Abstract
Background:
Tradescantia albiflora (TA) Kunth (Commelinaceae) has been used for treating gout and hyperuricemia as folklore remedies in Taiwan. Therefore, it is worthwhile to study the effect of TA extracts on lowering uric acid activity. The hypouricemic effects of TA extracts on potassium oxonate (PO)-induced acute hyperuricemia were investigated for the first time.
Materials and Methods:
All treatments at the same volume (1 ml) were orally administered to the abdominal cavity of PO-induced hyperuricemic rats. One milliliter of TA extract in n-hexane (HE), ethyl acetate (EA), n-butanol (BuOH), and water fractions has 0.28, 0.21, 0.28, and 1.03 mg TA, respectively; and the plasma uric acid (PUA) level was measured for a consecutive 4 h after administration.
Results:
All four fractions' extracts derived from TA were observed to significantly reduce PUA compared with the PO group. The EA-soluble fraction (TA-EA) exhibited the best xanthine oxidase (XO) inhibitory activity. Following column chromatography, 12 phytochemicals were isolated and identified from the EA fraction. The IC50 values of isolated phytochemicals indicated that bracteanolide A (AR11) showed the remarkable XO inhibitory effect (IC50 value of 76.4 μg/ml). These findings showed that the in vivo hypouricemic effect in hyperuricemic rats was consistent with in vitro XO inhibitory activity, indicating that TA extracts and derived phytochemicals could be potential candidates as hypouricemic agents.
SUMMARY
Tradescantia albiflora extracts possess in vivo hypouricemic action in hyperuricemic rats
T. albiflora extracts exhibited strong inhibitory activity against xanthine oxidase (XO)
Butenolide may play an important role in XO inhibition
The extract bracteanolide A was demonstrated potent XO inhibitory activity in vitro.
Abbreviations used: TA: Tradescantia albiflora, PO: potassium oxonate, HE: n-hexane, EA: ethyl acetate, BuOH: n-butanol, PUA: plasma uric acid, XO: xanthine oxidase, MeOH: methanol, IP: intraperitoneal
Keywords: Hyperuricemia, Tradescantia albiflora Kunth, uric acid, xanthine oxidase
INTRODUCTION
Tradescantia albiflora (TA) Kunth (Commelinaceae) is a facultative shade plant among a series of weed species of world importance belonging to the Commelinaceae. It is native to tropical rainforests and a persistent invasive weed of natural areas where it carpets the ground and prevents native regeneration.[1] TA has been used for treating gout and hyperuricemia as folklore remedies in Taiwan. Previous investigation on the whole plant of TA showed the presence of a variety of amides, apocarotenoids, aromatics, chlorophyll, flavonoid, steroids, triterpenoids, and other chemicals.[2] Literature records concerning the carpetweed are very limited. There have been no pharmacological studies performed and no research into potential treatments. In the course of our program to screen for xanthine oxidase (XO) inhibitors from botanical plants, it was found that a methanol (MeOH) extract from TA was one of the positive samples to have a potent XO inhibitory effect in comparison to Glinus oppositifolius, Morus alba twigs, and leaves, etc.
XO, which catalyzes the oxidation of hypoxanthine to xanthine and of xanthine to uric acid, plays a vital role in metabolic disorders as hyperuricemia and gout; the crystal deposition disease is characterized by the overproduction and/or underexcretion of uric acid resulting in inflammation and consequently, tissue damage. Accordingly, the use of XO inhibitor decreases uric acid level as well as results in an antihyperuricemic effect.[3] Among the known XO inhibitors, allopurinol has been used widely for the treatment of hyperuricemia and gout. However, its use is limited by unwanted side effects including hepatitis, nephropathy, and allergic reactions.[4,5] Thus, the search for a potent XO inhibitor with greater efficacy and fewer side effects remains a major need. In recent years, attentions have been focused on traditional herbal plants,[6,7,8,9,10,11,12,13,14] which possess the capacity to inhibit XO activity and reduce urate levels.
Our preliminary screening study revealed that a MeOH extract of the TA exhibited in vitro inhibitory activities. This study intended the isolation of 12 aromatic compounds from the MeOH extract of TA and the evaluation of its possible inhibitory activity against XO. Furthermore, we investigated the hypouricemic effect of TA extracts in potassium oxonate (PO)-treated rats for the first time.
MATERIALS AND METHODS
Chemicals
XO purified from bovine milk, xanthine, PO, and allopurinol were all purchased from Sigma-Aldrich (St. Louis, MO, USA). The other chemicals and solvents used in this experiment were of analytical grade.
Plant material
Mature leaves of TA Kunth (Commelinaceae) collected from greenhouse-grown plants were sliced into white and green portions with a blade. The plant material was taxonomically identified and authenticated by the botanist and a voucher specimen (TAIF-PLANT-199332) has been retained at the Herbarium of Taiwan Forestry Research Institute, Taipei, Taiwan. All the materials were air-dried at ambient temperature (25°C) to constant weight.
Preparation of extracts
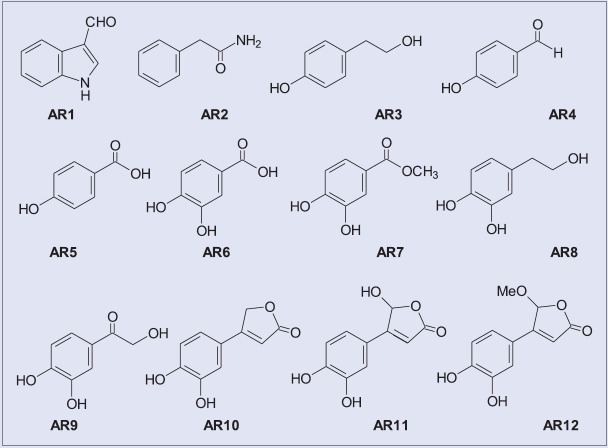
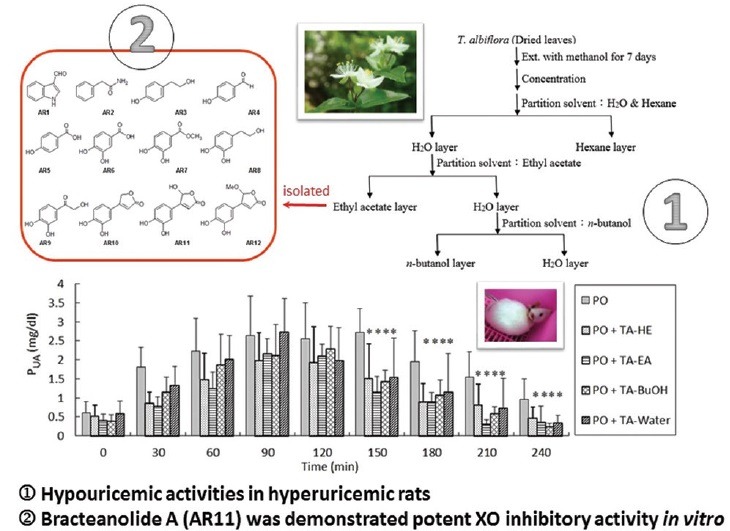
Air-dried leaves of TA (100 g) were extracted with MeOH at ambient temperature for 7 days and concentrated in a rotary vacuum evaporator. The resulting extract was fractionated successively with n-hexane (HE), ethyl acetate (EA), n-butanol (BuOH), and distilled water to yield soluble fractions. The scheme of separation is shown in Figure 1. In addition, repeated column chromatography of the EA subfractions resulting in the purification of phytochemicals AR1–AR12 [Figure 2] was prepared by the Department of Chinese Pharmaceutical Sciences and Chinese Medicine Resources, China Medical University. The 12 compounds were identified by high-resolution electrospray ionization mass spectrometry and nuclear magnetic resonance, and all spectral data were consistent with those reported in the literature[2] [Figures 1 and 2].
Figure 1.
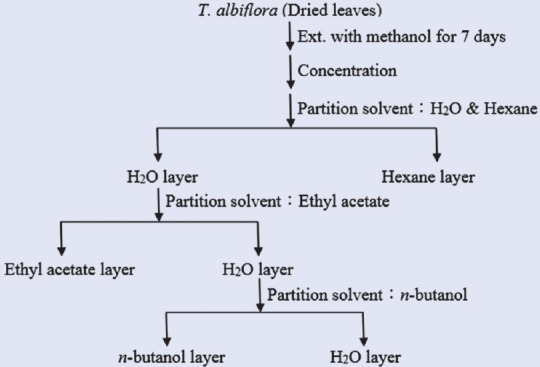
Flow chart for extraction of T. albiflora leaves
Figure 2.
Aromatic compounds isolated from EA subfractions of T. albiflora extract: AR1, indole-3-aldehyde; AR2, 2-phenylacetamide; AR3, tyrosol; AR4, p-hydroxybenzaldehyde; AR5, p-hydroxybenzoic acid; AR6, protocatechuic acid; AR7, 3,4-dihydroxymethylbenzoate; AR8, hydroxytyrosol; AR9, 2-hydroxy- 3’,4’-dihydroxyacetophenone; AR10, 3-(3’,4’-dihydroxyphenyl)-butenolide; AR11, bracteanolide A; AR12, bracteanolide B
Animals
Male Wistar rats (8–10 weeks old) weighing 250–280 g, breeding from the Laboratory Animal Center in National Taiwan University, College of Medicine (Taipei, Taiwan), were used in this study. They were allowed 1 week to adapt to the environment before testing. The animals were caged in a fully ventilated room, were maintained in a 12:12 h light and dark cycle, and were housed at temperature of 23 ± 2°C. They had free access to a standard chow diet and water ad libitum. All experiments were performed with the approval of the Institutional Animal Care and Use Committee (IACUC) of National Taiwan University (IACUC approval number: NTU-98-EL-105) and were carried out in accordance with the current guidelines for the care of laboratory animals and the ethical guidelines for investigations of experimental pain in conscious animals.[15]
Potassium oxonate-induced hyperuricemia in rats
Experimental hyperuricemia was induced in rats by intraperitoneal (IP) injections of the uricase inhibitor PO (250 mg/kg body weight) as previous procedure.[16] The rats were divided into five groups for treatment (n = 8 per group): (1) PO; (2) PO + TA-HE; (3) PO + TA-EA; (4) PO + TA-BuOH; (5) PO + TA-water. Besides the controls (Group 1), the treatments groups were IP injected with PO (250 mg/kg) 1 h before oral administration of TA extracts (1 ml of TA extract in HE, EA, BuOH, and water fractions, respectively).
Measurement of plasma uric acid level
The following procedure was carried out at 0.5 h increments after oral administration for a consecutive 4 h. Serial blood samples were collected from an indwelling right carotid artery cannula every 30 min. The same animal was submitted to 8 specimen withdrawals, and the uric acid level in plasma was determined using a commercial Ektachem clinical chemistry slides from Johnson & Johnson clinical diagnostics Inc. (Rochester, NY, USA).
Xanthine oxidase inhibition in vitro assay
The XO inhibitory activity was assayed spectrophotometrically based on the previous report.[17] The assay mixture, consisting of 50 µl of test solution, 35 µl of 50 mM phosphate buffer (pH 7.5), and 30 µl of XO solution (0.1 U/ml in 50 mM phosphate buffer, pH 7.5), was prepared immediately before use. After preincubation at RT (25°C) for 15 min, the reaction was initiated by the addition of 60 µl of substrate solution (150 µM xanthine in the same buffer). The assay mixture was incubated at RT for 30 min. Afterward, 25 µl of stop solution (1 N HCl) was added, and the absorbance values were measured at 290 nm with a microplate reader (µQuant™, BioTek Instruments Inc., Winooski, VT, USA). Allopurinol, a known inhibitor of XO, was used as a positive control. The increased ultraviolet absorption at 290 nm indicated the formation of uric acid, and all determinations were performed in triplicate. The XO inhibition was calculated as a percentage (%) = (1 − B/A) × 100, where A is the change in absorbance per min without the test sample and B is the change in absorbance per min with the test material. The concentrations of samples required to inhibit 50% of XO activity (IC50) were estimated from the % inhibition versus concentration plot using a linear regression algorithm.[9]
Statistical analysis
All results are expressed as mean ± standard deviation (n = 3). The significance of difference was calculated by Duncan's new multiple range test, and values P < 0.05 were considered statistically significant.
RESULTS
Hypouricemic activities in hyperuricemic rats
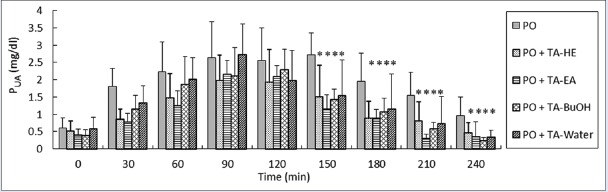
An experiment was conducted to examine the hypouricemic effects of TA on the plasma uric acid (PUA) of hyperuricemic rats. The concentration of uric acid of each plasma sample at different time points was determined after oral administration and point 0 min is from TA but 1 h from PO injections. The hypouricemic effects of HE, EA, BuOH, and water fractions on PO-induced hyperuricemic rats are shown in Figure 3. IP injections of PO markedly increased the PUA levels, and reached a Cmax of 2.63 ± 0.08 mg/dl after 2 h, followed by a slow decrease in PUA levels. The time course of response of PUA levels in hyperuricemic animals was consistent with the previous report.[18] Compared with the PO group, the remaining four groups exhibited the significant reducing effects of TA on uric acid in hyperuricemic rat plasma after TA administration within 2.5 h (P < 0.05). Based on the reducing trend of uric acid, TA-EA showed strong XO inhibition 3.5 h after administration. Phytochemicals isolated from the most inhibitory extracts of TA, TA-EA, were then examined for their potential effects of XO inhibitory activities. Recently, Xu et al. reported that the administration of Rhizoma smilacis glabrae extract (1 ml/100 g) in hyperuricemic rats significantly reduced serum UA levels within 12 h.[14] The comparison of their results in lowering PUA with ours indicates that TA extracts exhibit remarkable hyporuricemic effects [Figure 3].
Figure 3.
Uric acid lowering effect by the TA extracts in PO-induced hyperuricemic rats. Results are mean ± SD of eight rats. *, P < 0.05, compared with the PO group. PUA measurements after treatments administration and point 0 min are from TA but 1 h from PO (250 mg/kg) injections. One milliliter of TA extract of HE, EA, BuOH and water fractions has 0.28, 0.21, 0.28 and 1.03 mg TA, respectively
XO inhibitory activities of phytochemicals from TA extracts
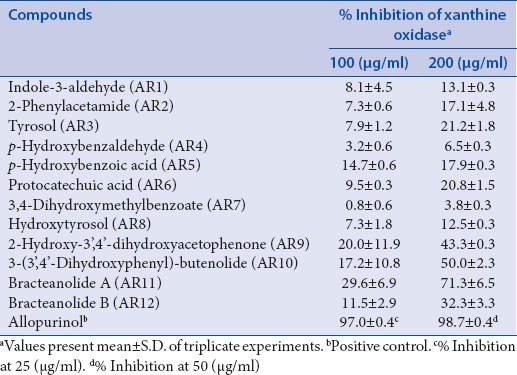
The XO inhibitory activity of major phytochemicals from TA extracts was compared with allopurinol, which is clinically used as an XO inhibitor, as shown in Table 1. In the presence of test samples, AR1–AR12 obtained from the EA fraction at a concentration of 200 µg/ml, AR11 (bracteanolide A) exhibited the best XO inhibitory activity (71.3%), followed by AR10 (3-(3′,4′-dihydroxyphenyl)-butenolide) (50.0%); other compounds showed weak inhibitory effects, with percent inhibition values from 3.8% to 43.3% at a dose of 200 µg/ml. The IC50 values of AR11 and allopurinol were measured to be 76.4 and 0.46 µg/ml, respectively. These data suggest that the butenolide plays a very important role in XO inhibition, which is similar to that the acyl group obtained by Ngoc et al. derived from Cinnamomum cassia (Lauraceae) twigs, and is an essential structural component that helps determine the XO inhibitory activity.[13] Others yield weak or negligible inhibitory activity against XO [Table 1].
Table 1.
Inhibitory effects of isolated phytochemicals AR1-12 from EA soluble fraction of T. albiflora extracts against XO
DISCUSSION
It is noteworthy that two hydroxybutenolides, bracteanolide A (AR11) and B (AR12) were isolated, identified, and first reported in the plant of study from Murdannia bracteata (Commelinaceae) using nitric oxide (NO) production assay.[19] The extract bracteanolide A was found the most potent and selective for inducible NO synthase, which may have potential anti-inflammatory properties. Moreover, our studies showed that bracteanolide A (AR11) was found to inhibit XO in vitro.
CONCLUSION
To the best of our knowledge, this is the first report on the scientific rationale of TA for anti-hyperuricemic medicinal use. Based on the results presented here, 12 compounds isolated from the TA-EA fraction of TA were evaluated for their XO inhibitory activity, and TA extracts were found to possess in vivo hypouricemic effects. The effective compound bracteanolide A (AR11), isolated from TA extracts, could be developed as a potent XO inhibitor.
Financial support and sponsorship
This research project was supported in part by China Medical University under the Aim for Top University Plan of the Ministry of Education, Taiwan, and Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence (MOHW105-TDU-B-212-133019).
Conflicts of interest
There are no conflicts of interest.
ABOUT AUTHORS

Yueh-Hsiung Kuo
Prof. Yueh-Hsiung Kuo, graduated from Agriculture of Chemistry National Taiwan University and get BS degree in 1964. He obtained his Ph.D. degree in 1973 from Department of Chemistry, Osaka City University. He retired from the Department of Chemistry, National Taiwan University as Emeritus professor since 2007. Then he continued to serve in China Medical University as Chair of Tsuzuki Institute for Traditional Medicine for continuing teaching and research. His research field was as follows: natural product, natural medicinal product, synthetic medicine and sensitizer photooxidation. He had published about 500 papers including many fields such as natural medicinal synthesis, anti-cancer, natural products’ elucidation, anti-diabetes, liver, lung and kidney protecting. His current research was focus in Antrodia camphorata, an edible and a medicinal fountional fungi.

Chun-Hsu Yao
Prof. Chun-Hsu Yao, obtained his Ph.D. degree in 1997 from Department of Chemistry, Chung Yuan Christian University, Taiwan (Ph.D. thesis accomplished in Center for Biomedical Engineering, NTU under supervision of Prof. Feng-Huei Lin). He is positioned as Dean of Academia-Industry Cooperation Office and Professor of Department of Biological Imaging and Radiological Science in China Medical University. He is also a Joint Professor of Department of Bioinformatics and Medical Engineering in Asia University. To biomaterials and regenerative medicine fields, he has contributed more than 100 SCI papers.
Figure.

Tzong-Fu Kuo
Prof. Tzong-Fu Kuo D.V.M. obtained his Ph.D. degree in 1989 from the School of Veterinary Medicine, National Taiwan University. He is positioned as Chief Executive Officer of Chinese Society of Traditional Veterinary Science (2009–2012) and Vice President of Asian Society of Traditional Veterinary Medicine (2010–2014). He is also Professor of School of Veterinary Medicine in National Taiwan University and School of Post-Baccalaureate Veterinary Medicine in Asia University. To veterinary herbal medicine fields, he has contributed more than 100 term papers in Chinese. His current research has a focus on bone marrow stem cells and growth factors in regenerative medicine.
REFERENCES
- 1.Pereira OL, Barreto RW, Waipara N. from Brazil for classical biocontrol of Tradescantia fluminensis. In: Julien MH, Sforza R, Bon MC, Evans HC, Hatcher PE, Hinz HL, editors. Proceedings of the XII International Symposium on Biological Control of Weeds. Wallingford: CAB International; 2008. pp. 195–9. [Google Scholar]
- 2.Tseng HC. Studies on the Chemical Constituents from Tradescantia albiflora Kunth. Taipei, Taiwan: Master's Thesis, National Taiwan University; 2008. [Google Scholar]
- 3.Harris MD, Siegel LB, Alloway JA. Gout and hyperuricemia. Am Fam Physician. 1999;59:925–34. [PubMed] [Google Scholar]
- 4.Wallach SL. The side effects of allopurinol. Hosp Pract. 1998;33:22. [PubMed] [Google Scholar]
- 5.Horiuchi H, Ota M, Nishimura S, Kaneko H, Kasahara Y, Ohta T, et al. Allopurinol induces renal toxicity by impairing pyrimidine metabolism in mice. Life Sci. 2000;66:2051–70. doi: 10.1016/s0024-3205(00)00532-4. [DOI] [PubMed] [Google Scholar]
- 6.Kong LD, Cai Y, Huang WW, Cheng CH, Tan RX. Inhibition of xanthine oxidase by some Chinese medicinal plants used to treat gout. J Ethnopharmacol. 2000;73:199–207. doi: 10.1016/s0378-8741(00)00305-6. [DOI] [PubMed] [Google Scholar]
- 7.Kong LD, Yang C, Ge F, Wang HD, Guo YS. A Chinese herbal medicine Ermiao wan reduces serum uric acid level and inhibits liver xanthine dehydrogenase and xanthine oxidase in mice. J Ethnopharmacol. 2004;93:325–30. doi: 10.1016/j.jep.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 8.Unno T, Sugimoto A, Kakuda T. Xanthine oxidase inhibitors from the leaves of Lagerstroemia speciosa (L.) Pers. J Ethnopharmacol. 2004;93:391–5. doi: 10.1016/j.jep.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 9.Chien SC, Yang CW, Tseng YH, Tsay HS, Kuo YH, Wang SY. Lonicera hypoglauca inhibits xanthine oxidase and reduces serum uric acid in mice. Planta Med. 2009;75:302–6. doi: 10.1055/s-0029-1185300. [DOI] [PubMed] [Google Scholar]
- 10.Tung YT, Chang ST. Inhibition of xanthine oxidase by Acacia confusa extracts and their phytochemicals. J Agric Food Chem. 2010;58:781–6. doi: 10.1021/jf901498q. [DOI] [PubMed] [Google Scholar]
- 11.Chen L, Yin H, Lan Z, Ma S, Zhang C, Yang Z, et al. Anti-hyperuricemic and nephroprotective effects of Smilax china L. J Ethnopharmacol. 2011;135:399–405. doi: 10.1016/j.jep.2011.03.033. [DOI] [PubMed] [Google Scholar]
- 12.Ho ST, Tung YT, Huang CC, Kuo CL, Lin CC, Yang SC, et al. The hypouricemic effect of Balanophora laxiflora extracts and derived phytochemicals in hyperuricemic mice. Evid Based Complement Alternat Med. 2012;2012:910152. doi: 10.1155/2012/910152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ngoc TM, Khoi NM, Ha do T, Nhiem NX, Tai BH, Don DV, et al. Xanthine oxidase inhibitory activity of constituents of Cinnamomum cassia twigs. Bioorg Med Chem Lett. 2012;22:4625–8. doi: 10.1016/j.bmcl.2012.05.051. [DOI] [PubMed] [Google Scholar]
- 14.Xu WA, Yin L, Pan HY, Shi L, Xu L, Zhang X, et al. Study on the correlation between constituents detected in serum from Rhizoma Smilacis Glabrae and the reduction of uric acid levels in hyperuricemia. J Ethnopharmacol. 2013;150:747–54. doi: 10.1016/j.jep.2013.09.024. [DOI] [PubMed] [Google Scholar]
- 15.Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–10. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]
- 16.Kong L, Zhou J, Wen Y, Li J, Cheng CH. Aesculin possesses potent hypouricemic action in rodents but is devoid of xanthine oxidase/dehydrogenase inhibitory activity. Planta Med. 2002;68:175–8. doi: 10.1055/s-2002-20262. [DOI] [PubMed] [Google Scholar]
- 17.Nguyen MT, Awale S, Tezuka Y, Tran QL, Watanabe H, Kadota S. Xanthine oxidase inhibitory activity of Vietnamese medicinal plants. Biol Pharm Bull. 2004;27:1414–21. doi: 10.1248/bpb.27.1414. [DOI] [PubMed] [Google Scholar]
- 18.Huang CG, Shang YJ, Zhang J, Zhang JR, Li WJ, Jiao BH. Hypouricemic effects of phenylpropanoid glycosides acteoside of Scrophularia ningpoensis on serum uric acid levels in potassium oxonate-pretreated Mice. Am J Chin Med. 2008;36:149–57. doi: 10.1142/S0192415X08005667. [DOI] [PubMed] [Google Scholar]
- 19.Wang GJ, Chen SM, Chen WC, Chang YM, Lee TH. Selective inducible nitric oxide synthase suppression by new bracteanolides from Murdannia bracteata. J Ethnopharmacol. 2007;112:221–7. doi: 10.1016/j.jep.2007.02.025. [DOI] [PubMed] [Google Scholar]