Abstract
Background:
Alcohol addiction is a social problem leading to both loss of health and economic prosperity among addicted individuals. Common properties of anti-addictive compounds include anti-anxiety, anticonvulsants, anti-depressant, and nootropic actions primarily through modulation of gamma-aminobutyric acid (GABA) and serotonergic systems.
Objective:
Here, we screen ashwagandha and shilajit known ethnopharmacologically as nervine tonic and adaptogenic herbs for possible anti-addictive potential.
Materials and Methods:
Effect of ashwagandha churna and shilajit was measured on ethanol withdrawal anxiety using elevated plus maze. Role of ashwagandha and shilajit on chronic ethanol consumption (21 days) was measured using two bottle choice protocol of voluntary drinking. We also measured the effect of the above herbs on corticohippocampal GABA, dopamine, and serotonin levels.
Results:
Both ashwagandha and shilajit were found to reduce alcohol withdrawal anxiety in a dose-dependent manner. These herbs alone or in combination also decreased ethanol intake and increased water intake significantly after 21 days of chronic administration. Chronic administration of ashwagandha was found to significantly increase GABA and serotonin levels whereas shilajit altered cortico-hippocampal dopamine in mice.
Conclusion:
These central nervous system active herbs alone or in combination reduced both alcohol dependence and withdrawal thus showing promising anti-addictive potential.
SUMMARY
Withinia Somnifera alone and in combination with Shilajeet prevented ethanol withdrawal and alcohol addiction
Abbreviations used: GABA: Gama aminobutyric acid, CNS: Central Nervous System, CPP:Condition place preference, DA: Dopamine, 5-HT: 5-hydroxytryptamine, NMDA:N-methyl-D-aspartate
Keywords: Addiction, alcohol, ashwagandha, mice, shilajit, withdrawal
INTRODUCTION
Alcohol addiction is a worldwide problem. In 2012, 3.3 million deaths have been reported due to alcohol consumption and is the fifth leading risk factor for premature death and disability. Alcohol contributes to over 200 diseases and injury-related health conditions, most notably alcohol dependence, liver cirrhosis, cancers, and injuries.[1,2]
Alcohol dependence is a complex and dynamic process. Many neurobiological and environmental factors influence drinking habits.[3] An individual's tendency to imbibe to alcohol is primarily a balance between alcohol's rewarding effects and its negative consequences like withdrawal symptoms. Association of these good/bad feelings with environmental clues may influence alcohol intake.[4] Alcohol withdrawal symptoms include emotional changes such as irritability, agitation, anxiety, sleep disturbances, and reduced pain threshold. These symptoms have also been observed in animals using various models of dependence.[5] To date, three medications-disulfiram, naltrexone, and acamprosate have been approved by the U.S. Food and Drug Administration to treat alcohol dependence. However, they have various side effects such as palpitation, flushing, nausea, vomiting, and headache is common following disulfiram therapy whereas nausea followed by headache, anxiety, and sedation on naltrexone treatment and transient diarrhea after acamprosate administration.
The use of herbal medicines worldwide is an excellent opportunity for India to look for therapeutic lead compounds from our ancient systems of medicines including Ayurveda. It can be used for the development of new therapeutically active compounds. Over 50% of all modern drugs have natural origin and play an important role in drug development.[6] Ashwagandha (Withania somnifera, family. Solanaceae) is commonly known as “Indian Winter cherry” or “Indian Ginseng.” Among its various physiological actions, its action on central nervous system (CNS) primarily involves its effect against cognitive impairment, anxiety, and depression[7,8,9] due to its anti-oxidant,[10] gamma-aminobutyric acid (GABA) mimetic,[11] and dopaminergic actions.[12] Shilajit is a blackish-brown powder or an exudate from high mountain rocks, especially found in the Himalayan Mountains between India and Nepal. Shilajit has been known and used for centuries in Ayurvedic medicine, as a rejuvenator and as anti-aging compound.[13,14] Considering its unique composition as a phytocomplex, very rich in fulvic acid, researchers hypothesize that Shilajit is produced by the decomposition of plant material from species such as Euphorbia royleana and Trifolium repens.[15,16] Various pharmacological properties for shilajit including anti-inflammatory[17] anti-oxidant,[18] immunomodulatory,[17] aphrodisiac[18] are well documented. In the CNS, it primarily acts as a memory enhancer,[19] neuroprotective,[19] and anxiolytic[20] agent. Shilajit has also been shown to have parasympathetic and GABA-mimetic actions.[21,22] Ashwagandha and a polyherbal formulation NR-ANX-C containing ashwagandha and shilajit may prevent alcohol-induced withdrawal anxiety in rats.[23,24]
Here, we report that acute administration of ashwagandha, shilajit, and their combination may prevent alcohol withdrawal anxiety whereas their chronic administration (15 days) may reduce alcohol consumption in mice. Chronic administration of these herbal extracts may also alter cortical GABA, 5-HT, and dopamine levels, which may be responsible for their anti-addictive properties.
MATERIALS AND METHODS
Animals
Swiss albino mice (20–30 g) were used in this study. Animals were issued from the Institutional Animal House (Reg. No. 621/02/AC/CPCSEA) of Birla Institute of Technology, Mesra. All animals were kept in polyacrylic cages and maintained under standard conditions (room temperature 24–27°C and humidity 60–65% with 12:12 light:dark cycles). The food was provided in the form of dry pellets and water ad libitum. The animals were allowed to get acclimatized to the laboratory conditions for 7 days before the commencement of the experiment. All experiments involving animals complied with the ethical standards of animal handling and approved by the Institutional Animal Ethics Committee (BIT/PH/IAEC/28/2013).
Estimation of blood alcohol levels
Blood was collected by retro-orbital bleeding with animals in light ether anesthesia after 20 min of ethanol administration. Ethanol levels were measured using ultraviolet (UV) assay kit for alcohol estimation based on manufacturer's protocol (NZY Tech Genes and Enzymes Portugal).
Development of conditioned place preference model
Apparatus
The conditioned place preference (CPP) apparatus contain three compartments. The two end compartments (30.5 cm × 26.5 cm × 37 cm) were connected by a central corridor (12.75 cm × 23 cm × 15.25 cm). The compartment on the left had black walls with a perforated stainless steel floor with round holes on staggered centers. The central corridor was transparent with a smooth plexiglass floor, and the right compartment had white walls with a stainless steel mesh floor.
CPP was performed as described[25,26] with slight modifications. It mainly consists of three phases:
Preconditioning phase: (1st–2nd day) The animals were placed in the middle chamber and allowed to explore both the chambers for 30 min
Conditioning phase: (3rd–10th day) Each mouse was treated for eight consecutive sessions with alternate oral administration of alcohol and saline. On day 3, 5, 7, and 9, the animals were administered ethanol (2 g/kg b.w.; i.p. 10% [v/v]) and placed in one compartment for 30 min. In addition, on day 4, 6, 8, and 10, the animals were administered saline and placed in opposite compartment
Postconditioning phase: (11th–12th day) Mice were placed in the middle chamber and allowed free access to both chamber for 30 min. Time spent in ethanol and saline-paired chamber was measured
-
Treatment protocol: After development of withdrawal (15th day), the following treatment schedule was followed:
- Group 1: Saline
- Group 2: Ethanol (2 g/kg, i.p. 10% v/v Bengal Chemical, Kolkata)
- Group 3: Ethanol (2 g/kg, i.p. 10% v/v) till day 10 followed by ashwagandha churna (50 mg/kg, b.w., oral in 2% Tween 80; Withania somnifera root extracts; Vyas Pharmaceuticals, Haridwar) on the 15th day
- Group 4: Ethanol (2 g/kg, i.p. 10% v/v) till day 10 followed by ashwagandha churna (100 mg/kg, oral in 2% Tween 80) on the 15th day
- Group 5: Ethanol (2 g/kg, i.p. 10% v/v) till day 10 followed by ashwagandha churna (200 mg/kg, oral in 2% Tween 80) on the 15th day
- Group 6: Ethanol (2 g/kg, i.p. 10% v/v) till day 10 followed by ashwagandha churna (500 mg/kg, oral in 2% Tween 80) on the 15th day
- Group 7: Ethanol (2 g/kg, i.p. 10% v/v) till day 10 followed by shilajit (10 mg/kg, oral in 2% Tween 80; Shuddha Shilajit, Baidyanath, Kolkata) on the 15th day
- Group 8: Ethanol (2 g/kg, i.p. 10% v/v) till day 10 followed by shilajit (25 mg/kg, oral in 2% Tween 80) on the 15th day
- Group 9: Ethanol (2 g/kg, i.p. 10% v/v) till day 10 followed by shilajit (50 mg/kg, oral in 2% Tween 80) on the 15th day
- Group 10: Ethanol (2 g/kg, i.p. 10% v/v) till day 10 followed by ashwagandha churna (500 mg/kg, oral) + shilajit (25 mg/kg, oral) on the 15th day
- Group 11: Ethanol (2 g/kg, i.p. 10% v/v) till day 10 followed by diazepam (1 mg/kg, i.p.) on the 15th day.
60 min after oral drug administration and 30 min after intraperitoneal administration, the behavioral tests were performed.
Behavioral studies to measure alcohol withdrawal anxiety
Elevated plus maze
Model has been validated pharmacologically and currently considered the “gold standard” test of anxiety-related behavior. Elevated plus maze (EPM) was performed as described by Kokare et al.[27] In summary, after drug treatment, individual mice was placed at the center of the maze, head facing an open arm. During the 5 min test period, the number of entries and time spent on the open arm were recorded automatically (Medicraft Electromedicals, Lucknow, India).
Chronic-treatment study to measure alcohol intake
Two-bottle choice ethanol drinking
We used the standard two-bottle choice protocol, which is a widely used animal model to capture aspects of voluntary alcohol consumption in humans.[28] Following 7 days of acclimatization, animals were subjected to an ethanol drinking acquisition regimen. The animals remained in their home cages at all times throughout the study but had their water bottles removed during a 4 h and ethanol presentation period. During this period, animals were exposed to a free choice between ethanol (15% v/v) and water for 20 days but with no drug pretreatment.
After 20 days of ethanol administration, animals were divided into different groups for 10 days of treatment. Each day, the bottles were weighed before and after 4 h of limited access period and the differences were used to calculate the water and ethanol intake. The mean intake was expressed as g/kg body weight/day of water and g/kg body weight/day of ethanol intake. All animals were given unrestricted food access. Every 2 days, the bottles were switched to eliminate place preference.[29,30]
After 20 days of pretreatment with ethanol (15% v/v), the animals were divided into different treatment groups (n = 7/group) as follows:
Group 1: (Control) received only water
Group 2: (Positive control) received free choice ethanol (15%v/v)/water
Group 3: Received free choice ethanol (15%v/v)/water and ashwagandha (500 mg/kg oral) 21st–30th day
Group 4: Received free choice ethanol (15%v/v)/water and shilajit (25 mg/kg oral) 21st–30th day
Group 5: Received free choice ethanol (15%v/v)/water and combination (ashwagandha 500 mg/kg, oral + shilajit 25 mg/kg oral) 21st–30th day
Group 6: Received free choice ethanol (15%v/v)/water and diazepam (10 mg/kg oral) 21st–30th day.
After the above experimental protocol of 30 days, 4 animals/group were sacrificed under ether anesthesia by cervical dislocation for biochemical estimation.
Estimation of gamma-aminobutyric acid levels from brain tissue
Brain tissue was homogenized in 5 ml of 0.01 M HCl. In this homogenate, 8 ml of ice cold ethanol was added and kept for 1 h at 0°C. The contents were centrifuged for 10 min at 16,000 rpm and supernatant was collected in a Petridish. The precipitate was washed 3 times with 5 ml of 75% ethanol. The washes were combined with supernatant and evaporated to dryness. To the dry mass 1 ml water and 2 ml chloroform were added and centrifuged at 2000 rpm. Upper phase containing GABA was separated, and 10 μl of it was applied as spot on Whatman filter paper. The mobile phase consisted of n-butanol, acetic acid, water in 4:1:5 ratios. The chamber was saturated for half an hour with mobile phase. The paper chromatogram was developed with ascending technique. The paper was dried in a hot air oven and then sprayed with 0.5% ninhydrin solution in 95% ethanol. The paper was dried. Blue color spot developed on paper which was cut and heated with 2 ml ninhydrin solution on water bath at 60–65°C. Water was added to the solution and kept for 1 h and supernatant was used. Absorbance was measured at 570 nm on UV-visible spectrophotometer.
Determination of DA and 5-HT
Corticolimbic slices were homogenized in 600 μL of ice cold solution of 0.4 M perchloric acid containing 0.4 mM sodium metabisulfite and disodium ethylenediaminetetraacetic acid (EDTA), and centrifuged at 5000 g for 20 min at 4°C; supernatants were filtered through 0.45 μm cellulose membranes. The monoamine content (DA, 5-HT) of the corticolimbic region was simultaneously detected. The mobile phase consisted of 6.74 g citric acid, 4.81 g sodium citrate, 47 mg EDTA, 200 mg heptasulfonic acid, 1.15 ml glacial acetic acid, 3 ml tetrahydrofuran, and 25 ml high-performance liquid chromatography (HPLC)-grade methanol. It was made up to 1 liter with HPLC-grade water and then brought to pH 4.9 using 10 mol/l NaOH. The HPLC system (Waters HPLC systems, Milford, MA, USA) equipped with electrochemical detector was used. The flow rate was set at 1 ml/min and temperature at 35°C.[31,32,33]
RESULTS
Conditioned place preference
In CPP test, the animal's choice to spend more time in either environment provides a direct measure of the conditioned reinforcing effect of a drug. In our study, on day 11, control group (saline-treated animals) showed little increase in the preference for the ethanol-paired chamber as compared to the saline-paired chamber. However, ethanol-treated animals showed a significant (P < 0.001, n = 7) increase in preference for the ethanol-paired chamber as compared to the saline-paired chamber. A similar preference was also observed on day 12 [Figure 1]. Ethanol levels in the blood samples were found to be 45 mg/dl (n = 5).
Figure 1.
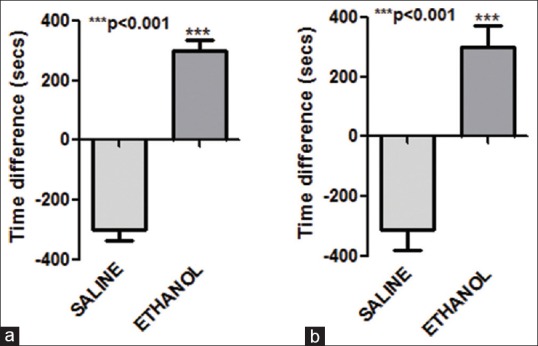
Conditioned place preference on ethanol administration. Time spent in alcohol and saline administration chamber on (a) day 11 (b) day 12 by control and ethanol-treated group. On both days, ethanol-treated animals showed significant increase in (P < 0.001) time spent in ethanol-paired alcohol compared to saline-paired chamber. Values represent mean ± standard error of the mean n = 7
Effect of ashwagandha on withdrawal anxiety
Ethanol consumption lead to increase in time spent in open arm of EPM in mice due to anxiolytic action of ethanol (data not shown).[34] Five days postabstinence animals showed significant (P < 0.001; n = 7) decrease in time spent in the open arm of EPM as compared to animals on ethanol suggesting withdrawal anxiety. Ashwagandha led to significant increase in time spent in the open arm in a dose-dependent manner (50 mg/kg P < 0.01, 100 mg/kg P < 0.001, 200 mg/kg P < 0.001, and 500 mg/kg P < 0.001, n = 7/dose) thus suggesting reversal of ethanol withdrawal anxiety. Moreover, 200 mg/kg and 500 mg/kg treatment with ashwagandha showed a significant increase in time spend in open arm (P < 0.01 and P < 0.001, respectively; n = 7) compared to animals on ethanol suggesting intrinsic and more potent anxiolytic action of ashwagandha over ethanol. At higher doses of 200 and 500 mg/kg, ashwagandha also increased (P < 0.001; n = 7) the number of open arm entries over 5 days ethanol abstinent animals, thus confirming its action against ethanol-abstinent anxiety [Figure 2].
Figure 2.
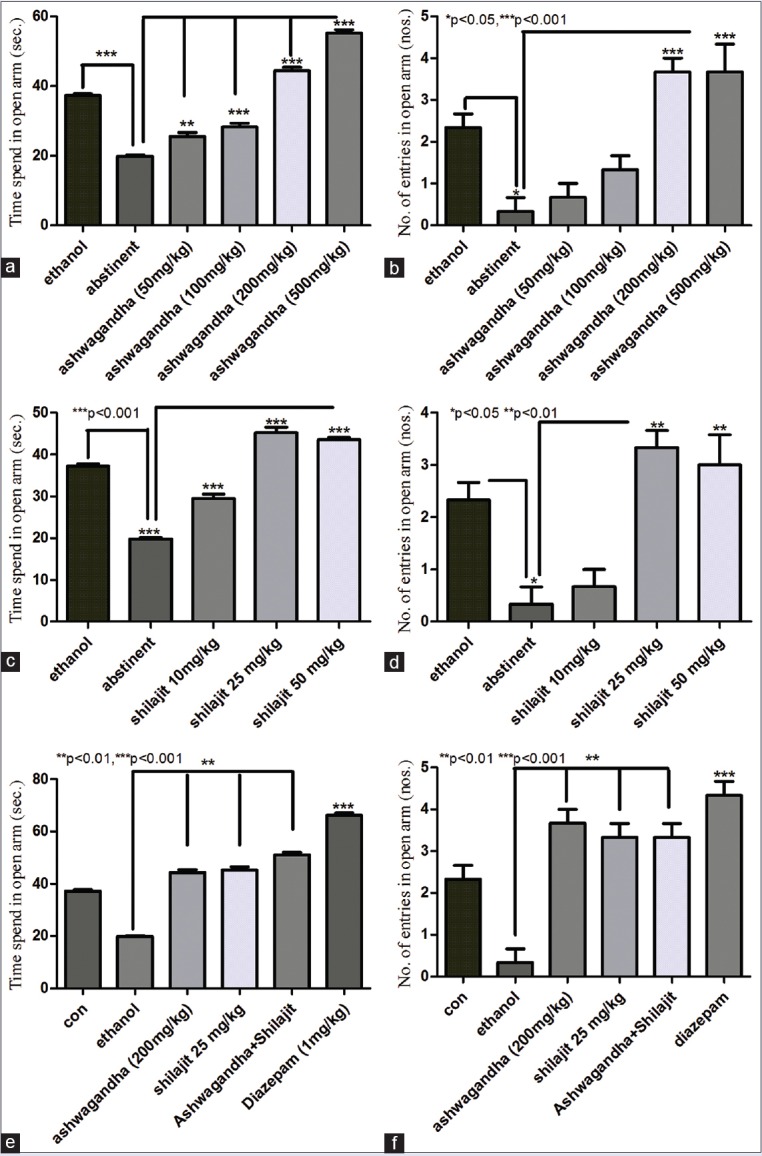
Effect of ashwagandha and shilajit on ethanol withdrawal anxiety using elevated plus maze. (a) Ethanol abstinence significantly decreased (P < 0.001) time spend in open arm compared to ethanol-treated animals. Ashwagandha treatment to abstinent animals significantly (50 mg/kg P < 0.01 and 100 mg/kg, 200 mg/kg, 500 mg/kg P < 0.001) increased the time spend in open arm compared to ethanol abstinent animals. (b) Ethanol abstinence significantly decreased (P < 0.001) the number of entries in open arm compared to animals on ethanol. Ashwagandha treatment (200 mg/kg and 500 mg/kg) significantly (P < 0.001) increased the number of open arm entries compared to abstinent animals. (c) Ethanol abstinence significantly decreased (P < 0.001) time spend in open arm compared to ethanol-treated animals. Shilajit treatment to abstinent animals significantly (10 mg/kg, 25 mg/kg, 50 mg/kg P < 0.001) increased the time spend in open arm compared to ethanol abstinent animals. (d) Ethanol abstinence significantly decreased (P < 0.05) the number of entries in open arm compared to animals on ethanol. Shilajit treatment (25 mg/kg and 50 mg/kg) significantly (P < 0.01) increased the number of open arm entries compared to abstinent animals. Values (e) Ashwagandha (200 mg/kg) and shilajit (25 mg/kg) together significantly increased (P < 0.01) the time spend in open arm compared to ethanol abstinent animals. However, this increase was comparable (P > 0.05) with ashwagandha (200 mg/kg) and shilajit (25 mg/kg) treatments alone. Diazepam also significantly increased time spend in open arm over ethanol abstinent and ethanol-treated groups (P < 0.001). (f) Ashwagandha (200 mg/kg) and shilajit (25 mg/kg) together significantly increased (P < 0.01) the number of entries into the open arm when compared with ethanol abstinent animals. However, this increase was comparable (P > 0.05) with ashwagandha (200 mg/kg) and shilajit (25 mg/kg) treatments alone. Diazepam also significantly increased the number of open arm entries over ethanol abstinent and ethanol-treated groups (P < 0.001). Values represent mean ± standard error of the mean n = 7
Effect of shilajit on withdrawal anxiety
Administration of shilajit to postabstinent animals led to significant increase in time spent in the open arm in a dose-dependent manner (P < 0.001 for 10 mg/kg, 25 mg/kg and 50 mg/kg; n = 7) thus reversing ethanol withdrawal anxiety. The time spent in the open arm was also significantly higher (P < 0.001) than animals on ethanol suggesting the intrinsic anxiolytic activity of shilajit. At doses of 25 and 50 mg/kg, shilajit also significantly increased (P < 0.01; n = 7) the number of entries in the open arm of EPM in ethanol abstinent animals [Figure 2].
Based on the above results, we studied the effect of optimal dose of ashwagandha (200 mg/kg), shilajit (25 mg/kg) and their combination on withdrawal anxiety. However, the combination of ashwagandha (200 mg/kg) and shilajit (25 mg/kg) did not show any significant increase in time spent or the number of entries in the open arm (P > 0.05, n = 7) as compared to ashwagandha or shilajit alone in ethanol-withdrawn animals [Figure 2].
Effect of ashwagandha and Shilajit on alcohol consumption
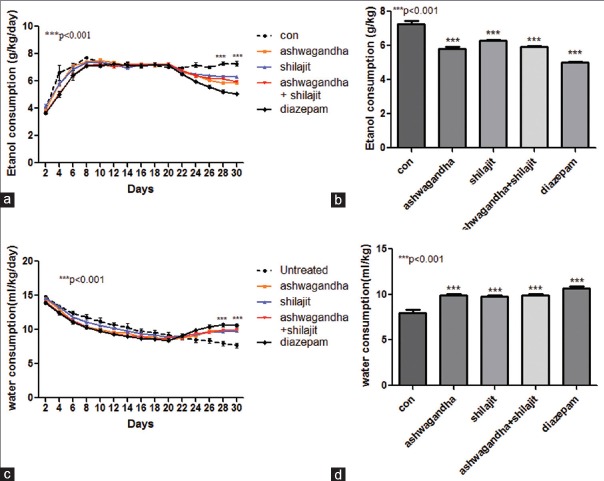
Both ashwagandha (500 mg/kg) and shilajit (50 mg/kg) and their combination treated animals showed a significant (P < 0.001, n = 7) decrease in ethanol intake and a significant increase in (P < 0.001, n = 7) water intake as compared to control group after the 28th day or after 8 days of therapy. This was comparable with diazepam-treated animals which also showed a significant decrease (P < 0.001, n = 7) in ethanol intake and an increase in water intake after day 28 compared to control group [Figure 3]. Hence, we conclude that both ashwagandha and shilajit significantly decreased ethanol intake in mice.
Figure 3.
Effect of ashwagandha and shilajit on chronic ethanol intake. (a) Changes in the ethanol intake before and after treatment. Ashwagandha (500 mg/kg), shilajit (50 mg/kg), ashwagandha + shilajit, and diazepam-treated animals showed a significant decrease in ethanol intake compared to untreated ethanol consuming control animals from day 28 (P < 0.001 n = 7) or after 8 days of treatment. (b) Changes in ethanol intake on day 28 of the 30 days study. All treatment groups showed significant (P < 0.001) decrease in alcohol intake. (c) Change in the water intake before and after treatment. Ashwagandha (500 mg/kg), Shilajit (50 mg/kg), Ashwagandha + shilajit and diazepam-treated animals showed a significant increase in water intake compared to untreated ethanol consuming control animals from day 28 (P < 0.001 n = 7) or after 8 days of treatment. (d) Change in alcohol intake on day 28 of the 30 days study. All treatment groups showed significant (P < 0.001) increase in water intake. Values represent mean ± standard error of the mean n = 7
Effect of ashwagandha and shilajit on corticohippocampal monoamine levels
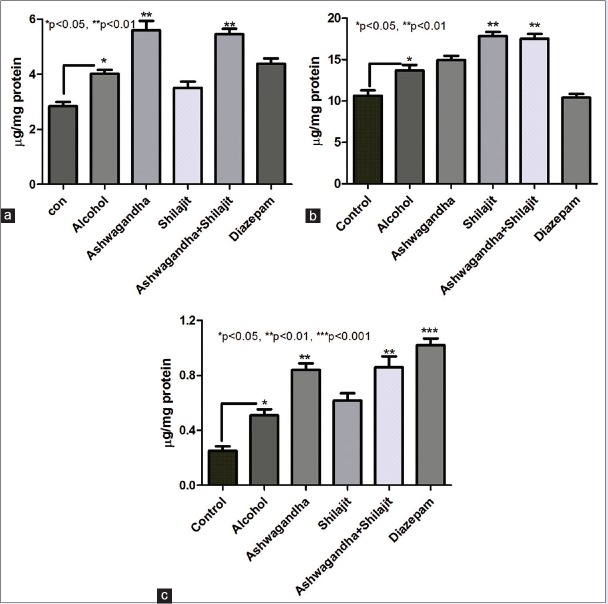
Animals on free access to alcohol for 30 days showed a significant increase in serotonin as well as dopamine (P < 0.05, n = 5) compared to control animals. While animals with free access to ethanol (30 days) and receiving ashwagandha (500 mg/kg) or ashwagandha (500 mg/kg) and shilajit (50 mg/kg) for the last 10 days of this 30 days period showed a significant (P < 0.01, n = 5) increase in corticohippocampal serotonin compared to animals on ethanol (30 days). While shilajit treated animals did not show any significant increase in serotonin levels. However, treatment with shilajit (50 mg/kg) as well as ashwagandha and shilajit combination leads to a significant increase (P < 0.01) in dopamine levels compared to animals on ethanol (30 days). Diazepam treatment had little effect on dopamine and serotonin levels [Figure 4].
Figure 4.
Effect of ashwagandha and shilajit on central nervous system neurotransmitter levels. (a) Changes in serotonin levels before and after treatment. Ashwagandha (500 mg/kg) as well as combined ashwagandha and shilajit treatment (day 21–30) lead to significant increase (P < 0.01) increase in corticohippocampal serotonin compared to untreated animals on ethanol (30 days). However, treatment with shilajit (50 mg/kg) alone or diazepam failed to increase serotonin levels compared to alcohol treated group. (b) Changes in dopamine levels before and after treatment. Shilajit (50 mg/kg) as well as ashwagandha and shilajit combination treatment (day 21–30) led to significant increase (P < 0.01) in corticohippocampal dopamine compared to untreated animals on ethanol (30 days). However, treatment with ashwagandha (500 mg/kg) alone or diazepam failed to increase serotonin levels compared to alcohol treated group. (c) Changes in gamma-aminobutyric acid levels before and after treatment. Ashwagandha (500 mg/kg) as well as combined ashwagandha and shilajit treatment (day 21–30) led to significant increase (P < 0.01) increase in corticohippocampal gamma-aminobutyric acid levels compared to untreated animals on ethanol (30 days). However, treatment with shilajit (50 mg/kg) alone failed to increase serotonin levels compared to alcohol-treated group. Diazepam treatment also showed a significant increase (P < 0.001) increase in corticohippocampal gamma-aminobutyric acid levels. Values represent mean ± standard error of the mean n = 5
Effect of ashwagandha and shilajit on gamma-aminobutyric acid levels
After 30 days of alcohol treatment, animals showed a significant (P < 0.05, n = 5) increase in GABA as compared to control group. Ashwagandha (500 mg/kg) treatment showed a significant (P < 0.01, n = 5) increase in GABA as compared to alcohol consuming animals. While ashwagandha and shilajit in combination also showed significant (P < 0.01, n = 5) increase in GABA level in the brain as compared to the alcohol group. This significance was further increased (P < 0.001, n = 5) in diazepam treated group as compared to alcohol group [Figure 4].
DISCUSSION
In the present study, using conditioned place preference model, addiction to alcohol developed after 10 days of ethanol administration with animals spending 66% of time in ethanol-paired chamber over saline chamber. Chronic and excessive ethanol consumption followed by withdrawal results in abstinence syndrome.[35,36] The predominant feature of alcohol withdrawal is anxiety, which is also considered to be the most important negative experience to revert to alcohol use.[37] This may perturb central neurotransmitters and ion channel activity. Evidences indicate that during ethanol withdrawal, there is an upregulation of excitatory N-methyl-D-aspartate receptor (NMDA) receptors[38] and a downregulation of inhibitory GABAA receptors.[39] An important characteristic for most anti-addictive compounds is the elimination of withdrawal syndrome. Usually, a drug that either facilitates GABA action or decreases glutamate activity is effective against ethanol withdrawal-induced anxiety behavior. In our study, 5 days of abstinence postaddiction lead to the development of withdrawal anxiety. The anxiety reflected in reduction in time spent in open arm and number of open arm entries in EPM. Acute treatment with ashwagandha and shilajit or their combination reversed the ethanol withdrawal anxiety. The downregulation of GABAA receptor and/or decrease in the GABAergic transmission has been implicated in the withdrawal symptoms of ethanol.[24] GABA mimetic effect of ashwagandha may prevent the declining GABA activity during alcohol withdrawal.[24] Ashwagandha may also act as anxiolytic by reducing the levels of tribulin, an endocoid marker of clinical anxiety and corticotrophin in the brain.[18] Shilajit known for its anxiolytic and nootropic actions may increase neuronal dopamine levels partly responsible for its anxiolytic actions.[40,41,42] Their amelioration of withdrawal anxiety was comparable to diazepam. Diazepam is a benzodiazepine moiety known for its positive allosteric modulator of GABAA receptor hence its anxiolytic actions.[43]
Next, we determined the effect of ashwagandha and shilajit on alcohol consumption in mice. The two bottle choice protocol is a widely used model that captures aspects of voluntary alcohol consumption in humans.[44] After 30 days of chronic ethanol intake, animals showed increased corticohippocampal levels of GABA. Decrease in GABAergic function after chronic administration of alcohol in experimental animals have been largely attributed to decrease in GABAA receptor expression and function.[44] Ashwagandha treatment significantly increased GABA levels in the corticohippocampal lysates over both control and ethanol-treated animals. Ashwagandha has been previously reported to have GABA-mimetic activity by acting on both GABAA and GABAB receptors and may also have glycine mimetic action.[45,46] Alcohol is an indirect GABA agonist. A plasma and CSF level of GABA is higher after initial withdrawal than after longer periods of abstinence.[47] Diazepam, an established GABAA agonist and anti-addictive compound, also showed increase in corticohippocampal GABA levels. Similar to previous reports, we also found an increase in serotonin levels in the corticohippocampal lysates of ethanol-exposed animals[44,48] suggesting either excess release or reduced uptake of serotonin. Serotonergic system plays an important role in the ethanol intake, preference, and dependence via central mechanisms.[49,50,51] Ashwagandha treatment showed an increase in cortical serotonin level over alcohol group. Others have shown that 5-HT2 receptor expression may also increase in animals receiving alcohol for several weeks. Increased serotonin activity at the 5-HT2 receptor caused by chronic alcohol exposure may also contribute to the alcohol withdrawal syndrome.[52] 5-HT1B knockout mice show less evidence of alcohol tolerance.[53] Diazepam was also found to increase the serotonin level in alcohol consuming brain. Alcohol consumption may induce a dopamine surge in the brain, turning it into a pleasurable experience thus reinforcing the action, and increasing consumption.[54,55] Even low alcohol doses can increase dopamine release in parts of the nucleus accumbens. In contrast to other stimuli, alcohol-related stimuli maintain their motivational significance even after repeated alcohol administration, which may contribute to the craving for alcohol observed in alcoholics.[56,57] In our study, chronic alcohol exposure increased corticohippocampal dopamine levels as compared to saline group while treatment with shilajit potentiated this effect further. Dopaminergic signal transmission may also be serotonin-mediated. 5-HT2 and 5-HT3 receptor signaling may stimulate dopaminergic neurons to release dopamine.[58,59,60]
CONCLUSION
Here, we show ashwagandha and shilajit mediated dose-dependent decrease in ethanol withdrawal anxiety as well as a reduction in ethanol intake in mice. The primary mechanism responsible for anti-addictive activity of ashwagandha was found via GABAergic and serotonergic system while shilajit primarily mediated its effect by modulating dopamine levels in mice.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
ABOUT AUTHOR

Sugato Banerjee
Dr. Sugato Banerjee, is Assistant Professor at Department of Pharmaceutical Sciences and Technology, BIT, Mesra, Ranchi. His research interest includes screening of natural compounds with anti-addictive potential and to study the molecular mechanisms involved in the process.
REFERENCES
- 1.WHO Press; 2014. [Last accessed on 2016 Feb 18]. World Health Organization. Global Status Report on Alcohol and Health; p. XIV. Available from: apps.who.int/iris/bitstream/10665/112736/1/9789240692763_eng.pdf . [Google Scholar]
- 2.WHO Press; 2014. [Last accessed on 2016 Feb 18]. World Health Organization. Global Status Report on Alcohol and Health; p. XIII. Available from: add apps.who.int/iris/bitstream/10665/112736/1/9789240692763_eng.pdf . [Google Scholar]
- 3.Weiss F. Neurobiology of craving, conditioned reward and relapse. Curr Opin Pharmacol. 2005;5:9–19. doi: 10.1016/j.coph.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 4.Vengeliene V, Bilbao A, Molander A, Spanagel R. Neuropharmacology of alcohol addiction. Br J Pharmacol. 2008;154:299–315. doi: 10.1038/bjp.2008.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Becker HC. Animal models of alcohol withdrawal. Alcohol Res Health. 2000;24:105–13. [PMC free article] [PubMed] [Google Scholar]
- 6.Baker JT, Borris RP, Carté B, Cordell GA, Soejarto DD, Cragg GM, et al. Natural product drug discovery and development: New perspectives on international collaboration. J Nat Prod. 1995;58:1325–57. doi: 10.1021/np50123a003. [DOI] [PubMed] [Google Scholar]
- 7.Singh N. Stress disease and herbal medicine. J Biotechnol Med Plant Res. 1987;3:2–4. [Google Scholar]
- 8.Dickson TC, Vickers JC. The morphological phenotype of beta-amyloid plaques and associated neuritic changes in Alzheimer's disease. Neuroscience. 2001;105:99–107. doi: 10.1016/s0306-4522(01)00169-5. [DOI] [PubMed] [Google Scholar]
- 9.Bhattacharya SK, Bhattacharya A, Sairam K, Ghosal S. Anxiolytic-antidepressant activity of Withania somnifera glycowithanolides: An experimental study. Phytomedicine. 2000;7:463–9. doi: 10.1016/S0944-7113(00)80030-6. [DOI] [PubMed] [Google Scholar]
- 10.Singh N, Bhalla M, de Jager P, Gilca M. An overview on ashwagandha: A Rasayana (rejuvenator) of ayurveda. Afr J Tradit Complement Altern Med. 2011;8 5 Suppl:208–13. doi: 10.4314/ajtcam.v8i5S.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mehta AK, Binkley P, Gandhi SS, Ticku MK. Pharmacological effects of Withania somnifera root extract on GABAA receptor complex. Indian J Med Res. 1991;94:312–5. [PubMed] [Google Scholar]
- 12.Singh N. Effect of Withania somnifera and Panax ginseng on dopaminergic receptors in rat brain during stress. J Nat Prod. 1988;56:28–35. [Google Scholar]
- 13.Ghosal S. Chemistry of shilajit, an immunomodulatory ayurvedic Rasayan. Pure Appl Chem. 1990;62:1285–8. [Google Scholar]
- 14.Mittal P, Kaushik D, Gupta V, Bansal P, Khokra S. Therapeutic potential of “Shilajit Rasayana” – A review. IJPCR. 2009;1:47–9. [Google Scholar]
- 15.Chopra RN, Chopra IC, Handa KL, Kapoo KD. Indigenous drugs of India. Phytother Res. 2005;21:335–43. [Google Scholar]
- 16.Agarwal SP, Khanna R, Karmarkar R, Anwer MK, Khar RK. Shilajit: A review. Phytother Res. 2007;21:401–5. doi: 10.1002/ptr.2100. [DOI] [PubMed] [Google Scholar]
- 17.Acharya SB, Frotan MH, Goel RK, Tripathi SK, Das PK. Pharmacological actions of Shilajit. Indian J Exp Biol. 1988;26:775–7. [PubMed] [Google Scholar]
- 18.Park JS, Kim GY, Han K. The spermatogenic and ovogenic effects of chronically administered Shilajit to rats. J Ethnopharmacol. 2006;107:349–53. doi: 10.1016/j.jep.2006.03.039. [DOI] [PubMed] [Google Scholar]
- 19.Bhattacharya SK, Sen AP. Effects of shilajit on biogenic free radicals. Phytother Res. 1995;9:56–9. [Google Scholar]
- 20.Cornejo A, Jiménez JM, Caballero L, Melo F, Maccioni RB. Fulvic acid inhibits aggregation and promotes disassembly of tau fibrils associated with Alzheimer's disease. J Alzheimers Dis. 2011;27:143–53. doi: 10.3233/JAD-2011-110623. [DOI] [PubMed] [Google Scholar]
- 21.Ghosal S, Lal J, Singh SK, Goel RK, Jaiwal AK, Bhattacharya SK. The need for formulation of shilajit by its isolated active constituents. Phytother Res. 1991;5:211–6. [Google Scholar]
- 22.Yin H, Yang EJ, Park SJ, Han SK. Glycine- and GABA-mimetic actions of shilajit on the substantia gelatinosa neurons of the trigeminal subnucleus caudalis in mice. Korean J Physiol Pharmacol. 2011;15:285–9. doi: 10.4196/kjpp.2011.15.5.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaur S, Kumar P, Kumar D, Kharya MD, Singh N. Parasympathomimetic effect of shilajit accounts for relaxation of rat corpus cavernosum. Am J Mens Health. 2013;7:119–27. doi: 10.1177/1557988312462738. [DOI] [PubMed] [Google Scholar]
- 24.Ruby B, Benson MK, Kumar EP, Sudha S, Wilking JE. Evaluation of ashwagandha in alcohol withdrawal syndrome. Asian Pac J Trop Dis. 2012;2:S856–60. [Google Scholar]
- 25.Gupta GL, Rana AC. Effect of Withania somnifera dunal in ethanol-induced anxiolysis and withdrawal anxiety in rats. Indian J Exp Biol. 2008;46:470–5. [PubMed] [Google Scholar]
- 26.Thanos PK, Kim R, Cho J, Michaelides M, Anderson BJ, Primeaux SD, et al. Obesity-resistant S5B rats showed greater cocaine conditioned place preference than the obesity-prone OM rats. Physiol Behav. 2010;101:713–8. doi: 10.1016/j.physbeh.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kokare DM, Chopde CT, Subhedar NK. Participation of alpha-melanocyte stimulating hormone in ethanol-induced anxiolysis and withdrawal anxiety in rats. Neuropharmacology. 2006;51:536–45. doi: 10.1016/j.neuropharm.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 28.Cunningham CL, Fidler TL, Hill KG. Animal models of alcohol's motivational effects. Alcohol Res Health. 2000;24:85–92. [PMC free article] [PubMed] [Google Scholar]
- 29.McBride WJ, Li TK. Animal models of alcoholism: Neurobiology of high alcohol-drinking behavior in rodents. Crit Rev Neurobiol. 1998;12:339–69. doi: 10.1615/critrevneurobiol.v12.i4.40. [DOI] [PubMed] [Google Scholar]
- 30.Sharma PV. Varanasi: Chaukhambha Viashwabharti; 1999. Ashwagandha, Dravyaguna Vijana; pp. 763–5. [Google Scholar]
- 31.Kunin D, Gaskin S, Rogan F, Smith BR, Amit Z. Caffeine promotes ethanol drinking in rats. Examination using a limited-access free choice paradigm. Alcohol. 2000;21:271–7. doi: 10.1016/s0741-8329(00)00101-4. [DOI] [PubMed] [Google Scholar]
- 32.Yilmaz B, Gilmore DP, Wilson CA. Inhibition of the preovulatory LH surge in the rat by central noradrenergic mediation: Involvement of an anaesthetic (urethane) and opioid receptor agonists. Biogenic Amines. 1996;12:423–35. [Google Scholar]
- 33.Yilmaz B, Gilmore DP, Wilson CA. Effects of DPDPE (a specific delta-opioid receptor agonist) and naloxone on hypothalamic monoamine concentrations during the pre-ovulatory LH surge in the rat. Eur J Endocrinol. 1998;139:546–51. doi: 10.1530/eje.0.1390546. [DOI] [PubMed] [Google Scholar]
- 34.Mishra N, Sasmal D. Modulations of brain amines and dopaminergic behavior by a novel, reversible and selective MAO-B inhibitor. Brain Res. 2012;1470:45–51. doi: 10.1016/j.brainres.2012.06.037. [DOI] [PubMed] [Google Scholar]
- 35.Escrig MA, Pardo M, Aragon CM, Correa M. Anxiogenic and stress-inducing effects of peripherally administered acetaldehyde in mice: Similarities with the disulfiram-ethanol reaction. Pharmacol Biochem Behav. 2012;100:404–12. doi: 10.1016/j.pbb.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 36.Schukitt MA. Alcohol and alcoholism. In: Braunwald E, editor. Harrison's Principles of Internal Medicine. McGraw-Hill: New York; 1987. pp. 2562–6. [Google Scholar]
- 37.Charness ME, Simon RP, Greenberg DA. Ethanol and the nervous system. N Engl J Med. 1989;321:442–54. doi: 10.1056/NEJM198908173210706. [DOI] [PubMed] [Google Scholar]
- 38.Schulteis G, Liu J. Brain reward deficits accompany withdrawal (hangover) from acute ethanol in rats. Alcohol. 2006;39:21–8. doi: 10.1016/j.alcohol.2006.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Whittington MA, Lamberd JD, Little HJ. Increased NMDA receptor and calcium channel activity underlying ethanol withdrawal hyperexcitability. Alcohol Alcohol. 1995;30:105–14. [PubMed] [Google Scholar]
- 40.Littleton J. Neurochemical mechanisms underlying alcohol withdrawal. Alcohol Health Res World. 1998;22:13–24. [PMC free article] [PubMed] [Google Scholar]
- 41.Jaiswal AK, Bhattacharya SK. Effects of shilajit on memory, anxiety and brain monoamines in rats. Indian J Pharmacol. 1992;24:12–7. [Google Scholar]
- 42.Schliebs R, Liebmann A, Bhattacharya SK, Kumar A, Ghosal S, Bigl V. Systemic administration of defined extracts from Withania somnifera (Indian Ginseng) and shilajit differentially affects cholinergic but not glutamatergic and GABAergic markers in rat brain. Neurochem Int. 1997;30:181–90. doi: 10.1016/s0197-0186(96)00025-3. [DOI] [PubMed] [Google Scholar]
- 43.Diana M, Pistis M, Muntoni A, Gessa G. Mesolimbic dopaminergic reduction outlasts ethanol withdrawal syndrome: Evidence of protracted abstinence. Neuroscience. 1996;71:411–5. doi: 10.1016/0306-4522(95)00482-3. [DOI] [PubMed] [Google Scholar]
- 44.Davies M. The role of GABAA receptors in mediating the effects of alcohol in the central nervous system. J Psychiatry Neurosci. 2003;28:263–74. [PMC free article] [PubMed] [Google Scholar]
- 45.Valenzuela CF. Alcohol and neurotransmitter interactions. Alcohol Health Res World. 1997;21:144–8. [PMC free article] [PubMed] [Google Scholar]
- 46.Besheer J, Lepoutre V, Mole B, Hodge CW. GABAA receptor regulation of voluntary ethanol drinking requires PKCepsilon. Synapse. 2006;60:411–9. doi: 10.1002/syn.20314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bhattarai JP, Park SJ, Han SK. Potentiation of NMDA receptors by Withania somnifera on hippocampal CA1 pyramidal neurons. Am J Chin Med. 2013;41:503–13. doi: 10.1142/S0192415X13500365. [DOI] [PubMed] [Google Scholar]
- 48.Adinoff B, Kramer GL, Petty F. Levels of gamma-aminobutyric acid in cerebrospinal fluid and plasma during alcohol withdrawal. Psychiatry Res. 1995;59:137–44. doi: 10.1016/0165-1781(95)02739-4. [DOI] [PubMed] [Google Scholar]
- 49.LeMarquand D, Pihl RO, Benkelfat C. Serotonin and alcohol intake, abuse, and dependence: Clinical evidence. Biol Psychiatry. 1994;36:326–37. doi: 10.1016/0006-3223(94)90630-0. [DOI] [PubMed] [Google Scholar]
- 50.Pessoa-Mahana H, Recabarren-Gajardo G, Temer JF, Zapata-Torres G, Pessoa-Mahana CD, Barría CS, et al. Synthesis, docking studies and biological evaluation of benzo[b] thiophen-2-yl-3-(4-arylpiperazin-1-yl)-propan-1-one derivatives on 5-HT1A serotonin receptors. Molecules. 2012;17:1388–407. doi: 10.3390/molecules17021388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grochans E, Grzywacz A, Malecka I, Samochowiec A, Karakiewicz B, Samochowiec J. Research on associations between selected polymorphisms of genes DRD2, 5HTT, GRIK3, ADH4 and alcohol dependence syndrome. Psychiatr Pol. 2011;45:325–35. [PubMed] [Google Scholar]
- 52.Thompson RD, Heffner JL, Strong JA, Blom TJ, Anthenelli RM. Relationship between the serotonin transporter polymorphism and obsessive-compulsive alcohol craving in alcohol-dependent adults: A pilot study. Alcohol. 2010;44:401–6. doi: 10.1016/j.alcohol.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Buck KJ, Reilly MT, Rogers LM, Szeliga K, Grant K, Brodie MS. Serotonin 5-HT2 receptors and alcohol: Reward, withdrawal and discrimination. Alcohol Clin Exp Res. 2004;28:211–6. doi: 10.1097/01.alc.0000113423.40075.a3. [DOI] [PubMed] [Google Scholar]
- 54.Crabbe JC, Phillips TJ, Feller DJ, Hen R, Wenger CD, Lessov CN, et al. Elevated alcohol consumption in null mutant mice lacking 5-HT1B serotonin receptors. Nat Genet. 1996;14:98–101. doi: 10.1038/ng0996-98. [DOI] [PubMed] [Google Scholar]
- 55.Lovinger DM. Serotonin's role in alcohol's effects on the brain. Alcohol Health Res World. 1997;21:114–20. [PMC free article] [PubMed] [Google Scholar]
- 56.Berridge KC. The debate over dopamine's role in reward: The case for incentive salience. Psychopharmacology (Berl) 2007;191:391–431. doi: 10.1007/s00213-006-0578-x. [DOI] [PubMed] [Google Scholar]
- 57.Di Chiara G. Alcohol and dopamine. Alcohol Health Res World. 1997;21:108–14. [PMC free article] [PubMed] [Google Scholar]
- 58.Brodie MS, Trifunovic RD, Shefner SA. Serotonin potentiates ethanol-induced excitation of ventral tegmental area neurons in brain slices from three different rat strains. J Pharmacol Exp Ther. 1995;273:1139–46. [PubMed] [Google Scholar]
- 59.Campbell AD, McBride WJ. Serotonin-3 receptor and ethanol-stimulated dopamine release in the nucleus accumbens. Pharmacol Biochem Behav. 1995;51:835–42. doi: 10.1016/0091-3057(95)00050-7. [DOI] [PubMed] [Google Scholar]
- 60.Grant KA. The role of 5-HT3 receptors in drug dependence. Drug Alcohol Depend. 1995;38:155–71. doi: 10.1016/0376-8716(95)01120-n. [DOI] [PubMed] [Google Scholar]