Abstract
Background:
Dianthus superbus, one of traditional herbal medicine, is widely used to treat urethritis, carbuncles and carcinoma.
Objective:
A simultaneous determination method was established for controlling the quality of D. superbus using the eight compounds, (E)-methyl-4-hydroxy-4-(8a-methyl-3-oxodecahydronaphthalen-4a-yl) (1), diosmetin-7-O(2'',6''-di-O-α-L-rhamnopyranosyl)-β-D-glucopyranoside (2), vanillic acid (3), 4-hydroxyphenyl acetic acid (4), 4-methoxyphenyl acetic acid (5), (E)-4-methoxycinnamic acid (6), 3-methoxy-4-hydroxyphenylethanol (7), and methyl hydroferulate (8) isolated from D. superbus.
Materials and Methods:
This analysis method was developed using high performance liquid chromatography coupled with diode array detector with a Shishedo C18 column at a column temperature of 3°C. The mobile phase was composed of 0.1% trifluoroacetic acid in water and acetonitrile. The flow rate was 1 ml/min and detection wavelength was set at 205 nm and 280 nm. Validation was performed in order to demonstrate selectivity, accuracy and precision of the method.
Results:
The calibration curves showed good linearity (R2 > 0.99). The limits of detection and limits of quantification were within the ranges 0.0159–0.6205 μg/ml and 0.3210–1.8802 μg/ml, respectively. Moreover, the relative standard deviations of intra- and inter-day precision were both <2.98%. The overall recoveries were in the range of 96.23–109.87%. Quantitative analysis of eight compounds in 12 D. superbus samples (D-1–D-12) from various regions were analyzed and compared by developed method.
Conclusion:
As a result, this established method was accurate and sensitive for the quality evaluation of eight compounds isolated from D. superbus and may provide a new basis for quality control of D. superbus.
SUMMARY
A simultaneous determination method of eight compounds in Dianthus superbus was established by high performance liquid chromatography-diode array detector
Developed analysis method is validated with linearity, precious and accuracy
The newly established method was successfully evaluated contents of eight compounds in 12 D. superbus samples (D.1.D.12) from various regions and compared.
Abbreviations used: HPLC: High performance liquid chromatography, LOD: Limits of detection, LOQ: Limits of quantification, RSD: Relative standard deviation.
Keywords: Dianthus superbus, quality control, simultaneous determination
INTRODUCTION
Dianthus superbus belonging to the Caryophyllaceae family is widely distributed in China and Korea. D. superbus is a traditional herbal medicine for treating urethritis, carbuncles and carcinoma. Previous studies on D. superbus showed various biologically activities, including antioxidant, antimicrobial, anticancer, and anti-inflammatory activity.[1,2,3] D. superbus also showed immunosuppressive effects, osteoblastic proliferative, suppress immunoglobulin E production and prevent peanut-induced anaphylaxis.[4,5,6,7] Especially, ethyl acetate fraction of D. superbus was found to possess the cytotoxic activity against cancer cells.[8] Dianthosaponins, dianthramide, flavonoid, coumarin, triterpenoid, pyran type glycoside, and cyclic peptides have been isolated from D. superbus.[9,10,11,12,13,14,15] We isolated eight compounds, (E)-methyl-4-hydroxy-4-(8a-methyl-3- oxodecahydronaphthalen-4a-yl) (1), diosmetin-7-O (2'',6''-di-O-α-L- rhamnopyranosyl)-β-D- glucopyranoside (2), vanillic acid (3), 4-hydroxyphenyl acetic acid (4), 4-methoxyphenyl acetic acid (5), (E)-4-methoxycinnamic acid (6), 3-methoxy-4-hydroxyphenylethanol (7), and methyl hydroferulate (8) from D. superbus and these compounds were used for simultaneous determination of D. superbus.
Herbal medicines are composed with various chemical compounds such as flavonoids, terpenoids, saponins, and phenols. These diversity compounds exhibited therapeutic efficacy of herbal medicines, but make some difficulties at quality control of herbal medicine. The contents of bioactive compounds were depended on the location, climate, and other cultivation factor. Considering these various factors, the quality control of the raw herbal medicines such as D. superbus has become important. Recently, many researchers develop analytical methods for quality control due to increase use of herbal medicines. Among the analytical methods, high performance liquid chromatography (HPLC) is the most popular one, because of its easy operation, side suitability, and high accuracy.[16]
In this paper, a simple and accurate HPLC-diode array detector (DAD) method was firstly developed and validated for the simultaneous determination of the eight compounds in D. superbus. This method was successfully applied to simultaneous quantification of D. superbus samples.
MATERIALS AND METHODS
Chemicals and reagents
The herbal samples of D. superbus were obtained from Kyungdong traditional herbal market in Seoul, Korea and identified by Dr. Young Bae Seo, a professor of the College of Oriental Medicine, Daejeon University, Korea. A voucher specimen (CJ004M) has been deposited in the natural products laboratory, the Kangwon National University, Chuncheon, Korea. D-1–D-7 and D-8–D-12 samples were originated from Korea and China, respectively.
HPLC grade water and acetonitrile was purchased from J.T. Baker. Analytical grade trifluoroacetic acid (TFA) was obtained from Dae Jung.
Isolation of compounds
(E)-methyl-4-hydroxy-4-(8a-methyl-3-oxodecahydronaphthalen-4a-yl) (1), diosmetin-7-O (2'',6''-di-O-α-L-rhamnopyranosyl)-β-D- glucopyranoside (2), vanillic acid (3), 4-hydroxyphenyl acetic acid (4), 4-methoxyphenyl acetic acid (5), (E)-4-methoxycinnamic acid (6), 3-methoxy-4- hydroxyphenylethanol (7), and methyl hydroferulate (8) were isolated in our laboratory [Figure 1]. The structures were confirmed by comparing their mass spectrometer and nuclear magnetic resonance data with literature data.[17,18,19,20,21,22,23,24] The purities of the eight standard compounds were above 98%.
Figure 1.
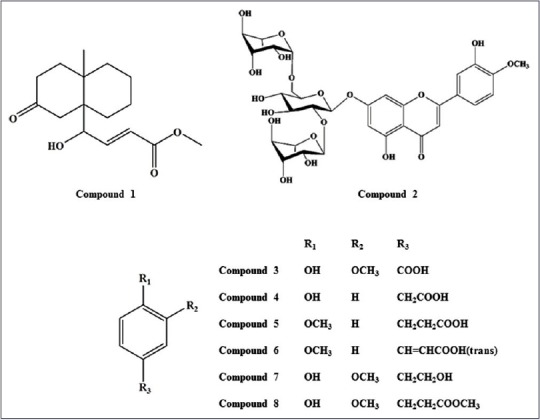
Chemical structures of compounds 1-8. 1: (E)-methyl-4-hydroxy-4-(8a-methyl-3-oxodecahydronaphthalen-4a-yl); 2: Diosmetin-7-O (2’’,6’’-di-O-α-L-rhamnopyranosyl)-β-D-glucopyranoside; 3: Vanillic acid; 4: 4-hydroxyphenyl acetic acid; 5: 4-methoxyphenyl acetic acid; 6: (E)-4-methoxycinnamic acid, 7: 3-methoxy-4-hydroxyphenylethanol 8: Methyl hydroferulate
Eight compound was isolated to a silica gel open column chromatography (CC) (90 cm × 10 cm, 70–230 mesh) using a gradient of n-hexane-EtOAc (100:1 → 0:1, v/v) on the EtOAc fraction.
7 fractions (A–G) were obtained from EtOAC fraction. Fraction F was performed to silica gel CC (EtOAc-MeOH from 50:1–0:1, v/v) to obtained 9 fractions (F1–F9).
Fraction F7 was subjected to medium-pressure liquid chromatography (MPLC) and four sub-fractions were collected. Compound 1 (51.3 mg) was purified with the Sephadex LH-20 and the MPLC.
Among the compounds, compound 1, (E)-methyl-4-hydroxy-4- (8a-methyl-3-oxodecahydronaphthalen- 4a-yl) was first isolated. (E)-methyl-4-hydroxy-4-(8a-methyl-3-oxodecahydronaphthalen-4a-yl) Yellow oil; 1H NMR (CD3 OD 400 MHz): δ 0.94 (3H, s, H-11), δ 1.17–2.31 (10H, m, H-4, 5, 6, 7, 9), δ 2.40–2.77 (4H, m, H-2, 10), δ 3.73 (3H, s, H-16), δ 3.89 (1H, dd, J = 7.58, 2.85 Hz, H-12), δ 6.01 (1H, dd, J = 15.26, 5.43 Hz, H-13), δ 6.16 (1H, dd, J = 15.26, 1.06 Hz, H-14).13 C NMR (CD3 OD 100 MHz) δ 18.20 (C-11), δ 23.01 (C-5), δ 23.77 (C-6), δ 29.00 (C-4), δ 29.14 (C-7), δ 33.77 (C-8), δ 36.85 (C-9), δ 42.28 (C-10), δ 50.99 (C-16), δ 52.27 (C-3), δ 52.95 (C-2), δ 77.40 (C-12), δ 139.67 (C-13), δ 124.67 (C-14), δ 174.99 (C-15), δ 210.25 (C-1).
Standard solution and Sample preparation
Eight Standard compounds, (E)-methyl-4-hydroxy-4-(8a-methyl-3- oxodecahydronaphthalen-4a-yl) (200 μg/ml), diosmetin- 7-O (2'',6''-di-O-α-L-rhamnopyranosyl)-β-D-glucopyranoside (200 μg/ml), vanillic acid (200 μg/ml), 4-hydroxyphenyl acetic acid (200 μg/ml), 4-methoxyphenyl acetic acid (200 μg/ml), (E)-4-methoxycinnamic acid (200 μg/ml), and 3-methoxy-4-hydroxyphenylethanol (200 μg/ml) were accuracy weighed and prepared in methanol. These were diluted to prepare solutions with six different concentrations for the establishment of calibration curves. Solutions were filtered through 0.45 µm membrane filter before HPLC analysis. Then, all solutions were stored in the refrigerator at 4°C.
Dried D. superbus extract powders were correctly weighed (10 mg) and dissolved in methanol (1 ml). These extract solutions also were filtered through a 0.45 µm membrane filter before sample injection.
High performance liquid chromatography-diode array detector analysis
The HPLC system is consisted of Dionex system with LPG 3X00 pump, ACC-3000 auto sampler, column oven, and DAD-3000(RS) DAD ultraviolet (UV)–visible. Separation was accomplished using a Shiseido C18 column (4.6 mm ID × 250 mm, 5 µm pore size). Column temperature was maintained at 30°C. The mobile phase was composed of 0.1% TFA aqueous solution (A) and acetonitrile (B) at a flow rate of 1.0 ml/min. The gradient flows were as follows: 15% B at 0–10 min, 15–25% B at 10–15 min, 25–70% B at 15–40 min, 70% B at 40–45 min. The injection volume was 20 µL. The wavelength of UV detection was set at 205 and 280 nm, respectively.
Calibration curves and limits of detection and quantification
The stock standard mixture was diluted with methanol to appropriate concentration ranges for the construction of calibration curves. Calibration curves were established by plotting the peak area (y) versus concentration (x) of each analyte. The linearity was measured by correlation coefficient (R2) values. Limit of detection (LOD) and quantification (LOQ) were determined using signal-to-noise ratios (S/N) of 3 and 10, respectively.
Precision and recovery
Intra- and inter-day test were conducted to determine the precision of the developed method. Validation of HPLC method with precision and recovery was performed according to the International Conference on Harmonization guidelines.[25] The intraday test was analyzed by standard solutions at three different concentrations on the single day and the inter-day test was performed on the three different days (1, 3, 5 days). In order to confirm the repeatability, each sample was analyzed 5 times as described above. The relative standard deviation (RSD) was taken as a measure of repeatability. RSD value determined repeatability of method.
Recovery test was used to investigate the accuracy of this analysis method. The standard solutions with known three different concentrations (5.56, 2.78, and 1.39 µg/mL) were added to D. superbus sample and then analyzed in 3 times. Recovery was calculated by the equation.
Recovery = (Amount found − original amount)/amount spiked × 100
RESULTS AND DISCUSSION
Method development
To obtain optimal analytic conditions, various HPLC parameters were tested, including column, mobile phase, and gradient elution system and flow rate. Based on the resolution, baseline and retention time, the analytical conditions were optimized. To improve the peak shape, TFA was added to the water. The detection wavelength was optimized at 205 nm for (E)-methyl-4-hydroxy-4-(8a-methyl-3- oxodecahydronaphthalen-4a-yl), diosmetin-7-O (2'',6''-di-O-α-L-rhamnopyranosyl)-β-D- glucopyranoside, vanillic acid, 3-methoxy-4-hydroxyphenylethanol and methyl hydroferulate and 280 nm for 4-hydroxy-phenylacetic acid, 4-methoxyphenylacetic acid, (E)-4-methoxycinnamic acid. About 20 μl was found to be the suitable injection volume. All peak of each compound were separated successful within 45 min. HPLC chromatogram of the eight compounds is shown in Figure 2. The peaks of each compound were confirmed by comparing their retention time in the HPLC chromatogram and the UV spectrum.
Figure 2.
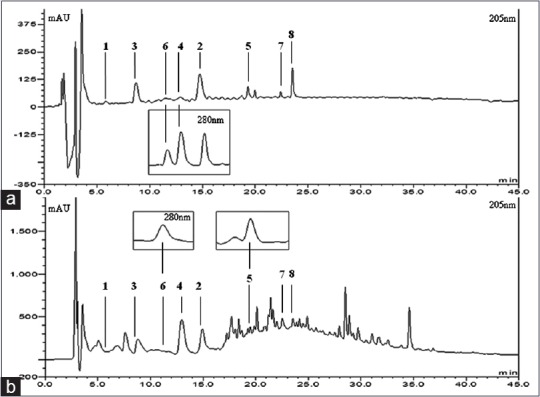
Chromatograms of standard compounds (a) and extract from Dianthus superbus (b). 1: (E)-methyl-4-hydroxy-4-(8a-methyl-3-oxodecahydronaphthalen-4a-yl); 2: Diosmetin-7-O (2’’,6’’-di -O-α-L-rhamnopyranosyl)-β-D-glucopyranoside; 3: Vanillic acid; 4: 4-hydroxyphenyl acetic acid; 5: 4-methoxyphenyl acetic acid; 6: (E)-4-methoxycinnamic acid; 7: 3-methoxy-4-hydroxyphenylethanol; 8: Methyl hydroferulate
Method validation
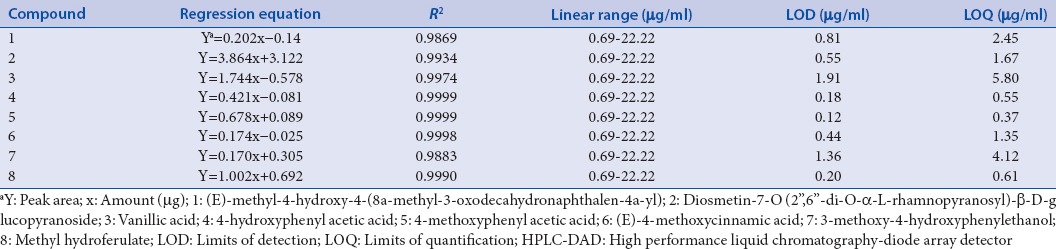
Calibration curves with six concentrations of standard solution were conducted in the concentration range of 0.69–22.22 µg/ml. The calibration data of the eight compounds showed a good linearity (R2 > 0.98). LOD and LOQ, which were measured by calibration curve, were in the range 0.12–1.91 μg/ml and 0.37–5.80 μg/ml, respectively [Table 1].
Table 1.
The regression data, limits of detection and limits of quantifications for eight compounds analyzed by HPLC-DAD
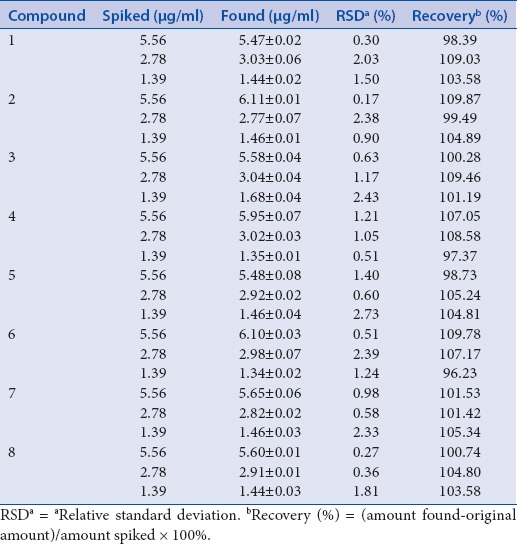
In inter- and intra-day test, the RSD values were taken as a measure of precision. As a result, the RSD values of the intra- and inter-day tests were found to be within the ranges 0.32–2.81% and 0.25–2.92%, respectively. And accuracy of intra- and inter-day was ranged from 91.14–109.70% and 93.61–108.49%, respectively [Table 2]. The recovery of the selected maker compounds ranged from 96.23 to 109.87%, and their RSD values were <2.73% [Table 3].
Table 2.
Intra- and inter-day precision of eight compounds. (E)-methyl-4-hydroxy-4-(8a-methyl-3-oxodecahydronaphthalen-4a-yl) (1), diosmetin-7-O (2“,6“-di-O-α-L-rhamnopyranosyl)-α-D-glucopyranoside (2), vanillic acid (3), 4-hydroxyphenyl acetic acid (4), 4-methoxyphenyl acetic acid (5), (E)-4-methoxycinnamic acid (6), 3-methoxy-4-hydroxyphenylethanol (7), and methyl hydroferulate (8)
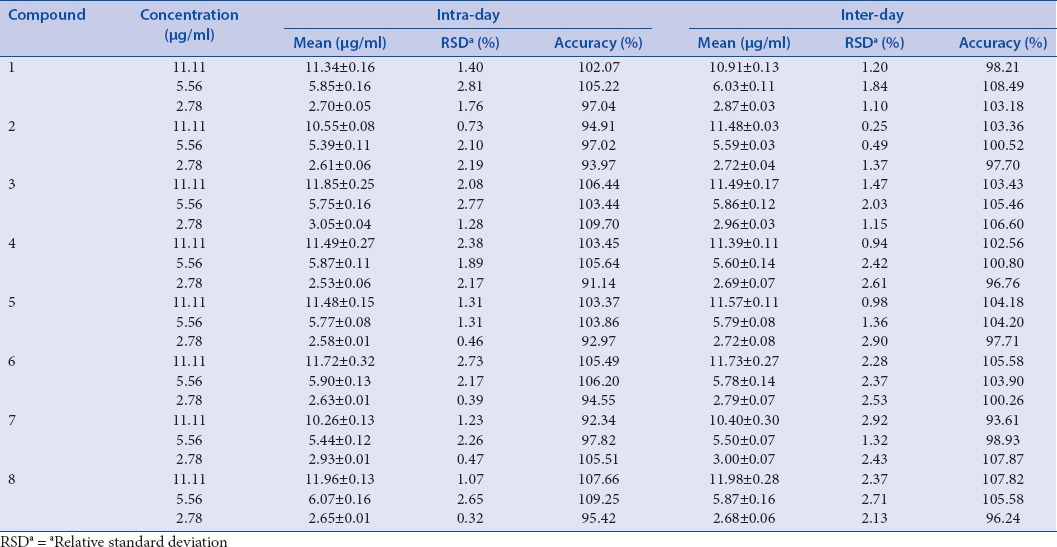
Table 3.
Recovery of the eight compounds. (E)-methyl-4-hydroxy-4-(8a-methyl-3-oxodecahydronaphthalen-4a-yl) (1), diosmetin-7-O (2“,6“-di-O-α-L-rhamnopyranosyl)-β-D-glucopyranoside (2), vanillic acid (3), 4-hydroxyphenyl acetic acid (4), 4-methoxyphenyl acetic acid (5), (E)-4-methoxycinnamic acid (6), 3-methoxy-4-hydroxyphenylethanol (7), and methyl hydroferulate (8)
Dianthus superbus samples analysis and cluster analysis
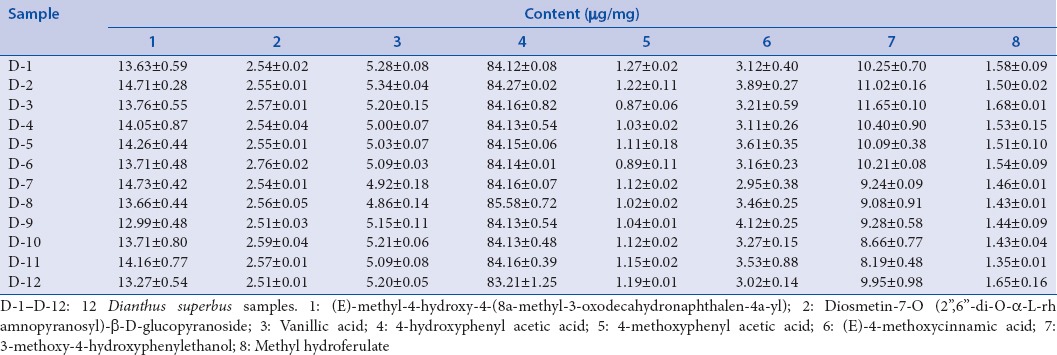
The newly established analytical method was applied to the analysis of eight compounds in 12 D. superbus samples (D-1–D-12) from various regions. The samples were analyzed using the optimized HPLC condition in triplicate to determine the mean content. The contents of eight compounds in D. superbus were calculated from the calibration curves of standard. Table 4 exhibited the results of sample analysis. The results show that 4-hydroxy-phenylacetic acid was the highest content (83.21–85.58 μg/mg) among the isolated compounds in D. superbus sample. Contents of 4-methoxyphenylacetic acid (0.87–1.27 μg/mg) and methyl hydroferulate (1.35–1.68 μg/mg) were lower than other compounds in 12 D. superbus samples.
Table 4.
Contents of eight compounds in 12 Dianthus superbus samples
We performed hierarchical cluster analysis (HCA) using IBM SPSS Statistics 21 (SPSS Inc., an IBM Company). Samples were investigated based on contents of eight compounds. HCA analysis has shown difference among different D. superbus sample origins. Difference from samples was represented by Euclidean distance [Figure 3]. Samples were divided into three clusters (I, II, III). Cluster I consisted of samples originated from Korea exclude D-10 and D-11 samples. Cluster II and III consisted of samples originated from China exclude D-3 sample. The samples clustered one group was associated with similar content of compounds. The result showed contents of compounds in D. superbus samples is different by cultivation environment and provided reference for the quality control of D. superbus.
Figure 3.
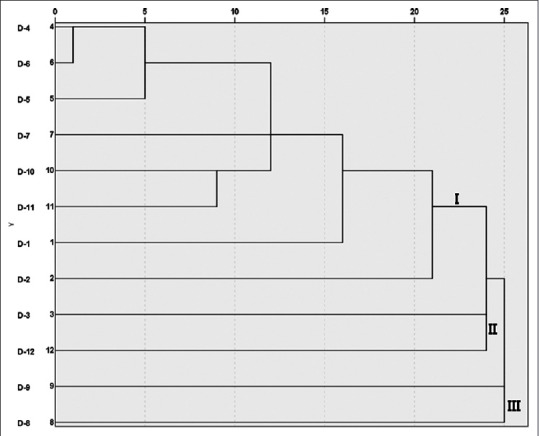
Dendrogram of cluster analysis for 12 Dianthus superbus samples
Simultaneous determination of eight compounds, (E)-methyl-4-hydroxy-4-(8a-methyl-3- oxodecahydronaphthalen-4a-yl) (1), diosmetin-7-O (2'',6''-di-O-α-L-rhamnopyranosyl)-β-D-glucopyranoside (2), vanillic acid (3), 4-hydroxyphenyl acetic acid (4), 4-methoxyphenyl acetic acid (5), (E)-4-methoxycinnamic acid (6), 3-methoxy-4- hydroxyphenylethanol (7), and methyl hydroferulate (8) in D. superbus was established for quantitative analysis of D. superbus and method validation including linearity, precision and accuracy. The results of linearity indicate that this established method has high sensitivity. Intra- and inter-day variability assays were used to determine the precision of the developed method and this result demonstrated good reproducibility of this analytical method. Recovery test was performed to evaluate the accuracy of this method. The results showed that the established method was reliable and accurate.
This developed method was successfully analyzed contents of eight compounds in 12 D. superbus samples. In addition, we confirmed that content of D. superbus is different between cultural environment, such as area and climate.
CONCLUSION
In this study, we developed the simultaneous determination of eight compounds, (E)-methyl-4-hydroxy-4- (8a-methyl-3- oxodecahydronaphthalen-4a-yl) (1), diosmetin-7-O (2'',6''-di-O-α-L-rhamnopyranosyl)-β-D-glucopyranoside (2), vanillic acid (3), 4-hydroxyphenyl acetic acid (4), 4-methoxyphenyl acetic acid (5), (E)-4-methoxycinnamic acid (6), 3-methoxy-4- hydroxyphenylethanol (7), and methyl hydroferulate (8) in D. superbus for quality control using HPLC-DAD.
This HPLC-DAD method was applied for simultaneous determination of D. superbus sample under optimized HPLC conditions. The results of method validation demonstrated that this developed method was sensitive, reliable and reproducible for simultaneous determination of D. superbus. Thus, this study may be provided analysis method for quality evaluation and identification of medicinal effect of D. superbus by using a HPLC-DAD.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
ABOUT AUTHOR

Choong Je Ma
Choong Je Ma has completed his PhD at the age of 32 years from Seoul National University and postdoctoral studies from University of Michigan. He is the professor of Department of Medical Biomaterials Engineering, College of Biomedical science, Kangwon National University, Korea. He has published more than 20 papers in reputed journals.
Acknowledgments
This research was supported by a Basic Science Research Program grant from the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology (2010-0005149).
REFERENCES
- 1.Ding C, Zhang W, Li J, Lei J, Yu J. Cytotoxic constituents of ethyl acetate fraction from Dianthus superbus. Nat Prod Res. 2013;27:1691–4. doi: 10.1080/14786419.2012.763127. [DOI] [PubMed] [Google Scholar]
- 2.Yu JO, Liao ZX, Lei JC, Hu XM. Antioxidant and cytotoxic activities of various fractions of ethanol extract of Dianthus superbus. Food Chem. 2007;104:1215–9. [Google Scholar]
- 3.Gou J, Zou Y, Ahn J. Enhancement of antioxidant and antimicrobial activities of Dianthus superbus, Polygonum aviculare, Sophora flavescens, and Lygodium japonicum by pressure-assisted water extraction. Food Sci Biotechnol. 2011;20:83–7. [Google Scholar]
- 4.López-Expósito I, Castillo A, Yang N, Liang B, Li XM. Chinese herbal extracts of Rubia cordifolia and Dianthus superbus suppress IgE production and prevent peanut-induced anaphylaxis. Chin Med. 2011;6:35. doi: 10.1186/1749-8546-6-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shin IS, Lee MY, Ha H, Jeon WY, Seo CS, Shin HK. Dianthus superbus fructus suppresses airway inflammation by downregulating of inducible nitric oxide synthase in an ovalbumin-induced murine model of asthma. J Inflamm (Lond) 2012;9:41. doi: 10.1186/1476-9255-9-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tong Y, Luo JG, Wang R, Wang XB, Kong LY. New cyclic peptides with osteoblastic proliferative activity from Dianthus superbus. Bioorg Med Chem Lett. 2012;22:1908–11. doi: 10.1016/j.bmcl.2012.01.058. [DOI] [PubMed] [Google Scholar]
- 7.Reid-Adam J, Yang N, Song Y, Cravedi P, Li XM, Heeger P. Immunosuppressive effects of the traditional Chinese herb Qu Mai on human alloreactive T cells. Am J Transplant. 2013;13:1159–67. doi: 10.1111/ajt.12180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu JQ, Yin Y, Lei JC, Zhang XQ, Chen W, Ding CL, et al. Activation of apoptosis by ethyl acetate fraction of ethanol extract of Dianthus superbus in HepG2 cell line. Cancer Epidemiol. 2012;36:e40–5. doi: 10.1016/j.canep.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 9.Shimizu M, Hayashi T, Shimizu K, Morita N. A pyran-type glycoside from Dianthus superbus var.longicalycinus. Phytochemistry. 1982;21:245–7. [Google Scholar]
- 10.Wang YC, Tan NH, Zhou J, Wu H. Cyclopeptides from Dianthus superbus. Phytochemistry. 1998;49:1453–6. [Google Scholar]
- 11.Hsieh PW, Chang FR, Wu CC, Wu KY, Li CM, Chen SL, et al. New cytotoxic cyclic peptides and dianthramide from Dianthus superbus. J Nat Prod. 2004;67:1522–7. doi: 10.1021/np040036v. [DOI] [PubMed] [Google Scholar]
- 12.Hsieh PW, Chang FR, Wu CC, Li CM, Wu KY, Chen SL, et al. Longicalycinin A, a new cytotoxic cyclic peptide from Dianthus superbus var. longicalycinus (MAXIM.) WILL. Chem Pharm Bull (Tokyo) 2005;53:336–8. doi: 10.1248/cpb.53.336. [DOI] [PubMed] [Google Scholar]
- 13.Dahiya R. Synthesis and biological activity of a cyclic hexapeptide from Dianthus superbus. Chem Pap. 2008;62:527–35. [Google Scholar]
- 14.Chen X, Luo JG, Kong LY. Two new triterpenoid saponins from Dianthus superbus L. J Asian Nat Prod Res. 2010;12:458–63. doi: 10.1080/10286020.2010.493326. [DOI] [PubMed] [Google Scholar]
- 15.Luo JG, Chen X, Kong LY. Three new triterpenoid saponins from Dianthus superbus. Chem Pharm Bull (Tokyo) 2011;59:518–21. doi: 10.1248/cpb.59.518. [DOI] [PubMed] [Google Scholar]
- 16.Jiang Y, David B, Tu P, Barbin Y. Recent analytical approaches in quality control of traditional Chinese medicines – A review. Anal Chim Acta. 2010;657:9–18. doi: 10.1016/j.aca.2009.10.024. [DOI] [PubMed] [Google Scholar]
- 17.Hon YS, Lu L, Chang RC, Linb SW, Sunb PP, Leea CF. Syntheses of α, β-unsaturated carbonyl compounds from the reactions of monosubstituted ozonides with stable phosphonium ylides. Tetrahedron. 2000;56:9269–79. [Google Scholar]
- 18.Emam AM, Elias R, Moussa AM, Faure R, Debrauwer L, Balansard G. Two flavonoid triglycosides from Buddleja madagascariensis. Phytochemistry. 1998;48:739–42. doi: 10.1016/s0031-9422(97)01043-1. [DOI] [PubMed] [Google Scholar]
- 19.Huang Z, Dostal L, Rosazza JP. Mechanisms of ferulic acid conversions to vanillic acid and guaiacol by Rhodotorula rubra. J Biol Chem. 1993;268:23954–8. [PubMed] [Google Scholar]
- 20.Lehnert W, Hunkler D. Possibilities of selective screening for inborn errors of metabolism using high-resolution 1H-FT-NMR spectrometry. Eur J Pediatr. 1986;145:260–6. doi: 10.1007/BF00439397. [DOI] [PubMed] [Google Scholar]
- 21.Rosecke J, Konig WA. Odorous compounds from the fungus Gloeophyllum odoratum. Flavour Fragr J. 2000;15:315–9. [Google Scholar]
- 22.Yu J, Whitney PS, Spencer JB. Direct comparison between the mechanism of hydrometalation and β-elimination in heterogeneous and homogeneous hydrogenation. J Mol Catal A Chem. 1999;146:199–210. [Google Scholar]
- 23.Shimoji Y, Tamura Y, Nakamura Y, Nanda K, Nishidai S, Nishikawa Y, et al. Isolation and identification of DPPH radical scavenging compounds in Kurosu (Japanese unpolished rice vinegar) J Agric Food Chem. 2002;50:6501–3. doi: 10.1021/jf020458f. [DOI] [PubMed] [Google Scholar]
- 24.Doyle MP, Hu W, Valenzuela MV. Total synthesis of (S)-(+)-imperanene.Effective use of regio- and enantioselective intramolecular carbon-hydrogen insertion reactions catalyzed by chiral dirhodium (II) carboxamidates. J Org Chem. 2002;67:2954–9. doi: 10.1021/jo016220s. [DOI] [PubMed] [Google Scholar]
- 25.Anonymous. ICH Guidelines: Validation of Analytical Procedures. Methodology. 2003;2 [Google Scholar]


