Abstract
Background:
Longan is a fruit tree known to contain many phenolic components, which are capable of protecting people from oxidative damage through an anti-inflammatory mechanism. It may be also worthwhile to study the effect on lowering uric acid activity.
Materials and Methods:
This study investigates the lowering of uric acid using longan extracts, including flowers, pericarps, seeds, leaves, and twigs, on potassium-oxonate-induced hyperuricemia mice and its inhibitory actions against xanthine oxidase (XO) activities.
Results:
The findings revealed that ethyl acetate fraction of longan extracts exhibited strong XO-inhibitory activity, and the flower extracts (IC50 = 115.8 μg/mL) revealed more potent XO-inhibitory activity to those of pericarps (118.9 μg/mL), twigs (125.3 μg/mL), seeds (262.5 μg/mL), and leaves (331.1 μg/mL) in vitro. In addition, different dosages of longan extract (50–100 mg/kg) were administered to hyperuricemic mice. The lowering effect of longan extracts on uric acid at 75 mg/kg markedly reduced plasma uric acid levels in decreasing order: Flowers (80%) > seeds (72%) > pericarps (64%) > twigs (59%) > leaves (41%), compared with allopurinol (89%). Finally, 10 isolated phytochemicals from longan flowers were then examined in vitro. The results indicated that proanthocyanidin A2 and acetonylgeraniin A significantly inhibited XO activity in vitro. This is the first report providing new insights into the urate-reducing effect of phenolic dimer and hydrolyzable tannin, which can be developed to potential hypouricemic agents.
SUMMARY
Longan flower extracts possess more potent XO-inhibitory activity than pericarps, twigs, seeds, and leaves in vitro
The lowering effect of longan flowers and seeds extracts markedly reduced plasma uric acid levels as compared to allopurinol in vivo
The extract proanthocyanidin A2 and acetonylgeraniin A were demonstrated potent XO inhibitory activity in vitro
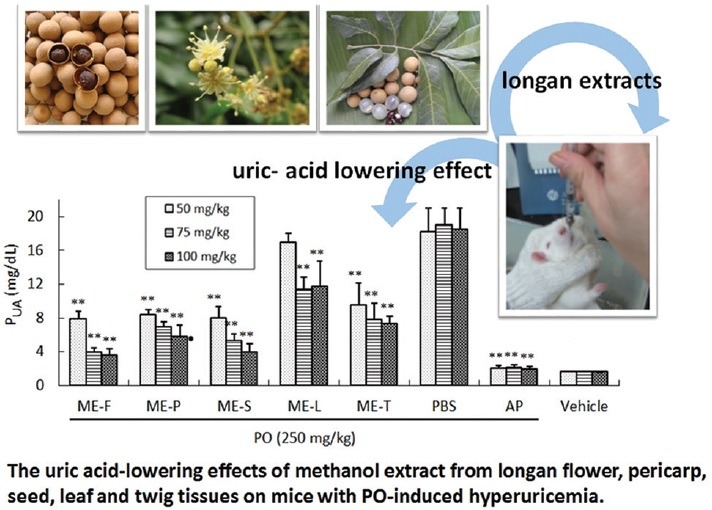
Abbreviations used: PO: Potassium-oxonate, XO: xanthine oxidase, HE: n-hexane, EA: ethyl acetate, i.p.: intraperitoneal, PBS: phosphate-buffered saline, AP: allopurinol, PUA: plasma uric acid.
Keywords: Dimocarpus longan Lour, hypouricemic effect, xanthine oxidase inhibition
INTRODUCTION
Longan (Dimocarpus longan Lour.) is an evergreen fruit tree belonging to the Sapindaceae family. It is native to temperate and tropical Asia and widely cultivated in Southern China and Southeast Asia. Many people love to eat longan due to its delicate flavor and sweet taste; the fruit is also used as a medicament by tonifying heart blood, spleen qi, strengthening postpartum weakness, and calming the spirit, especially when dried.
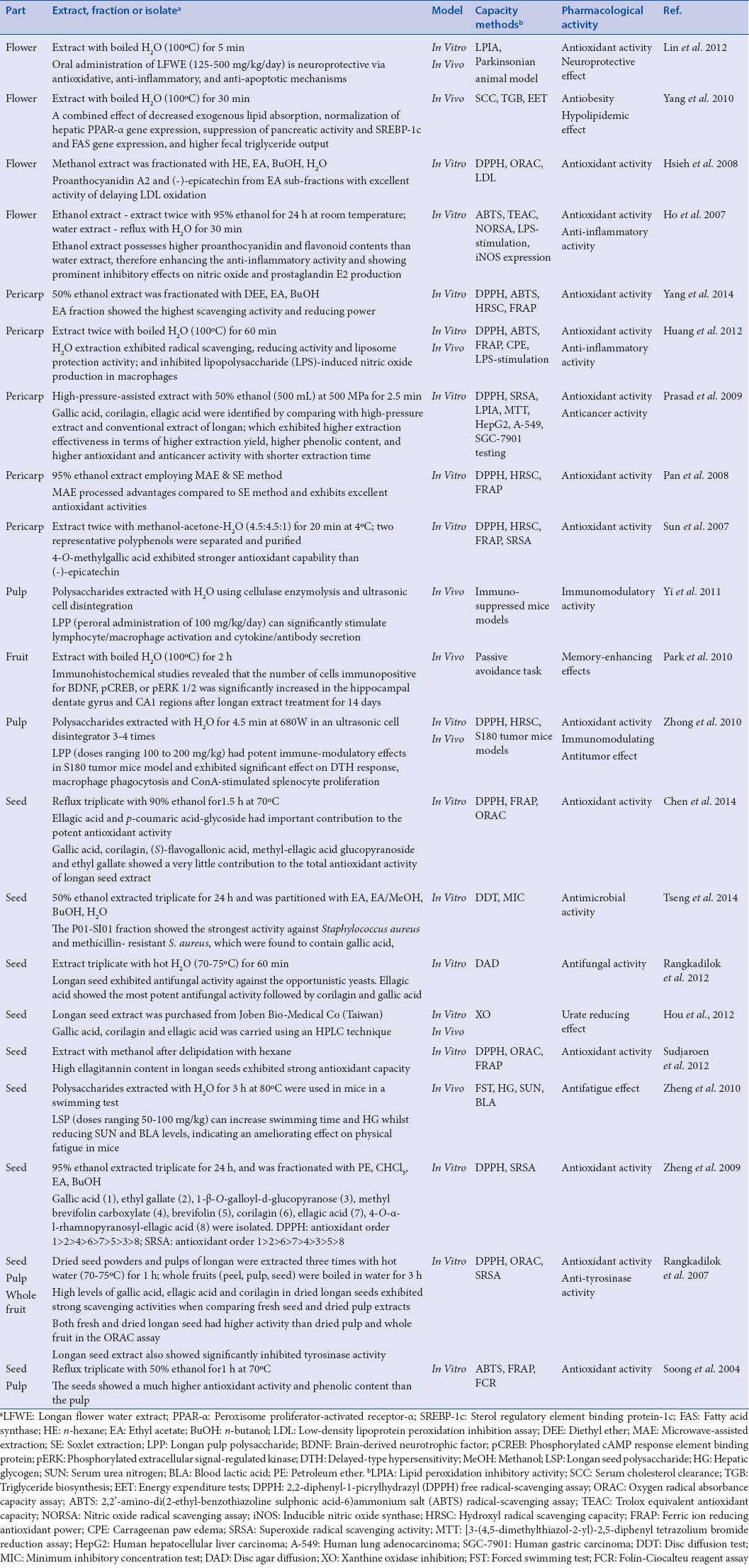
Previous reports depicted that longan flowers, fruits, pericarps, pulps, and seeds are known to contain many phenolic components, which are capable of protecting people from oxidative damage through an anti-inflammatory mechanism.[1,2] Several scientific studies validated that longan extract with different extraction technologies display a wide range of therapeutic activities as reviewed herein [Table 1] (i.e., antifungal,[3] antimicrobial,[4] anti-inflammatory,[1,2] antioxidant,[1,2,5,6,7,8,9,10,11,12,13,14,15,16] antiobesity,[17] hypolipidemic,[17] antifatigue,[18] anticancer,[9] antitumor,[16] neuroprotective,[7] immunomodulatory,[16,19] anti-tyrosinase,[10] memory-enhancing,[20] and urate-reducing effect)[21] [Table 1].
Table 1.
Pharmacological activities of Dimocarpus longan Lour. in different experimental models reported by previous investigators
To our knowledge, longan extracts, rich in phenolics, have been well characterized chemically; in this regard, most of the therapeutic properties of natural phenolics and flavonoids have been ascribed to their enzyme inhibitory and antioxidant activity.[22,23,24] Taking into account the high levels of phenolics and flavonoids in longans,[10,25] this in vivo investigation performed the uric acid-lowering effect of longan extracts (including flowers, pericarps, seeds, leaves, and twigs) in potassium-oxonate (PO) treated mice. According to our preliminary screening, longan flower extracts have been shown to contribute beneficially to lowering the levels of uric acid. In an attempt to pursue this anti-gout effect, we isolated 10 compounds from longan flower [Figure 1], such as acetonylgeraniin A, chebulagic acid, chebulinic acid, corilagin, (-)-epicatechin, gallic acid, geraniin, proanthocyanidin A2, procyanidin B2, and protocatechuic acid. Therefore, this study also intended the evaluation of longan flower extracts for their possible inhibitory activity against xanthine oxidase (XO) in vitro [Figure 1].
Figure 1.
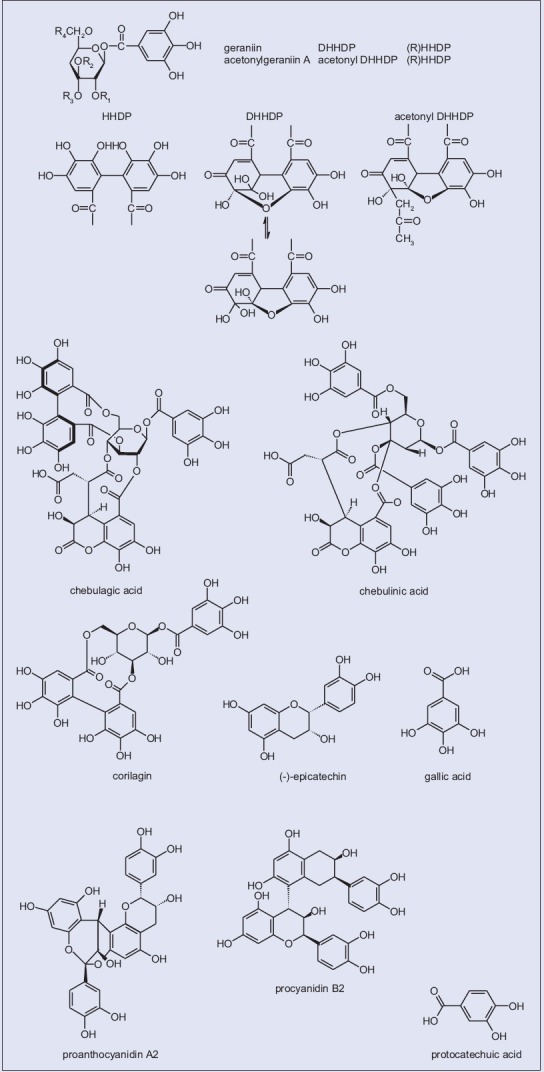
Chemical structures of typical constituents isolated from Dimocarpus longan Lour.
MATERIALS AND METHODS
Chemicals
XO purified from bovine milk, allopurinol, PO, and xanthine were purchased from Sigma-Aldrich (St. Louis, MO, US). The solvents used for extraction and column chromatography, including methanol, n-hexane, ethyl acetate (EA), n-butanol, and acetone, were of analytical grade and supplied by J.T. Baker (Phillipsburg, NJ, US).
Plant material and extractions
The male flowers, pericarps, seeds, leaves, and twigs of Dimocarpus longan Lour. were collected from private farms in Hongjia (Xuejia District, Tainan City, Taiwan). Dried materials (flowers, pericarps, seeds, and twigs) were ground into powder and extracted with 95% methanol at room temperature for 24 h, and then passed through a Whatman no. 1 filter paper. This procedure was repeated 3 times for the residues, and the filtrates were combined. All solvents were removed under reduced pressure for isolation of the extract. Longan leaf powders were extracted with 70% acetone triplicate. The total acetone extracts were filtered and evaporated in a vacuum. The percentage yield so obtained was 10.2%, 11.9%, 10.2%, 26.7%, and 4.5% toward flower, pericarp, seed, leaf, and twig samples, respectively. To these extracts, H2O was added individually and the resulting extracts were successively partitioned with HE, EA and distilled water to yield soluble fractions. All the fractions were further concentrated under vacuum at 50°C; the crude dried extracts obtained were used directly for XO assay. In context, the chemical constituents identified in longan flower have been characterized with those reported.[6,21] Briefly, the freeze-dried methanol extract was re-dissolved in methanol and then sequentially partitioned with HE, EA, n-butanol, and H2O. The EA fraction that showed prominent antioxidant activity was further chromatographed on Sephadex LH-20 (Pharmacia) with H2O-MeOH (2:3) and MCI-CHP-20P (Mitsubishi) with H2O-MeOH (1:0–1:1). The structures of some of the compounds isolated are given in Figure 1.
Determination of xanthine oxidase-inhibitory activity
XO-inhibitory activity was measured spectrophotometrically based on the procedure reported by Nguyen et al.[26] The reaction mixture consisted of 50 μL of test samples or compounds, 35 μL of 50 mM phosphate buffer (pH 7.5), and 30 μL of XO solution (0.1 U/mL in 50 mM phosphate buffer, pH 7.5) and was prepared immediately before use. After preincubation at RT (25°C) for 15 min, 60 μL of substrate solution (150 μM xanthine in the same buffer) was added to the mixture to initiate the reaction. The assay mixture was then incubated at RT for 30 min. Afterward, 25 μL of stop solution (1 N HCl) was added, and the absorbance values were measured at 290 nm with a microplate reader (μQuant™, BIO-TEK Instruments Inc., USA). Allopurinol was used as a positive control. Three replicates were performed for each test sample, and the increased ultraviolet absorption at 290 nm indicated the formation of uric acid. The percentage inhibition ratio was calculated according to the following equation: % inhibition = (1 − B/A) × 100, where A is the change in absorbance per min without the test sample and B is the change in absorbance per min with the test material. The concentration of samples required to inhibit 50% of XO activity (IC50) was estimated from the % inhibition versus concentration plot using a linear regression algorithm.[27]
Hyperuricemia model in mice
About 6–8-week-old male ICR mice weighing 25–30 g were purchased from BioLASCO Taiwan Co., Ltd. (Yilan, Taiwan), maintained in 12 h light/dark cycles, and housed at 23 ± 2°C for at least 1 week prior to the experiment. Animals were provided with a rodent diet and clean water ad libitum, except 1 h prior to drug administration when access to food was restricted. Animal tests used in this study were conducted under the guidelines of the International Association for the Study of Pain.[28] The experimental protocol was approved by the Institutional Animal Care and Use Committee (IACUC) of National Taiwan University (IACUC Approval No: NTU-101-EL-14).
Experimental hyperuricemia was induced in mice by intraperitoneal (i.p.) injections of the uricase inhibitor PO as described previously.[29] The mice were i.p. injected with phosphate-buffered saline (PBS) containing PO (250 mg/kg) 1 h after administration of test samples to increase blood urate levels. The names of the samples are as follows: Methanol extracts of the flower (F), pericarp (P), seed (S), leaf (L), and twig (T) of longan were shortened for ME-F, ME-P, ME-S, ME-L, and ME-T, respectively. Mice were randomly assigned into the following seven groups for different treatments (n = 6): (1) Vehicle group (normal control); (2) PO + allopurinol (AP, 10 mg/kg) group (positive control); (3) PO + PBS group (negative); (3) PO + ME-F group; (4) PO + ME-P group; (5) PO + ME-S group; (6) PO + ME-L group; (7) PO + ME-T group. For the comparative study, three dosages (100, 75, and 50 mg/kg) were delivered to ensure the utilization of this extract.
Measurement of plasma uric acid level
Uric acid in tail vein blood was measured 2 h after PO injection using commercial Ektachem clinical chemistry slides from Johnson & Johnson clinical diagnostics (US).
Statistical analysis
All data were expressed as the mean ± standard deviation (n = 3). The significance of difference was performed with Duncan's new multiple range test, and *P < 0.05 and **P < 0.01 were considered statistically significant.
RESULTS
Time course potassium-oxonate effects on plasma uric acid levels in mice
The effects of PO on mice plasma uric acid (PUA) levels are shown in Figure 2. Uric acid levels in nonhyperuricemic, vehicle-treated mice were 1.6 ± 0.05 mg/dL. However, treatment with uricase inhibitor PO resulted in a significant elevation of PUA levels reaching to 18 ± 2.8 mg/dL after 2 h, followed by slow decrease in urate levels 8 h postinjection [Figure 2].
Figure 2.
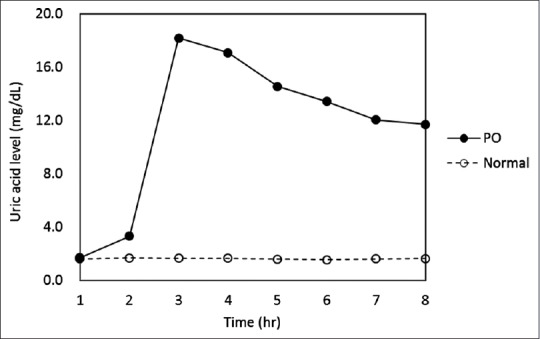
Time-course effect of potassium oxonate on hyperuricemic mice uric acid levels
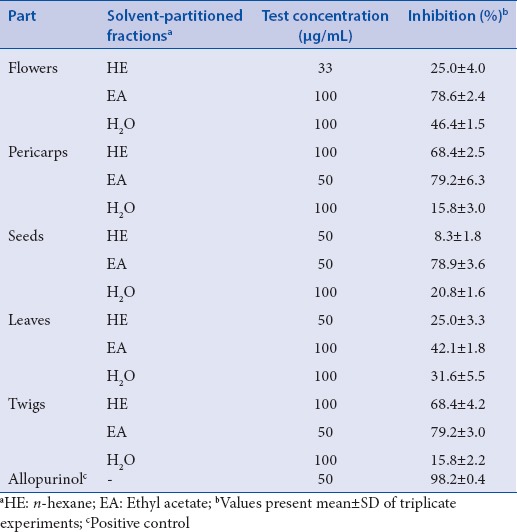
Xanthine oxidase-inhibitory activity of longan crude extracts and derived soluble fractions
The tested longan crude extracts inhibited XO in a concentration-dependent manner. Longan flowers showed the best XO-inhibitory activity, with an IC50 value of 115.8 μg/mL, followed by pericarps (118.9 μg/mL), twigs (125.3 μg/mL), seeds (262.5 μg/mL), and leaves (331.1 μg/mL), respectively. These results are in accordance with Hou et al.,[21] who reported that longan seed extract had dose-dependent XO-inhibitory activity with an IC50 value of 277.8 μg/mL. Comparisons of XO-inhibitory activity results [Table 2] in various derived soluble fractions from longan extracts indicated that there are abundant XO-inhibitory phytochemicals present in longan extracts, especially in the EA fraction [Table 2].
Table 2.
XO-inhibitory activitiy of derived soluble fractions of Dimocarpus longan Lour
Xanthine oxidase-inhibitory activity of phytochemicals from longan flower extracts
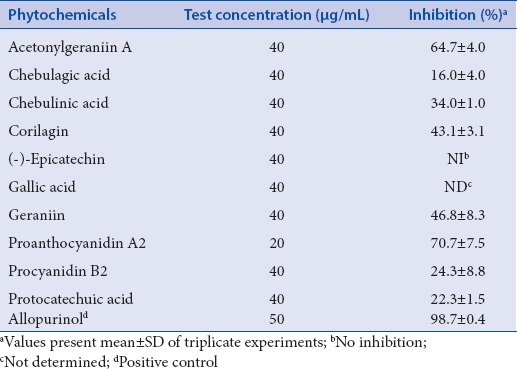
The inhibitory effects of isolated 10 phytochemicals [Figure 1] from longan flower extracts against XO are given in Table 3. The experimental evidence indicates that proanthocyanidin A2 and acetonylgeraniin A showed an excellent activities' profile for inhibition to XO compared to that of the standard, allopurinol, as indicated by inhibition (%). In addition, proanthocyanidin A2, a phenolic dimer belonging to the class of condensed tannins, has been shown to display superior antioxidant activity to that of ascorbic acid in previous research works;[6,30] while some studies revealed that acetonylgeraniin A, a hydrolyzable tannin, has an antihypertensive effect.[31,32] In this study, proanthocyanidin A2 and acetonylgeraniin A were found to inhibit XO in vitro, which may be potentially useful for the treatment of gout [Table 3].
Table 3.
Inhibitory effects of isolated 10 phytochemicals from longan flower extracts against XO
In vivo hypouricemic effect determined in mice with potassium-oxonate-induced hyperuricemia
To further confirm the capabilities of methanol extract of longan flower, pericarp, seed, leaf, and twig tissues to reduce the uric acid level in vivo, a PO-induced hyperuricemia mice model was investigated. In vehicle control mice, the PUA level was 1.6 ± 0.03 mg/dL. After 2 h of PO treatment, the level of PUA had increased to 18.5 ± 0.4 mg/dL. As shown in Figure 3, oral administration of ME-F, ME-P, ME-S, ME-L, and ME-T (50, 75 and 100 mg/kg) significantly reduced plasma urate levels in hyperuricemic mice in a dose-dependent manner, as well as the reference (AP) group. The administration of allopurinol (PO + AP group) significantly reduced the level of PUA by (2.0 ± 0.1 mg/dL) 89% as compared with the PO + PBS group (P < 0.01). At doses of 75 mg/kg of ME-F, ME-P, ME-S, ME-L, ME-T, or above, plasma urate levels of the PO-treated mice were significantly reduced by 80%, 64%, 72%, 41%, and 59%, respectively, relative to the PO + PBS group (P < 0.01). No significant difference existed between the dosages of longan extracts at 75 and 100 mg/kg. Conversely, the lowering effect on uric acid by longan extracts at 50 mg/kg on PO-induced hyperuricemic mice was found to be weaker than that observed for 75 and 100 mg/kg dosages. It is noteworthy that comparisons of these results indicate that ME-F and ME-S exhibit excellent hypouricemic effects. Remarkably, the methanol extract of the flowers was more potent than seeds in uric acid-lowering effects in vivo [Figure 3].
Figure 3.
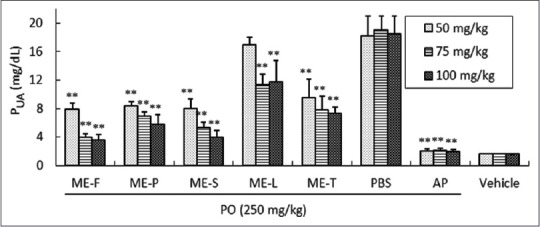
The uric acid-lowering effects of methanol extract from longan flower, pericarp, seed, leaf and twig tissues (50, 75, 100 mg/kg) on mice with PO-induced hyperuricemia. The results are presented as the mean ± SD (n = 6). **P < 0.01 compared to the PO-treated group
DISCUSSION
In recent years, there is an increasing interest in finding herbal plants and phytochemicals which possess the capacity to inhibit XO activity and reduce urate levels. Longan is a fruit used in herbal preparations in China, and though unpollinated longan flowers and nonedible fruit seeds are generally regarded as disposable byproducts, studies show that longan flowers, pericarps, and seeds contain high levels of phenolics and flavonoids, which exhibit high antioxidant activity and may be rendered suitable as protective agents [Table 1]. The activities of extracts from its flowers, pericarps, leafs, and twigs against XO are reported here for the first time. A study was demonstrated that longan seed extract and its active components (corilagin, gallic acid, and ellagic acid) inhibited XO dose dependently in vitro, but were less potent than allopurinol.[21] Our findings indicate that extracts from longan flowers have a great potential for preventing diseases caused by the XO-inhibitory activity in vitro, with an IC50 value of 115.8 μg/mL, followed by pericarps (118.9 μg/mL), twigs (125.3 μg/mL), seeds (262.5 μg/mL), and leaves (331.1 μg/mL). For the in vivo study, longan extract (75 mg/kg) was able to reduce PUA levels and XO activities in hyperuricemic mice in a decreasing order: ME-F (80%) > ME-S (72%) > ME-P (64%) > ME-T (59%) > ME-L (41%), compared with allopurinol (89%). Meanwhile, 10 phytochemicals were identified from longan flower, and a superior XO-inhibitory activity in the type of phenolics was observed. The in vitro inhibitions of XO by proanthocyanidin A2 and acetonylgeraniin A are high when compared to allopurinol, which possess the hypouricemic activities for the first time. Others yield weak inhibitory activity against XO.
A toxicological study revealed no toxic effects of oral administration of longan seed extract during acute and repeated doses (4 and 13 weeks).[33] Besides, longan seed extract inhibited uric acid production and XO activity in normal liver cells (clone-9 cells) and was not cytotoxic under the concentration of 200 μg/ml.[21] The results suggested that its urate-reducing effect might be due to modulation of urate transporters (GLUT1 and GLUT9) and inhibition of circulating XO. It is of great interest that the XO-inhibitory effect of longan flowers and seeds, the byproduct materials, may provide some choices for prevention and/or treatment of hyperuricemia as valuable functional ingredients.
CONCLUSION
It can be concluded from the present finding that longan extracts possess potent in vivo hypouricemic effects in hyperuricemic rats pretreated with oxonate. The use of longan flowers and seeds in the treatment of gout could be attributed to its inhibitory effect on XO. These results also suggest that proanthocyanidin A2 and acetonylgeraniin A extractions from Dimocarpus longan Lour. flowers could be developed as potent XO inhibitors for clinical application.
Financial support and sponsorship
Nil.
Conflicts of interest
All authors declare that they have no conflicts of interest.
ABOUT AUTHORS
Figure.

Chun-Hsu Yao
Prof. Chun-Hsu Yao, obtained his Ph.D. degree in 1997 from Department of Chemistry, Chung Yuan Christian University, Taiwan (Ph.D. thesis accomplished in Center for Biomedical Engineering, NTU under supervision of Prof. Feng-Huei Lin). He is positioned as Dean of Academia-Industry Cooperation Office and Professor of Department of Biological Imaging and Radiological Science in China Medical University. He is also a Joint Professor of Department of Bioinformatics and Medical Engineering in Asia University. To biomaterials and regenerative medicine fields, he has contributed more than 100 SCI papers.
Figure.

Feng-Lin Hsu
Prof. Feng-Lin Hsu, obtained his Ph.D. degree in 1985 from Division of Pharmaceutical Sciences, Graduate School Kyushu University, Fukuoka, Japan. He is positioned as Professor Emeritus of Graduate Institute of Pharmacology, Taipei Medical University. In his research career, Prof. Hsu has been able to successfully carry out extensive basic and applied research investigations in the chemistry of natural products of biological relevance. He is a specialist in new drug discovery from natural products, structure-activity relationship studies and standardization of herbal medicines of commercial importance. He has successfully isolated several new bioactive molecules from natural resources.
Figure.

Tzong-Fu Kuo
Prof. Tzong-Fu Kuo, D.V.M. obtained his Ph.D. degree in 1989 from School of Veterinary Medicine, National Taiwan University. He is positioned as Chief Executive Officer of Chinese Society of Traditional Veterinary Science (2009-2012) and Vice President of Asian Society of Traditional Veterinary Medicine (2010-2014). He is also Professor of School of Veterinary Medicine in National Taiwan University and School of Post-Baccalaureate Veterinary Medicine in Asia University. To veterinary herbal medicine fields, he has contributed more than 100 term papers in Chinese. His current research has a focus on bone marrow stem cells and growth factors in regenerative medicine.
REFERENCES
- 1.Ho SC, Hwang LS, Shen YJ, Lin CC. Suppressive effect of a proanthocyanidin-rich extract from longan (Dimocarpus longan Lour.) flowers on nitric oxide production in LPS-stimulated macrophage cells. J Agric Food Chem. 2007;55:10664–70. doi: 10.1021/jf0721186. [DOI] [PubMed] [Google Scholar]
- 2.Huang GJ, Wang BS, Lin WC, Huang SS, Lee CY, Yen MT, et al. Antioxidant and anti-inflammatory properties of longan (Dimocarpus longan Lour.) Pericarp. Evid Based Complement Alternat Med. 2012;2012:709483. doi: 10.1155/2012/709483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rangkadilok N, Tongchusak S, Boonhok R, Chaiyaroj SC, Junyaprasert VB, Buajeeb W, et al. In vitro antifungal activities of longan (Dimocarpus longan Lour.) seed extract. Fitoterapia. 2012;83:545–53. doi: 10.1016/j.fitote.2011.12.023. [DOI] [PubMed] [Google Scholar]
- 4.Tseng HC, Wu WT, Huang HS, Wu MC. Antimicrobial activities of various fractions of longan (Dimocarpus longan Lour.Fen Ke) seed extract. Int J Food Sci Nutr. 2014;65:589–93. doi: 10.3109/09637486.2014.886181. [DOI] [PubMed] [Google Scholar]
- 5.Chen J, Ge ZZ, Zhu W, Xu Z, Li CM. Screening of key antioxidant compounds of longan (Dimocarpus longan Lour.) seed extract by combining online fishing/knockout, activity evaluation, Fourier transform ion cyclotron resonance mass spectrometry, and high-performance liquid chromatography electrospray ionization mass spectrometry methods. J Agric Food Chem. 2014;62:9744–50. doi: 10.1021/jf502995z. [DOI] [PubMed] [Google Scholar]
- 6.Hsieh MC, Shen YJ, Kuo YH, Hwang LS. Antioxidative activity and active components of longan (Dimocarpus longan Lour.) flower extracts. J Agric Food Chem. 2008;56:7010–6. doi: 10.1021/jf801155j. [DOI] [PubMed] [Google Scholar]
- 7.Lin AM, Wu LY, Hung KC, Huang HJ, Lei YP, Lu WC, et al. Neuroprotective effects of longan (Dimocarpus longan Lour.) flower water extract on MPP -induced neurotoxicity in rat brain. J Agric Food Chem. 2012;60:9188–94. doi: 10.1021/jf302792t. [DOI] [PubMed] [Google Scholar]
- 8.Pan YM, Wang K, Huang SQ, Wang HS, Mu X, He CH, et al. Antioxidant activity of microwave-assisted extract of longan (Dimocarpus Longan Lour.) peel. Food Chem. 2008;106:1264–70. [Google Scholar]
- 9.Prasad KN, Hao J, Shi J, Liu T, Li J, Wei X, et al. Antioxidant and anticancer activities of high pressure-assisted extract of longan (Dimocarpus longan Lour.) fruit pericarp. Innov Food Sci Emerg Technol. 2009;10:413–9. [Google Scholar]
- 10.Rangkadilok N, Sitthimonchai S, Worasuttayangkurn L, Mahidol C, Ruchirawat M, Satayavivad J. Evaluation of free radical scavenging and antityrosinase activities of standardized longan fruit extract. Food Chem Toxicol. 2007;45:328–36. doi: 10.1016/j.fct.2006.08.022. [DOI] [PubMed] [Google Scholar]
- 11.Soong YY, Barlow PJ. Antioxidant activity and phenolic content of selected fruit seeds. Food Chem. 2004;88:411–7. [Google Scholar]
- 12.Sudjaroen Y, Hull WE, Erben G, Würtele G, Changbumrung S, Ulrich CM, et al. Isolation and characterization of ellagitannins as the major polyphenolic components of Longan (Dimocarpus longan Lour) seeds. Phytochemistry. 2012;77:226–37. doi: 10.1016/j.phytochem.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 13.Sun J, Shi J, Jiang Y, Xue SJ, Wei X. Identification of two polyphenolic compounds with antioxidant activities in longan pericarp tissues. J Agric Food Chem. 2007;55:5864–8. doi: 10.1021/jf070839z. [DOI] [PubMed] [Google Scholar]
- 14.Yang X, Yan F, Huang SR, Fu C. Antioxidant activities of fractions from longan pericarps. Food Sci Technol. 2014;34:341–5. [Google Scholar]
- 15.Zheng G, Xu L, Wu P, Xie H, Jiang Y, Chen F, Wei X. Polyphenols from longan seeds and their radical-scavenging activity. Food Chem. 2009;116:433–6. [Google Scholar]
- 16.Zhong K, Wang Q, He Y, He X. Evaluation of radicals scavenging, immunity-modulatory and antitumor activities of longan polysaccharides with ultrasonic extraction on in S180 tumor mice models. Int J Biol Macromol. 2010;47:356–60. doi: 10.1016/j.ijbiomac.2010.05.022. [DOI] [PubMed] [Google Scholar]
- 17.Yang DJ, Chang YY, Hsu CL, Liu CW, Lin YL, Lin YH, et al. Antiobesity and hypolipidemic effects of polyphenol-rich longan (Dimocarpus longans Lour.) flower water extract in hypercaloric-dietary rats. J Agric Food Chem. 2010;58:2020–7. doi: 10.1021/jf903355q. [DOI] [PubMed] [Google Scholar]
- 18.Zheng SQ, Jiang F, Gao HY, Zheng JG. Preliminary observations on the antifatigue effects of longan (Dimocarpus longan Lour.) seed polysaccharides. Phytother Res. 2010;24:622–4. doi: 10.1002/ptr.2963. [DOI] [PubMed] [Google Scholar]
- 19.Yi Y, Liao ST, Zhang MW, Zhang RF, Deng YY, Yang B, et al. Immunomodulatory activity of polysaccharide-protein complex of longan (Dimocarpus longan Lour.) pulp. Molecules. 2011;16:10324–36. doi: 10.3390/molecules161210324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park SJ, Park DH, Kim DH, Lee S, Yoon BH, Jung WY, et al. The memory-enhancing effects of Euphoria longan fruit extract in mice. J Ethnopharmacol. 2010;128:160–5. doi: 10.1016/j.jep.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 21.Hou CW, Lee YC, Hung HF, Fu HW, Jeng KC. Longan seed extract reduces hyperuricemia via modulating urate transporters and suppressing xanthine oxidase activity. Am J Chin Med. 2012;40:979–91. doi: 10.1142/S0192415X12500723. [DOI] [PubMed] [Google Scholar]
- 22.Nile SH, Park SW. Total phenolics, antioxidant and xanthine oxidase inhibitory activity of three colored onions (Allium cepa L.) Front Life Sci. 2013;7:224–8. [Google Scholar]
- 23.Santas J, Carbó R, Gordon MH, Almajano MP. Comparison of the antioxidant activity of two Spanish onion varieties. Food Chem. 2008;107:1210–6. [Google Scholar]
- 24.Tapas AR, Sakarkar DM, Kakde RB. Flavonoids as nutraceuticals: A review. Trop J Pharm Res. 2008;7:1089–99. [Google Scholar]
- 25.Wisitsak P, Nimkamnerd J, Thitipramote N, Saewan N, Chaiwut P, Pintathong P. In: Proceedings of the 1st Mae Fah Luang University International Conference (1st MFUIC) Chiang Rai: Thailand; 2012. Comparison the bioactive compounds and their activities between longan and litchi seeds extracts. [Google Scholar]
- 26.Harris MD, Siegel LB, Alloway JA. Gout and hyperuricemia. Am Fam Physician. 1999;59:925–34. [PubMed] [Google Scholar]
- 27.Horiuchi H, Ota M, Nishimura S, Kaneko H, Kasahara Y, Ohta T, et al. Allopurinol induces renal toxicity by impairing pyrimidine metabolism in mice. Life Sci. 2000;66:2051–70. doi: 10.1016/s0024-3205(00)00532-4. [DOI] [PubMed] [Google Scholar]
- 28.Worasuttayangkurn L, Watcharasit P, Rangkadilok N, Suntararuks S, Khamkong P, Satayavivad J. Safety evaluation of longan seed extract: Acute and repeated oral administration. Food Chem Toxicol. 2012;50:3949–55. doi: 10.1016/j.fct.2012.07.068. [DOI] [PubMed] [Google Scholar]
- 29.Nguyen MT, Awale S, Tezuka Y, Tran QL, Watanabe H, Kadota S. Xanthine oxidase inhibitory activity of Vietnamese medicinal plants. Biol Pharm Bull. 2004;27:1414–21. doi: 10.1248/bpb.27.1414. [DOI] [PubMed] [Google Scholar]
- 30.Chien SC, Yang CW, Tseng YH, Tsay HS, Kuo YH, Wang SY. Lonicera hypoglauca inhibits xanthine oxidase and reduces serum uric acid in mice. Planta Med. 2009;75:302–6. doi: 10.1055/s-0029-1185300. [DOI] [PubMed] [Google Scholar]
- 31.Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–10. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]
- 32.Kong L, Zhou J, Wen Y, Li J, Cheng CH. Aesculin possesses potent hypouricemic action in rodents but is devoid of xanthine oxidase/dehydrogenase inhibitory activity. Planta Med. 2002;68:175–8. doi: 10.1055/s-2002-20262. [DOI] [PubMed] [Google Scholar]
- 33.Mandell BF. Clinical manifestations of hyperuricemia and gout. Cleve Clin J Med. 2008;75(Suppl 5):S5–8. doi: 10.3949/ccjm.75.suppl_5.s5. [DOI] [PubMed] [Google Scholar]


