-proliferative activities of polyphenols. RSC Adv. 2015;5:3260–8. [Google Scholar]
- 42.Payne MJ, Hurst WJ, Miller KB, Rank C, Stuart DA. Impact of fermentation, drying, roasting, and Dutch processing on epicatechin and catechin content of cacao beans and cocoa ingredients. J Agric Food Chem. 2010;58:10518–27. doi: 10.1021/jf102391q. [DOI] [PubMed] [Google Scholar]
- 43.Khoddami A, Wilkes MA, Roberts TH. Techniques for analysis of plant phenolic compounds. Molecules. 2013;18:2328–75. doi: 10.3390/molecules18022328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ali F, Ranneh Y, Ismail A, Esa NM. Identification of phenolic compounds in polyphenols-rich extract of Malaysian cocoa powder using the HPLC-UV-ESI-MS/MS and probing their antioxidant properties. J Food Sci Technol. 2015;52:2103–11. doi: 10.1007/s13197-013-1187-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim M, Wu X, Song I, Fu M, Chang SH, Lisanti MP, et al. Selective cytotoxicity of synthesized procyanidin 3-O-galloylepicatechin-4b, 8-3-O-galloylcatechin to human cancer cells. Cell Cycle. 2008;7:1648–57. doi: 10.4161/cc.7.11.5980. [DOI] [PubMed] [Google Scholar]
- 46.Roberts D, Schick J, Conway S, Biade S, Laub PB, Stevenson JP, et al. Identification of genes associated with platinum drug sensitivity and resistance in human ovarian cancer cells. Br J Cancer. 2005;92:1149–58. doi: 10.1038/sj.bjc.6602447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Macedo NJ, Neto CC, Liberty AM, Ferreira TL. Zebrafish as an in vivo screen for early black cranberry proanthocyanidin biomolecular activity. Am J Mol Biol. 2014;4:37–48. [Google Scholar]
- 48.Giampieri F, Alvarez-Suarez JM, Mazzoni L, Forbes-Hernandez TY, Gasparrini M, Gonzàlez-Paramàs AM, et al. Polyphenol-rich strawberry extract protects human dermal fibroblasts against hydrogen peroxide oxidative damage and improves mitochondrial functionality. Molecules. 2014;19:7798–816. doi: 10.3390/molecules19067798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schwartz GK, Shah MA. Targeting the cell cycle: A new approach to cancer therapy. J Clin Oncol. 2005;23:9408–21. doi: 10.1200/JCO.2005.01.5594. [DOI] [PubMed] [Google Scholar]
- 50.Singh AP, Singh RK, Kim KK, Satyan KS, Nussbaum R, Torres M, et al. Cranberry proanthocyanidins are cytotoxic to human cancer cells and sensitize platinum-resistant ovarian cancer cells to paraplatin. Phytother Res. 2009;23:1066–74. doi: 10.1002/ptr.2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Januchowski R, Wojtowicz K, Sujka-Kordowska P, Andrzejewska M, Zabel M. MDR gene expression analysis of six drug-resistant ovarian cancer cell lines. Biomed Res Int. 2013;2013:241763. doi: 10.1155/2013/241763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Martin MA, Goya L, Ramos S. Potential for preventive effects of cocoa and cocoa polyphenols in cancer. Food Chem Toxicol. 2013;56:336–51. doi: 10.1016/j.fct.2013.02.020. [DOI] [PubMed] [Google Scholar]