Abstract
Background:
Oxidative stress has been implicated in the pathogenesis of several acute and chronic diseases of the heart as a result of indiscriminate exposure to cardiotoxic heavy metals. The study reported here was designed to evaluate the possible ameliorative effect of aqueous extracts from Sesamum indicum (SI) seeds on oxidative stress induced by cadmium (Cd) in Wistar rats.
Materials and Methods:
Daily administration of Cd (200 mg/L Cd as CdCl2) in the animals’ main drinking water for 21 days led to oxidative stress. Thereafter, the ameliorative effects were assessed by measuring biochemical parameters such as extent of lipid peroxidation (LPO), lipid profile, and enzymatic and nonenzymatic antioxidants, as well as serum aminotransferase activities.
Results:
Treatment with SI extract elicited notable reduction in serum total cholesterol, triglyceride, and low-density lipoprotein cholesterol levels as well as concomitant increase in high-density lipoprotein cholesterol. SI extract also reversed the elevations witnessed in serum aminotransferase activities, LPO level, and ameliorated enzymatic and nonenzymatic antioxidant status in the heart of Cd-exposed rats.
Conclusion:
Thus, SI appears to be an attractive candidate with potential for the novel treatment of cardiotoxicity and management of oxidative stress arising from Cd exposure.
SUMMARY
Cadmium (200 mg/L) exposure in drinking water caused pronounced oxidative stress and cardiac tissue damage in animal model
Aqueous extract of Sesamum indicum (SI) seeds at a dose of 200 or 400 mg/kg body weight exhibited a significant reversal effect in all biochemical parameters measured such as extent of lipid peroxidation, lipid profile, and enzymatic and nonenzymatic antioxidants, as well as serum aminotransferase activities
Aqueous extract of SI seeds possess antioxidant and cardioprotective potential in a dose-dependent manner, thus conferring protection against oxidative stress induced by cadmium.
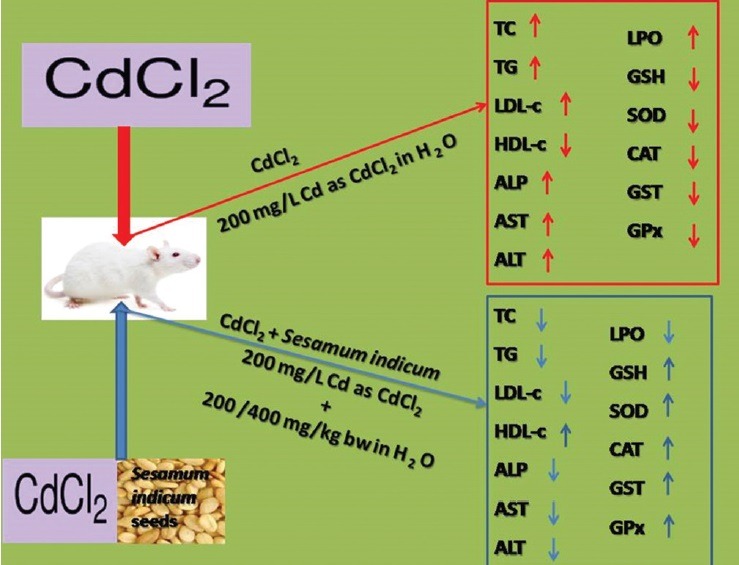
Abbreviation used: SI: Sesamum indicum, Cd: Cadmium, CdCl2: Cadmium chloride, LPO: Lipid peroxidation, TBA: Thiobarbituric acid, ALT: Alanine aminotransferase, AST: Aspartate aminotransferase, ALP: Alkaline phosphatise, TC: Total cholesterol, TG: Triglyceride, HDL-C: Highdensity lipoprotein cholesterol, LDL-C: Low-density lipoprotein cholesterol, SD: Standard deviation, GSH: Glutathione, SOD: Superoxide dismutase, CAT: Catalase, GST: Glutathione-S-transferase, GPx: Glutathione peroxidise.
Keywords: Antioxidant, cadmium, cardiotoxicity, oxidative stress, Sesamum indicum
INTRODUCTION
Indiscriminate exposure to cadmium (Cd), a known environmental biohazard contaminant, cardiotoxic, and carcinogenic heavy metal, is associated with the pathogenesis of oxidative dysfunction in various animal and human organs notably the liver, kidney, brain, testis, and heart as a result of excessive generation of free radicals, chiefly reactive oxygen species, leading to induce damage and lipid peroxidation (LPO) even at extremely small concentrations.[1,2,3] To a great extent, some toxic heavy metals are known to be concentrated in the food chain, Cd among them, and which is not degradable; consequently, it is easily transported from soil to plants, which animals and humans largely depend on for survival.[4]
Upon exposure, regardless of the route, Cd elicits its deleterious effects by impeding cellular energy metabolism; it disrupts calcium actions, generates free radicals, and causes alterations in antioxidant defense mechanisms, thereby inducing oxidative stress and damage, which affect cellular machinery involving such as proteins, DNA, and lipids.[4,5] In the recent years, the prevalence of cardiovascular diseases has been on the increase globally, accounting for major causes of morbidity and mortality.[6] Because the cardiovascular system is not the primary target of Cd toxicity, the exact influence of the metal on the cardiovascular system remains controversial. However, experimental evidence revealed that cumulative Cd exposure is associated with diabetes, hypertension, and cardiovascular disorders.[7]
Although the underlying molecular modes of action involving Cd toxicity are believed to be multifactorial, these mechanisms are poorly understood.[2,8] Recent interest has shifted to the role played by medicinal plants and natural products in mitigating and ameliorating cardiotoxicity because of their safety profiles, associated with minimal transient side effects, as well as their antioxidant and powerful bioactive compounds.[9] Sesamum indicum (SI) (sesame seed or benne seed in West Africa) is an example of such a plant common in southern temperate, subtropical, and tropical regions, predominantly in Africa, China, India, and South America. It plays an important role in human nutrition, especially because of its high content of edible oil and protein.[10] Previous studies have shown that SI possesses hepatoprotective, hypoglycemic, antihypertensive, antitumorigenic, and anti-estrogenic properties as well as its ability to increase Vitamin E concentration without the use of Vitamin E supplements and its beneficial effect in treating Parkinson's disease.[11,12] It has been reported recently to contain free radical scavengers such as flavonoids, saponins, tannins, and phenolic compounds in appreciable quantities.[11]
Little information is available on any possible cardioprotective effect of SI seed on Cd-induced cardiotoxicity. We demonstrate here that aqueous extract of SI at a dose of 200 or 400 mg/kg body weight (BW) exhibited a significant reversal effect in all biochemical parameters (notably, LPO and aspartate aminotransferase [AST]) measured in serum and heart tissues of a Cd-exposed animal model. Thus, in the treatment of cardiotoxicity, SI seeds confer protection against oxidative stress induced by Cd.
MATERIALS AND METHODS
Plant material and preparation of extract
Fresh plant material in the form of SI seeds was obtained from a local market in Ibadan, Nigeria. The air-dried seeds were grounded into a fine powder using an electric blender. At room temperature with the aid of a magnetic stirrer, 1 kg of the pulverized seed was defatted in 2000 ml of n-hexane. The resulting mixture was filtered; thereafter, 600 g of the defatted residue was air-dried for 24 h followed by maceration for 72 h in 1500 ml of distilled water. Afterward, the extract was filtered and concentrated using a rotary evaporator and dried at 60°C to constant mass to yield a brown extract. These extracts were stored in a clean sample bottle at 4°C until required.
Experimental animals
Twenty-four healthy male Wistar albino rats (weighing 140–160 g), raised in the animal house at Afe Babalola University, were used for the study. They were divided into four groups namely Group I (control) served as the control and received tap water only, Group II (CdCl2) served as Cd control while Group III (CdT1) and Group IV (CdT2) served as the treatment groups and received 200 and 400 mg/kg BW of the extract, respectively. Prior to the commencement of the experiment, the animals were fed on standard feed obtained from Ladokun Feeds, Ibadan, and allowed free access to drinking water ad libitum for 7 days for their acclimatization. In the treatment groups (CdT1 and CdT2), SI extracts (200 and 400 mg/kg BW) were given orally by gavage for 21 days. Cd was administered daily (200 mg/L Cd as CdCl2) in the animals’ (Groups II, III, and IV) main drinking water per day for 21 days to induce oxidative stress. Animal experiments were confirmed to the protocols for the Care and Use of Laboratory Animals established by the National Institutes of Health (NIH Publication No. 85–23 revised 1985) and the protocol was approved by the Institutional Animal Care and Use Committee of Afe Babalola University (ABUAD-SCIREC04/14/02/023).
Chemicals
Adrenaline, bovine serum albumin, Ellman's reagent, glutathione (GSH), and thiobarbituric acid (TBA) were from Sigma Chemical Co (St. Louis, MO, USA). Alanine aminotransferase (ALT), AST, alkaline phosphatase (ALP), total cholesterol (TC), and triglyceride (TG) assay kits were from Randox Laboratories, Ardmore, Co., Antrim, UK. All other chemicals were of the highest purity commercially available.
Biochemical analyses and histological examination of tissues
At the end of treatment period (21 days), animals of all groups were sacrificed 24 h after the last dose. The heart tissue was excised quickly and washed in ice-cold 1.15% KCl solution, dried using filter paper, and weighed. The tissue homogenate was prepared in 4 volumes of 56 mM Tris-HCl buffer (pH 7.4) containing 1.15% KCl and then centrifuged at 10,000 × g for 15 min. The supernatant was collected and stored until needed for assays. Small samples of heart tissues were fixed in 10% normal saline. Sections were cut and stained with hematoxylin and eosin.
Biochemical assays
ALT, AST, ALP, TC, and TG were evaluated by routine enzymatic methods using Randox Commercial Kits. LPO was determined by measuring TBA-reactive substances as described by Varshney and Kale;[13] superoxide dismutase (SOD) activity was assayed according to the method of Misra and Fridovich.[14] Catalase (CAT) activity was determined by adopting the method described by Sinha.[15] Glutathione peroxidase (GPx) was assayed by the method of Hafeman et al., based on the degradation of H2O2 in the presence of GSH.[16] Glutathione-S-transferase (GST) activity was determined according to Habig et al.[17] Reduced GSH level was estimated using the method described by Beutler et al. at 412 nm.[18]
Statistical analysis
All data are expressed as the mean ± standard deviation of six animals. Differences between the groups were assessed by a one-way analysis of variance (ANOVA) followed by the post-hoc LSD test for analysis of biochemical data using SPSS version 10.0 (SPSS Inc., Chicago, IL, USA) statistical software. P < 0.05 was considered statistically significant.
RESULTS
Extent of lipid peroxidation and lipid profile
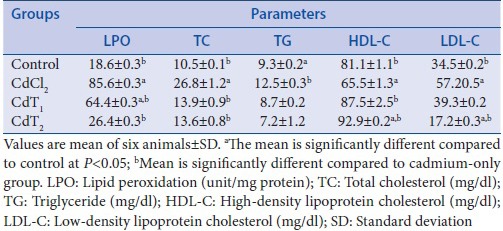
The effect of Cd exposure and SI supplementation on LPO, TC, TG, high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C) levels is shown in Table 1. There were significant elevations in the LPO level of rats exposed to Cd alone when compared with the control. SI supplementation in the treatment groups (200 or 400 mg/kg BW) considerably modulated the elevated levels. In that manner, there was a corresponding increase in the levels of TC, TG, and LDL-C associated with a concomitant reduction in the level of HDL-C in Cd-exposed rats alone when compared with the control. Interestingly, administration of SI (200 or 400 mg/kg BW) lowered TC, TG, and LDL-C levels in serum of rats treated and also increased the level of HDL-C.
Table 1.
Effect of cadmium exposure of four experimental groups on lipid peroxidation, total cholesterol, triglyceride, high-density lipoprotein cholesterol, and low-density lipoprotein cholesterol
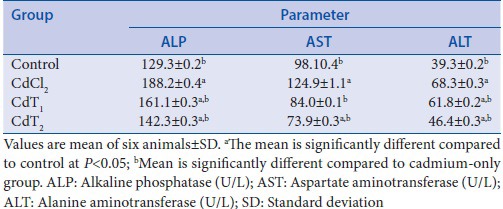
Sesamum indicum treatment alleviated serum aminotransferase activities
Cd exposure was accompanied by a significant elevation in serum aminotransferase activities [Table 2]. The activities of ALP, AST, and ALT were raised significantly in rats challenged with Cd alone compared with the control animals. SI treatment (200 or 400 mg/kg BW) countered the elevation witnessed in Cd-challenged rats alone and restored normalcy compared to control.
Table 2.
Effect of cadmium exposure of four experimental groups on alkaline phosphatase, aspartate aminotransferase, and alanine aminotransferase
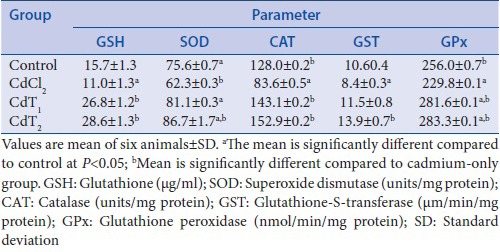
Sesamum indicum ameliorated oxidative stress induced by cadmium exposure
The induction of oxidative stress by exposure to Cd was associated with conspicuous suppression of enzymatic and nonenzymatic antioxidants in the heart homogenates [Table 3]. GSH levels as well as SOD, CAT, GST, and GPx activities in heart homogenate were significantly decreased in Cd-alone treated rats compared with the control animals. However, treatment with SI (200 or 400 mg/kg BW) resulted in a significant increase in various enzymatic and nonenzymatic antioxidants examined compared with the control group.
Table 3.
Effect of cadmium exposure of four experimental groups on glutathione, superoxide dismutase, catalase, glutathione-S-transferase, and glutathione peroxidase
Cadmium exposure alters normal heart architecture
The results obtained from the histological studies of the heart tissue showing histopathological alterations are presented in Figure 1.
Figure 1.
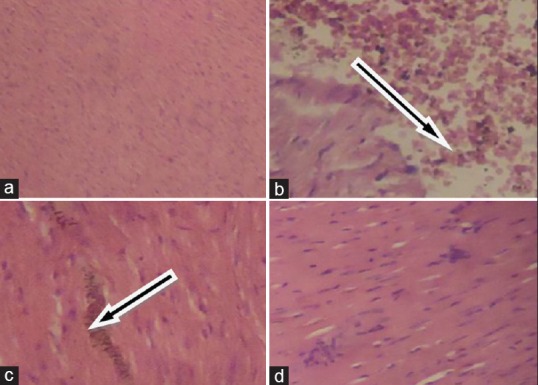
Histological illustration of rat heart sections stained with H and E, ×400. (a) Control: Normal cardiac architecture, showing no lesions or abnormalities; (b) CdCl2 (cadmium alone): Cardiac fiber degeneration characterized by extensive edema and infiltration of inflammatory cells; (c) CdT1 (200 mg/kg body weight): Illustration of mild mononuclear cellular infiltration; (d) CdT2 (400 mg/kg body weight): Edema and mononuclear cellular infiltration were hardly detected, illustrating improved cardiac architecture. Arrows in B and C indicate the regions with pronounced damage in cadmium-challenged rat heart
DISCUSSION
The present study demonstrated that aqueous extract of SI (200 or 400 mg/kg BW) significantly reversed all indices of changes in all biochemical parameters evaluated in serum and heart homogenates of Cd-challenged rats. This complemented previously identified protective roles of SI in human health and disease.
The role of cumulative Cd exposure in the induction of oxidative stress is well established and documented in the literature.[19,20] The extent of LPO plays a significant role in the measurement of toxicity of many xenobiotics and also serves as one of the most important manifestations of oxidative stress and damage. An increased LPO level in heart homogenates in this study was an indication of damage and dysfunction in the organ. This was previously demonstrated in the earlier studies on the liver, kidney, testis, and blood of rats.[19,21] LPO products are extremely reactive and exhibit distinct biological effects, depending upon their concentration, and may result in selective changes in cell signaling mechanisms as well as enhancing protein and DNA damage, and promote cytotoxicity. Elevated levels of lipid peroxides and aldehydes have been observed in atherosclerosis, ischemia-reperfusion, heart failure, Alzheimer's disease, rheumatic arthritis, cancer, and other immunological disorders.[22]
Alteration in the lipid profile in the Cd-challenged rats was evidenced by a significant elevation in serum TC, TG, and LDL-C levels as well as a corresponding reduction in HDL-C level. Other studies have reported similar results.[19,23,24] The changes observed in the lipid profile of the Cd-challenged rats are an indication of abnormalities in lipoprotein metabolism. Lipoproteins are involved in diverse processes such as immune reactions, coagulation, and tissue repair. They are macromolecules of lipid and protein which transport lipids (including cholesterol and TGs) through the vascular and extravascular fluids in the body. Oxidative alteration of lipoproteins, particularly the LDL-C, which commonly follow the reduction of lipoprotein antioxidants, especially Vitamin E, is identified to cause accumulation of cholesterol and enhance vulnerability of atherosclerosis.[24] Generally, increased levels of HDL-C and a concomitant reduction in TG, TC, and LDL-C levels are an indication of reduced risk of vulnerability to coronary artery disease.[25]
Enhancement of LPO in this study on Cd exposure may be attributed to damage in the heart membrane. Cd in its role as a cardiotoxic agent[26] has the ability to directly destroy myocardial cells and release diagnostic biomarkers of myocardial tissue damage such as lactate dehydrogenase, creatine kinase, ALP, AST, and ALT into the bloodstream. Elevations in the levels of these enzymes are connected with certain types of heart damage such as myocardial infarction, myocarditis, and heart failure. The increase observed in serum ALP, AST, and ALT activities in this study was an outcome of this phenomenon.[27] Treatment with our plant extract prevented the activities of ALP, AST, and AST from being altered in the serum of Cd-exposed rats. Thus, the protection provided by the extract suggests that it was responsible for restricting the leakage of these biomarkers due to its membrane stabilizing ability, which may be attributed to its bioactive constituents, and which was known to reduce the risk of heart failure.[28]
It is generally accepted that antioxidant enzymes are the first line of defense in response to oxidative challenges to protect cellular integrity and the pathogenesis of various degenerative diseases. During oxidative stress, depending on the extent of disturbances in the normal redox state within the cells, there is an overwhelming effect on the status of the enzymatic antioxidant enzymes notably SOD, CAT, GST, and GPx as well as the nonenzymatic antioxidant, GSH, due to overproduction of free radicals. Oxidative stress has been implicated in playing a significant role in all inflammatory diseases, alcoholism, smoking-related disorders, ischemic diseases, and many others.[5,19,29] The general reduction observed in the status of enzymatic and nonenzymatic antioxidants in the heart homogenates in Cd-treated rats in the present study was an indication of a net suppression of the total antioxidant capacity in the tissue. This is in agreement with other reports.[30] Treatment with our plant extract resulted in a significant reversal in antioxidant status, indicating the ameliorative potential of the extract. This result suggests that the plant extract contains bioactive constituents that are capable of donating hydrogen ions to free radicals, thereby scavenging them and consequently preventing their potential of inducing cellular damage. This affirms the protective influence of the extract on oxidative stress induced by Cd exposure.
In summary, Cd (200 mg/L) exposure in drinking water caused pronounced oxidative stress and cardiac tissue damage in our animal model. Our study proposes that SI possesses antioxidant and cardioprotective potential in a dose-dependent manner—400 mg/kg BW was identified as having particular efficacy with no evidence of toxicity. SI may seem therefore to have potential in the novel treatment of cardiotoxicity and management of oxidative stress arising from Cd exposure.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors hereby acknowledged the support of the University of Zululand Research Committee.
ABOUT AUTHOR

Abidemi Paul Kappo
Dr. Abidemi Paul Kappo is a Senior Lecturer in the Department of Biochemistry and Microbiology, University of Zululand, South Africa. He leads a vibrant group, Biotechnology and Structural Biochemistry (BSB) Research Group, with an interest in the development of capacity in phytotherapeutics derived from indigenous plants of South Africa, aimed at improving or preventing the development of metabolic diseases. He is a member of the South African Society of Biochemistry and Molecular Biology.
REFERENCES
- 1.Milton Prabu S, Muthumani M, Shagirtha K. Protective effect of Piper betle leaf extract against cadmium-induced oxidative stress and hepatic dysfunction in rats. Saudi J Biol Sci. 2012;19:229–39. doi: 10.1016/j.sjbs.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mitra E, Ghosh AK, Ghosh D, Mukherjee D, Chattopadhyay A, Dutta S, et al. Protective effect of aqueous Curry leaf (Murraya koenigii) extract against cadmium-induced oxidative stress in rat heart. Food Chem Toxicol. 2012;50:1340–53. doi: 10.1016/j.fct.2012.01.048. [DOI] [PubMed] [Google Scholar]
- 3.Onwuka FC, Erhabor O, Eteng MU, Umoh IB. Protective effects of ginger toward cadmium-induced testes and kidney lipid peroxidation and hematological impairment in albino rats. J Med Food. 2011;14:817–21. doi: 10.1089/jmf.2010.0106. [DOI] [PubMed] [Google Scholar]
- 4.Del Pino J, Zeballos G, Anadon MJ, Capo MA, Díaz MJ, García J, et al. Higher sensitivity to cadmium induced cell death of basal forebrain cholinergic neurons: A cholinesterase dependent mechanism. Toxicology. 2014;325:151–9. doi: 10.1016/j.tox.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 5.Dailiah Roopha P, Padmalatha C. Effect of herbal preparation on heavy metal (cadmium) induced antioxidant system in female Wistar rats. J Med Toxicol. 2012;8:101–7. doi: 10.1007/s13181-011-0194-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tellez-Plaza M, Guallar E, Howard BV, Umans JG, Francesconi KA, Goessler W, et al. Cadmium exposure and incident cardiovascular disease. Epidemiology. 2013;24:421–9. doi: 10.1097/EDE.0b013e31828b0631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alissa EM, Ferns GA. Heavy metal poisoning and cardiovascular disease. J Toxicol. 2011;2011:870125. doi: 10.1155/2011/870125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Newairy AA, El-Sharaky AS, Badreldeen MM, Eweda SM, Sheweita SA. The hepatoprotective effects of selenium against cadmium toxicity in rats. Toxicology. 2007;242:23–30. doi: 10.1016/j.tox.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 9.Zarei M, Javarappa KK, Zarei M, Baker S. Cardioprotective effect of the root extract of Hemidesmus indicus against doxorubicin-induced oxidative stress in mice. Pharm Lett. 2013;5:334–9. [Google Scholar]
- 10.Kanu PJ. Biochemical analysis of black and white sesame seeds from China. Am J Biochem Mol Biol. 2011;1:145–57. [Google Scholar]
- 11.Oyinloye BE, Nwozo SO, Amah GH, Awoyinka AO, Ojo OA, Ajiboye BO, et al. Prophylactic effect of aqueous extract of Sesamum indicum seeds on ethanol-induced toxicity in male rats. Nutr Res Pract. 2014;8:54–8. doi: 10.4162/nrp.2014.8.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kumar M, Anjoo K, Sidhraj S. Hepatoprotective activity of Sesamum indicum Linn. against CCl4-induced hepatic damage in rats. Int J Pharm Biol Arch. 2011;2:710–5. [Google Scholar]
- 13.Varshney R, Kale RK. Effects of calmodulin antagonists on radiation-induced lipid peroxidation in microsomes. Int J Radiat Biol. 1990;58:733–43. doi: 10.1080/09553009014552121. [DOI] [PubMed] [Google Scholar]
- 14.Misra HP, Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972;247:3170–5. [PubMed] [Google Scholar]
- 15.Sinha AK. Colorimetric assay of catalase. Anal Biochem. 1972;47:389–94. doi: 10.1016/0003-2697(72)90132-7. [DOI] [PubMed] [Google Scholar]
- 16.Hafeman DG, Sunde RA, Hoekstra WG. Effect of dietary selenium on erythrocyte and liver glutathione peroxidase in the rat. J Nutr. 1974;104:580–7. doi: 10.1093/jn/104.5.580. [DOI] [PubMed] [Google Scholar]
- 17.Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974;249:7130–9. [PubMed] [Google Scholar]
- 18.Beutler E, Duron O, Kelly BM. Improved method for the determination of blood glutathione. J Lab Clin Med. 1963;61:882–8. [PubMed] [Google Scholar]
- 19.Prabu SM, Muthumani M, Shagirtha K. Quercetin potentially attenuates cadmium induced oxidative stress mediated cardiotoxicity and dyslipidemia in rats. Eur Rev Med Pharmacol Sci. 2013;17:582–95. [PubMed] [Google Scholar]
- 20.Dzobo K, Naik YS. Effect of selenium on cadmium-induced oxidative stress and esterase activity in rat organs. S Afr J Sci. 2013;109:1–8. [Google Scholar]
- 21.Ognjanovic BI, Markovic SD, Ethordevic NZ, Trbojevic IS, Stajn AS, Saicic ZS. Cadmium-induced lipid peroxidation and changes in antioxidant defense system in the rat testes: Protective role of coenzyme Q(10) and Vitamin E. Reprod Toxicol. 2010;29:191–7. doi: 10.1016/j.reprotox.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 22.Ramana KV, Srivastava S, Singhal SS. Lipid peroxidation products in human health and disease. Oxid Med Cell Longev. 2013;2013:583438. doi: 10.1155/2013/583438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ige SF, Akhigbe RE. Common onion (Allium cepa) extract reverses cadmium-induced organ toxicity and dyslipidaemia via redox alteration in rats. Pathophysiology. 2013;20:269–74. doi: 10.1016/j.pathophys.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 24.Olayinka ET, Ore A, Akinnawo OO. Protective role of ethanolic extract of Sorghum bicolor leaf sheath against cadmium-induced oxidative stress in rats. Int J Pharm. 2011;2:254–60. [Google Scholar]
- 25.Choi EM, Hwang JK. Effect of some medicinal plants on plasma antioxidant system and lipid levels in rats. Phytother Res. 2005;19:382–6. doi: 10.1002/ptr.1464. [DOI] [PubMed] [Google Scholar]
- 26.Finsterer J, Ohnsorge P. Influence of mitochondrion-toxic agents on the cardiovascular system. Regul Toxicol Pharmacol. 2013;67:434–45. doi: 10.1016/j.yrtph.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 27.Koti BC, Nagathan S, Vishwanathswamy A, Gadad PC, Thippeswamy A. Cardioprotective effect of Vedic guard against doxorubicin-induced cardiotoxicity in rats: A biochemical, electrocardiographic, and histopathological study. Pharmacogn Mag. 2013;9:176–81. doi: 10.4103/0973-1296.111287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mirmiran P, Bahadoran ZM, Golzarand M, Rajab A, Azizi FA. Ardeh (Sesamum indicum) could improve serum triglycerides and atherogenic lipid parameters in type 2 diabetic patients: A randomized clinical trial. Arch Iran Med. 2013;16:652–6. [PubMed] [Google Scholar]
- 29.Lobo V, Patil A, Phatak A, Chandra N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn Rev. 2010;4:118–26. doi: 10.4103/0973-7847.70902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ahmed MA, Hassanein KM. Cardio protective effects of Nigella sativa oil on lead induced cardio toxicity: Anti-inflammatory and antioxidant mechanism. J Physiol Pathol. 2013;4:72–80. [Google Scholar]


