Abstract
Objective:
The present study sought to detect spinal sirtuin 1 (SIRT1) and acetylation of histone H3 (Ac-H3) expression in rats with burn injury pain (BIP model).
Procedures and Results:
A BIP model was first established. BIP rats showed lower paw withdrawal threshold (PWT) from day 1, which persisted for 21 days following the burn injury. Spinal SIRT1/Ac-H3 expression increased following burn injury. The intrathecal use of resveratrol increased PWT and SIRT1 expression but induced down-regulation of Ac-H3 expression. We first demonstrated that the inhibition of SIRT1 significantly induced mechanical allodynia in naïve rats. The preinjection of SIRT1 inhibitor partly antagonized the analgesic effects of resveratrol in BIP rats.
Conclusion:
Inhibition of SIRT1 produces pain facilitation in the naïve rats. The expression of spinal SIRT1 increased after burn injury in the BIP model. The activation of spinal SIRT1 might mediate the resveratrol-induced analgesic effects.
SUMMARY
Burn injury resulted in pain facilitation
Resveratrol attenuates pain facilitation induced by burn injury
Intrathecal injection of resveratrol attenuates burn injury pain by increasing spinal sirtuin 1 (SIRT1) expression
Inhibition of SIRT1 by selisistat, an SIRT1 inhibitor attenuated analgesic effects of resveratrol

Abbreviations used: SIRT1: Sirtuin 1, Ac-H3: Acetylation of histone H3, SD: Sprague-Dawley, EX527: Selisistat, an SIRT1 inhibitor, BIP: Burn injury pain, DMSO: Dimethyl sulfoxide, PWTs: Paw withdrawal thresholds
Keywords: Burn-induced pain, mechanical allodynia, resveratrol, sirtuin 1
INTRODUCTION
Burn injury-induced pain (BIP) is intensely painful and often long-lasting. Treatments for BIP are often ineffective. As a result, studies of the mechanism of BIP and optimized analgesic strategies are important.[1,2] Previous studies have demonstrated that molecular changes (TrkA, µ-opioid receptors, PKCγ, and p38-MAPK)[3,4,5] in the nervous system mediate burn injury-induced nociceptive sensitization. Stimulations from chronic pain may induce varieties of changes in pain-related gene expressions in the spinal and supraspinal neuronal systems. Recent studies have shown that abnormal changes of histone acetylation are important epigenetic mechanisms that may be involved in nociceptive information transmission.[6,7]
Sirtuin 1 (SIRT1) is a type of histone deacetylases that are involved in the development of several diseases through the deacetylation of histones.[8] In the SOD1G93A mouse model, spinal SIRT1 was up-regulated.[8,9] In addition, SIRT1 was decreased in the L4–L5 segments of chronic constriction injury (CCI) animals.[10,11] Resveratrol is a phytoalexin that can be derived from natural foods. It can bind to the N-terminus of SIRT1 and result in the activation of SIRT1, which may have a meaningful role in several physiopathological disorders.[8,9,10,11] The anti-inflammatory effect and anti-tumor effect of resveratrol are receiving an increasing amount of attention. Recent studies have demonstrated that intrathecal (i.t) injection of resveratrol antagonized CCI-induced pain hypersensitivities by increasing spinal SIRT1 expression and inhibiting the acetylation of histone H3 (Ac-H3).[10,11]
So far, it is not clear whether resveratrol plays a meaningful role in burn injury-induced pain. To answer this question, the present study intends to understand the nature of spinal SIRT1 and Ac-H3 expression changes after burn injury and whether the intrathecal administration of resveratrol attenuates BIP and affects spinal SIRT1 and Ac-H3 expression in BIP rats.
PROCEDURES
Animal and intrathecal injection
Male Sprague-Dawley (SD) rats (230–300 g) were chosen. The experimental procedures complied with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and the approval of the Scientific Investigation Board of Shandong University and Xuzhou Medical College.
After anesthetizing the rats with sevoflurane, a microinjection syringe needle was used to insert into the interspaces of L5 and L6. When tail- or paw-flick responses were observed, prefilled drugs were injected. We deleted rats with motor dysfunctions.[12] Dosages of resveratrol (Sigma) and selisistat, an SIRT1 inhibitor (EX527, Selleck), were chosen according to a recent study[11] and our preliminary experiments.
Burn-pain model
The method followed Chang's description.[13] Briefly, after baseline response evaluation and 3% sevoflurane anesthesia, the right hind paw was placed on a metal block (85 ± 5°C) that was heated via thermostatic feedback to a bath that circulates water through the metal block. A 10-g sandbag was placed on top of the paw (heel region) to maintain a consistent pressure between the plantar surface and the metal surface for 15 s. This produced injury to the plantar surface of the paw and a burn area of approximately 1% of the total body surface. To prevent infection, silver sulfadiazine ointment was applied to the injured site twice daily until scar tissue formed (approximately 6–7 days after injury).
Von Frey test
As previously described,[14] SD rats were individually housed in the single cage (26 cm3 × 20 cm3 × 14 cm3) with a 5 mm2 × 5 mm2 wire mesh grid floor. The hind paw withdrawal threshold (PWT) was utilized to observe the allodynia after accommodation for 30 min. A series of filaments (0.40, 0.60, 1.4, 2.0, 4.0, 6.0, 8.0, 10.0, and 15.0 g) were utilized to perform these tests. We placed von Frey hairs for approximately 6–8 s. Each stimulus was used 5 times at 30-s intervals. Furthermore, 50% of PWTs were calculated according to the up-down method.
Western blot analysis
The right L4–L5 spinal segments were collected for analyses. The protocols were similar to those described previously. Rabbit anti-SIRT1 (1:500), rabbit anti-H3 (1:2000) and Ac-H3 (1:1000), and mouse anti-GAPDH primary antibodies were used for incubation. The secondary antibody was alkaline phosphatase-conjugated goat anti-rabbit (1:4000). The blots were repeated 3 times, and the data were consistent among the experiments. The densities of the band area were calculated following a previously described method.[10,11]
Statistical analysis
The data are presented as the mean ± standard error of mean and all data were collected blindly. The Tukey test, SNK test in ANOVA, and repeated measures ANOVA were used to detect significant differences. P < 0.05 was considered statistically significant.
RESULTS
Burn injury resulted in pain facilitation in the Von Frey test
An increasing amount of evidence has proven that burn injury can induce pain hypersensitivities.[3,13] In Figure 1, rats were divided into three groups (naive group; sham group; BIP group). The rats developed a significantly lower PWTs in the Von Frey test after burn injury (P < 0.01), thereby indicating the development of mechanical allodynia. The PWTs became obvious from day 1, which persisted for 21 days following burn injury. Sham animals did not show remarkable changes of PWTs (P > 0.05).
Figure 1.

Burn injury-induced mechanical allodynia in the ipsilateral legs. Sham rats did not show a decrease in paw withdrawal thresholds. **P < 0.01 compared to day 0 (mean ± standard error of mean, n = 8)
Resveratrol attenuates pain facilitation induced by burn injury
To observe whether i.t injection of resveratrol can produce analgesic effects at the spinal level in the BIP model, we divided the rats into seven groups (sham group, sham + dimethyl sulfoxide (DMSO) group, sham + 400 µg resveratrol group, BIP group, BIP + DMSO group, BIP + 200 µg resveratrol group, and BIP + 400 µg resveratrol group) and intrathecally injected resveratrol or DMSO for three consecutive days after burn injury (7–9 days). As shown in Figure 2, the rats developed noticeably lower PWTs (P < 0.01). This indicated the development of pain hypersensitivity. When compared with BIP + DMSO rats, the PWTs of BIP + resveratrol rats decreased at 7–9 days following burn injury (P < 0.01). Repeated intrathecal infusions of resveratrol did not affect the PWTs of sham rats (P > 0.05).
Figure 2.
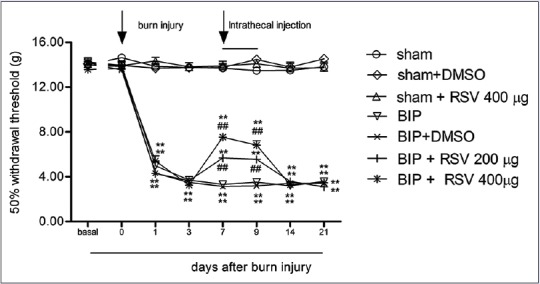
Intrathecal administration of resveratrol (200 and 400 μg) attenuated the mechanical allodynia that was induced by burn injury. Resveratrol (200 and 400 μg) or dimethyl sulfoxide was used (day 7–9 following burn injury, intrathecal) (mean ± standard error of mean, n = 8). **P < 0.01, versus day 0; ##P < 0.01 versus the burn injury pain/dimethyl sulfoxide group
Intrathecal use of resveratrol attenuates burn injury pain by increasing spinal sirtuin 1 expression
Previous studies have shown that decreased spinal SIRT1 expression and increased Ac-H3 expression are involved in the development of neuropathic rats. The SIRT1 agonist (NAD or resveratrol) attenuated established pain facilitation and increased SIRT1 expression in CCI rats.[10,11] Resveratrol potentially represents a novel therapeutic strategy for neuropathic pain.
As shown in Figure 3, the rats were divided into six groups (naive group, sham group, and BIP 3, 7, 14, and 21 day groups). We first demonstrated that the intensities of specific SIRT1 and Ac-H3 bands increased after burn injury (P < 0.01) and that the time course of the increase in SIRT1 and Ac-H3 expression matched closely the development of pain facilitation. Figure 4 indicates that the rats were divided into five groups (naive group, sham group, BIP group, BIP + DMSO group, and BIP + 400 µg resveratrol group). In contrast to the BIP/DMSO rats, resveratrol (400 µg/day) increased SIRT1 expression and decreased Ac-H3 expression in the spine of BIP/resveratrol rats (P < 0.01).
Figure 3.
(a) The increase of sirtuin 1 expression in L4-5 segments after burn injury; (b) The increase of acetylation of histone H3 expression in L4-5 segments after burn injury
Figure 4.
(a) Resveratrol increased sirtuin 1 expression in the spine of burn injury pain/resveratrol rats. (b) Resveratrol decreased acetylation of histone H3 expression in the spine of burn injury pain/resveratrol rats
Inhibition of sirtuin 1 by selisistat, an SIRT1 inhibitor attenuated the analgesic effects of resveratrol
Previous studies have reported that the decrease of SIRT1 expression paralleled the development of neuropathic pain.[10,11] To investigate whether the inhibition of SIRT1 resulted in pain facilitation, we injected different doses of EX527 into the L5–L6 interspaces in naïve rats.
As shown in Figure 5, the rats were divided into six groups (naive group, DMSO group, EX527 6.4 µg group, EX527 8 µg group, EX527 10 µg group, and EX527 12.5 µg group). We first demonstrated that EX527 (10, 12.5 µg) induced robustly lower PWTs in naïve rats. The decrease in PWTs lasted for approximately 90 min. EX527-induced pain facilitation broadened previous studies, which indicated that the inhibition of SIRT1 resulted in pain facilitation in naïve rats. As shown in Figure 6, the rats were divided into five groups (sham + DMSO + DMSO group, BIP + DMSO + DMSO group, BIP + DMSO + 8 µg EX527 group, BIP + 400 μg resveratrol + DMSO group, and BIP + 400 μg resveratrol + 8 µg EX527 group). We found that pretreatment with EX527 (8 µg) or the vehicle did not affect PWTs when compared to baseline values (P > 0.05). Meanwhile, the injection of resveratrol (400 μg) robustly increased pain thresholds and pretreatment with EX527 (8 µg) partly attenuated the analgesia that was induced by resveratrol in BIP rats (P < 0.01).
Figure 5.

Inhibition of sirtuin 1 produced mechanical hyperalgesia in naïve rats. To investigate whether the inhibition of sirtuin 1 contributed to pain facilitation, we evaluated the effect of selisistat, an SIRT1 inhibitor (6.4, 8, 10, 12.5 µg) on the mechanical withdrawal threshold. Intrathecal injection of selisistat, an SIRT1 inhibitor (10 and 12.5 µg), significantly decreased paw withdrawal thresholds (P < 0.05). In contrast, no differences in paw withdrawal thresholds were observed in dimethyl sulfoxide group and selisistat, an SIRT1 inhibitor (6.4 and 8 µg) groups (P > 0.05). **P < 0.01, versus-1 h; ##P < 0.01 versus dimethyl sulfoxide group (mean ± standard error of mean, n = 8)
Figure 6.

Pretreatment with selisistat, an SIRT1 inhibitor (8 µg) reversed the analgesic effects that were induced by resveratrol. Rats exhibited marked mechanical allodynia following burn injury. Pretreatment with selisistat, an SIRT1 inhibitor (8 µg) or the vehicle did not affect PWTs when compared with the-1 h. Injection of resveratrol (400 μg) robustly increased pain thresholds, and pretreatment with selisistat, an SIRT1 inhibitor (8 µg) partly attenuated the analgesia that was induced by resveratrol. *P < 0.05, **P < 0.01, versus-1 h; #P < 0.05, ##P < 0.01 versus the burn injury pain + resveratrol + selisistat, an SIRT1 inhibitor group (comparing between the burn injury pain + resveratrol + dimethyl sulfoxide group and the burn injury pain + resveratrol + selisistat, an SIRT1 inhibitor group); &P < 0.05, &&P < 0.01 versus the burn injury pain + dimethyl sulfoxide + selisistat, an SIRT1 inhibitor group (comparing between the burn injury pain + dimethyl sulfoxide + selisistat, an SIRT1 inhibitor group and the burn injury pain + resveratrol + selisistat, an SIRT1 inhibitor group) (mean ± standard error of mean, n = 8)
DISCUSSION
Burn injury-induced pain can be observed frequently and persists for 2–3 months.[1,5,13] In this experiment, we placed the hind paw on a metal block (85 ± 50°C) for 15 s. The rats demonstrated rapid pain facilitation in the Von Frey test until 3 weeks following burn injury. In this study, elevated SIRT1 and Ac-H3 expression was observed until 21 days after burn injury. The time-course of elevation in these substances of expression on the ipsilateral side of the burn injury matched closely with the development of allodynia after burn injury. The intrathecal administration of resveratrol, an SIRT1 agonist, attenuated the established pain facilitation that was induced by burn injury. Resveratrol also increased SIRT1 expression but decreased Ac-H3 expression. Bai et al. recently demonstrated that the expression of SIRT1 increased upon burn-induced lung injury. Resveratrol enhanced SIRT1 expression but attenuated the upregulation of tumor necrosis factor-alpha and interleukin-1 beta in rats with burn-induced lung injuries. The results from Bai's study are in accordance with our data.[15] It is thought that along with the impairment of the body, the expression of SIRT1 increased through a negative feedback loop to relieve pulmonary inflammation and burn-induced pain, thereby promoting DNA repair and inhibiting extensive tissue impairment.
The anatomical analysis of SIRT1 immunoreactivity highlighted the presence of SIRT1 in all four major divisions of both adult rodent and human spinal cords, including the cervical, thoracic, lumbar, and sacral columns.[16] Previous studies have shown that the SIRT1 expression decreased in CCI models and that SIRT1 agonists (nicotinamide adenine dinucleotide [NAD] or resveratrol) attenuated the established pain facilitation following sciatic nerve ligation.[10,11] Inhibition of SIRT1 by EX527 can antagonize the analgesic effects of NAD + or resveratrol in a neuropathic pain model. The above results suggested that SIRT1 and its substrates might mediate signal transduction in the nervous system of neuropathic pain models. Different types of pain stimulations after nerve injury may remodel information transmission between nerve cells, which involves several pain-related transcription factors. Furthermore, alternations in their gene and mRNA expression might also be regulated by SIRT1.[10,17,18] According to the above information, we speculated that as a negative feedback factor to burn injury, the increase in spinal SIRT1 may promote the DNA repair and inhibit pain-related transcription factors, which can further mediate the development of BIP.
We first demonstrated that EX527 significantly induced mechanical allodynia in the naïve rats, which indicates that the inhibition of SIRT1 can result in pain facilitation in naïve rats. Previous studies have reported that SIRT1 expression decreased in CCI-induced neuropathic pain models. Data for CCI models indicate that the down-regulation of SIRT1 was observed following CCI surgery.[10,11] Our data also indicated a robust increase of spinal SIRT1 expression in BIP rats. As an SIRT1 agonist, resveratrol induced increased SIRT1 expression and pain thresholds and the SIRT1 inhibitor partly antagonized the resveratrol-induced analgesia, which indicated that resveratrol attenuates this pain due to an increase of spinal SIRT1 expression. Our data and previous studies have demonstrated that spinal SIRT1 increased in the BIP model while SIRT1 decreased in CCI models. We speculate the probable mechanisms of these differences may be that the roles of SIRT1 in different pain models are different.
CONCLUSION
Our data suggested that spinal SIRT1 and Ac-H3 increased following burn injury and that spinal SIRT1 and Ac-H3 may be involved in the resveratrol-induced analgesia. The mechanisms of BIP that result in the activation of SIRT1 and energy metabolism pathways in the spinal levels require further research.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (31100801, 81200858).
ABOUT AUTHOR

Zhi-Jian Fu
Prof. Zhi-Jian Fu obtained her Ph.D. degree in 1999 from Tongji Medical University, China. Currently, she is the chairperson of pain management of the Shandong Provincial Hospital affiliated to Shandong University. She studies various aspects of pain disorders, including neuropathic pain, spinal pain, and cancer pain.
REFERENCES
- 1.Summer GJ, Dina OA, Levine JD. Enhanced inflammatory hyperalgesia after recovery from burn injury. Burns. 2007;33:1021–6. doi: 10.1016/j.burns.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 2.Summer GJ, Puntillo KA, Miaskowski C, Green PG, Levine JD. J Pain. 2007. Burn injury pain: The continuing challenge; pp. 533–48. [DOI] [PubMed] [Google Scholar]
- 3.Wang S, Lim G, Yang L, Zeng Q, Sung B, Jeevendra Martyn JA, et al. A rat model of unilateral hindpaw burn injury: Slowly developing rightwards shift of the morphine dose-response curve. Pain. 2005;116:87–95. doi: 10.1016/j.pain.2005.03.044. [DOI] [PubMed] [Google Scholar]
- 4.Summer GJ, Puntillo KA, Miaskowski C, Dina OA, Green PG, Levine JD. TrkA and PKC-epsilon in thermal burn-induced mechanical hyperalgesia in the rat. J Pain. 2006;7:884–91. doi: 10.1016/j.jpain.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 5.Sorkin L, Svensson CI, Jones-Cordero TL, Hefferan MP, Campana WM. Spinal p38 mitogen-activated protein kinase mediates allodynia induced by first-degree burn in the rat. J Neurosci Res. 2009;87:948–55. doi: 10.1002/jnr.21905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Uchida H, Matsushita Y, Ueda H. Epigenetic regulation of BDNF expression in the primary sensory neurons after peripheral nerve injury: Implications in the development of neuropathic pain. Neuroscience. 2013;240:147–54. doi: 10.1016/j.neuroscience.2013.02.053. [DOI] [PubMed] [Google Scholar]
- 7.Kiguchi N, Kobayashi Y, Maeda T, Fukazawa Y, Tohya K, Kimura M, et al. Epigenetic augmentation of the macrophage inflammatory protein 2/C-X-C chemokine receptor type 2 axis through histone H3 acetylation in injured peripheral nerves elicits neuropathic pain. J Pharmacol Exp Ther. 2012;340:577–87. doi: 10.1124/jpet.111.187724. [DOI] [PubMed] [Google Scholar]
- 8.Finkel T, Deng CX, Mostoslavsky R. Recent progress in the biology and physiology of sirtuins. Nature. 2009;460:587–91. doi: 10.1038/nature08197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee JC, Shin JH, Park BW, Kim GS, Kim JC, Kang KS, et al. Region-specific changes in the immuno reactivity of SIRT1 expression in the central nervous system of SOD1 (G93A) transgenic mice as an in vivo model of amyotrophic lateral sclerosis. Brain Res. 2012;1433:20–8. doi: 10.1016/j.brainres.2011.11.019. [DOI] [PubMed] [Google Scholar]
- 10.Shao H, Xue Q, Zhang F, Luo Y, Zhu H, Zhang X, et al. Spinal SIRT1 activation attenuates neuropathic pain in mice. PLoS One. 2014;9:e100938. doi: 10.1371/journal.pone.0100938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yin Q, Lu FF, Zhao Y, Cheng MY, Fan Q, Cui J, et al. Resveratrol facilitates pain attenuation in a rat model of neuropathic pain through the activation of spinal Sirt1. Reg Anesth Pain Med. 2013;38:93–9. doi: 10.1097/AAP.0b013e3182795b23. [DOI] [PubMed] [Google Scholar]
- 12.Xu JJ, Walla BC, Diaz MF, Fuller GN, Gutstein HB. Intermittent lumbar puncture in rats: A novel method for the experimental study of opioid tolerance. Anesth Analg. 2006;103:714–20. doi: 10.1213/01.ane.0000226100.46866.ea. [DOI] [PubMed] [Google Scholar]
- 13.Chang YW, Tan A, Saab C, Waxman S. Unilateral focal burn injury is followed by long-lasting bilateral allodynia and neuronal hyperexcitability in spinal cord dorsal horn. J Pain. 2010;11:119–30. doi: 10.1016/j.jpain.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 14.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 15.Bai X, Fan L, He T, Jia W, Yang L, Zhang J, et al. SIRT1 protects rat lung tissue against severe burn-induced remote ALI by attenuating the apoptosis of PMVECs via p38 MAPK signaling. Sci Rep. 2015;5:10277. doi: 10.1038/srep10277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zakhary SM, Ayubcha D, Dileo JN, Jose R, Leheste JR, Horowitz JM, et al. Distribution analysis of deacetylase SIRT1 in rodent and human nervous systems. Anat Rec (Hoboken) 2010;293:1024–32. doi: 10.1002/ar.21116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Borrelli E, Nestler EJ, Allis CD, Sassone-Corsi P. Decoding the epigenetic language of neuronal plasticity. Neuron. 2008;60:961–74. doi: 10.1016/j.neuron.2008.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Imai S, Saeki M, Yanase M, Horiuchi H, Abe M, Narita M, et al. Change in microRNAs associated with neuronal adaptive responses in the nucleus accumbens under neuropathic pain. J Neurosci. 2011;31:15294–9. doi: 10.1523/JNEUROSCI.0921-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]




