Abstract
Background:
Proteins present in the latex of Calotropis procera have been shown to produce anti-inflammatory effect and to afford protection in various disease models.
Objectives:
To determine the efficacy of high molecular weight protein sub-fraction (LPPI) of latex of C. procera in ameliorating joint inflammation and hyperalgesia in a preclinical model of arthritis.
Materials and Methods:
Monoarthritis was induced in rats by intra-articular injection of Freund's complete adjuvant (FCA) and the effect of two doses of LPPI (5 and 25 mg/kg) and diclofenac (5 mg/kg) was evaluated on joint swelling, stair climbing ability, motility, and dorsal flexion pain on day 3. The rats were sacrificed on day 3 to measure tissue levels of reduced glutathione (GSH) and thiobarbituric acid reactive substances (TBARS). Evaluation of joint histology was also made.
Results:
Intra-articular injection of FCA produced joint swelling and difficulty in stair climbing ability, motility, and pain on flexion of the joint as revealed by scores obtained for these functional parameters. LPPI produced a dose-dependent decrease in joint swelling and improved joint functions. Arthritic rats also revealed altered oxidative homeostasis where joint tissue GSH levels were decreased and TBARS levels were increased as compared to normal rats. The levels of these oxidative stress markers were near normal in arthritic rats treated with LPPI. Moreover, treatment with LPPI also maintained the structural integrity of the joint. The protective effect of LPPI was comparable to the standard anti-inflammatory drug, diclofenac.
Conclusion:
The findings of the present study show that LPPI fraction comprising high molecular weight proteins could be used for the alleviation of arthritic symptoms.
SUMMARY
High molecular weight protein sub-fraction of latex of Calotropis procera (LPPI) reduced joint swelling and hyperalgesia in arthritic rats
LPPI produced a significant improvement in stair climbing ability and motility in arthritic rats
LPPI normalized the levels of oxidative stress markers in the arthritic joints
Treatment with LPPI reduced neutrophil influx and edema in the arthritic joints
Abbreviations used: FCA: Freund's complete adjuvant, GSH: Glutathione, TBARS: Thiobarbituric acid reactive substances, TBA: Thiobarbituric acid, MDA: Malondialdehyde, LPPI: Latex protein fraction PI.
Keywords: Arthritis, hyperalgesia, inflammation, latex proteins, oxidative stress
INTRODUCTION
Arthritis is an inflammatory condition of the joints associated with pain and functional limitations that affect the quality of life. The goal of treatment in this condition is to provide symptomatic relief and to arrest the further progression of disease. The drug therapy for arthritis includes the long-term use of nonsteroidal anti-inflammatory drugs and steroids, both of which are known to produce serious side effects.[1] In view of this, plants have been considered as a safer alternative source of medicinal agents that have been shown to provide relief both in experimental and clinical set up.[2] According to WHO, the primary health care need of a large proportion of world population is being met through the use of herbal preparations.[3] Hence, there is a need to explore such preparations having better efficacy and safety profiles though their standardization remains a big challenge. Studies carried out in in vivo models provide a scientific basis for the clinical use of plant-derived preparations and validate their traditional use.
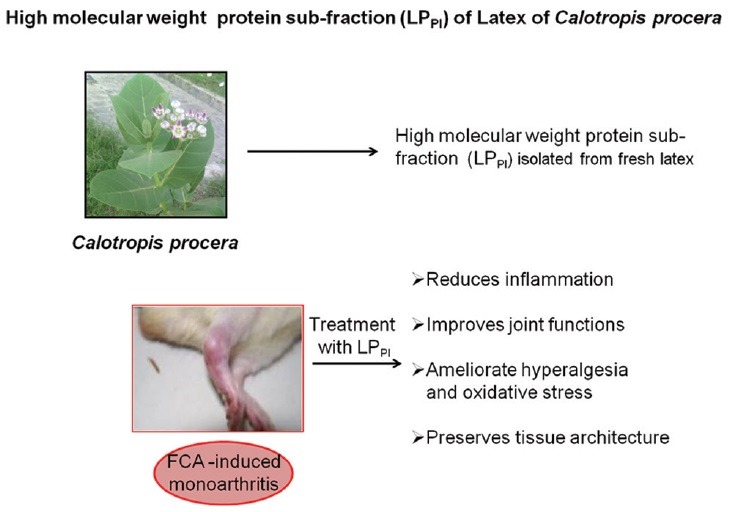
Calotropis procera (Ait) R. Br. is a perennial shrub that grows in wild and belongs to the family Apocynaceae. This plant is found throughout the tropics of Asia and Africa and has been widely used in various traditional medicinal systems.[4] In last two decades, a number of in vivo and in vitro studies have been carried out that provide scientific evidence for the medicinal properties of C. procera.[5,6,7,8] This plant yields large quantities of milky white latex synthesized by specialized laticifer cells that is a tremendous source of various biologically active metabolites, enzymes, natural polymers, defense proteins, and nonprotein constituents.[9,10,11,12] It also contains rubber (poly-isoprene) responsible for the undesirable effects produced by the latex. Recently, proteins separated from the remaining constituents of the latex have been found to exhibit potent anti-inflammatory and anti-hyperalgesic properties in the rodent model of arthritis.[13] Further segregation of these proteins by ion-exchange chromatography provided a sub-fraction as peak I (LPPI) comprising high molecular weight proteins that have been shown to alleviate edema formation and neutrophilic influx in acute inflammation.[14,15] The present study was carried out to evaluate the efficacy of these proteins in ameliorating joint inflammation and dysfunction in a rat model of monoarthritis.
MATERIALS AND METHODS
Animals
Wistar albino rats of either sex, weighing 150–180 g, were used for the study. The animals were kept in the departmental animal house under ambient environmental conditions with free access to food and water. The study was conducted in accordance with the guidelines of Institutional Animal Ethics Committee.
Reagents and drugs
Freund's complete adjuvant (FCA) and diclofenac were purchased from Sigma-Aldrich Co., St. Louis, MO, USA, and Biochem Pharmaceutical Industries Ltd., New Delhi, India, respectively. All other chemicals were of analytical grade and of highest purity commercially available.
Plant material and preparation of high molecular weight protein fraction
The plant C. procera was identified by a taxonomist and a voucher specimen has been retained in Prisco Bezerra Herbarium of the Universidade Federal do Ceará, Brazil. The fresh latex collected in distilled water (1:1, v/v) was centrifuged to separate the rubber-like material and the supernatant was dialyzed using a membrane having a cut-off value of 8000 Da. The nondialyzable fraction comprising proteins was freeze dried and subjected to ion-exchange chromatography where three peaks (LPPI, LPPII, and LPPIII) were obtained.[15] The peak LPPI revealed the presence of high molecular weight proteins by sodium dodecyl sulfate- polyacrylamide gel electrophoresis and was used to carry out the present study.
Experimental design
To study the joint function-related parameters, the overnight fasted rats were trained to climb a staircase (steps at 5, 10, and 15 cm) having water at 2nd step and food at 3rd step for around 1 week. The trained rats were randomly divided into five groups (n = 6): Group I served as normal control (NC), Group II served as FCA control (FC) whereas arthritic rats in Groups III and IV received LPPI at 5 and 25 mg/kg doses (LPPI-5 and LPPI-25), respectively, and Group V received standard anti-inflammatory drug diclofenac at 5 mg/kg dose (D5). All the drug solutions were prepared in normal saline. The standard anti-inflammatory drug diclofenac was given orally 1 h before whereas LPPI was given intravenously 30 min before the induction of arthritis and then daily till the end of the study.
Induction and assessment of joint inflammation
Monoarticular arthritis in rats was induced by injecting 0.1 mL of 0.1% FCA into the intra-articular space of right ankle joint according to the method described by Butler et al.[16] Joint inflammation was induced within 24 h and reached its peak on day 3 as described earlier.[13] Joint diameter was measured just before the adjuvant challenge (day 0) and daily till the time of peak inflammation (day 3) by using micrometer screw gauge. The extent of inflammation was determined by subtracting the joint diameter of day 0 from day 3 and expressed in mm. After evaluating all the joint function-related parameters on day 3, the animals were sacrificed and the joint tissue was preserved at −80°C to measure the oxidative stress markers. The effect of these proteins was also evaluated on joint histology in a separate set of experiment.
Stair climbing ability
The ability of trained arthritic rats to climb the staircase was scored as: 0 - if the rats did not climb, 1 - if the rats climbed to step one, 2 - if the rats climbed up to step two, and 3 - if the rats climbed all the three steps.[13]
Motility
The arthritic rats were allowed to move freely on the floor for 5 min and their motility pattern was scored as: 0 - if the rats avoided touching the floor, 1 - if rats walked with difficulty with toes touching the floor, and 2 - if rat walked normally.[13]
Dorsal flexion pain
The ankle joint of rat was flexed dorsally until the toe touched the anterior part of the leg. The leg was flexed five times at an interval of 5 s and squeaking and leg withdrawal with each flexion was counted. A score of 0 was given if there was no squeaking and no leg withdrawal, 1, if either squeaking or leg withdrawal was present, or 2, if both squeaking and leg withdrawal were present.[13]
Estimation of tissue glutathione level
Reduced glutathione reacts with 5-5-dithiobis 2-nitrobenzoic acid at alkaline pH to form thionitrobenzoic acid, a yellow-colored compound that gives absorbance at 412 nm. The joint tissue was homogenized in 5% trichloroacetic acid and centrifuged at 3000 rpm for 10 min. The supernatant was used to evaluate the tissue glutathione (GSH) levels according to the method of Ellman.[17] The standard curve was prepared by using various concentrations of reduced glutathione. Tissue GSH level was determined from the standard curve and expressed as μg/g tissue.
Estimation of tissue thiobarbituric acid reactive substances level
Thiobarbituric acid (TBA) reacts with lipid peroxides and aldehydes (malondialdehyde [MDA], N-aldehyde, and α, β aldehyde) to form colored chemical compound that gives absorbance at 532 nm. The joint tissue was homogenized in 0.15 M KCl and the homogenate was used to evaluate the tissue MDA levels according to the method of Ohkawa et al.[18] The standard curve was prepared by using various concentrations of 1, 1, 3, 3-tetraethoxypropane. Tissue MDA level was determined from the standard curve and expressed as nM/g tissue.
Histological analysis
The arthritic joint was removed and fixed in 1% formaldehyde in saline. The fixed joints were decalcified in ethylenediaminetetraacetic acid, processed for paraffin embedding, and sectioned. The mounted slides were stained with eosin and hematoxylin to examine the inflammatory changes.[19]
Statistical analysis
Increase in joint diameter and oxidative stress parameters are presented as mean ± standard error of mean and one-way ANOVA followed by post hoc test (least significant difference) was used to compare the treated groups with FC group. The joint functional parameters, i.e., stair climbing ability, motility, and dorsal flexion pain are expressed as median score, and Fisher's exact test was used to compare the treated groups with FC group. The statistical analysis was carried out by SPSS program version 17 (SPSS Inc. Chicago) and STATA version 11 (Stata Corp LP, College Station, Texas). The statistical significance was considered at P < 0.05.
RESULTS
Effect of latex protein fraction PI on joint inflammation
The intra-articular injection of FCA in rat ankle joint produced a peak inflammatory response on day 3 with increase in joint diameter of 4.04 ± 0.17 mm. However, injection of 0.1 mL normal saline produced a marginal increase in joint diameter of 0.12 ± 0.01 mm on day 3. Treatment with LPPI produced a dose-dependent inhibition of joint swelling, and increase in diameter was 2.81 ± 0.12 and 1.97 ± 0.13 mm in LPPI-5 and LPPI-25 groups, respectively (30% and 51% inhibition). The inhibitory effect of LPPI-25 was found comparable to that of D5 group where the increase in joint diameter was 1.90 ± 0.20 mm (53% inhibition) [Figure 1].
Figure 1.
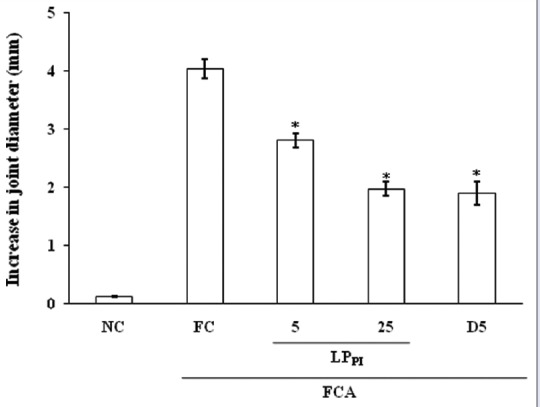
Effect of LPPI on increase in joint diameter in monoarthritic rats. n = 6. *P < 0.05 versus Freund's complete adjuvant control group, ANOVA followed by least significant difference post hoc test
Effect of latex protein fraction PI on stair climbing ability
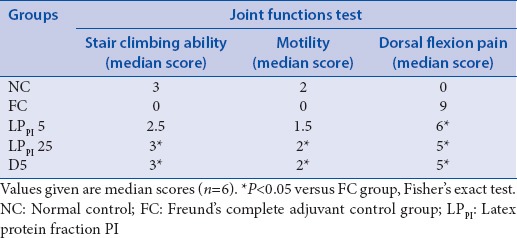
The rats in FC group experienced difficulty in climbing the staircase, and a median score of 0 was obtained in this group in comparison with median score of 3 in NC group on day 3. Treatment with LPPI improved the stair climbing ability of the arthritic rats, and median scores of 2.5 and 3 were obtained in LPPI-5 and LPPI-25 groups, respectively. The effect of LPPI-25 was found comparable to that of standard anti-inflammatory drug diclofenac, where the median score was 3 [Table 1].
Table 1.
Effect of LPPI on joint functions
Effect of latex protein fraction PI on motility
The intra-articular injection of FCA affected the motility of rats, and a median score of 0 was obtained in FC group as compared with NC group having median score of 2. Treatment with LPPI at 5 and 25 mg/kg doses improved the motility of arthritic rats, and median scores of 1.5 and 2 were obtained on day 3 at the two doses [Table 1].
Effect of latex protein fraction PI on dorsal flexion pain
The inflamed joints of arthritic rats were flexed and scored to assess the effect of LPPI on hyperalgesia. The injection of FCA lowered the threshold of pain in FC group, and a median score of 9 was obtained on day 3. Treatment with LPPI afforded protection against pain, and median scores of 6 and 5 were obtained in LPPI-5 and LPPI-25 groups, respectively [Table 1].
Effect of latex protein fraction PI on tissue glutathione level
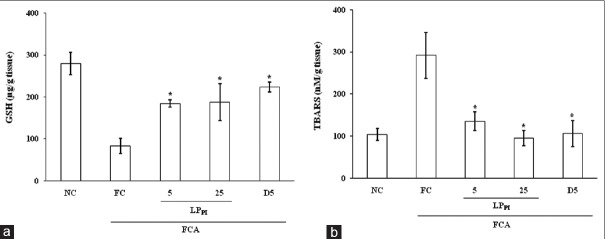
Intra-articular injection of FCA produced a marked decrease in tissue GSH level (83.33 ± 18.16 μg/g) in FC group in comparison with NC group (279.16 ± 26.74 μg/g tissue). Treatment with LPPI increased the tissue levels of GSH and in LPPI-5 and LPPI-25 groups, the tissue GSH levels were 184.37 ± 8.80 and 187.50 ± 43.69 μg/g tissue, respectively [Figure 2a].
Figure 2.
Effect of LPPI on the levels of glutathione (a) and thiobarbituric acid reactive substances (b) in joint tissue of monoarthritic rats. n = 6. *P < 0.05 versus Freund's complete adjuvant control group, ANOVA followed by least significant difference post hoc test
Effect of latex protein fraction PI on tissue thiobarbituric acid reactive substances level
The injection of FCA produced a marked increase in TBA reactive substances (TBARS) level (291.66 ± 54.67 nM/g tissue) as compared with NC group (104.16 ± 14.28 nM/g tissue). Treatment with LPPI dose dependently decreased the tissue TBARS levels to 135.00 ± 22.32 and 95.00 ± 18.16 nM/g tissue in LPPI-5 and LPPI-25 groups, respectively. The TBARS level in D5 group was 105.83 ± 31.31 nM/g tissue [Figure 2b].
Effect of latex protein fraction PI on joint histology
The intra-articular injection of FCA in rat joint produced peak inflammation in the joint on day 3, which is characterized by neutrophilic infiltration and tissue edema. Treatment with LPPI maintained joint tissue integrity and its effect was comparable to that of diclofenac [Figure 3].
Figure 3.
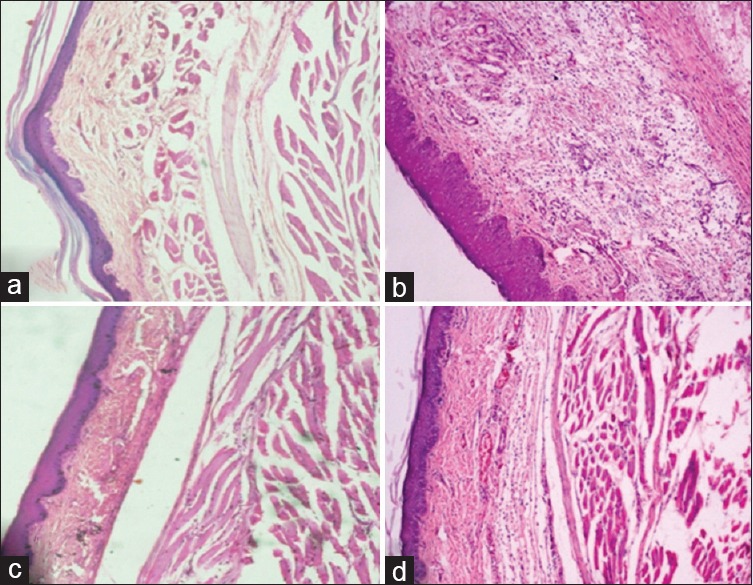
Effect of LPPI on joint histology in arthritic rats. Normal control (a), Freund's complete adjuvant control (b), Diclofenac 5 mg/kg (c) and LPPI-25 mg/kg (d) (X = 100)
DISCUSSION
Preparations derived from different parts of plants have been used for medicinal purpose from time immemorial. C. procera is a wild growing plant that has been shown to possess numerous medicinal properties and it has been used in the traditional medicinal system for treating various diseases.[4] The plant produces latex when it gets injured and this latex has also been shown to afford protection in several disease models.[20,21,22,23] Recently, it has been shown that the latex of this plant comprises both nonprotein and protein constituents that inhibit inflammation induced in an animal model.[5,6] The proteins present in latex have been separated into three fractions by ion-exchange chromatography, and the fraction comprising high molecular weight proteins (LPPI) has been demonstrated to possess anti-inflammatory property.[15] The present study was aimed to evaluate the protective effect of LPPI against inflammatory hyperalgesia, functional disability, and oxidative stress in monoarthritic rat. Upon intra-articular injection, FCA induced inflammation of the joint as evident by swelling, redness, and pain. Treatment with LPPI suppressed the joint inflammation and decreased the joint swelling in a dose-dependent manner. Recently, this fraction has been shown to inhibit edema formation in the rat model of acute inflammation.[14]
As inflammation and associated pain are characteristic features of the arthritic joint that affect daily activity, the present study also evaluated the effect of LPPI on joint functions. Compared to arthritic rats, LPPI treated rats were able to climb and move like normal rats and their pain threshold was also elevated as revealed by dorsal flexion pain test. In this regard, the present study shows that the beneficial effect of LP in arthritis could be due to the proteins present in its sub-fraction LPPI. Further, it is interesting to note that the proteins derived from the latex inhibit the release of tumor necrosis factor alpha (TNF-α), prostaglandin E2, and inflammatory response elicited by other mediators, which have a significant role in pain perception.[24] Earlier LP has been reported to exhibit anti-nociceptive property that has been found to be comparable to that of morphine.[20]
It is well established that oxidative homeostasis gets altered in inflammatory conditions and free radicals generated in the swollen joints produce damage to the cellular components such as proteins, DNA, and membrane lipids. Unchecked production of free radicals leads to disease progression and destruction of cartilage in arthritic conditions.[25] In the present study, FCA injection into the joint increased the levels of TBARS, a marker of lipid peroxidation, along with reduction in the level of GSH, an intracellular antioxidant. Treatment with LPPI and diclofenac maintained the tissue levels of these oxidative stress markers in monoarthritic rats. Several reports on the antioxidant property of proteins derived from latex, roots, and explant culture of C. procera are available.[8,22,26] Besides, antioxidants have also been shown to ameliorate arthritic dysfunction by scavenging free radicals.[27]
Histological analysis of the arthritic joints revealed that the FCA-induced inflammation was associated with intense neutrophil infiltration and tissue edema. The protection afforded by LPPI was also evident from a marked reduction in neutrophil infiltration as observed in treated groups. The protective effect of LPPI was found to be better than that of diclofenac. The anti-infiltrative and anti-edematogenic property of latex proteins have been well established in animal models.[14,15] The activated neutrophils are known to contribute to arthritic conditions by the release of inflammatory mediators, oxygen radicals, and tissue degrading enzymes. In addition, neutrophils synthesize eicosanoids and pro-inflammatory cytokines that eventually degrade the matrix components.[28] The inhibitory effect of latex proteins has been demonstrated on the expression of iNOS, COX-2, TNF-α, and interleukin (IL-1β) in hamster model of oral mucositis.[21]
Thus, the findings of present study indicate that LPPI has a therapeutic potential in the treatment of arthritis.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
ABOUT AUTHOR

Vijay L. Kumar
Vijay L. Kumar is a Professor in the Department of Pharmacology, All India Institute of Medical Sciences, New Delhi. She has been working in the area of molecular mechanisms affecting drug response, anti-inflammatory properties of natural products and synthetic compounds, medicinal properties of the latex of Calotropis procera.
Acknowledgments
Part of the study has been supported by grants from National Council for Research and Development (CNPq), Brazil. This study is part of the consortium Molecular Biotechnology of Plant Latex. The Senior Research Fellowship to PC from DBT, New Delhi, is acknowledged.
REFERENCES
- 1.Dequeker J. NSAIDs/corticosteroids – Primum non nocere. Adv Exp Med Biol. 1999;455:319–25. [PubMed] [Google Scholar]
- 2.Maroon JC, Bost JW, Maroon A. Natural anti-inflammatory agents for pain relief. Surg Neurol Int. 2010;1:80. doi: 10.4103/2152-7806.73804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization; 2002. WHO. WHO Traditional Medicine Strategy 2002-2005. [Google Scholar]
- 4.Vol. 3. New Delhi: Information and Publication Directorate, CSIR; 1992. Wealth of India. Raw Materials; pp. 78–84. [Google Scholar]
- 5.Kumar VL, Roy S. Calotropis procera latex extract affords protection against inflammation and oxidative stress in Freund's complete adjuvant-induced monoarthritis in rats. Mediators Inflamm. 2007;2007:47523. doi: 10.1155/2007/47523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alencar NM, Figueiredo IS, Vale MR, Bitencurt FS, Oliveira JS, Ribeiro RA, et al. Anti-inflammatory effect of the latex from Calotropis procera in three different experimental models: Peritonitis, paw edema and hemorrhagic cystitis. Planta Med. 2004;70:1144–9. doi: 10.1055/s-2004-835842. [DOI] [PubMed] [Google Scholar]
- 7.Ibrahim SR, Mohamed GA, Shaala LA, Moreno L, Banuls Y, Kiss R, et al. Proceraside A, a new cardiac glycoside from the root barks of Calotropis procera with in vitro anticancer effects. Nat Prod Res. 2014;28:1322–7. doi: 10.1080/14786419.2014.901323. [DOI] [PubMed] [Google Scholar]
- 8.Samy RP, Rajendran P, Li F, Anandi NM, Stiles BG, Ignacimuthu S, et al. Identification of a novel Calotropis procera protein that can suppress tumor growth in breast cancer through the suppression of NF-κB pathway. PLoS One. 2012;7:e48514. doi: 10.1371/journal.pone.0048514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mossa JS, Tariq M, Mohsin A, Ageel AM, al-Yahya MA, al-Said MS, et al. Pharmacological studies on aerial parts of Calotropis procera. Am J Chin Med. 1991;19:223–31. doi: 10.1142/S0192415X91000302. [DOI] [PubMed] [Google Scholar]
- 10.Ramos MV, Araújo ES, Jucá TL, Monteiro-Moreira AC, Vasconcelos IM, Moreira RA, et al. New insights into the complex mixture of latex cysteine peptidases in Calotropis procera. Int J Biol Macromol. 2013;58:211–9. doi: 10.1016/j.ijbiomac.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 11.Kumar VL, Basu N. Anti-inflammatory activity of the latex of Calotropis procera. J Ethnopharmacol. 1994;44:123–5. doi: 10.1016/0378-8741(94)90078-7. [DOI] [PubMed] [Google Scholar]
- 12.Ramos MV, Bandeira Gde P, de Freitas CD, Nogueira NA, Alencar NM, de Sousa PA, et al. Latex constituents from Calotropis procera (R. Br.) display toxicity upon egg hatching and larvae of Aedes aegypti (Linn) Mem Inst Oswaldo Cruz. 2006;101:503–10. doi: 10.1590/s0074-02762006000500004. [DOI] [PubMed] [Google Scholar]
- 13.Kumar VL, Chaudhary P, Ramos MV, Mohan M, Matos MP. Protective effect of proteins derived from the latex of Calotropis procera against inflammatory hyperalgesia in monoarthritic rats. Phytother Res. 2011;25:1336–41. doi: 10.1002/ptr.3428. [DOI] [PubMed] [Google Scholar]
- 14.Chaudhary P, Viana CA, Ramos MV, Kumar VL. Anti-edematogenic and anti-oxidant effects of high molecular weight protein fraction of Calotropis procera latex proteins in rat. J Basic Clin Pharm. 2015;6:69–73. doi: 10.4103/0976-0105.152098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ramos MV, Oliveira JS, Figueiredo JG, Figueiredo IS, Kumar VL, Bitencourt FS, et al. Involvement of NO in the inhibitory effect of Calotropis procera latex protein fractions on leukocyte rolling, adhesion and infiltration in rat peritonitis model. J Ethnopharmacol. 2009;125:387–92. doi: 10.1016/j.jep.2009.07.030. [DOI] [PubMed] [Google Scholar]
- 16.Butler SH, Godefroy F, Besson JM, Weil-Fugazza J. A limited arthritic model for chronic pain studies in the rat. Pain. 1992;48:73–81. doi: 10.1016/0304-3959(92)90133-V. [DOI] [PubMed] [Google Scholar]
- 17.Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70–7. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 18.Ohkawa H, Ohishi N, Yagi K. Assay of lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–8. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 19.Anderson GD, Hauser SD, McGarity KL, Bremer ME, Isakson PC, Gregory SA. Selective inhibition of cyclooxygenase (COX)-2 reverses inflammation and expression of COX-2 and interleukin 6 in rat adjuvant arthritis. J Clin Invest. 1996;97:2672–9. doi: 10.1172/JCI118717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soares PM, Lima SR, Matos SG, Andrade MM, Patrocínio MCA, de Freitas CDT, et al. Antinociceptive activity of Calotropis procera latex in mice. J Ethnopharmacol. 2005;99:125–9. doi: 10.1016/j.jep.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 21.Freitas AP, Bitencourt FS, Brito GA, de Alencar NM, Ribeiro RA, Lima-Júnior RC, et al. Protein fraction of Calotropis procera latex protects against 5-fluorouracil-induced oral mucositis associated with downregulation of pivotal pro-inflammatory mediators. Naunyn Schmiedebergs Arch Pharmacol. 2012;385:981–90. doi: 10.1007/s00210-012-0778-3. [DOI] [PubMed] [Google Scholar]
- 22.Roy S, Sehgal R, Padhy BM, Kumar VL. Antioxidant and protective effect of latex of Calotropis procera against alloxan-induced diabetes in rats. J Ethnopharmacol. 2005;102:470–3. doi: 10.1016/j.jep.2005.06.026. [DOI] [PubMed] [Google Scholar]
- 23.Padhy BM, Srivastava A, Kumar VL. Calotropis procera latex affords protection against carbon tetrachloride induced hepatotoxicity in rats. J Ethnopharmacol. 2007;113:498–502. doi: 10.1016/j.jep.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 24.Kumar VL, Guruprasad B, Chaudhary P, Fatmi FMA, Oliveira RSB, Ramos MV. Protective effect of proteins derived from Calotropis procera latex against acute inflammation in rat. Auton Autacoid Pharmacol. 2015;35:1–8. doi: 10.1111/aap.12022. [DOI] [PubMed] [Google Scholar]
- 25.Trachootham D, Lu W, Ogasawara MA, Nilsa RD, Huang P. Redox regulation of cell survival. Antioxid Redox Signal. 2008;10:1343–74. doi: 10.1089/ars.2007.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kumar VL, Sharma N, Souza IC, Ramos MV, da Silveira Carvalho CP. Proteins derived from in vitro culture of the callus and roots of Calotropis procera ameliorate acute inflammation in the rat paw. Appl Biochem Biotechnol. 2015;175:1724–31. doi: 10.1007/s12010-014-1361-9. [DOI] [PubMed] [Google Scholar]
- 27.van Vugt RM, Rijken PJ, Rietveld AG, van Vugt AC, Dijkmans BA. Antioxidant intervention in rheumatoid arthritis: Results of an open pilot study. Clin Rheumatol. 2008;27:771–5. doi: 10.1007/s10067-008-0848-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaplan MJ. Role of neutrophils in systemic autoimmune diseases. Arthritis Res Ther. 2013;15:219. doi: 10.1186/ar4325. [DOI] [PMC free article] [PubMed] [Google Scholar]