Abstract
Objective:
The migration and invasion features, which were associated with inflammatory response, acted as vital roles in the development of colon cancer. Quercetin, a bioflavonoid compound, was widely spread in vegetables and fruits. Although quercetin exerts antioxidant and anticancer activities, the molecular signaling pathways in human colon cancer cells remain unclear. Hence, the present study was conducted to investigate the suppression of quercetin on migratory and invasive activity of colon cancer and the underlying mechanism.
Materials and Methods:
The effect of quercetin on cell viability, migration, and invasion of Caco-2 cells was analyzed by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay, wound-healing assay, and transwell chambers assay, respectively. The protein expressions of toll-like receptor 4 (TLR4), nuclear factor-kappa B (NF-κB) p65, mitochondrial membrane potential-2 (MMP-2), and MMP-9 were detected by Western blot assay. The inflammatory factors, such as tumor necrosis factor-α (TNF-α), cyclooxygenase-2 (Cox-2), and interleukin-6 (IL-6), in cell supernatant were detected by enzyme-linked immunosorbent assay.
Results:
The concentration of quercetin <20 μM was chosen for further experiments. Quercetin (5 μM) could remarkably suppress the migratory and invasive capacity of Caco-2 cells. The expressions of metastasis-related proteins of MMP-2, MMP-9 were decreased, whereas the expression of E-cadherin protein was increased by quercetin in a dose-dependent manner. Interestingly, the anti-TLR4 (2 μg) antibody or pyrrolidine dithiocarbamate (PDTC; 1 μM) could affect the inhibition of quercetin on cell migration and invasion, as well as the protein expressions of MMP-2, MMP-9, E-cadherin, TLR4, and NF-κB p65. In addition, quercetin could reduce the inflammation factors production of TNF-α, Cox-2, and IL-6.
Conclusion:
The findings suggested for the 1st time that quercetin might exert its anticolon cancer activity via the TLR4- and/or NF-κB-mediated signaling pathway.
SUMMARY
Quercetin could remarkably suppress the migratory and invasive capacity of Caco-2 cells
The expressions of metastasis-related proteins of mitochondrial membrane potential-2 (MMP-2), MMP-9 were decreased, whereas the expression of E-cadherin protein was increased by quercetin in a dose-dependent manner
The anti-toll-like receptor 4 (TLR4) antibody or pyrrolidine dithiocarbamate affected the inhibition of quercetin on cell migration and invasion, as well as the protein expressions of MMP-2, MMP-9, E-cadherin, TLR4, and nuclear factor-kappa B p65
Quercetin could reduce the inflammation factors production of tumor necrosis factors-α, cyclooxygenase-2, and interleukin-6.
Abbreviations used: MTT: 3-(4,5-dimethylthiazol-2-yl)- 2,5-diphen yltetrazolium bromide, TLR4: Toll-like receptor 4, NF-κB: Nuclear factor-kappa B, MMP-2: Mitochondrial membrane potential-2, MMP-9: Mitochondrial membrane potential-9, TNF-α: Tumor necrosis factor-α, Cox-2: Cyclooxygenase-2, IL-6: Interleukin-6, ELISA: Enzyme-linked immunosorbent assay, PDTC: Pyrrolidine dithiocarbamate, ROS: Reactive oxygen species, DMSO: Dimethyl sulfoxide, FBS: Fetal bovine serum, DMEM: Dulbecco modified Eagle medium, OD: Optical density, IPP: Image Pro-plus, PBS: Phosphate buffered saline, SD: Standard deviation, ANOVA: One-way analysis of variance, SPSS: Statistical Package for the Social Sciences, ECM: Extracellular matrix, TLRs: Toll-like receptors, LPS: Lipopolysaccharide.
Keywords: Colon cancer, invasion, migration, quercetin, toll-like receptor 4
INTRODUCTION
Colon cancer, one of the most frequent malignant tumors worldwide, causes relatively high rates of morbidity and mortality.[1,2] Patients suffering from colon cancer often receive radiotherapy, hormonal therapy, immune therapy, chemotherapy, and symptomatic and supportive therapy. However, the current treatment was usually inefficient or caused a serious side effect. Therefore, it was urgent to explore the pathological mechanism of colon cancer and search effective, safe, and natural product to treat this malignancy.[3,4]
The relationship between colitis and the occurrence of colon cancer has been well-recognized.[5,6,7] It has been reported that the inflammatory bowel disease was widely acknowledged as the precancerous lesion associated with two-thirds of colitis-associated colon cancer.[8] Toll-like receptor 4 (TLR4), one mechanism for chronic inflammation in colon cancer tumorigenesis and progression, has been reported to be responsible for carcinogenesis, invasion, metastasis, and cancer progression.[9] TLR4 expression could be a useful prognostic marker in colon cancer.[10] TLR4/nuclear factor-kappa B (NF-κB) signaling pathway, frequently overexpressed in human colon cancer, plays crucial roles in colon cancer development.[11,12] Accumulating literatures demonstrated that the increased level of NF-kB phosphorylation in cancer tissues was associated with the clinical stage, migration, and invasion of colon cancer, indicating an important role of NF-kB activation in mediating the signaling event.[13,14]
Quercetin (3,3',4',5,7-pentahydroxyflavone, molecular weight = 302 g/mol), a bioactive flavonoid, is widely spread in various edible plants, and the primary sources are red onions, apples, and tea.[15,16] It was reported that quercetin-induced apoptosis through increased intracellular ROS in colon cancer cells.[17] In addition, the anti-invasion and antimigration ability of quercetin has been explored in other cells, such as human oral squamous cell carcinoma cell line SAS.[18] However, the underlying mechanism of quercetin suppressing the migration and invasion characteristics of colon cancer cells remains poorly understood.
In the current study, we explored the regulation effect of quercetin on the migration and invasion of colon cancer Caco-2 cells and investigated its role on TLR4/NF-κB pathway. The effect of quercetin on metastasis-related proteins and inflammatory factors was also evaluated.
MATERIALS AND METHODS
Chemicals and reagents
Quercetin (purity: ≥98%) was purchased from Sigma Chemical Co. (St. Louis, MO), and it was dissolved in dimethyl sulfoxide (DMSO) for the next experiments. Fetal bovine serum (FBS) and Dulbecco modified Eagle medium (DMEM) were obtained from GIBCO BRL Co. (Gaithersburg, MD, USA). Rabbit antihuman TLR4 antibody was obtained from AMS Biotechnology (Abington, UK), whereas pyrrolidine dithiocarbamate (PDTC; an NF-κB inhibitor) was purchased from Beyotime Institute of Biotechnology (Nantong, China). The antibodies to TLR4, NF-κB p65, mitochondrial membrane potential-2 (MMP-2), and MMP-9 were obtained from Cell Signaling Technology (Beverly, MA, USA). The enzyme-linked immunosorbent assay (ELISA) kits for tumor necrosis factor-α (TNF-α), cyclooxygenase-2 (Cox-2), and interleukin-6 (IL-6) were purchased from KeyGEN Biotech. Co., LTD. (Nanjing, China). Growth factor-reduced Matrigel was purchased from Becton Dickinson (BD Biosciences, San Jose, USA). 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was purchased from Sigma Chemical Co.(St. Louis, MO, USA). All other materials were from commercial sources.
Cell culture
The Caco-2 colon cancer cell line was obtained from the American Type Culture Collection (Manassas, VA, USA). Caco-2 cells were cultured in high glucose DMEM supplemented with 10% FBS, 80 units/ml of penicillin/streptomycin, 1% glutamine, and 1% nonessential amino acid. Cells were maintained on plastic cell culture flasks in a humidified atmosphere of 5% CO2 at 37°C. Medium was changed every 2 days. Cells were applied to the experiments when 80–90% confluent layer was generated.
Cell viability assay by 3-(4,5-dimethylthiazol-2-yl)-2,5- diphenyltetra zolium bromide
The effect of quercetin on cell viability was determined by MTT assay.[19] Briefly, Caco-2 cells were seeded in 96 well-plate (5 × 103 cells/well) and treated with quercetin (5, 10, and 20 μM) for 24 h. Then, the medium was removed and incubated with 10 μl of MTT (0.5 mg/ml) for 4 h. The formed crystals were dissolved by 100 μl of DMSO, and the optical density (OD) was measured at 490 nm on a Multiskan spectrum microplate reader (Thermo, Waltham, MA, USA). Cell viability was calculated by the following equation: Cell viability (%) = (ODquercetin–ODblank)/(ODcontrol–ODblank) ×100%. The test wasparallel conducted in triplicate.
Wound-healing assay
Caco-2 cells (1 × 106 cells/well) were seeded in 24 well-plates and allowed to grow until they reached confluence. Subsequently, the cells were scratched by 100 μl pipette tips and then cell debris was removed by washing with phosphate-buffered saline (PBS), and the wounded cell monolayer was cultured in FBS-free medium with quercetin or other agents for 24 h. Photographs of the wound area were captured at the 0 and 24 h with C × 31-32RFL microscope (Olympus, Tokyo, Japan). The relative wound area was obtained by quantitatively analyzing the areas in the scratch not overlapped by Caco-2 cells using Image Pro-plus (IPP) software (Media Cybernetics, Rockville, MD, USA).[20]
Invasion assay of Caco-2 cells
Cell invasion assay was performed by Matrigel-coated transwell cell culture chambers (8 μm pore size). Caco-2 cells were cultured in FBS-free DMEM overnight, then were collected and resuspended. The upper side of the membranes in the transwell chamber was coated with Matrigel (30 mg/L) in 50 μL ice-cold serum-free medium and air dried at the room temperature. The cell suspension of 250 μL at a density of 1 ns105 cell/well was added to the upper and 10% FBS DMEM medium of 500 μL was added to the lower chamber. Caco-2 cells were treated with quercetin, anti-TLR4 antibody or PDTC for 24 h. Subsequently, the transwell chamber was removed, and the medium in the upper chamber was washed twice with PBS. The nonpenetrated cells were removed by a cotton swab. Then, the transwell chamber was dried at room temperature. The invasive cells in the back of the membrane were stained with 0.1% crystal violet. The invasiveness of Caco-2 cells was defined according to the total number of cells in 3 randomly selected microscopic fields. Finally, the membrane was washed in 96 well plates with 100 μl acetic acid (30%). The OD value of washing solution was detected at 580 nm with a microplate reader.[21]
Western blot analysis
To explore the effect of quercetin on colon cancer-related protein expression and investigate the underlying mechanism, Western blot analysis was conducted for the expressions of MMP-2, MMP-9, E-cadherin, TLR4, and NF-κB p65.[22] After treatment with quercetin and other agents, Caco-2 cells were washed twice with PBS. Cell lysates were centrifuged at 13,000 × g at 4°C for 10 min to extract proteins. Proteins were separated by 10% SDS-PAGE gel and transferred onto a polyvinylidene difluoride membrane. In addition, membranes were blocked with 5% skimmed milk at room temperature for 1 h. Subsequently, the membranes were probed with 1:1000 diluted primary antibodies including MMP-2, MMP-9, E-cadherin, TLR4, and NF-κB p65 at 37°C for another 2 h. Membranes were rinsed with TBST for 4 times and then incubated with the horseradish peroxidase bound secondary antibody (1:5000) in a shaker. Finally, membranes were washed with PBS for 3 times and then chemoluminescence reagents were added for the visualization of the protein bands. The quantification of proteins was analyzed by IPP software (Media Cybernetics, Rockville, MD, USA).
Determination of tumor necrosis factor-α, cyclooxygenase-2, and interleukin-6 by enzyme-linked immunosorbent assay kits
The levels of inflammatory cytokines, such as TNF-α, Cox-2, and IL-6, in cells culture supernatant, were determined by ELISA kits (KeyGEN, Nanjing, China). Finally, the absorbance of each sample was read at 450 nm with a microplate reader within 3 min.[23] The content of TNF-α, Cox-2, and IL-6 were calculated according to the standard curve.
Statistical analysis
All values in this study were taken from three independent experiments and expressed as means ± standard deviation (SD). The statistical significance was analyzed using the one-way analysis of variance with the Statistical Package for the Social Sciences (SPSS, 13.0) software (Chicago, IL, USA). Differences with P < 0.05 were considered statistically significant.
RESULTS
Quercetin inhibited the viability of Caco-2 cells
In the experiment, the effect of quercetin on Caco-2 cell viability was estimated by MTT assay. Caco-2 cells were treated with various concentration of quercetin ranging from 0 μM to 100 μM for 24 h. As it can be seen in Figure 1, the viability of Caco-2 cells could be markedly inhibited when the concentration of quercetin was more than 20 μM. Moreover, the viability of Caco-2 cells did not remarkably change when the concentration of quercetin was <20 μM. Thus, the dose of quercetin <20 μM was chosen for further experiments.
Figure 1.
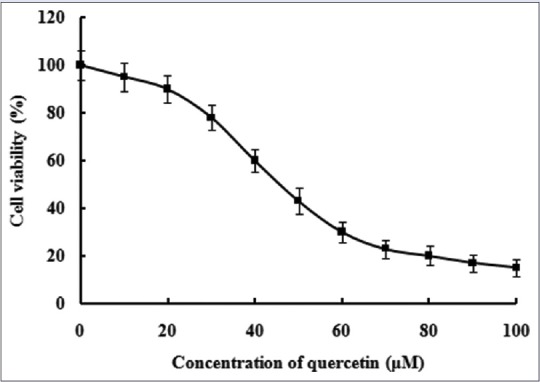
Effect of quercetin on cell viability of Caco-2 cells. The data were obtained from triplicate experiments and expressed as the means ± standard deviation (n = 3)
Quercetin suppressed the migratory ability of Caco-2 cells
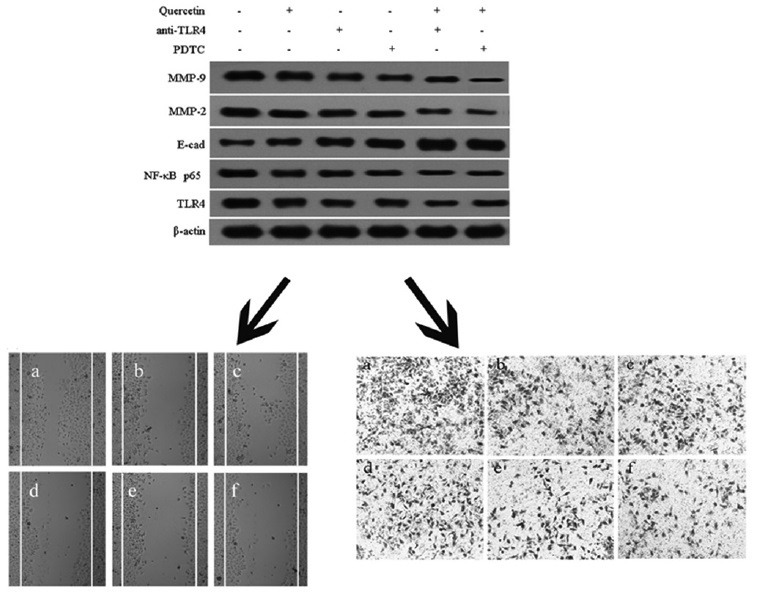
To investigate the ability of quercetin to possibly suppress cell migration, confluent monolayers of Caco-2 cells were scratched as described above. Subsequently, the cells were treated with quercetin and other agents for 24 h. The closure rate of Caco-2 cells in each group was evaluated by IPP software. As depicted in Figure 2, the Caco-2 cells in control blank group migrated and covered most of the wound area during the 24 h of incubation, and its wound closure was 75.2 ± 5.3%, which was significantly larger than the cells exposed to 5 μM quercetin, whose closure rate was 42.6 ± 4.4% (P< 0.01). To verify whether quercetin affected the migratory capacity of Caco-2 cells via TLR4 or NF-κB-mediated signaling pathway, Caco-2 cells were pretreated with anti-TLR4 antibody (2 μg) or PDTC (1 μM) for 1 h and then incubated in the presence or absence of quercetin (5 μM) for 24 h. Both anti-TLR4 antibody and PDTC significantly diminished the closure rate of Caco-2 cells (P< 0.05, P < 0.01). Moreover, there was a statistical difference between the co-incubation group and the alone one. Interestingly, the incubation of quercetin in the presence of anti-TLR4 antibody or PDTC could also decrease the closure rate (P< 0.05). The above evidence suggested that the inhibition of quercetin on the migration of Caco-2 cells might be related to TLR4-and/or NF-κB-mediated signaling pathway.
Figure 2.
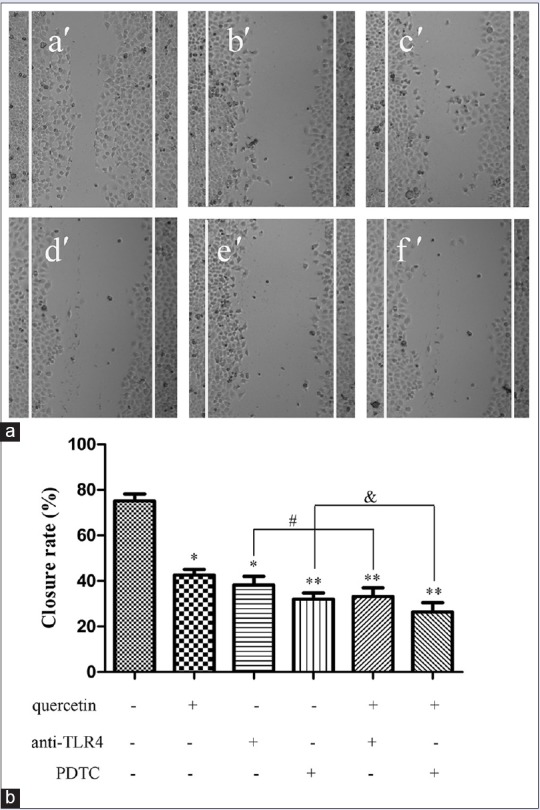
Effect of quercetin on migration of Caco-2 cells. (a) Cells were incubated in 24 well plates and treated with quercetin (5 μM) for 24 h in the presence or absence of anti-toll-like receptor 4 antibody (2 μg) or pyrrolidine dithiocarbamate (1 μM). a’: Blank control; b’: Quercetin of 5 μM; c’: Anti-toll-like receptor 4 antibody (2 μg); d’: Pyrrolidine dithiocarbamate (1 μM); e’: Quercetin (5 μM) + anti-toll-like receptor 4 antibody (2 μg); f’: Quercetin (5 μM) + pyrrolidine dithiocarbamate (1 μM). (b) The closure rate of cells was recorded, and the data were obtained from triplicate experiments and expressed as the means ± standard deviation (n = 3). *P < 0.05, **P < 0.01, versus blank control; #P < 0.05, quercetin (5 μM) + anti-toll-like receptor 4 versus anti-toll-like receptor 4 alone; &P < 0.05, quercetin (5 μM) + pyrrolidine dithiocarbamate versus pyrrolidine dithiocarbamate alone
Quercetin inhibited the invasive ability of Caco-2 cells
In this study, transwell invasion assay was performed to evaluate the effect of quercetin on the invasive capability of Caco-2 cells. The invasive cells in the lower surface of the filter were stained and counted. As it can be seen from Figure 3, the concentration of quercetin at 5 μM could remarkably reduced the number of penetrated cells compared with the control blank group (P< 0.05). As expected, the anti-TLR4 antibody (2 μg) and NF-κB inhibitor PDTC (1 μM) could also significantly inhibit the number of penetrated cells. Furthermore, quercetin of 5 μM in the presence of anti-TLR4 antibody (2 μg) or PDTC (1 μM) evidently prevented the cells penetrating through the Matrigel compared with the control blank group (P< 0.05). Furthermore, there was a significant statistical difference of the number of penetrated cells between the co-incubation of quercetin (5 μM) and anti-TLR4 antibody group and anti-TLR4 antibody alone group (P< 0.05). Interestingly, the same result could be seen in the co-incubation of quercetin (5 μM) and PDTC group and PDTC alone group (P< 0.01). The above results suggested that quercetin could suppress the invasive capability of Caco-2 cells. This suppression might be associated with the TLR4-and/or NF-κB-mediated signaling pathway.
Figure 3.
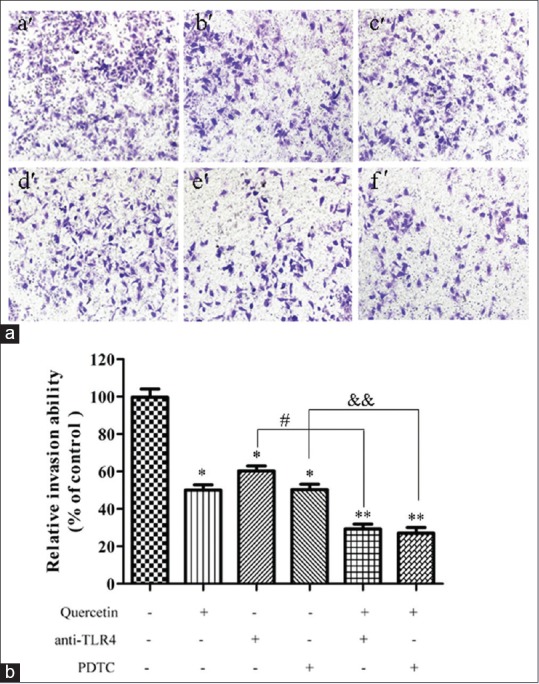
Effect of quercetin on invasion of Caco-2 cells. (a) The invasion of Caco-2 cells was evaluated by cell invasion assay, which was performed on 8 μm polycarbonate Matrigel-coated transwell cell culture chambers. Cells were incubated in 24 well plates and treated with quercetin (5 μM) for 24 h in the presence or absence of anti-toll-like receptor 4 antibody (2 μg) or pyrrolidine dithiocarbamate (1 μM). a’: Blank control; b’: Quercetin of 5 μM; c’: Anti-toll-like receptor 4 antibody (2 μg); d’: Pyrrolidine dithiocarbamate (1 μM); e’: Quercetin (5 μM) + anti-toll-like receptor 4 antibody (2 μg); f’: Quercetin (5 μM) + pyrrolidine dithiocarbamate (1 μM). (b) Optical density for the permeated cells. Data were obtained from triplicate experiments and expressed as the means ± standard deviation (n = 3). **P < 0.01, versus blank control; #P < 0.05, quercetin (5 μM) + anti-toll-like receptor 4 versus. anti-toll-like receptor 4 alone; &&P < 0.05, quercetin (5 μM) + pyrrolidine dithiocarbamate versus pyrrolidine dithiocarbamate alone
Effect of quercetin on the protein expressions of mitochondrial membrane potential-2, mitochondrial membrane potential-9, E-cadherin, toll-like receptor 4, and nuclear factor-kappa B p65 in Caco-2 cells
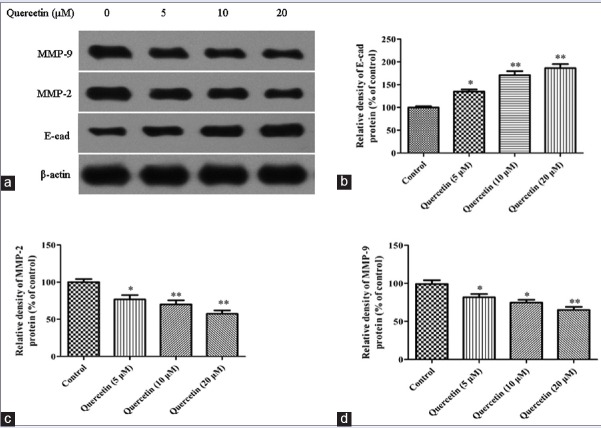
The degradation of extracellular matrix played an important role in the migration of tumor cells, which suggested that matrix-degrading proteinases were required.[24,25] To investigate whether or not quercetin suppressed the migration of Caco-2 cells via the inhibition of related proteins, the expressions of E-cadherin, MMP-2, and MMP-9 protein were evaluated by Western blot analysis. As presented in Figure 4, the Caco-2 cells after exposure to 5, 10, and 20 μM quercetin for 24 h, the expression of E-cadherin protein was 134.7 ± 8.1%, 171.4 ± 13.6%, and 186.3 ± 15.3% compared with the control blank group, respectively. The overexpression of MMP-2 and MMP-9 had been found to contribute to the migration of tumor cells. With the increment of quercetin dose from 5 μM to 20 μM, the expressions of MMP-2 and MMP-9 protein could be significantly decreased (P< 0.05, P < 0.01).
Figure 4.
Effect of quercetin on the expressions of metastasis-related protein with Western blot assay. (a) Western blot for the expressions of E-cadherin, mitochondrial membrane potential-2 and mitochondrial membrane potential-9 in Caco-2 cells. (b) E-cadherin protein level; (c) mitochondrial membrane potential-2 protein level. (d) Mitochondrial membrane potential-9 protein level. Data were expressed as the means ± standard deviation (n = 3). *P < 0.05, **P < 0.01 versus control blank group
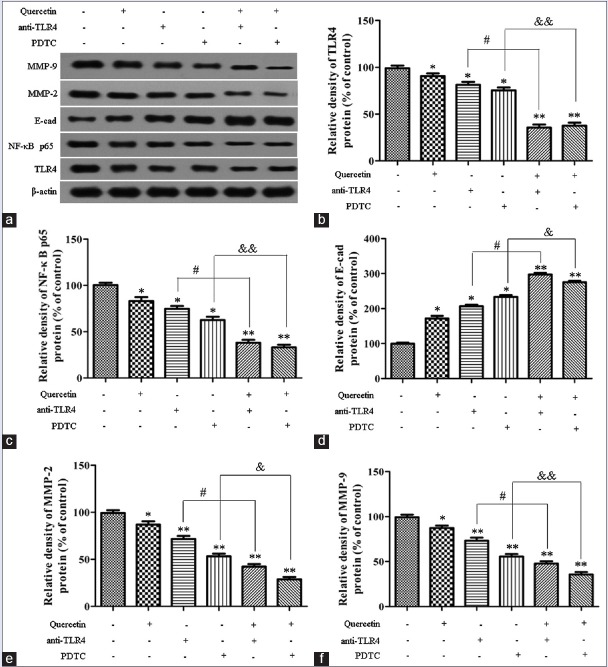
To further determine the specific effect of quercetin on TLR4/NF-κB pathway, Caco-2 cells were pretreated with anti-TLR4 antibody (2 μg) or PDTC (1 μM) for 1 h and then incubated in the presence or absence of quercetin (5 μM) for 24 h. As shown in Figure 5, the expressions of MMP-2, MMP-9, TLR4, and NF-κB p65 protein could be significantly diminished, and the expression of E-cadherin protein was evidently increased when the Caco-2 cells were incubated with anti-TLR4 antibody (2 μg) or PDTC (1 μM), compared with the control blank group (P< 0.05, P < 0.01). In addition, the co-treatment of quercetin and anti-TLR4 or quercetin and PDTC could markedly reduce the expressions of MMP-2, MMP-9, TLR4, and NF-κB p65 protein and increase the expression of E-cadherin protein, compared with the Caco-2 cells only been treated with quercetin (P< 0.05, P < 0.01). These findings indicated that the suppression of quercetin on migratory and invasive ability of Caco-2 cells through regulating the levels of MMP-2, MMP-9, and E-cadherin protein, which were affected by quercetin via TLR4-and/or NF-κB-mediated signaling pathway.
Figure 5.
Effect of quercetin on the toll-like receptor 4/nuclear factor-kappa B pathway in Caco-2 cells. (a) Western blot for the expressions of toll-like receptor 4, nuclear factor-kappa B p65, mitochondrial membrane potential-2, mitochondrial membrane potential-9, and E-cadherin; (b) toll-like receptor 4 protein level; (c) nuclear factor-kappa B p65 protein level; (d) E-cadherin protein level; (e) mitochondrial membrane potential-2 protein level; (f) mitochondrial membrane potential-9 protein level. Data were expressed as the means ± standard deviation (n = 3). *P < 0.05, **P < 0.01, versus blank control; #P < 0.05, quercetin (5 μM) + anti-toll-like receptor 4 versus anti-toll-like receptor 4 alone; &P < 0.05, &&P < 0.01, quercetin (5 μM) + pyrrolidine dithiocarbamate versus pyrrolidine dithiocarbamate alone
Regulation of quercetin on the levels of inflammation factors in Caco-2 cells
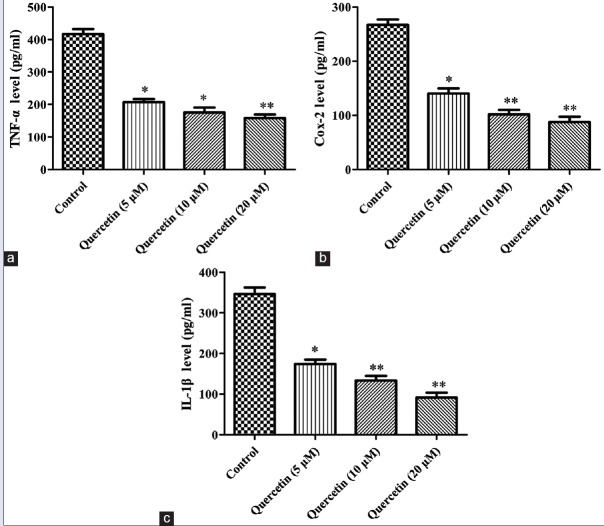
It has been reported that inflammation factors, such as TNF-α, Cox-2, and IL-6, played crucial roles in cancer cell migration.[26,27,28] ELISA kits assay was carried out to determine the levels of the inflammation factors. As presented in Figure 6, quercetin of different concentrations significantly decreased the levels of TNF-α, Cox-2, and IL-6, compared with the control blank group (P< 0.05, P < 0.01), which was in accordance with the effect of quercetin on migration and invasion of Caco-2 cells. The results demonstrated that the development of colon cancer Caco-2 cells was associated with the inflammatory response.
Figure 6.
Effect of quercetin on the expression of inflammation factors with enzyme-linked immunosorbent assay. (a) Tumor necrosis factor-α content in the cell supernatant; (b) cyclooxygenase-2 content in the cell supernatant; (c) interleukin-6 content in the cell supernatant. Data were expressed as the means ± standard deviation (n = 3). *P < 0.05, **P < 0.01 versus control blank group
DISCUSSION
Recently, medical scientists paid more attention to natural chemical compounds, which were vital targets in anticancer field, because of the drug resistance and toxic side effects of current chemotherapy.[29] Growing evidence indicated that quercetin could fight against different types of cancer, including Dalton's lymphoma, prostate cancer, hepatocellular carcinoma, breast cancer, glioma, and so on.[30,31,32,33,34] Quercetin has been reported to possess antiproliferation and antimigration capacity of cancer via EGFR/PI3K/Akt pathway, NF-κB, and matrix metalloproteinase-2/-9 signaling pathways.[18,35] However, to the best of our knowledge, the antimigratory and anti-invasive activity and the underlying mechanism of quercetin on Caco-2 cells remains unclear. The present study found that quercetin could suppress the migration and invasion of Caco-2 cells through the TLR4/NF-κB pathway to regulate migration and invasion-related protein expressions, including E-cadherin and MMPs.
Tumor metastasis was the greatest reasons of mortality. The complex processes of tumor invasion and metastasis included cell migration, adhesion, and invasion. E-cadherin, a key cell-to-cell adhesion molecule, was relevant to the invasion and metastasis of cancer cells and could promote the development of cancer.[36] MMPs, especially MMP-2 and MMP-9, were considered to be one of the most important intracellular factors responsible for the migration and invasion of cancer cells.[37] Chien et al. has proved that galangin could attenuate metastatic feature through inhibiting MMP-2 and MMP-9 enzyme activity in HepG2 cells.[38] In the present study, we also found that the expressions of E-cadherin, MMP-2, and MMP-9 could be suppressed by quercetin in a concentration-dependent manner as well as migration and invasion. The results suggested that the antimetastatic feature of quercetin on Caco-2 cells was associated with its suppression on E-cadherin, MMP-2, and MMP-9 expression.
Colonic inflammation could promote colon cancer development, especially for colitis-associated cancer.[8,39] TNF-α was a pro-inflammatory cytokine, which played an important role in tumor malignancy, including tumor cell motility, invasion, and metastasis.[40] High expression of TNF-α in the tumor microenvironment was a common characteristic of numerous malignant tumors. It has been reported that the increment of MMP-9 activity and expression was associated with the stimulation of TNF-α in cancer cells.[41] Furthermore, evidence suggested that the inflammatory cytokine of COX-2 could promote tumor angiogenesis and tumor invasion, and the downregulation of COX-2 inhibited cancer metastasis through preventing migration and invasion.[42,43] IL-6, linking the relationship between inflammation and cancer, was an indispensable inflammatory cytokines for cancer cell proliferation and invasion.[44,45] In the present study, we demonstrated that the levels of inflammation factors of TNF-α, COX-2, and IL-6 in cell supernatant were suppressed by quercetin in a dose-dependent manner. The result suggested that the attenuation of quercetin on the inflammatory responses could prevent the development of Caco-2 cells.
TLRs mediated signaling was thought to be closely relevant with cell migration and invasion during the development and progression of cancer.[46] TLR4, an important member of TLRs family, was known to recognize lipopolysaccharide on the surface of bacteria. TLR4 signaling pathway on colon cancer cell facilitated evasion of immune surveillance and mediated tumor progression, which was considered as one of the underlying mechanisms for chronic inflammation in colon cancer tumorigenesis and progression.[47] It has been reported that TLR4 signaling pathway was one of the most important pathways to regulate the proteinase activation of E-cadherin, MMP-2, and MMP-9.[48,49] The research of Byun et al. indicated that quercetin could negatively regulate TLR4 signaling in inflammatory disease.[50] Although the model was different, the above literature evidenced our results that quercetin could decrease the protein expression of TLR4. In the present study, the treatment of anti-TLR4 could obviously affect the migration and invasion of Caco-2 cells, and the expressions of E-cadherin, MMP-2, and MMP-9 protein. The results demonstrated that quercetin might play its anti-migratory and anti-invasive effect via TLR4-mediated signaling pathway.
NF-κB was the key downstream signal transducer of TLR4. Transcriptional factor NF-κB plays an important role in human colon cancer cells, and its constitutive activity has been found in cancer cell migration and invasion.[13] A growing body of evidence indicated that quercetin could efficiently down-regulate the phosphorylation of NF-κB to inhibit cancer migration and invasion through the decrement of MMP-2/MMP-9 protein expression in cancer cells.[18] Bhaskar et al. demonstrated that quercetin inhibited TLR4/NF-κB signaling pathway, thereby modulated the cytokine production and downregulated the activity of inflammatory enzymes, thus had protective effect against the ox-LDL-induced inflammation in PBMCs.[51] Wang et al. indicated that isoquercetin, a flavonoid similar to quercetin, protected cortical neurons from oxygen-glucose deprivation-reperfusion induced injury via suppression of TLR4-NF-κB signal pathway.[52] A study by Kaneko et al. showed that quercetin suppressed the TLR4/NF-κB signaling pathway to decrease the accumulation of lipid rafts.[53] The similar results were present in our observations. Quercetin could inhibit the activation of TLR4 and NF-κB p65. Our results indicated that the suppression of quercetin on migration and invasion of Caco-2 cells might be relevant to TLR4-and/or NF-κB-mediated signaling pathway.
CONCLUSION
Our findings indicated that quercetin could inhibit the migration and invasion of Caco-2 cells. The treatment with quercetin in the presence or absence of anti-TLR4 antibody and NF-κB inhibitor (PDTC) could decrease the expressions of MMP-2, MMP-9, TLR4, and NF-κB p65 protein, and increase the expression of E-cadherin protein. The results demonstrated that quercetin could inhibit the migration and invasion of Caco-2 cells by inhibiting TLR4/NF-κB signaling pathway. These results might provide new insight into the underlying molecular mechanisms of quercetin on treating colon cancer. Consequently, we advise that quercetin could be a promising candidate for the treatment of colon cancer.
Conflicts of interest
There are no conflicts of interest.
ABOUT AUTHOR
Figure.

Yucheng Song
Yucheng Song, deputy chief physician, graduated from Zhongshan University in 2005, receiving a doctorate in oncology and worked in Henan Province People's Hospital.
Acknowledgment
This project was funded by the Health and Family Planning Commission of Henan Province (201403190).
REFERENCES
- 1.Zhang XA, Zhang S, Yin Q, Zhang J. Quercetin induces human colon cancer cells apoptosis by inhibiting the nuclear factor-kappa B Pathway. Pharmacogn Mag. 2015;11:404–9. doi: 10.4103/0973-1296.153096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shen Y, Lu B, Zhang S, Ma ZJ. Diterpenoid C of Radix Curcumae : An inhibitor of proliferation and inducer of apoptosis in human colon adenocarcinoma cells acting via inhibiting MAPK signaling pathway. Pharm Biol. 2014;52:1158–65. doi: 10.3109/13880209.2013.879907. [DOI] [PubMed] [Google Scholar]
- 3.Guo S, Shan S, Jin X, Li Z, Li Z, Zhao L, et al. Water stress proteins from Nostoc commune Vauch.exhibit anti-colon cancer activities in vitro and in vivo. J Agric Food Chem. 2015;63:150–9. doi: 10.1021/jf503208p. [DOI] [PubMed] [Google Scholar]
- 4.Pandurangan AK, Kumar SA, Dharmalingam P, Ganapasam S. Luteolin, a bioflavonoid inhibits azoxymethane-induced colon carcinogenesis: Involvement of iNOS and COX-2. Pharmacogn Mag. 2014;10 Suppl 2:S306–10. doi: 10.4103/0973-1296.133285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Swann JB, Vesely MD, Silva A, Sharkey J, Akira S, Schreiber RD, et al. Demonstration of inflammation-induced cancer and cancer immunoediting during primary tumorigenesis. Proc Natl Acad Sci U S A. 2008;105:652–6. doi: 10.1073/pnas.0708594105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Asquith M, Powrie F. An innately dangerous balancing act: Intestinal homeostasis, inflammation, and colitis-associated cancer. J Exp Med. 2010;207:1573–7. doi: 10.1084/jem.20101330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Greten FR, Eckmann L, Greten TF, Park JM, Li ZW, Egan LJ, et al. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118:285–96. doi: 10.1016/j.cell.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 8.Terzic J, Grivennikov S, Karin E, Karin M. Inflammation and colon cancer. Gastroenterology. 2010;138:2101–14.e5. doi: 10.1053/j.gastro.2010.01.058. [DOI] [PubMed] [Google Scholar]
- 9.Liu WT, Jing YY, Yu GF, Han ZP, Yu DD, Fan QM, et al. Toll like receptor 4 facilitates invasion and migration as a cancer stem cell marker in hepatocellular carcinoma. Cancer Lett. 2015;358:136–43. doi: 10.1016/j.canlet.2014.12.019. [DOI] [PubMed] [Google Scholar]
- 10.Eiró N, González L, González LO, Fernandez-Garcia B, Andicoechea A, Barbón E, et al. Toll-like receptor-4 expression by stromal fibroblasts is associated with poor prognosis in colorectal cancer. J Immunother. 2013;36:342–9. doi: 10.1097/CJI.0b013e31829d85e6. [DOI] [PubMed] [Google Scholar]
- 11.Tye H, Jenkins BJ. Tying the knot between cytokine and toll-like receptor signaling in gastrointestinal tract cancers. Cancer Sci. 2013;104:1139–45. doi: 10.1111/cas.12205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duan H, Qu L, Shou C. Mycoplasma hyorhinis induces epithelial-mesenchymal transition in gastric cancer cell MGC803 via TLR4-NF-κB signaling. Cancer Lett. 2014;354:447–54. doi: 10.1016/j.canlet.2014.08.018. [DOI] [PubMed] [Google Scholar]
- 13.Zhang D, Mi M, Jiang F, Sun Y, Li Y, Yang L, et al. Apple polysaccharide reduces NF-Kb mediated colitis-associated colon carcinogenesis. Nutr Cancer. 2015;67:177–90. doi: 10.1080/01635581.2015.965336. [DOI] [PubMed] [Google Scholar]
- 14.Qazi AK, Hussain A, Aga MA, Ali S, Taneja SC, Sharma PR, et al. Cell specific apoptosis by RLX is mediated by NFκB in human colon carcinoma HCT-116 cells. BMC Cell Biol. 2014;15:36. doi: 10.1186/1471-2121-15-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He Y, He Z, He F, Wan H. Determination of quercetin, plumbagin and total flavonoids in Drosera peltata Smith var. glabrata Y.Z.Ruan. Pharmacogn Mag. 2012;8:263–7. doi: 10.4103/0973-1296.103649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen X. Protective effects of quercetin on liver injury induced by ethanol. Pharmacogn Mag. 2010;6:135–41. doi: 10.4103/0973-1296.62900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim GT, Lee SH, Kim YM. Quercetin regulates sestrin 2-AMPK-mTOR signaling pathway and induces apoptosis via increased intracellular ROS in HCT116 colon cancer cells. J Cancer Prev. 2013;18:264–70. doi: 10.15430/JCP.2013.18.3.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lai WW, Hsu SC, Chueh FS, Chen YY, Yang JS, Lin JP, et al. Quercetin inhibits migration and invasion of SAS human oral cancer cells through inhibition of NF-κB and matrix metalloproteinase-2/-9 signaling pathways. Anticancer Res. 2013;33:1941–50. [PubMed] [Google Scholar]
- 19.Russo A, Caggia S, Piovano M, Garbarino J, Cardile V. Effect of vicanicin and protolichesterinic acid on human prostate cancer cells: Role of Hsp70 protein. Chem Biol Interact. 2012;195:1–10. doi: 10.1016/j.cbi.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 20.Cheng X, Gu J, Zhang M, Yuan J, Zhao B, Jiang J, et al. Astragaloside IV inhibits migration and invasion in human lung cancer A549 cells via regulating PKC-a-ERK1/2-NF-κB pathway. Int Immunopharmacol. 2014;23:304–13. doi: 10.1016/j.intimp.2014.08.027. [DOI] [PubMed] [Google Scholar]
- 21.Banyard J, Chung I, Migliozzi M, Phan DT, Wilson AM, Zetter BR, et al. Identification of genes regulating migration and invasion using a new model of metastatic prostate cancer. BMC Cancer. 2014;14:387. doi: 10.1186/1471-2407-14-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scieglinska D, Piglowski W, Chekan M, Mazurek A, Krawczyk Z. Differential expression of HSPA1 and HSPA2 proteins in human tissues; tissue microarray-based immunohistochemical study. Histochem Cell Biol. 2011;135:337–50. doi: 10.1007/s00418-011-0791-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cui L, Feng L, Zhang ZH, Jia XB. The anti-inflammation effect of baicalin on experimental colitis through inhibiting TLR4/NF-κB pathway activation. Int Immunopharmacol. 2014;23:294–303. doi: 10.1016/j.intimp.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 24.Peng PL, Hsieh YS, Wang CJ, Hsu JL, Chou FP. Inhibitory effect of berberine on the invasion of human lung cancer cells via decreased productions of urokinase-plasminogen activator and matrix metalloproteinase-2. Toxicol Appl Pharmacol. 2006;214:8–15. doi: 10.1016/j.taap.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 25.Okada N, Ishida H, Murata N, Hashimoto D, Seyama Y, Kubota S. Matrix metalloproteinase-2 and -9 in bile as a marker of liver metastasis in colorectal cancer. Biochem Biophys Res Commun. 2001;288:212–6. doi: 10.1006/bbrc.2001.5741. [DOI] [PubMed] [Google Scholar]
- 26.Singh K, Poteryakhina A, Zheltukhin A, Bhatelia K, Prajapati P, Sripada L, et al. NLRX1 acts as tumor suppressor by regulating TNF-a induced apoptosis and metabolism in cancer cells. Biochim Biophys Acta. 2015;1853:1073–86. doi: 10.1016/j.bbamcr.2015.01.016. [DOI] [PubMed] [Google Scholar]
- 27.Wang JY, Sun J, Huang MY, Wang YS, Hou MF, Sun Y, et al. STIM1 overexpression promotes colorectal cancer progression, cell motility and COX-2 expression. Oncogene. 2015;34:4358–67. doi: 10.1038/onc.2014.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Di GH, Liu Y, Lu Y, Liu J, Wu C, Duan HF. IL-6 secreted from senescent mesenchymal stem cells promotes proliferation and migration of breast cancer cells. PLoS One. 2014;9:e113572. doi: 10.1371/journal.pone.0113572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li X, Yang G, Li X, Zhang Y, Yang J, Chang J, et al. Traditional Chinese medicine in cancer care: A review of controlled clinical studies published in Chinese. PLoS One. 2013;8:e60338. doi: 10.1371/journal.pone.0060338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maurya AK, Vinayak M. Quercetin regresses Dalton's lymphoma growth via suppression of PI3K/AKT signaling leading to upregulation of p53 and decrease in energy metabolism. Nutr Cancer. 2015;67:354–63. doi: 10.1080/01635581.2015.990574. [DOI] [PubMed] [Google Scholar]
- 31.Wang P, Henning SM, Heber D, Vadgama JV. Sensitization to docetaxel in prostate cancer cells by green tea and quercetin. J Nutr Biochem. 2015;26:408–15. doi: 10.1016/j.jnutbio.2014.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee RH, Cho JH, Jeon YJ, Bang W, Cho JJ, Choi NJ, et al. Quercetin induces antiproliferative activity against human hepatocellular carcinoma (HepG2) cells by suppressing specificity protein 1 (Sp1) Drug Dev Res. 2015 doi: 10.1002/ddr.21235. doi: 10.1002/ddr.21235. [DOI] [PubMed] [Google Scholar]
- 33.Li SZ, Qiao SF, Zhang JH, Li K. Quercetin increase the chemosensitivity of breast cancer cells to doxorubicin via PTEN/Akt pathway. Anticancer Agents Med Chem. 2015;15:1185–9. doi: 10.2174/1871520615999150121121708. [DOI] [PubMed] [Google Scholar]
- 34.Pan HC, Jiang Q, Yu Y, Mei JP, Cui YK, Zhao WJ. Quercetin promotes cell apoptosis and inhibits the expression of MMP-9 and fibronectin via the AKT and ERK signalling pathways in human glioma cells. Neurochem Int. 2015;80:60–71. doi: 10.1016/j.neuint.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 35.Bhat FA, Sharmila G, Balakrishnan S, Arunkumar R, Elumalai P, Suganya S, et al. Quercetin reverses EGF-induced epithelial to mesenchymal transition and invasiveness in prostate cancer (PC-3) cell line via EGFR/PI3K/Akt pathway. J Nutr Biochem. 2014;25:1132–9. doi: 10.1016/j.jnutbio.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 36.Fan L, Wang H, Xia X, Rao Y, Ma X, Ma D, et al. Loss of E-cadherin promotes prostate cancer metastasis via upregulation of metastasis-associated gene 1 expression. Oncol Lett. 2012;4:1225–33. doi: 10.3892/ol.2012.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guruvayoorappan C, Kuttan G. Amentoflavone inhibits experimental tumor metastasis through a regulatory mechanism involving MMP-2, MMP-9, prolyl hydroxylase, lysyl oxidase, VEGF, ERK-1, ERK-2, STAT-1, NM23 and cytokines in lung tissues of C57BL/6 mice. Immunopharmacol Immunotoxicol. 2008;30:711–27. doi: 10.1080/08923970802278276. [DOI] [PubMed] [Google Scholar]
- 38.Chien ST, Shi MD, Lee YC, Te CC, Shih YW. Galangin, a novel dietary flavonoid, attenuates metastatic feature via PKC/ERK signaling pathway in TPA-treated liver cancer HepG2 cells. Cancer Cell Int. 2015;15:15. doi: 10.1186/s12935-015-0168-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Macarthur M, Hold GL, El-Omar EM. Inflammation and cancer II.Role of chronic inflammation and cytokine gene polymorphisms in the pathogenesis of gastrointestinal malignancy. Am J Physiol Gastrointest Liver Physiol. 2004;286:G515–20. doi: 10.1152/ajpgi.00475.2003. [DOI] [PubMed] [Google Scholar]
- 40.Balkwill F. TNF-alpha in promotion and progression of cancer. Cancer Metastasis Rev. 2006;25:409–16. doi: 10.1007/s10555-006-9005-3. [DOI] [PubMed] [Google Scholar]
- 41.Kim S, Choi JH, Kim JB, Nam SJ, Yang JH, Kim JH, et al. Berberine suppresses TNF-alpha-induced MMP-9 and cell invasion through inhibition of AP-1 activity in MDA-MB-231 human breast cancer cells. Molecules. 2008;13:2975–85. doi: 10.3390/molecules13122975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lasalandra C, Coviello M, Falco G, Divella R, Trojano G, Laterza AM, et al. Serum vascular endothelial growth factor and adiponectin levels in patients with benign and malignant gynecological diseases. Int J Gynecol Cancer. 2010;20:507–12. doi: 10.1111/IGC.0b013e3181c54fc5. [DOI] [PubMed] [Google Scholar]
- 43.Zheng H, Li Y, Wang Y, Zhao H, Zhang J, Chai H, et al. Downregulation of COX-2 and CYP 4A signaling by isoliquiritigenin inhibits human breast cancer metastasis through preventing anoikis resistance, migration and invasion. Toxicol Appl Pharmacol. 2014;280:10–20. doi: 10.1016/j.taap.2014.07.018. [DOI] [PubMed] [Google Scholar]
- 44.Park BK, Zhang H, Zeng Q, Dai J, Keller ET, Giordano T, et al. NF-kappaB in breast cancer cells promotes osteolytic bone metastasis by inducing osteoclastogenesis via GM-CSF. Nat Med. 2007;13:62–9. doi: 10.1038/nm1519. [DOI] [PubMed] [Google Scholar]
- 45.Karin M, Cao Y, Greten FR, Li ZW. NF-kappaB in cancer: From innocent bystander to major culprit. Nat Rev Cancer. 2002;2:301–10. doi: 10.1038/nrc780. [DOI] [PubMed] [Google Scholar]
- 46.Song EJ, Kang MJ, Kim YS, Kim SM, Lee SE, Kim CH, et al. Flagellin promotes the proliferation of gastric cancer cells via the Toll-like receptor 5. Int J Mol Med. 2011;28:115–9. doi: 10.3892/ijmm.2011.656. [DOI] [PubMed] [Google Scholar]
- 47.Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13:1050–9. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- 48.Liu CY, Xu JY, Shi XY, Huang W, Ruan TY, Xie P, et al. M2-polarized tumor-associated macrophages promoted epithelial-mesenchymal transition in pancreatic cancer cells, partially through TLR4/IL-10 signaling pathway. Lab Invest. 2013;93:844–54. doi: 10.1038/labinvest.2013.69. [DOI] [PubMed] [Google Scholar]
- 49.Yang H, Wang B, Wang T, Xu L, He C, Wen H, et al. Toll-like receptor 4 prompts human breast cancer cells invasiveness via lipopolysaccharide stimulation and is overexpressed in patients with lymph node metastasis. PLoS One. 2014;9:e109980. doi: 10.1371/journal.pone.0109980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Byun EB, Yang MS, Choi HG, Sung NY, Song DS, Sin SJ, et al. Quercetin negatively regulates TLR4 signaling induced by lipopolysaccharide through Tollip expression. Biochem Biophys Res Commun. 2013;431:698–705. doi: 10.1016/j.bbrc.2013.01.056. [DOI] [PubMed] [Google Scholar]
- 51.Bhaskar S, Shalini V, Helen A. Quercetin regulates oxidized LDL induced inflammatory changes in human PBMCs by modulating the TLR-NF-κB signaling pathway. Immunobiology. 2011;216:367–73. doi: 10.1016/j.imbio.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 52.Wang CP, Li JL, Zhang LZ, Zhang XC, Yu S, Liang XM, et al. Isoquercetin protects cortical neurons from oxygen-glucose deprivation-reperfusion induced injury via suppression of TLR4-NF-κB signal pathway. Neurochem Int. 2013;63:741–9. doi: 10.1016/j.neuint.2013.09.018. [DOI] [PubMed] [Google Scholar]
- 53.Kaneko M, Takimoto H, Sugiyama T, Seki Y, Kawaguchi K, Kumazawa Y. Suppressive effects of the flavonoids quercetin and luteolin on the accumulation of lipid rafts after signal transduction via receptors. Immunopharmacol Immunotoxicol. 2008;30:867–82. doi: 10.1080/08923970802135690. [DOI] [PubMed] [Google Scholar]