Abstract
Low-frequency repetitive transcranial magnetic stimulation (rTMS) of the unaffected hemisphere can enhance function of the paretic hand in patients with mild motor impairment. Effects of low-frequency rTMS to the contralesional motor cortex at an early stage of mild to severe hemiparesis after stroke are unknown. In this pilot, randomized, double-blind clinical trial we compared the effects of low-frequency rTMS or sham rTMS as add-on therapies to outpatient customary rehabilitation, in 30 patients within 5–45 days after ischemic stroke, and mild to severe hand paresis. The primary feasibility outcome was compliance with the interventions. The primary safety outcome was the proportion of intervention-related adverse events. Performance of the paretic hand in the Jebsen–Taylor test and pinch strength were secondary outcomes. Outcomes were assessed at baseline, after ten sessions of treatment administered over 2 weeks and at 1 month after end of treatment. Baseline clinical features were comparable across groups. For the primary feasibility outcome, compliance with treatment was 100% in the active group and 94% in the sham group. There were no serious intervention-related adverse events. There were significant improvements in performance in the Jebsen–Taylor test (mean, 12.3% 1 month after treatment) and pinch force (mean, 0.5 Newtons) in the active group, but not in the sham group. Low-frequency rTMS to the contralesional motor cortex early after stroke is feasible, safe and potentially effective to improve function of the paretic hand, in patients with mild to severe hemiparesis. These promising results will be valuable to design larger randomized clinical trials.
Keywords: Stroke, Transcranial magnetic stimulation, Rehabilitation, Hemiparesis, Neuromodulation
Introduction
Up to 65% of stroke survivors are not able to perform activities of daily living with the affected upper limb [1]. Even mild motor impairments of the upper limb negatively impact disability and quality of life in these patients [2]. A systematic review concluded that arm function can be improved by constraint-induced movement therapy, but no intervention was found to consistently decrease hand disability [3].
According to the model of interhemispheric competition, excessive inhibition of the lesioned hemisphere by the contralesional hemisphere may contribute to hand motor impairment in patients with mild motor deficits [4, 5]. Repetitive transcranial magnetic stimulation (rTMS) has emerged as a potential tool to restore interhemispheric balance and improve hand motor performance. While high-frequency rTMS (HF-rTMS) often increases M1 excitability, low-frequency rTMS (LF-rTMS) has the opposite effect [5, 6]. Up-regulation of excitability in the lesioned hemisphere by HF-rTMS or down-regulation of the contralesional hemisphere by LF-rTMS can facilitate movement of the paretic hand [6]. Both strategies, as well as their combination, have yielded encouraging results when applied to patients with mild hand motor impairment in the subacute and chronic stages after stroke [5–11].
However, until now few studies had targeted patients with moderate to severe hand motor impairment within the first months after stroke, and all of them applied HF-rTMS to the affected hemisphere [8, 12]. Furthermore, most rTMS studies excluded patients <6 months after stroke even though responsiveness to rehabilitation interventions is less prominent and less likely to occur in the chronic phase, compared to early stages [13].
Optimal timing and dosing of rehabilitation interventions are yet unresolved issues. For instance, high-intensity constraint-induced therapy, known to enhance activity in the lesioned hemisphere [14, 15] led to less improvement in upper extremity function compared to traditional therapy or low-intensity constraint-induced therapy, when administered early after stroke in the VECTORS study [16]. It has been argued that excessive disinhibition of the lesioned hemisphere early after stroke may be harmful [17, 18].
Here, we wanted to evaluate whether LF-rTMS of the contralesional hemisphere early after stroke is safe. We examined feasibility, safety, and preliminary efficacy of either active or sham LF-rTMS of the contralesional motor cortex as add-on therapies to outpatient customary rehabilitation, to patients with mild to severe hand paresis, at an early stage within 5–45 days after unilateral ischemic stroke.
Methods
Study design
In this pilot, randomized, double-blinded clinical trial we compared the feasibility, safety, and efficacy of either LF-rTMS of the contralesional hemisphere or sham rTMS as add-on therapies to outpatient customary rehabilitation, in patients within 5–45 days after stroke and mild to severe hand paresis (Medical Research Council scale, 4-0 in finger flexion or extension). This period is within the second epoch of post-stroke brain changes, at which most repair processes occur and during which most spontaneous behavior recovery is seen [19].
Participants
Patients aged 18–80 years, with ischemic stroke in the internal carotid artery territory within 5–45 days, confirmed by CT or MRI and leading to contralateral hand paresis, were eligible for the study. Exclusion criteria were: previous symptomatic strokes; contraindications to rTMS [20]; other neurological or severe chronic diseases; shoulder pain; joint deformity in the paretic upper limb; use of drugs that might interfere on cortical excitability [21]; inability to provide informed consent due to severe language or cognitive impairment. The protocol was approved by the local ethics committee and was therefore performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki. Informed consent was provided by patients or, for patients who could not write, by caregivers.
Baseline measures
The following characteristics were evaluated at baseline: age, gender, handedness [22], time from stroke, NIH Stroke Scale [23], infarct side/location. Infarcts were classified as cortico-subcortical or subcortical according to presence or absence of involvement of primary motor, primary somatosensory, supplementary motor, or premotor cortices on MRI (n = 28) or CT (n = 2).
Experimental design
Randomization, allocation concealment, and blinding
Patients were randomly assigned in blocks by the principal investigator with a basic random number computerized generator in a 1:1 ratio to receive, immediately before each 60-min rehabilitation treatment, either active or sham rTMS, five times per week, during 2 weeks (total, ten treatment sessions). The number of treatment sessions was the same described in a study that administered HF-rTMS to the lesioned hemisphere early after stroke [12].
This investigation was performed at Hospital das Clínicas/University of São Paulo on patients undergoing customary rehabilitative treatment for the appropriate stage of their diseases previously described [24] and administered by physical therapists blinded to the type of rTMS intervention. The same therapists evaluated behavioral endpoints. Patients were not aware of group assignment. To ensure anonymity, information about randomization and rTMS procedures was kept in printed and electronic formats in a locked cabinet, accessed only by researchers who performed rTMS. Patients did not discuss their experience during rTMS with therapists, or among each other.
rTMS interventions
In each treatment session, the optimal site of motor stimulation of the contralesional hemisphere was defined as the location where TMS elicited the largest motor evoked potentials (MEPs) in the nonparetic abductor pollicis brevis muscle (APB) with surface electrodes. The signal was amplified and filtered (10 Hz to 2 kHz) with an electromyography and evoked potential measuring unit (MEB-9104J, Nihon Kohden, Japan). Resting motor threshold was defined as the lowest intensity of TMS that evoked MEPs of at least 50 μV amplitude in the non-paretic abductor pollicis brevis muscle in at least 3/6 trials [25].
In both groups, 1 Hz rTMS was administered with a figure-of-eight coil (MCF B-65) at 90% of the nonparetic abductor pollicis brevis muscle resting motor threshold with a biphasic MagPro Compact stimulator (Alpine Biomed), for 25 min (1,500 pulses). These parameters led to increase in pinch acceleration of the paretic hand and decrease in interhemispheric inhibition from the contralesional hemisphere to the lesioned hemisphere when applied in patients in the chronic phase after stroke [7].
In the active group, the TMS coil was tangentially positioned to the scalp on the optimal site for the nonparetic APB in the contralesional hemisphere with the intersection of both wings at a 45° angle with the midline. In the sham group, the coil was held perpendicularly to the vertex. Patients were comfortably seated, wore earplugs, and were instructed to remain at rest during TMS.
Outcome measures
Primary outcome measures
The co-primary outcome measures were feasibility and safety. Therefore, the primary feasibility outcome was compliance with the interventions, measured by (number of planned sessions of treatment/number of attended sessions) × 100 (%).
The primary safety outcome was the proportion of intervention-related adverse events in the active and sham groups. After each treatment session, patients were asked about the presence of each of the following: headache or local pain under the coil, neck pain, dizziness, paresthesias, and sleepiness. Investigators also observed patients for the presence of seizures, syncope or behavioral changes, during treatment and 1 hour after treatment. Adverse events were registered in a log, at each treatment session. Heart rate, systolic and diastolic blood pressures were measured before and after each treatment session.
Secondary outcome measures
Efficacy, a secondary outcome measure, was evaluated with the Jebsen–Taylor test (JTT), that scores the time (in seconds) required to perform tasks that resemble daily life activities: picking up small objects, stacking checkers, among others [26]. The sentence writing task was not included in the score to avoid bias in evaluation of patients with language impairments. JTT is a commonly used scale in stroke rehabilitation studies, with moderate responsiveness to change in the first months after stroke [27–29]. The outcome was the JTT performance of the paretic upper limb (JTTper), compared to the nonparetic limb, and calculated as the percentage: (affected arm/unaffected arm) × 100. This calculation was implemented to characterize performance (0%) in patients with upper limb plegia who were not able to perform any of the JTT tasks at baseline, and thus whose performance could not be quantified in seconds.
Other secondary outcomes were: force of the lateral pinch of paretic hand, measured with a digital dynamometer (Kratos, São Paulo, Brazil) while the elbow was stabilized in extension by a splint [30]; Fugl-Meyer Assessment of Sensorimotor Recovery after stroke (sum of scores for the upper limb: passive range of motion, 24; joint pain, 24; sensation, 12; motor performance, 66; maximum score for the upper limb = 126) [31]; Ashworth Scale (median of elbow, wrist and fingers) [32] and modified Rankin scale (MRS) [23]. Clinical outcomes were assessed at baseline, after ten sessions of treatment administered over 2 weeks and at 1 month after end of treatment.
Sample size was not formally determined because this was a “hypothesis-generating” project carried out with the purpose of generating a power analysis for a more advanced investigation.
Analysis
Data are presented as mean (±standard deviation) or as median (range). Baseline characteristics were compared with the Mann–Whitney test or with the Fisher’s exact test. The Mann–Whitney test was also used to compare the number of tasks performed during rehabilitation therapy between the active and sham groups, as well as compliance with treatment in the active and sham groups. Rates of adverse events in each group were compared with Fisher’s exact tests. Changes in heart rate, systolic and diastolic blood pressures in each treatment session were compared, from the first through the tenth session of treatment in each group, with Friedman’s analysis of variance.
Secondary outcomes were also compared with per-protocol Friedman’s analysis of variance in the active and sham groups, given the pilot nature of this study. A 2-sided p value of 0.05 was considered statistically significant. Post hoc Wilcoxon tests with Bonferroni corrections were performed and a 2-sided p value of 0.017 was considered statistically significant for post hoc analysis. We chose the Friedman’s test over parametric tests due to the sample size and the pilot nature of this study.
Effect sizes for outcomes that improved significantly in only one of the groups were calculated as the ratio between the mean absolute change after treatment and the standard deviation at baseline [33]. SPSS (versions 10 and 16) software was used for statistical analysis.
Results
Between February 2008 and December 2010, 608 patients with ischemic stroke were screened for the study and 30 (4.7%) fulfilled inclusion criteria (Fig. 1). The main reason for exclusion was history of previous stroke.
Fig. 1.
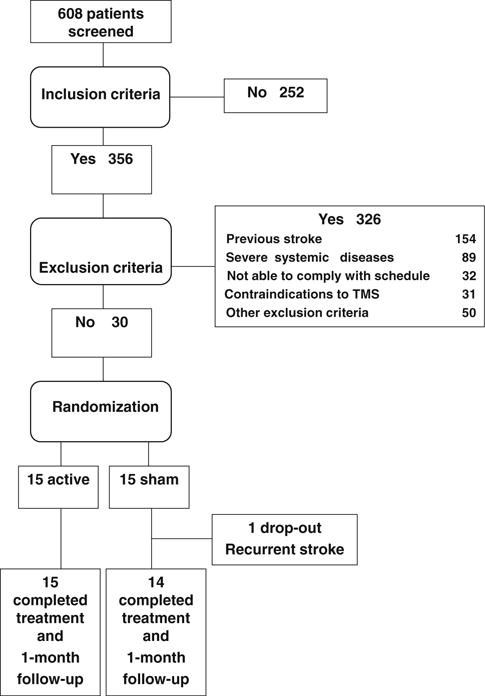
Flow diagram of patients through the trial
Fifteen patients were randomized to each group. There were no significant baseline differences between the two groups (Table 1), or differences between the number of tasks performed in the active group (median, 125.5; range, 82–174) and in the sham group (median, 121; range, 10–137) during therapy (p = 0.19).
Table 1.
Characteristics of the patients
| Characteristics | Active | Sham | p value |
|---|---|---|---|
| Age (years) | 54.8 ± 11.7 | 56.7 ± 14.8 | 0.376a |
| Gender (men/women) | 10/5 | 8/7 | 0.710b |
| Oldfield handedness inventory (%) | 85.6 ± 19.3 | 76.2 ± 36.2 | 0.801c |
| Time from stroke (days) | 27 ± 8.6 | 28.3 ± 10.5 | 0.529a |
| Affected hemisphere (right/left) | 8/7 | 7/8 | 1.0b |
| Lesion location (cortico-subcortical/subcortical) | 7/8 | 7/8 | 1.0b |
| NIH Stroke Scale | 5 (3–11) | 5 (1–11) | 0.753c |
| Modified Ashworth Scale | 0 (0–2) | 0 (0–3) | 0.067c |
| JTT (% affected hand/unaffected hand) | 40.7 ± 35.7 | 44.4 ± 39.4 | 0.673c |
| Pinch force, paretic hand (Newtons) | 0.29 ± 0.23 | 0.29 ± 0.27 | 0.926c |
| Fugl-Meyer (total score for the upper limb) | 108 (61–123) | 102 (50–119) | 0.561c |
| Modified Rankin scale | 3 (2–4) | 3 (0–4) | 0.580c |
Means ± standard deviations, counts or medians (ranges) are given
JTT Jebsen–Taylor test
Paired t test
Fisher’s exact test
Mann–Whitney test
Primary outcome measures: feasibility and safety
For the primary feasibility outcome, compliance with treatment was 100% in the active group and 94% in the sham group. One patient in the sham group had a recurrent stroke on the day before the second session. He was a 69-year old man with history of arterial hypertension, diabetes mellitus and hypercholesterolemia. In this patient, magnetic resonance angiography showed widespread intracranial atherosclerosis, known to be associated with a high risk of recurrent stroke [34].
There were no serious intervention-related adverse events. There were no significant differences in rates of sleepiness (86.7%, active and 78.6%, sham; p = 0.65) or headache, local or nuchal pain (33.3%, active and 28.6%, sham; p = 1.0) between the groups. Head or nuchal pain were mild and subsided spontaneously after a few minutes. None of the patients reported dizziness or paresthesias. No syncopes or seizures were observed. There were no significant differences in systolic (active group, p = 0.46; sham group, p = 0.21) or diastolic blood pressure (active group, p = 0.26; sham group, p = 0.96), and heart rate (active group, p = 0.76; sham group, p = 0.32), along the ten sessions of treatment (Online Resource 1).
Secondary outcome measures
JTTper improved significantly in the active group but not in the sham group (p = 0.46) (Table 2). Post hoc analysis showed that, in the active group, improvement was significant at the end of treatment compared to baseline (p = 0.05) and at 1 month after treatment compared to baseline (p = 0.01). At 1 month, patients in the active group had an absolute improvement of 12.3 ± 16.9% in performance compared to baseline and patients in the sham group, 5.5 ± 10.3%. In the active group, JTTper effect sizes were 0.16 at end of treatment and 0.36 at 1 month after end of treatment. In the sham group, effect sizes were 0.05 at end of treatment and 0.14 one month after end of treatment. A power analysis (1-tailed t test, α = 0.05, β = 0.2) indicated that 49 subjects per group would be needed to detect the difference between the groups 1 month after end of treatment.
Table 2.
Results, secondary outcomes
| Outcome | Baseline | Active
|
Baseline | Sham
|
||||
|---|---|---|---|---|---|---|---|---|
| End | 1 month | p value | End | 1 month | p value | |||
| JTTper (%) | 40.7 ± 35.7 | 46.1 ± 37.3 | 52.9 ± 31.9 | 0.03 | 44.4 ± 39.4 | 46.3 ± 38.6 | 50.3 ± 40.4 | 0.46 |
| PF (N) | 0.27 ± 0.23 | 0.31 ± 0.23 | 0.32 ± 0.32 | 0.008 | 0.29 ± 0.28 | 0.33 ± 0.31 | 0.34 ± 0.27 | 0.441 |
| AS | 0 (0–2) | 0 (0–2) | 1 (0–3) | 0.549 | 0 (0–3) | 0.5 (0–2) | 0.5 (0–3) | 0.846 |
| FM (UL) | 108 (61–123) | 114 (59–123) | 111 (62–120) | 0.038 | 97.5 (50–119) | 105.5 (58–121) | 107.5 (49–125) | 0.001 |
| MRS | 3 (2–4) | 3 (1–4) | 2 (1–4) | 0.016 | 3 (0–4) | 3 (0–4) | 3 (0–5) | 0.037 |
Means ± standard deviations, or medians (ranges) are shown. Friedman’s tests were performed
End At end of treatment, 1 month 1 month after end of treatment JTTper Jebsen–Taylor test (% affected hand/unaffected hand), PF pinch force of the paretic hand, N Newtons, FM (UL) Fugl-Meyer (upper limb, total score; maximum = 126), AS Ashworth Scale, MRS Modified Rankin Scale
p-values ≤ 0.05 are highlighted in bold
At baseline, five patients in each group presented hand plegia. One month after treatment, 3/5 patients from the active group, but none from the sham group, were able to perform hand movements. Post hoc analysis of JTT scores (in seconds) in the unaffected hand showed a trend for improvement that did not reach statistical significance in either active (p = 0.07) or sham (p = 0.06) groups (Online Resource 2).
Table 2 shows results of other secondary outcomes. Force of the paretic hand improved significantly in the active group (p = 0.008) but not in the sham group (p = 0.44). There was a trend towards improvement at 1 month after treatment compared to baseline (p = 0.02) in the active group. Effect size was 0.24 in the active group and 0.17 in the sham group at 1 month.
Ashworth scores did not change significantly in either group (Table 2). All other outcomes improved significantly in both groups.
Discussion
The development of interventions able to enhance hand motor function when administered early after stroke has been a challenge. Effects of LF-rTMS early after stroke in patients in deepest need of rehabilitative treatment had not yet been determined. In the present study, including patients with severe motor impairments within 5–45 days post-stroke, LF-rTMS of the contralesional hemisphere in combination with customary rehabilitation was well tolerated, feasible, and safe. Moreover, the ability to perform tasks related to activities of daily living and force of the paretic hand improved significantly in patients that received active, but not sham LF-rTMS. Beneficial effects of LF-rTMS lasted for at least 1 month after treatment. The magnitude of improvement in JTTper was 2.2 times greater in the active group compared to the sham group. The effect size was moderate at 1 month in the active group, but small in the sham group.
Our results build upon previous studies that administered five or ten sessions of LF-rTMS at earlier (7–20 days, average 6.5 days) [10] or later stages (2.3–13.5 months, average 6.5 months) [11] following stroke. These studies included patients who were able to perform grip or finger tapping movements with the paretic hand at baseline, and therefore did not have severe motor impairments. Our results are not directly comparable with those of the VECTORS study that included more severely affected patients, due to different outcomes and inclusion/exclusion criteria [16]. Still, the favorable safety profile of LF-rTMS and the beneficial effects on hand motor function in our patients encourage the careful design of larger clinical trials to further address efficacy of this treatment relatively early after stroke, extending to more severely affected patients under the criteria used in this study.
There were no significant differences in side effects between the active and sham groups. Sleepiness significantly increased in most of the patients, probably because of the monotonous nature of the active and sham LF-rTMS interventions.
The comparable Ashworth scores suggest that the changes in JTTper and pinch force in the active group were not merely related to decrease in spasticity, but to improvement in motor control. We found no evidence that inhibition of the contralesional hemisphere may have led to deterioration in JTT performance in the nonparetic hand, an issue not yet explored in previous reports.
In our study, both groups improved in Fugl-Meyer and MRS scores at end of treatment and 1 month later. It is not surprising that the sham group improved significantly at this early stage, because motor and functional improvements evolve dynamically during the first weeks after stroke [19]. In contrast, greater improvements in MRS scores were described in patients with less severe motor impairments that received active LF-rTMS, compared to sham [11]. This may be due to differences between patients’ characteristics or paradigms of rTMS [11]. Future studies should evaluate improvements in MRS and quality of life outcomes in the long-term, in patients more severely affected and treated early after stroke.
Mechanisms underlying benefits of LF-rTMS of the contralesional hemisphere in humans are not completely elucidated. Previous studies suggest the involvement of disinhibition of surviving neurons in perilesional areas related to a decrease in interhemispheric inhibition from the contralesional to the lesioned hemisphere [35]. The concept that disinhibition of perilesional areas early after stroke favors recovery is supported by the finding that, in mice, down-regulation of excessive GABAergic inhibition in the peri-infarct zone is associated with greater motor recovery [36]. Whether benefits of LF-rTMS of the contralesional hemisphere are restricted to patients with viable neurons in M1, similarly to results described after HF-rTMS of the lesioned hemisphere [37], remains to be determined. Furthermore, development of neuroimaging and/or TMS surrogate measures [38] to identify patients more likely to benefit from LF-rTMS, as well as fine adjustments in duration, intensity and number of sessions of rTMS according to different stages after stroke, in patients with different levels of impairment and corticospinal tract involvement [39] would be valuable to plan larger multicenter trials.
A limitation of the present study is the small number of screened patients that fulfilled inclusion criteria (4.7%), mainly because of history of previous strokes. This is a common issue in stroke rehabilitation, particularly in developing countries [40, 41].
Thrombolysis and multidisciplinary care in stroke units in the acute phase substantially changed stroke care and outcomes. Plastic processes that continue to occur during the first weeks and months after stroke offer a window of opportunity for therapies aimed at boosting effective connections in preserved tissue and improving functional outcomes [19]. The results of this pilot study point to LF-rTMS of the unaffected hemisphere within the first 45 days after stroke as a putative candidate intervention to improve hand motor function. Moreover, they encourage implementation of larger scale randomized trials to determine whether low-frequency repetitive transcranial magnetic stimulation of the unaffected hemisphere can help change the outcome of stroke disability for more severely affected patients.
Supplementary Material
Acknowledgments
This work was funded by grant 2006/55504-0 from São Paulo State’s Foundation for Research Support (FAPESP), a Brazilian governmental agency. The authors have no financial relationships with FAPESP. Scholarships from FAPESP were funded to Erina Nagaya (2009/51641-0 and 2010/12696-1) and Sarah dos Anjos (2010/15660-8). FAPESP had no roles in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. We thank David Luckenbaugh for helpful discussions about statistical analysis of the data.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00415-011-6364-7) contains supplementary material, which is available to authorized users.
Conflicts of interest The authors have no conflicts of interest.
Contributor Information
Adriana B. Conforto, Email: adriana.conforto@gmail.com, abconf@usp.br, Division of Clinical Neurology, Hospital das Clínicas, University of São Paulo, Av. Dr. Enéas C. Aguiar 255/5084, São Paulo 05403010, Brazil; The Instituto Israelita de Ensino e Pesquisa Albert Einstein, São Paulo, Brazil.
Sarah M. Anjos, Division of Clinical Neurology, Hospital das Clínicas, University of São Paulo, Av. Dr. Enéas C. Aguiar 255/5084, São Paulo 05403010, Brazil
Gustavo Saposnik, Stroke Outcomes Research Unit, St. Michael’s Hospital, University of Toronto, Toronto, Canada.
Eduardo A. Mello, Division of Clinical Neurology, Hospital das Clínicas, University of São Paulo, Av. Dr. Enéas C. Aguiar 255/5084, São Paulo 05403010, Brazil
Erina M. Nagaya, Division of Clinical Neurology, Hospital das Clínicas, University of São Paulo, Av. Dr. Enéas C. Aguiar 255/5084, São Paulo 05403010
Waldyr Santos, Jr., Division of Clinical Neurology, Hospital das Clínicas, University of São Paulo, Av. Dr. Enéas C. Aguiar 255/5084, São Paulo 05403010, Brazil
Karina N. Ferreiro, Division of Clinical Neurology, Hospital das Clínicas, University of São Paulo, Av. Dr. Enéas C. Aguiar 255/5084, São Paulo 05403010, Brazil
Eduardo S. Melo, Division of Clinical Neurology, Hospital das Clínicas, University of São Paulo, Av. Dr. Enéas C. Aguiar 255/5084, São Paulo 05403010, Brazil
Felipe I. Reis, Division of Clinical Neurology, Hospital das Clínicas, University of São Paulo, Av. Dr. Enéas C. Aguiar 255/5084, São Paulo 05403010, Brazil
Milberto Scaff, Division of Clinical Neurology, Hospital das Clínicas, University of São Paulo, Av. Dr. Enéas C. Aguiar 255/5084, São Paulo 05403010, Brazil.
Leonardo G. Cohen, The Human Cortical Physiology and Stroke Rehabilitation Section, National Institutes of Neurological Disorders and Stroke, Bethesda, USA
References
- 1.Kwakkel G, Kollen BJ, van der Grond J, Prevo AJ. Probability of regaining dexterity in the flaccid upper limb: impact of severity of paresis and time since onset in acute stroke. Stroke. 2003;34:2181–2186. doi: 10.1161/01.STR.0000087172.16305.CD. [DOI] [PubMed] [Google Scholar]
- 2.Lum PS, Mulroy S, Amdur RL, Requejo P, Prilutsky BI, Dromerick AW. Gains in upper extremity function after stroke via recovery or compensation: potential differential effects on amount of real-world limb use. Top Stroke Rehabil. 2009;16:237–253. doi: 10.1310/tsr1604-237. [DOI] [PubMed] [Google Scholar]
- 3.Langhorne P, Coupar F, Pollock A. Motor recovery after stroke: a systematic review. Lancet Neurol. 2009;8:741–754. doi: 10.1016/S1474-4422(09)70150-4. [DOI] [PubMed] [Google Scholar]
- 4.Murase N, Duque J, Mazzocchio R, Cohen LG. Influence of interhemispheric interactions on motor function in chronic stroke. Ann Neurol. 2004;55:400–409. doi: 10.1002/ana.10848. [DOI] [PubMed] [Google Scholar]
- 5.Nowak DA, Grefkes C, Dafotakis M, et al. Effects of low-frequency repetitive transcranial magnetic stimulation of the contralesional primary motor cortex on movement kinematics and neural activity in subcortical stroke. Arch Neurol. 2008;65:741–747. doi: 10.1001/archneur.65.6.741. [DOI] [PubMed] [Google Scholar]
- 6.Nowak DA, Grefkes C, Ameli M, Fink GR. Interhemispheric competition after stroke: brain stimulation to enhance recovery of function of the affected hand. Neurorehabil Neural Repair. 2009;23:641–656. doi: 10.1177/1545968309336661. [DOI] [PubMed] [Google Scholar]
- 7.Takeuchi N, Chuma T, Matsuo Y, Watanabe I, Ikoma K. Repetitive transcranial magnetic stimulation of contralesional primary motor cortex improves hand function after stroke. Stroke. 2005;36:2681–2686. doi: 10.1161/01.STR.0000189658.51972.34. [DOI] [PubMed] [Google Scholar]
- 8.Kakuda W, Abo M, Kaito N, Ishikawa A, Taguchi K, Yokoi A. Six-day course of repetitive transcranial magnetic stimulation plus occupational therapy for post-stroke patients with upper limb hemiparesis: a case series study. Disabil Rehabil. 2010;32:801–807. doi: 10.3109/09638280903295474. [DOI] [PubMed] [Google Scholar]
- 9.Takeuchi N, Tada T, Toshima M, Matsuo Y, Ikoma K. Repetitive transcranial magnetic stimulation over bilateral hemispheres enhances motor function and training effect of paretic hand in patients after stroke. J Rehabil Med. 2009;41:1049–1054. doi: 10.2340/16501977-0454. [DOI] [PubMed] [Google Scholar]
- 10.Khedr EM, Abdel-Fadeil MR, Farghali A, Qaid M. Role of 1 and 3 Hz repetitive transcranial magnetic stimulation on motor function recovery after acute ischemic stroke. Eur J Neurol. 2009;16:1323–1330. doi: 10.1111/j.1468-1331.2009.02746.x. [DOI] [PubMed] [Google Scholar]
- 11.Emara TH, Moustafa RR, ElNahas NM, et al. Repetitive transcranial magnetic stimulation at 1 Hz and 5 Hz produces sustained improvement in motor function and disability after ischaemic stroke. Eur J Neurol. 2010;17:1203–1209. doi: 10.1111/j.1468-1331.2010.03000.x. [DOI] [PubMed] [Google Scholar]
- 12.Khedr EM, Ahmed MA, Fathy N, Rothwell JC. Therapeutic trial of repetitive transcranial magnetic stimulation after acute ischemic stroke. Neurology. 2005;65:466–468. doi: 10.1212/01.wnl.0000173067.84247.36. [DOI] [PubMed] [Google Scholar]
- 13.Cramer SC, Sur M, Dobkin BH, et al. Harnessing neuroplasticity for clinical applications. Brain. 2011;134:1591–1609. doi: 10.1093/brain/awr039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liepert J, Bauder H, Miltner WHR, Taub E, Weiller C. Treatment-induced reorganization after stroke in humans. Stroke. 2001;31:1210–1216. doi: 10.1161/01.str.31.6.1210. [DOI] [PubMed] [Google Scholar]
- 15.Lin KC, Chung HY, Wu CY, et al. Constraint-induced therapy versus control intervention in patients with stroke: a functional magnetic resonance imaging study. Am J Phys Med Rehabil. 2010;89:177–185. doi: 10.1097/PHM.0b013e3181cf1c78. [DOI] [PubMed] [Google Scholar]
- 16.Dromerick AW, Lang CE, Birkenmeier RL, et al. Very early constraint-induced movement during stroke rehabilitation (VECTORS): a single-center RCT. Neurology. 2009;73:195–201. doi: 10.1212/WNL.0b013e3181ab2b27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Humm JL, Kozlowski DA, Bland ST, James DC, Schallert T. Use-dependent exaggeration of brain injury: is glutamate involved? Exp Neurol. 1999;157:349–358. doi: 10.1006/exnr.1999.7061. [DOI] [PubMed] [Google Scholar]
- 18.Bland ST, Schallert T, Strong R, Aronowski J, Grotta JC. Early exclusive use of the affected forelimb after moderate transient focal ischemia in rats: functional and anatomic outcome. Stroke. 2000;31:1144–1152. doi: 10.1161/01.str.31.5.1144. [DOI] [PubMed] [Google Scholar]
- 19.Cramer SC. Repairing the human brain after stroke: I. Mechanisms of spontaneous recovery. Ann Neurol. 2008;63:272–287. doi: 10.1002/ana.21393. [DOI] [PubMed] [Google Scholar]
- 20.Rossi S, Hallett M, Rossini PM, Pascual-Leone A, The Safety of TMS Consensus Group Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol. 2009;120:2008–2039. doi: 10.1016/j.clinph.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ziemann U. TMS and drugs. Clin Neurophysiol. 2004;115:1717–1729. doi: 10.1016/j.clinph.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 22.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 23.de Caneda MAG, Fernandes JG, de Almeida AG, Mugnol FE. Reliability of neurological assessment scales in patients with stroke. Arq Neuro-Psiquiatr. 2006;64:690–697. doi: 10.1590/s0004-282x2006000400034. [DOI] [PubMed] [Google Scholar]
- 24.Conforto AB, Ferreiro KN, Tomasi C, et al. Effects of somatosensory stimulation on motor function after subacute stroke. Neurorehabil Neural Repair. 2010;24:263–272. doi: 10.1177/1545968309349946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rossini PM, Barker AT, Berardelli A, et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical application. Report of an IFCN committee. Electroencephalogr Clin Neurophysiol. 1994;91:79–92. doi: 10.1016/0013-4694(94)90029-9. [DOI] [PubMed] [Google Scholar]
- 26.Jebsen RH, Taylor N, Trieschmann RB, Trotter MJ, Howard LA. An objective and standardized test of hand function. Arch Phys Med Rehabil. 1969;50:311–319. [PubMed] [Google Scholar]
- 27.Fregni F, Boggio PS, Valle AC, et al. A sham-controlled trial of a 5-day course of repetitive transcranial magnetic stimulation of the unaffected hemisphere in stroke patients. Stroke. 2006;37:2115–2122. doi: 10.1161/01.STR.0000231390.58967.6b. [DOI] [PubMed] [Google Scholar]
- 28.Hummel F, Celnik P, Giraux P, et al. Effects of noninvasive cortical stimulation on skilled motor function in chronic stroke. Brain. 2005;128:490–499. doi: 10.1093/brain/awh369. [DOI] [PubMed] [Google Scholar]
- 29.Beebe JA, Lang CE. Relationships and responsiveness of six upper extremity function tests during the first 6 months of recovery after stroke. J Neurol Phys Ther. 2009;33:96–103. doi: 10.1097/NPT.0b013e3181a33638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mathiowetz V, Rennells C, Donahoe L. Effect of elbow position on grip and key pinch strength. J Hand Surg Am. 1985;10:694–697. doi: 10.1016/s0363-5023(85)80210-0. [DOI] [PubMed] [Google Scholar]
- 31.Fugl-Meyer AR, Jaasko L, Leyman I, Olsson S, Steglind S. The post stroke hemiplegic patient. I. A method for evaluation of physical performance. Scand J Rehabil Med. 1975;7:13–31. [PubMed] [Google Scholar]
- 32.Bohannon RW, Melissa MB. Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys Ther. 1987;67:206–207. doi: 10.1093/ptj/67.2.206. [DOI] [PubMed] [Google Scholar]
- 33.Kazis LE, Anderson JJ, Meenan RF. Effect sizes for interpreting changes in health status. Med Care. 1989;27:S178–S189. doi: 10.1097/00005650-198903001-00015. [DOI] [PubMed] [Google Scholar]
- 34.Arenillas JF. Intracranial atherosclerosis: current concepts. Stroke. 2011;42:S20–S23. doi: 10.1161/STROKEAHA.110.597278. [DOI] [PubMed] [Google Scholar]
- 35.Hummel FC, Cohen LG. Non-invasive brain stimulation: a new strategy to improve neurorehabilitation after stroke? Lancet Neurol. 2011;5:708–712. doi: 10.1016/S1474-4422(06)70525-7. [DOI] [PubMed] [Google Scholar]
- 36.Clarkson AN, Huang BS, Macisaac SE, Mody I, Carmichael ST. Reducing excessive GABA-mediated tonic inhibition promotes functional recovery after stroke. Nature. 2010;468:305–309. doi: 10.1038/nature09511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ameli M, Grefkes C, Kemper F, et al. Differential effects of high-frequency repetitive transcranial magnetic stimulation over ipsilesional primary motor cortex in cortical and subcortical middle cerebral artery stroke. Ann Neurol. 2009;66:298–309. doi: 10.1002/ana.21725. [DOI] [PubMed] [Google Scholar]
- 38.Seitz RJ, Donnan GA. Role of neuroimaging in promoting long-term recovery from ischemic stroke. J Magn Reson Imaging. 2010;32:756–772. doi: 10.1002/jmri.22315. [DOI] [PubMed] [Google Scholar]
- 39.Sterr A, Shen S, Szameitat AJ, Herron KA. The role of corticospinal tract damage in chronic motor recovery and neurorehabilitation: a pilot study. Neurorehabil Neural Repair. 2010;24:413–419. doi: 10.1177/1545968309348310. [DOI] [PubMed] [Google Scholar]
- 40.Wolf SL, Winstein CJ, Miller JP, et al. Effect of constraint-induced movement therapy on upper extremity function 3 to 9 months after stroke: the EXCITE randomized clinical trial. JAMA. 2006;296:2095–2104. doi: 10.1001/jama.296.17.2095. [DOI] [PubMed] [Google Scholar]
- 41.Conforto AB, Paulo RB, Patroclo CB, et al. Stroke management in a university hospital in the largest South American city. Arq Neuro-Psiquiatr. 2008;66:308–311. doi: 10.1590/s0004-282x2008000300004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


