Abstract
Background:
Piper trichostachyon is a wild, endemic Piper species from Western Ghats of India. The folklore healers of Belagavi region use this plant, similar to Piper nigrum.
Aims:
The present study investigates the comparison between P. nigrum and P. trichostachyon using pharmacognostic parameters.
Materials and Methods:
Pharmacognostic evaluation was carried out in terms of morphological, microscopic characters, and phytochemical analysis using standard methods. Comparative physicochemical analysis between P. trichostachyon and P. nigrum was also carried out through estimation of micro-macro nutrients, high-performance thin layer chromatography (HPTLC) investigation and using piperine as a marker compound for reversed phase-ultra flow liquid chromatographic (RP-UFLC) technique.
Results:
P. trichostachyon grows in the forests, and the fruits are morphologically similar to P. nigrum fruits, so the name in Kannada “Kaadu Kalu menasu” (wild/forest black pepper). The microscopy revealed the presence of stone cells, starch grains, oil cells and globules, beaker cells, and yellowish brown pigment layer, parenchymatous cells. The presence of alkaloids, oil, and tannins were observed in P. trichostachyon fruits. The HPTLC studies visibly indicated differences among two species with 12 peaks and varied banding pattern. RP-UFLC results showed less amount of piperine in P. trichostachyon (0.05 ± 0.002 mg/g) than in P. nigrum (16.14 ± 0.807 mg/g).
Conclusion:
The study reports on pharmacognostic parameters of P. trichostachyon for the 1st time and will be useful for the identification and authentication. The comparative HPTLC and RP-UFLC studies resolve the differentiation impasse among two species. However, further biological efficacy studies are required to establish its use in traditional medicine.
SUMMARY
Piper trichostachyon grows in the forests, and the fruits are morphologically similar to Piper nigrum fruits
The microscopy of P. trichostachyon revealed the presence of stone cells, starch grains, oil cells and globules, beaker cells and yellowish brown pigment layer, parenchymatous cells
The high-performance thin layer chromatography studies visibly indicated differences among two species with varied banding pattern
Reversed phase-ultra flow liquid chromatographic results showed less amount of piperine in P. trichostachyon than in P. nigrum.
Abbreviation used: HPTLC: High Performance Thin Layer Chromatography, RP-UFLC: Reversed phase-ultra flow liquid chromatographic analysis, DST: Length of line, Maj: Length of large half axis for ellipse RDS - radius for circle, Rf: Retention Factor, TS: Transverse Section, TLC: Thin Layer Chromatography.
Keywords: High-performance thin layer chromatography, pharmacognosy, physicochemistry, Piper nigrum, Piper trichostachyon, reversed phase-ultra flow liquid chromatography
INTRODUCTION
Piper nigrum L., (family-Piperaceae) known as “King of spices” (vernacular: Kannada - Kari menasu/Kalu menasu, Marathi-Miri, English - black pepper) is one of the highly used medicinal plant by the healers of Belagavi region.[1] The literature also suggests P. nigrum (black pepper) to be a popular spice and a potent medicinal plant used in Ayurveda.[2] However, folklore healers of Belagavi region use a wild species, Piper trichostachyon (Miq.) C. DC., similar to P. nigrum.[1] Genus Piper is represented by about 13 species from the Western Ghats and 05 (02 wild; 03 cultivated) species are reported from Belagavi district.[3,4] P. trichostachyon is endemic to Western Ghats of India. It is locally called as “Kadukalu menasu,” (Kannada) or “Miri mirch” (Marathi) and in English it is known as “Pouched pepper.”[1,5]
Medicinal plants used in folklore practices are one of the major sources for drug development by the scientific community and industries. The heterogeneity of a less known medicinal plant may be treated as substitute or adulterant against a popular well-accepted species. The standardization and comparative analysis of medicinal plants used as herbal drugs is necessary and may also help to differentiate between two. Available literature revealed lack of pharmacognostic studies on the fruits of P. trichostachyon. Hence, the present investigation was undertaken with the objective to evaluate various pharmacognostic characters of P. trichostachyon fruits. Comparative phytochemical analysis between P. trichostachyon and P. nigrum was also carried out through the estimation of micro-macro nutrients, high-performance thin layer chromatography (HPTLC) investigation and using piperine as a marker compound for reversed phase-ultra flow liquid chromatographic (RP-UFLC) technique.
MATERIALS AND METHODS
Collection and authentication of plant material
Fruits of P. trichostachyon and P. nigrum were collected from Belagavi (N 15.63834°, E 074.27841°) and Uttara Kannada (N 14.4721°, E 074.5131°) regions of the Western Ghats in Karnataka state, India, respectively. The plant specimens were scientifically identified and herbaria prepared were deposited at the Regional Medical Research Centre (RMRC), (Indian Council of Medical Research), Belagavi, Karnataka, India, for future reference (voucher numbers - P. trichostachyon: RMRC 1072 and P. nigrum: RMRC 1205).
Macroscopic and microscopic analysis
Key morphological features of the fruits of P. trichostachyon were observed during macroscopic analysis using dissecting microscope (Labomed, India). Sections of the fruits were taken using Leica Germany (Model no - CM 1850) cryostat following the procedure described by Upadhya, et al.[6] The sections were of the thickness of 20 ± 2 μ. Histochemical and powder studies were carried out by using reagents and stains like iodine, concentrated sulphuric acid, concentrated hydrochloric acid (HCL), ferric chloride, Sudan III, ruthenium red, and phloroglucinol with concentrated HCL (1:1).[7] Similarly, organoleptic characters such as color, odor, and taste were determined for the fruit powder.[7]
Microphotography
Olympus America Inc., (Model no. BX 41) microscope with inbuilt analog camera (ProgRess C3-Jenoptik) was used for microphotography of the sections and powder. Computer images at ×4, ×10, and ×40 magnifications were captured using inbuilt software (ProgRes® CapturePro 2.1.1-Jenoptik laser optical system). Cell dimensions were represented as DST (length of line), Maj (length of large half axis for ellipse) and RDS (radius for circle) in microscopy as defined in ProgRes® CapturePro 2.1.1-Jenoptik, software.[6]
Preparation of extracts and preliminary phytochemical analysis
The dried fruit powder was serially extracted by the continuous shaking method given by Ankad et al., using different solvents.[8] The extracts were stored at 4°C until further use. These extracts were subjected for preliminary phytochemical screening as per standard pharmacognostic methods.[7]
Fluorescence analysis
Fruit powder (0.5 g) was mixed with 1.5 mL of respective reagent. The mixture was observed under visible light, short ultra-violet (UV) light (254 nm) and long UV light (365 nm) after a minute. Different reagents were used for the analysis of fluorescence activity.[8,9]
Comparative analysis between Piper trichostachyon and Piper nigrum
Physicochemical and nutritive content analysis
Powder physicochemical parameters viz., acid-insoluble ash, soluble extractive values, total ash, and water-soluble ash were determined as per standard methods.[7] The moisture content was estimated by loss on drying method.[7] Microelements (copper, iron, manganese, sodium, and zinc) and macroelements (calcium, magnesium, phosphorus, potassium, and sulfur) were estimated using atomic absorption techniques.[10,11] Nitrogen content was determined by Kjeldahl method.[10,11] Nutritive contents such as percent starch, total carbohydrates, reducing and nonreducing sugars were also estimated.[10,11] Protein content was calculated using the formula: % proteins = % nitrogen × 6.125.
High performance thin layer chromatography analysis
A CAMAG made HPTLC system was used during the study. Separation was achieved on a precoated HPTLC silica gel G60 F254 plates (MERCK, Germany). Bands of 6 mm length were applied using CAMAG Automated TLC Sampler (ATS-4) equipped with 25-μL syringe. Application settings given by Upadhya et al.[12] were followed. The plates were developed using 10 mL mobile phase toluene:ethyl acetate (7:3) to a distance of 80 mm in a CAMAG twin trough glass chamber (10 cm × 10 cm) saturated with the mobile phase at room temperature.[2] The developed plate was visualized and scanned at 254 nm using CAMAG TLC visualizer.[12]
Reversed phase-ultra flow liquid chromatographic analysis
Shimadzu chromatographic system (model number LC-20AD) was used to estimate the piperine content in Piper species using RP-UFLC technique as described by Upadhya et al.[13] Chromatographic separation of piperine was achieved on RP-18e (LiChrospher 100, 5 μm, 4.6 × 250 mm) with a mobile phase consisting of methanol:water (70:30). A chromatographic condition of 1.4 mL/min flow rate at 343 nm with 10 min retention time was set for piperine. The built-in LC-Solution software system was used for data processing.
One mg/mL stock solution of piperine in methanol was prepared. The stock solution was serially diluted with methanol to obtain working concentrations of 0.01, 1, 3, 10, 50, 100, 200, 400, and 1000 μg/L to obtain calibration curves. All the solutions were stored at 4°C until further use. The system suitability test, limit of detection (LOD), and limit of quantification (LOQ) were assessed followed by Upadhya et al.[13]
Chemicals
Solvents viz., methanol, water, toluene, ethyl acetate used in the analysis of HPTLC and RP-UFLC were of HPLC grade. HPLC grade piperine (97% pure) was procured from Sigma for RP-UFLC analysis. All other chemicals, reagents, and test solutions used in various physicochemical investigations were of analytical grade.
RESULTS
Morphological characters
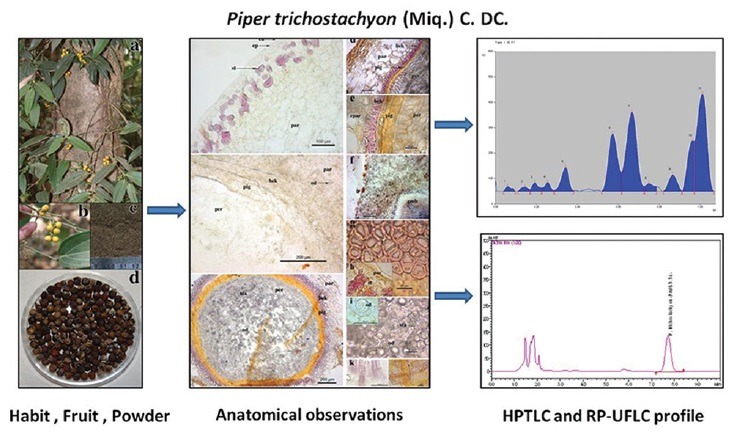
P. trichostachyon is a large woody climber [Figure 1a] found in moist deciduous forests to evergreen forests of Western Ghats. The leaves are elliptic to lanceolate, apex actue to acuminate and 3 nerved [Figure 1a]. Fruits are globose, 5–9 mm in diameter, yellow when ripe [Figure 1b].
Figure 1.

Piper trichostachyon - (a) habit; (b) fresh fruits; (c) dried fruits; (d) fruit powder
Medicinal uses and parts used
Fruits of P. trichostachyon were reported to be used in the treatment of cough, cold, fever, migraine, sperm count increase, toothache by the folklore practitioners of Belagavi district.[1]
Macro and microscopic analysis
Organoleptic characters
Fruits turned to grayish brown to black after drying with wrinkled surface [Figure 1c]. Powder showed Khaki gray nature with less pungent, peppery bitter taste compared to P. nigrum. The powder was coarse to touch [Figure 1d].
Anatomical description and powder microscopy
Fruit transverse section (TS) was circular in its outline and can be differentiated into pericarp, testa, and inner perisperm with embryo [Figure 2]. Pericarp includes outer epicarp, mesocarp and endocarp. The epicarp in TS showed a single layered epidermis covered by cuticle [Figure 2a and d]. The cells of the epicarp were oval or polygonal in nature followed by 2–4 layers of sub-epidermal layer made of thin walled parenchymatous cells with scattered, single or groups of stone cells [Figure 2a and d]. Stone cells showed heavily thickened elongated walls (thickness DST: 2.863–7.278 μm), with rectangle, round, and/or oval outline.
Figure 2.
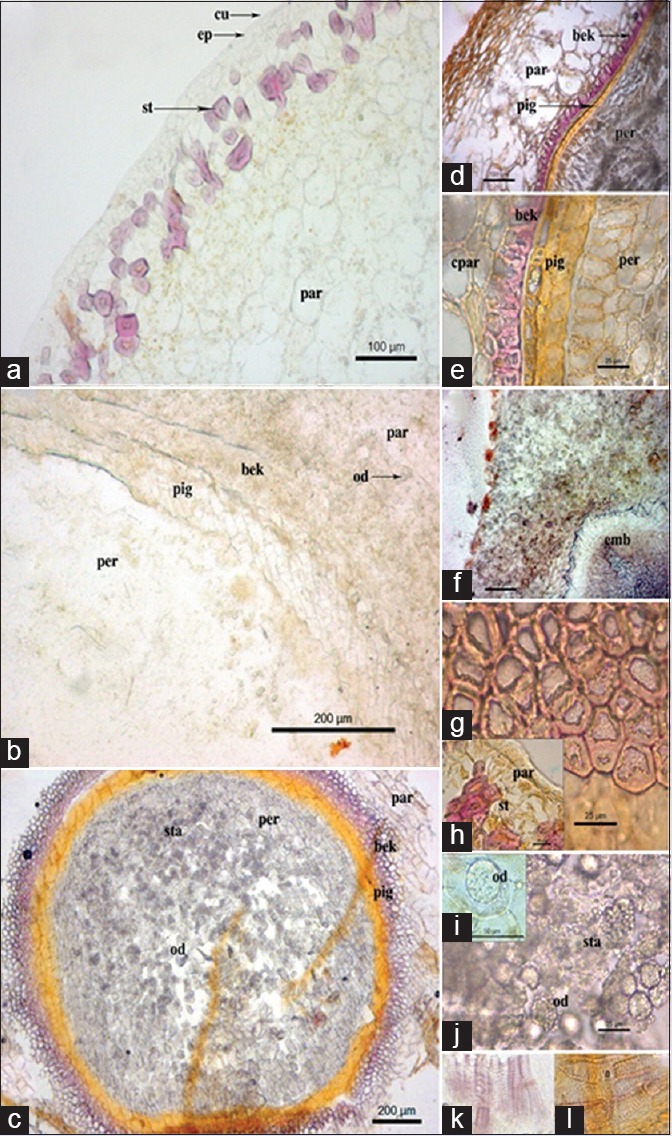
Piper trichostachyon - (a) transverse section of fruit showing epicarp region; (b) transverse section of fruit showing mesocarp region; (c) transverse section of fruit with seed region; (d) transverse section of the fruit; (e) transverse section of endocarp region; (f) transverse section showing embryo; (g and h) Stone cells; (i) oil globule; (j) Starch grains and oil globules; (k and l) Xylem vessels; bek: Beaker cells; cpar: Compressed parenchyma cells; cu: Cuticle; emb: Embryo; ep: Epidermis; od: Oil drops; par: Parenchyma cells; per: Perisperm; pig: Pigmented layer; st: Stone cells; sta: Starch grains
Mesocarp was largely composed of parenchymatous region [Figure 2b and c] consisting 6–10 layers with thin walled oval and/or polygonal cells [Figure 2b, c and e]. The cells showed accumulation of spheroidal starch grains within. Few parenchyma cells also contain prismatic calcium oxalate crystals. Isolated, elongated, irregular shaped, oleoresin, or oil cells were scattered in the mesocarp region. The inner region of the mesocarp showed compressed parenchymatous layers with scattered, small, 4–5 groups of circularly arranged, fibro-vascular bundles. Innermost layers of mesocarp were made of thick-walled parenchyma cells.
Endocarp [Figure 2c] was made up of a row of beaker cells (size WD 11.73–32.87 μm2) with greatly lignified cell walls on three sides (thickness DST 5.203–20.49 μm) than the outer layer, thus, appear as horseshoe. The beaker cells were followed by a layer of seed coat, formed by testa consisted of 2–3 layers of compressed, elongated cells, followed by a pigment layer, containing a dark – brown tannin substance (thickness DST 15.56–27.12 μm). Endosperm also contains small embryo at the central portion [Figure 2f]. Pericarp forms the larger part of the seed made up of thin-walled, rectangular and/or polygonal cells. Some of the cells contain aleurone grains and also calcium oxalate crystals. Inner portions of the pericarp consist oval, round, and/or polygonal shaped cells containing large oil globules [Figure 2b and c].
Powder microscopy showed the presence of varied size and shaped parenchymatous cells [Figure 2h]; stone cells [Figure 2d and g]; starch [grains Figure 2j]; and oil cells [Figure 2i and j]. The presence of horseshoe shaped beaker cells [Figure 2e] and brown polygonal cells of pigment layers were [Figure 2c and e] also observed. Fragments of spiral and pitted xylem vessels [Figure 2k and l]; cells containing oil drops [Figure 2i]; starch grains were seen in powder microscopy.
Histochemical and preliminary phytochemical analysis
Fruit sections of P. trichostachyon were treated with different reagents to know various cell components. The tests showed the presence of starch, oil components, lignin, and tannin clearly. The calcium oxalate crystals soluble in concentrated HCL were also occasionally observed.
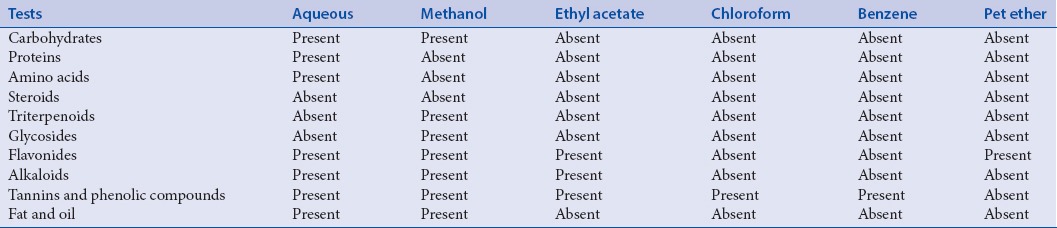
Preliminary phytochemical investigations against various extracts viz., aqueous, methanol, ethyl acetate, chloroform, benzene, and petroleum ether were carried out, and the results were presented in Table 1. Preliminary phytochemical analysis showed the presence of alkaloids, flavonoids, oil, tannins, and phenolic compounds. Maximum positive results for phytochemical tests were observed in aqueous and alcoholic extracts.
Table 1.
Preliminary phytochemical analysis of Piper trichostachyon
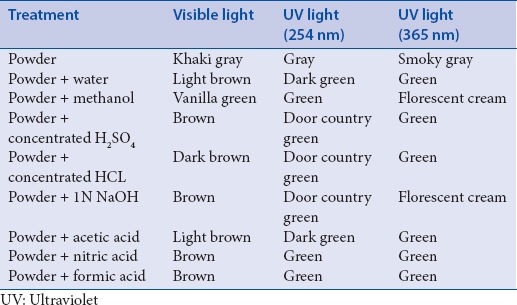
Fluorescence analysis
Fluorescence analysis of fruit powder was carried out after treating with several chemicals viz., water, methanol, acids, and sodium hydroxide. The observations were presented in Table 2.
Table 2.
Fluorescence analysis of Piper trichostachyon
Comparative physicochemical analysis
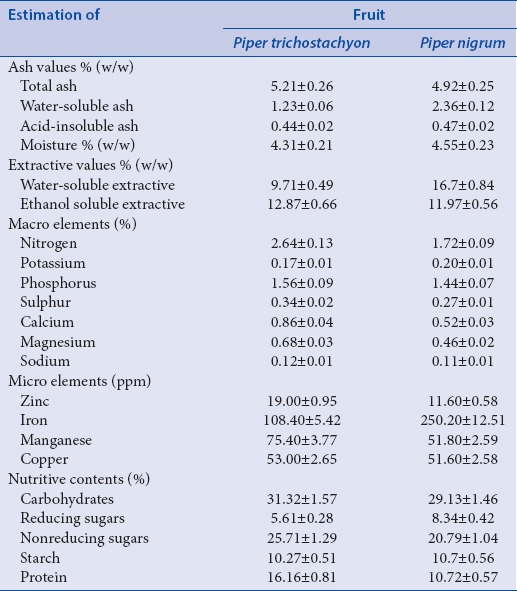
The quantitative parameters such as ash values, extractive values, estimation of micro- and macro-elements, and nutritive contents were studied for P. trichostachyon and P. nigrum. The results were represented in Table 3.
Table 3.
Estimation of ash values, moisture content, extractive values, micro, macro and nutritive content
Ash values, moisture content, and extractive values
The estimated ash values, moisture content, and extractive values were showed in Table 3 given as percent values (w/w). The estimated total ash values for both species were almost equal. The water-soluble ash and acid-insoluble ash values were higher in P. nigrum (2.36 ± 0.12% and 0. 47 ± 0.02%) than in P. trichostachyon (1.23 ± 0.06% and 0.44 ± 0.02%). The moisture contents in the fruits of both the species were within the range of standard deviation [Table 3]. The water-soluble extractive was higher in P. nigrum (16.7 ± 0.84%) whereas ethanol soluble extractive was higher in P. trichostachyon (12.87 ± 0.66%).
Estimation of micro, macro elements and nutritive contents
Estimation of macro elements (viz., nitrogen, potassium Phosphorus sulfur, calcium, magnesium, sodium), microelements (zinc, iron, copper, manganese) and nutritive contents were presented in Table 3. Nitrogen, Phosphorus, sulfur, calcium, magnesium, and sodium contents were higher in P. trichostachyon than in P. nigrum. Fruits of P. nigrum estimated to contain 2-fold more amount of iron content (250.20 ± 12.51 ppm) than that in P. trichostachyon (108.40 ± 5.42 ppm). Amount of copper was almost equal in both the fruits. The percent nutritive content was higher in the fruits of P. trichostachyon in terms of total carbohydrates and protein (31.32 ± 1.57% and 16.16 ± 0.81%) than that in P. nigrum (29.13 ± 1.46% and 10.72 ± 0.54%).
Comparative high-performance thin layer chromatography analysis
Methanol extracts were used for the HPTLC analysis. A better separation with toluene:ethyl acetate (7:1 v/v) was observed for band separation during the plate development. P. nigrum methanol extracts showed thick bands which were not differentiable from one another. So, the extracts were diluted 3 times with methanol to have the separated bands. The peaks were identified after the development at 254 nm without derivatization. The HPTLC densitogram and profile image of P. trichostachyon and P. nigrum were represented in Figure 3a–d.
Figure 3.
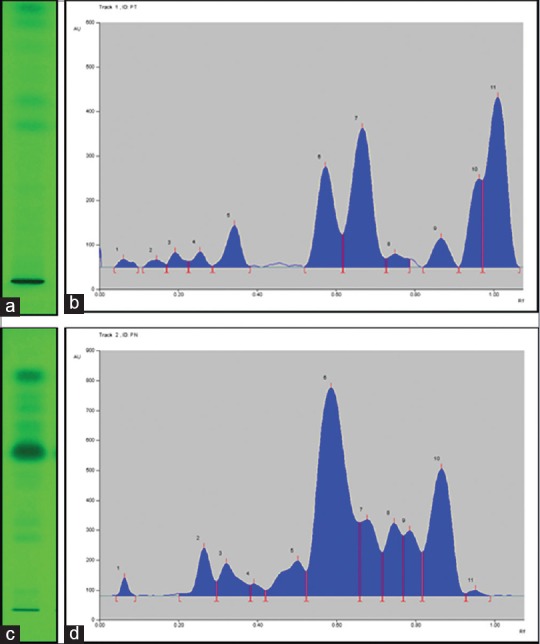
(a) Piper trichostachyon high performance thin layer chromatography profile; (b) Piper trichostachyon high performance thin layer chromatography densitogram; (c) Piper nigrum high performance thin layer chromatography profile; (d) Piper nigrum high performance thin layer chromatography densitogram
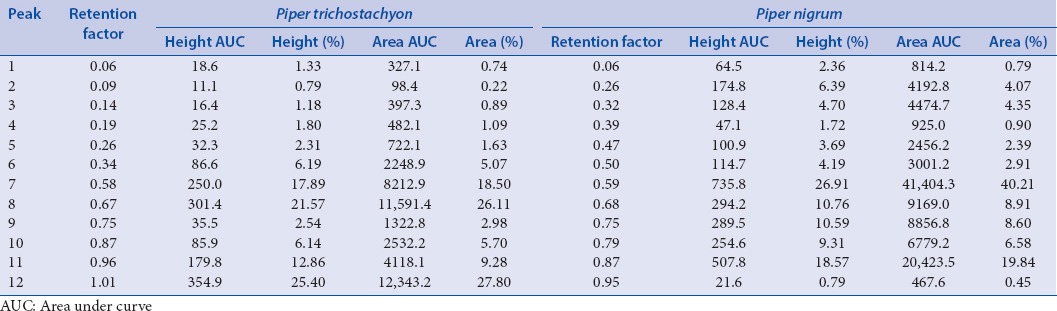
An autogenerated Table 4 shows the number of peaks, retention factor (Rf) values, area, and % area [Table 4]. Both the plant species showed 12 peaks. Three peaks at Rf 0.06, 0.75, and 0.87 were similar in P. trichostachyon and P. nigrum. The peaks at Rf 0.75 and 0.87 showed 2 times greater the percent area in P. nigrum compared to the peaks of P. trichostachyon. Other peaks showed different Rf values and percent height and area indicating the varied chemical nature among two species.
Table 4.
High performance thin layer chromatography peak table of Piper trichostachyon and Piper nigrum
Comparative quantification of piperine using reversed phase-ultra flow liquid chromatography
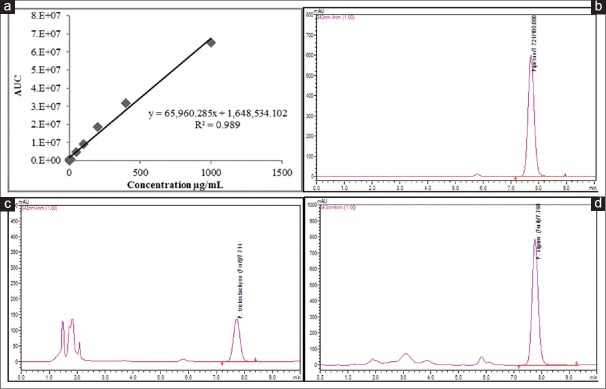
Methanol fruit extracts were used for quantitative determination of piperine, and results were expressed as mg/g on dry weight basis. Calibration curve was constructed from nine different concentrations of standard piperine against its area under the curve with a coefficient of determination (R2) 0.989 [Figure 4a]. The regression equitation (y = 65,960.285x + 1,648,534.102) showed significant relationship between peak areas and concentrations [Figure 4a]. This was used to estimate piperine content from sample extracts of both the species. LOD and LOQ of piperine were determined as 0.13 and 0.40 μg/mL, respectively. The lowest calibrator used during the study was 0.01 μg/mL. The relative standard deviation values were <2% indicating the method to be precise and reproducible. Validation of the method was carried out by spiking 50 μL (100 μg) of standard piperine to an equal volume of P. trichostachyon fruit extracts to obtain recovery within the range of 94–100%.
Figure 4.
(a) Standard piperine calibration curve; (b) Piperine reversed phase-ultra flow liquid chromatography chromatogram; (c) Piper trichostachyon reversed phase-ultra flow liquid chromatography chromatogram; (d) Piper nigrum reversed phase-ultra flow liquid chromatography chromatogram
Profiles with a retention time of 7.774 ± 0.09 min for standards and samples were obtained [Figure 4b–d]. Clear, sharp standard peaks ensured purity (98%) and also reduced compatibility issues between extraction solvent and mobile phase in the analysis. The autoscaled chromatograms were generated for standard piperine [Figure 4b] and fruit extracts of P. trichostachyon [Figure 4c] and P. nigrum [Figure 4d]. The higher content of piperine in P. nigrum fruit extract produced broad and flat-topped peak, which was resolved by further diluting the extract to 1.0% [Figure 4d]. Fruits of both the species showed the content of piperine. However, piperine content in fruits of P. nigrum (16.14 ± 0.81 mg/g) was 99 times higher than that in P. trichostachyon fruits (0.05 ± 0.01 mg/g).
DISCUSSION
According to Hegde et al., every plant shows unique nature in terms of its botany, chemistry, and therapeutic potency. Thus, it is essential to study pharmacognostic characters of a medicinal plant, not only for quality control standardization but also to understand its structure and biology.[14]
P. trichostachyon is wild, endemic species, seen in semievergreen forests; whereas P. nigrum is widely cultivated in Southern states of India.[3,4] However, wealth of India recorded grown P. trichostachyon along with the cultivated P. nigrum in Southern parts of Western Ghats during the early 20th century.[5] P. trichostachyon grows in the forests, and the fruits are morphologically very similar to the P. nigrum, so the name in Kannada “Kaadu Kalu menasu” (wild/forest black pepper).
The morphological and biosystematic studies suggested that P. trichostachyon is one of the putative parents of P. nigrum[15] with 75–90% similarity.[16,17] This may be the reason that dried fruits of both species looks similar and difficult to differentiate. However, the taste of P. trichostachyon fruits was not as pungent and spicy as that of P. nigrum. The fruits were bitter in nature than the fruits of P. nigrum.
The TS and powder microscopy revealed that P. trichostachyon had many similarities such as the presence of stone cells, starch grains, oil cells and globules, beaker cells, and yellowish brown pigment layer and parenchymatous cells compared to TS of P. nigrum fruits.[2] Preliminary phytochemical investigation showed the presence of alkaloids. Fluorescence study showed various colors indicating the presence of various inorganic and organic phytoconstituents.
Total ash, acid-insoluble ash, and water-soluble ash estimations indicate the presence of inorganic and silica components in the sample studied.[14] The comparative analysis of ash contents and extractive values in P. trichostachyon and P. nigrum lies within the range, similar to that of P. nigrum, as reported in Ayurvedic pharmacopeia.[2] Extractive values using water and ethanol indirectly indicated the presence of secondary metabolites in the powder sample.[14] Comparable amounts of nutritive contents were observed in both the species, whereas iron content was higher in P. nigrum than that of P. trichostachyon. P. trichostachyon was rich in total macronutrients compared to P. nigrum whereas it was vice versa for total micronutrient.
The analytical separation techniques such as HPTLC and HPLC were among most popular methods of choice used for quality control of raw material and finished herbal products.[18] These techniques were used because of their simplicity and reliability.[18] Results of HPTLC analysis from the fruit extracts of both species revealed variations in their densitograms. The HPTLC studies visibly indicate the differences among two species. Dried fruits of the species can be differentiated using this technique.
Results showed the presence of piperine in both P. trichostachyon and P. nigrum fruits. Present study results correlate with earlier reports by Upadhya et al.,[13] Parmar et al.,[19] and Jayashree et al.,[20] for the amount of piperine in P. nigrum. Piperine is the major chemical constituent responsible for the aromatic odor and spicy nature of P. nigrum. Thus, less amount of piperine in P. trichostachyon was indicative for its nonspiciness. However, earlier reports suggest P. trichostachyon to contain cyclopiperstachine, cyclostachine, I-piperettylpyrrolidine, trichoplein, trichonine, trichostachine, and cubebinin.[19] Further studies in P. trichostachyon are required to assess its biological efficacy to establish its use in traditional medicine.
CONCLUSION
The study reports macroscopic, microscopic, and physicochemical parameters for P. trichostachyon in comparison with P. nigrum. These parameters may help in differentiating among these species. Comparative HPTLC fingerprint analysis and estimation of marker compounds using RP-UFLC technique showed clear differentiation among P. trichostachyon and P. nigrum. Thus, both the techniques, combined with pharmacognostic parameters can serve as a tool for identification, authentication, and quality control between species of Piper.
Financial support and sponsorship
Senior Research Fellowship was awarded to first author (VU) by the Indian Council of Medical Research, New Delhi.
Conflicts of interest
There are no conflicts of interest.
ABOUT AUTHOR

Harsha V. Hegde
Dr. Harsha V. Hegde, is Scientist C, in Regional Medical Research Centre, ICMR, Belagavi. His research interest is in the areas of plant systematic, ethnomedicine and ethnopharmacology. He has expertise in natural products research, plant identification and herbarium, having 15 years experience.
Acknowledgments
Authors acknowledge Director-in-charge, Regional Medical Research Centre, Belagavi, for kind support. VU is thankful to Indian Council of Medical Research, New Delhi, for providing Indian Council of Medical Research-SRF grant for the study. Authors also acknowledge Mr. Venkatesh Millanatti, (Lab Assistant), for his assistance during the experiment.
REFERENCES
- 1.Upadhya V. Ph.D Thesis, Faculty of Science. Karnataka: KLE Deemed University, Belagavi; 2015. Ethnomedicobotany and Development of Quality Control Parameters for Selected Medicinal Plants of Belgaum Region. [Google Scholar]
- 2.Vol. 3. New Delhi (India): Ministry of Health and Family Welfare, Government of India; 2007. Department of ISM and H. The Ayurvedic Pharmacopeia of India Part 2; pp. 115–7. [Google Scholar]
- 3.Punekar SA, Lakshminarasimhan P. 1st ed. Pune, India: Biospheres Publications; 2011. Flora of Anashi National Park, Western Ghats – Karnataka. [Google Scholar]
- 4.Malpure NV. 1, 2. Kolhapur, Maharashtra: Ph D Thesis, Faculty of Botany, Shivaji University; 2010. Floristic Studies on Dicotyledones of Belgaum District. [Google Scholar]
- 5.Vol. 8. New Delhi (India): Government of India; 1996. Council of Scientific and Industrial Research. The Wealth of India: A Dictionary of Indian Raw Materials and Industrial Products; p. 117. [Google Scholar]
- 6.Upadhya V, Ankad GM, Pai SR, Hegde SV, Hegde HV. Preliminary pharmacognostic screening of Achyranthes coynei stem. J Ayurveda Integr Med. 2015;6:134–8. doi: 10.4103/0975-9476.159076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khandelwal KR. 10th ed. Pune: Nirali Publication; 2003. Practical Pharmacognosy. [Google Scholar]
- 8.Ankad GM, Pai SR, Upadhya V, Hurkadale PJ, Hegde HV. Pharmacognostic evaluation of Achyranthes coynei: Leaf. Egypt J Basic Appl Sci. 2015;2:25–31. [Google Scholar]
- 9.Kokoshi CJ, Kokoshi RJ, Salma FJ. Fluorescence of powdered vegetable drugs under ultraviolet radiation. J Pharm Sci. 1958;47:715–7. doi: 10.1002/jps.3030471010. [DOI] [PubMed] [Google Scholar]
- 10.Thimmaiah SK. 1st ed. Calcutta: Kalyani Publishers; 1999. Standard Methods of Biochemical Analysis. [Google Scholar]
- 11.Chopra SL, Kanwar JS. 1st ed. Calcutta: Kalyani Publishers; 1991. Analytical Agricultural Chemistry. [Google Scholar]
- 12.Upadhya V, Ankad G, Pai SR, Hegde HV. Comparative HPTLC analysis of stem and leaf of Achyranthes coynei with Achyranthes aspera. Plant Sci Today. 2015;2:7–10. [Google Scholar]
- 13.Upadhya V, Pai SR, Sharma AK, Hegde HV, Kholkute SD, Joshi RK. Compound specific extraction of camptothecin from Nothapodytes nimmoniana and piperine from Piper nigrum using accelerated solvent extractor. J Anal Methods Chem. 2014;2014:932036. doi: 10.1155/2014/932036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hegde SV, Hegde GR, Mulgund GS, Upadhya V. Pharmacognostic evaluation of leaf and fruit of Capsicum frutescens (Solanaceae) Pharmacognosy J. 2014;6:14–22. [Google Scholar]
- 15.Ravindran PN. Ph.D Thesis University of Calicut; 1991. Studies on Black Pepper and Some of Its Wild Relatives. [Google Scholar]
- 16.Parthasarathy U, Saji KV, Jayarajan SK, Parthasarathy VA. Biodiversity of piper in South India – Application of GIS and cluster analysis. Curr Sci. 2006;91:652–8. [Google Scholar]
- 17.Ravindran PN, Babu KN, Sasikumar B, Krishnamurthy KS. Botany and crop improvement of black pepper Black Pepper: Piper nigrum Amsterdam. In: Ravindran PN, editor. the Netherlands: Overseas Publishers Association; 2000. pp. 25–146. [Google Scholar]
- 18.Hariprasad PS, Ramakrishnan N. HPTLC fingerprint profile of Rumex vesicarius L. Asian J Pharm Clin Res. 2011;4:134–6. [Google Scholar]
- 19.Parmar VS, Jain SC, Bisht KS, Jain R, Taneja P, Jha A, et al. Phytochemistry of the genus Piper. Phytochemistry. 1977;46:591–673. [Google Scholar]
- 20.Jayashree E, John ZT, Gobinath P. Physico-chemical properties of black pepper from selected varieties in relation to market grades. J Food Sci Technol. 2009;46:263–5. [Google Scholar]