Abstract
Aims
Prenatal dysglycaemia is associated with adverse maternal and offspring outcomes. This study examined the association between dysglycaemia and multiple modifiable factors measured during pregnancy.
Methods
The Healthy Start Study collected self-reported data on modifiable factors in early and mid-pregnancy (median 17 and 27 weeks gestation, respectively) from 832 women. Women received 1 point for each optimal modifiable factor: diet quality (Healthy Eating Index ≥64), physical activity level (estimated energy expenditure ≥170 MET-hours/week), and mental health status (Perceived Stress <6 and Edinburgh Postnatal Depression <13). Dysglycaemia during pregnancy was defined as an abnormal glucose challenge, ≥1 abnormal results on an oral glucose tolerance test, or a clinical diagnosis of gestational diabetes. Logistic regression models estimated odds ratios for dysglycaemia as a function of each factor and the total score, adjusted for age, race/ethnicity, pre-pregnant body mass index, history of gestational diabetes, and family history of type 2 diabetes.
Results
In individual analyses, only physical activity was significantly associated with a reduced risk of dysglycaemia (adjusted OR 0.67, 95%CI 0.44–1.00). We observed a significant, dose-response association between increasing numbers of optimal factors and odds of dysglycaemia (adjusted p=0.01). Compared to having no optimal modifiable factors, having all 3 was associated with a 73% reduced risk of dysglycaemia (adjusted OR 0.27, 95%CI 0.08–0.95).
Conclusions
An increasing number of positive modifiable factors in pregnancy was associated with a dose-response reduction in risk of dysglycaemia. Our results support the hypothesis that modifiable factors in pregnancy are associated with the risk of prenatal dysglycaemia.
Introduction
Gestational diabetes (GDM) affects 9% of pregnancies in the United States [1]. GDM and milder glucose intolerance in pregnancy are associated with adverse health outcomes for mother and offspring [1–3]. The Nurses’ Health Study reported that 47.5% of GDM cases could be prevented if women maintained a healthy lifestyle before pregnancy with regard to weight, diet, physical activity, and smoking [4]. While dietary habits are relatively stable from preconception through pregnancy [5], physical activity declines [6] and prenatal mental health status may affect prenatal diet and activity [7, 8]. Understanding the degree to which modifiable factors (diet quality, physical activity levels, mental health status) during pregnancy are associated with prenatal dysglycaemia can be useful for developing prevention programs. We examined the association between multiple modifiable factors (diet quality, physical activity levels, mental health status) in early to mid-pregnancy with risk of prenatal dysglycaemia. We hypothesized that women meeting data-derived thresholds for all factors would have significantly a lower risk of dysglycaemia than women not meeting any thresholds.
Patients and Methods
Participants were from The Healthy Start Study, a pre-birth cohort of 1410 mother-offspring dyads in Colorado, USA. Pregnant women were recruited at the University of Colorado Anschutz Medical Campus obstetric clinic from 2010–2014. Eligibility criteria included age ≥16 years, <24 weeks gestation, singleton pregnancy, and no history of serious chronic disease (including pre-gestational diabetes), prior stillbirth, or previous delivery <25 weeks gestation. Participants completed research visits in early and mid-pregnancy (median 17 and 27 weeks, respectively). Women were eligible for the present analysis if they completed ≥1 visit after February 10, 2011 (when the mental health questionnaires were added), completed ≥1 dietary recall prior to 27 weeks gestation, and had clinical results of GDM screening/diagnosis (n=899). The Healthy Start Study was approved by the Colorado Multiple Institutional Review Board. All participants provided written informed consent.
Modifiable factors were assessed by self-report. Prenatal diet was assessed 1–7 times prior to 27 weeks gestation with Automated Self-Administered 24-hour dietary recalls. We calculated total energy, macro- and micronutrients, and MyPyramid Equivalents with the USDA Food and Nutrition Data System for Research. Recalls with daily energy <450 kilocalories or >5000 kilocalories were excluded (n=35, 1.6% of all recalls). We used the National Cancer Institute mixed-effects model to estimate typical intake, adjusting for pre-pregnancy body mass index (BMI), gravidity, and prenatal smoking [9]. MyPyramid Equivalents were used to calculate the Healthy Eating Index (HEI; scores range 0–100), an indicator of diet quality [10]. Physical activity level (metabolic equivalent task (MET) hours/week) was assessed with the Pregnancy Physical Activity Questionnaire [11]. Perceived stress and depressive symptoms were assessed with the 10-item Perceived Stress Scale (scores range 0–40) [12] and the Edinburgh Postnatal Depression Scale (scores range 0–30) [13], respectively.
Clinical routine GDM screening occurred at 24–28 weeks (mean 25.7 weeks) with a two-step procedure (50g glucose challenge + 100g oral glucose tolerance test) and results were abstracted from medical records. We classified women as having prenatal dysglycaemia when they had an abnormal glucose challenge, ≥1 abnormal value on the glucose tolerance test according to Carpenter and Coustan criteria, or a clinical diagnosis of GDM.
Covariate data (age, race/ethnicity, history of GDM, maternal family history of diabetes) were collected via self-report. Pre-pregnant BMI was abstracted from the medical record (91%) or calculated from self-reported data (9%). Weekly rates of gestational weight gain were predicted using mixed models as described previously [14]. Gestational weight gain through 26 weeks was classified according to the 2009 Institute of Medicine guidelines, which recommend a 1.1–4.4 lb gain in the first 13 weeks for all women and BMI-specific weekly rates of gain for the remaining 27 weeks (e.g. 0.4–0.6 lbs/week for BMI ≥30).
Statistical Analyses
Analyses were conducted in SAS 9.4 (SAS Institute, Cary, NC, USA). We determined the threshold for each modifiable factor using the Youden index [15]. For each predictor, the cut-off for categorization was chosen as the value which maximized the J statistic (sensitivity + specificity – 1) from a logistic regression with odds of dysglycaemia as outcome and the specified factor as predictor. Women received one point for each optimal factor: diet quality (HEI ≥threshold), physical activity level (MET-hours/week ≥threshold), mental health status (stress <threshold and depression scores <13 [the diagnostic threshold for probable depression [13]]).
Odds ratios (OR) and 95% confidence intervals (95%CI) of prenatal dysglycaemia for each individual modifiable factor and the score were assessed with logistic regression in unadjusted and adjusted models. Covariates included age (continuous), race/ethnicity (Hispanic, non-Hispanic white, non-Hispanic black, other), pre-pregnant BMI (continuous), GDM history (yes/no), and maternal family history of diabetes (yes/no). We considered p<0.05 statistically significant.
Results
Complete data were available for 832 of 899 eligible participants (4 fetal deaths, 32 missing lifestyle data, 18 missing GDM history, 13 missing diabetes family history). There were no clinically significant between the full cohort (n=1410), the eligible cohort (n=899), and the final analytic cohort (n=832) (data not shown). Sample characteristics are presented in Table 1.
Table 1.
Sample characteristics
| Normal n=713 | Dysglycaemia* n=119 | p-value | |
|---|---|---|---|
| Age (years) | 28.0 (6.1) | 29.5 (5.8) | 0.01 |
| Pre-pregnancy BMI (kg/m2) | 25.5 (6.2) | 27.6 (7.0) | 0.003 |
| Race (n) | |||
| Non-Hispanic White | 403 (57%) | 55 (46%) | 0.05 |
| Hispanic | 170 (24%) | 39 (33%) | |
| Black | 100 (14%) | 14 (12%) | |
| Other | 40 (6%) | 11 (9%) | |
| History of GDM (n) | 10 (1%) | 8 (7%) | 0.0002 |
| Family history of T2DM (n) | 67 (9%) | 22 (18%) | 0.003 |
| Education (n) | |||
| Less than 12th grade | 92 (13%) | 18 (15%) | 0.82 |
| High school degree or GED | 120 (17%) | 19 (16%) | |
| Some college or associate’s degree | 168 (24%) | 30 (25%) | |
| Four years of college (BA, BS) | 170 (24%) | 23 (19%) | |
| Graduate degree (Master’s, PhD) | 163 (23%) | 29 (24%) | |
| Employment (n) | |||
| Full-time student | 67 (9%) | 13 (11%) | 0.70 |
| Part-time student | 46 (6%) | 5 (4%) | |
| Currently employed | 405 (57%) | 76 (64%) | 0.20 |
| Household income (n) | |||
| < $40,000 | 200 (28%) | 40 (34%) | 0.26 |
| $40,000 – $70,000 | 121 (17%) | 24 (20%) | |
| > $70,000 | 136 (19%) | 16 (13%) | |
| Missing or don’t know | 256 (36%) | 37 (31%) | |
| Gravidity (n) | 1.3 (1.5) | 1.4 (1.5) | 0.45 |
| Smoking in pregnancy (n) | 52 (7%) | 7 (6%) | 0.58 |
| Gestational weight gain (kg)† | 7.4 (4.0) | 6.2 (4.2) | 0.01 |
| Inadequate (n) | 130 (18%) | 35 (29%) | 0.01 |
| Adequate (n) | 203 (28%) | 33 (28%) | |
| Excessive (n) | 380 (53%) | 51 (43%) | |
| Lifestyle factors (n) | |||
| HEI ≥64 | 300 (42%) | 41 (34%) | 0.11 |
| Physical activity ≥170 MET-hours/week | 375 (53%) | 51 (43%) | 0.05 |
| Stress <6 and Depression <13 | 167 (23%) | 21 (18%) | 0.14 |
| Lifestyle score (n) | |||
| 0 | 142 (20%) | 38 (32%) | 0.02 |
| 1 | 343 (48%) | 52 (44%) | |
| 2 | 185 (26%) | 26 (22%) | |
| 3 | 43 (6%) | 3 (3%) |
Values are mean (SD) or n (%). P-values determined with t-tests for continuous variables and the Cochran-Mantel-Haenszel test for categorical variables. BMI; body mass index; GDM, gestational diabetes; T2DM, type 2 diabetes mellitus; HEI, Healthy Eating Index; MET, metabolic equivalent of task; Stress, Perceived Stress Scale; Depression, Edinburgh Postnatal Depression Scale.
Dysglycaemia defined as abnormal glucose screen, ≥1 abnormal value on the 100g oral glucose tolerance test, or a clinical diagnosis of gestational diabetes.
Classified according to the 2009 Institute of Medicine recommendations through 26 weeks gestation.
The Youden index identified the following factor thresholds: HEI diet quality ≥64, physical activity level ≥170 MET-hours/week, and stress <6. In individual analyses, we observed a significantly reduced risk of dysglycaemia for physical activity level (adjusted OR 0.67, 95%CI 0.44–1.00), but not diet quality (adjusted OR 0.71, 95%CI 0.45–1.10) or mental health status (adjusted OR 0.77, 95%CI 0.46–1.29). For the score, we observed a statistically significant dose-response association with dysglycaemia risk (adjusted p for trend = 0.01; Fig. 1). Compared to women with a score of 0, women with a score of 3 had a 73% reduced risk of dysglycaemia (adjusted OR 0.27, 95%CI 0.08–0.95). Further adjustment for gestational weight gain through 26 weeks gestation did not substantially change the results.
Fig. 1.
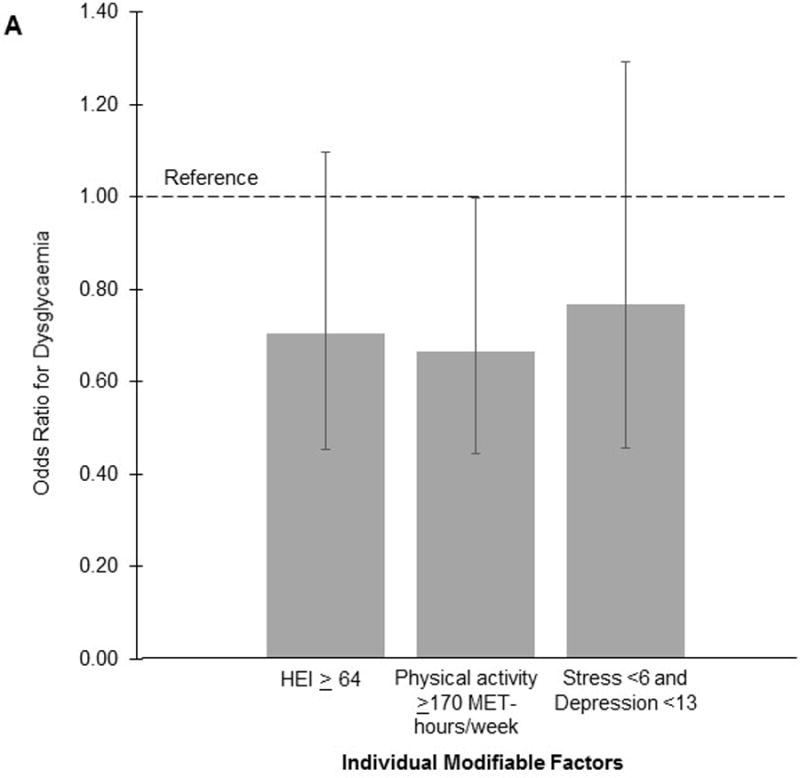
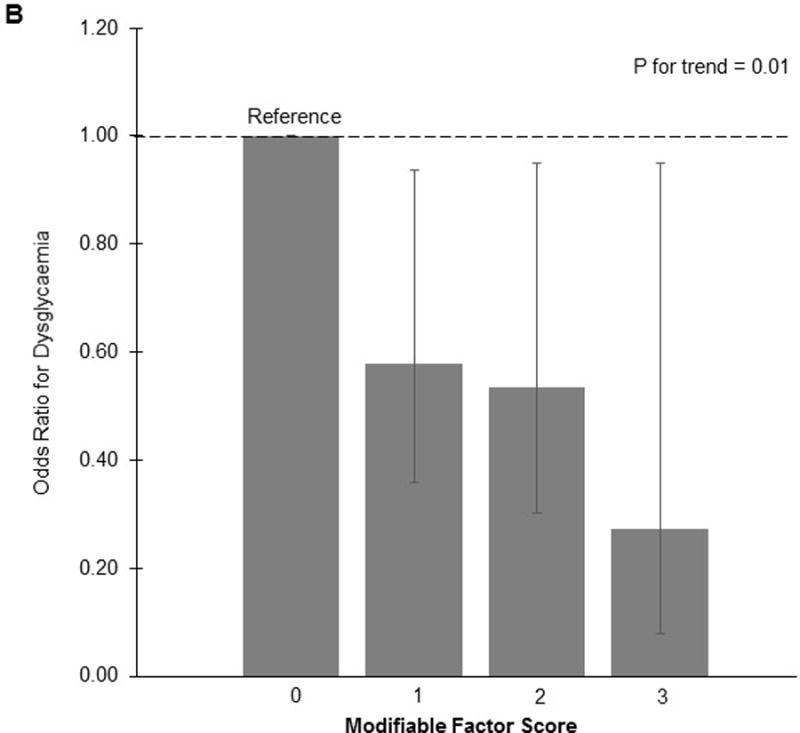
Odds of prenatal dysglycaemia according to individual modifiable factors (A) and total score (B) in early to mid-pregnancy
Discussion
We found that having multiple positive modifiable factors during pregnancy is associated with a 73% reduced risk of prenatal dysglycaemia in a sample of ethnically-diverse pregnant women. When examined individually, physical activity levels were marginally associated with reduced risk, while diet quality and mental health status were not. However, when examined collectively, the synergistic association of multiple modifiable factors with dysglycaemia was pronounced. Our results were independent of pre-pregnant BMI and not affected by further adjustment for gestational weight gain, suggesting that these modifiable factors in pregnancy may be related to glucose tolerance regardless of maternal weight and weight gain.
Our study is consistent with the Nurses’ Health Study, which reported that a healthy pre-pre-pregnancy lifestyle (non-smoker, BMI <25, top 40% of the Alternate HEI diet index, and moderate physical activity ≥150 min/week) was associated with a reduced risk of GDM by 52% [4]. Results of other observational studies have been mixed [16], potentially because most examined modifiable factors individually rather than collectively. Prenatal intervention trials have had varying degrees of success with diet, physical activity, or combined interventions [17]. The two largest intervention trials (LIMIT [18] and UPBEAT [19]) did not reduce GDM among overweight and/or obese pregnant women despite improvements in diet and physical activity. The smaller DALI Lifestyle Pilot study also observed no difference in fasting glucose at 24–28 weeks in obese women receiving diet, physical activity, or combined interventions [20].
There are several reasons why our results, which suggest that modifiable factors in early-to-mid pregnancy are related to dysglycaemia risk, differ from intervention trials, which have been largely ineffective in preventing dysglycaemia. First, the improvements in diet or physical activity achieved in intervention trials may not have been sufficient to influence glucose tolerance. For example, in the LIMIT trial, mean physical activity was low (<125 MET-hours/week) at baseline and declined in both the intervention and control groups as pregnancy progressed [18]. These activity levels were substantially lower than our data-driven physical activity threshold (170 MET-hours/week), suggesting that even a 6 MET-hour/week treatment effect (equal to 15–20 minutes of walking/day) was insufficient. Second, the timing of interventions may be important. Our results and those from the Nurses’ Health Study indicate that pre-pregnancy and early pregnancy lifestyle factors are related to risk of dysglycaemia. However, intervention studies face challenges in recruiting women early in pregnancy, obtaining baseline measurements, and then delivering an intervention quickly enough to produce meaningful changes in behaviors and outcomes by 24–28 weeks gestation. Interventions that begin at the first prenatal appointment, or even prior to conception for high-risk women, may have more potential for success.
Our study is limited by self-reported data, although prospectively-administered and validated questionnaires reduced potential for recall bias. The small sample size prevented examination of GDM separately from dysglycaemia; however, sub-clinical prenatal glucose intolerance is associated with adverse maternal and fetal outcomes [2, 3], and our combined approach suggests that an increasing number of optimal prenatal modifiable factors is related to all degrees of dysglycaemia risk. Study strengths include the diverse sample, prospective data collection, and consideration of multiple modifiable factors and pre-pregnancy characteristics.
In conclusion, we found that women with multiple positive modifiable factors in early-to-mid pregnancy have a significantly reduced risk of prenatal dysglycaemia. Our results support the hypothesis that modifiable factors in early pregnancy are associated with the risk of prenatal dysglycaemia.
Novelty statement.
We prospectively examined the association between multiple modifiable factors (diet quality, physical activity level, mental health status) in early to mid-pregnancy with risk of prenatal dysglycaemia among 832 women.
Compared to having no optimal modifiable factors, having any 1 optimal modifiable factor reduced the risk of dysglycaemia by 42% (OR 0.58, 95%CI 0.36–0.94), and having all 3 optimal modifiable factors reduced the risk by 73% (OR 0.27, 95%CI 0.08–0.95).
Our results support the hypothesis that modifiable factors in pregnancy are associated with the risk of prenatal dysglycaemia.
Acknowledgments
The authors thank the Healthy Start Study Project Coordinator, Mrs. Mercedes Martinez, and the study investigators, participants, and personnel.
Funding
This work was supported by the NIH (R01 DK076648 – DD; UL1 TR001082 - NIH/NCATS Colorado CTSA; P30 DK56350 – UNC Nutrition Obesity Research Center). KAS is supported by an NIH post-doctoral institutional training grant to the University of Colorado (T32 DK07658). The NIH was not involved in study design, data collection, data analysis, manuscript preparation and/or publication decisions. Contents are the authors’ sole responsibility and do not necessarily represent official NIH views.
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
References
- 1.DeSisto CL, Kim SY, Sharma AJ. Prevalence estimates of gestational diabetes mellitus in the United States, Pregnancy Risk Assessment Monitoring System (PRAMS), 2007–2010. Prev Chronic Dis. 2014;11:E104. doi: 10.5888/pcd11.130415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kramer CK, Swaminathan B, Hanley AJ, Connelly PW, Sermer M, Zinman B, et al. Each degree of glucose intolerance in pregnancy predicts distinct trajectories of beta-cell function, insulin sensitivity, and glycemia in the first 3 years postpartum. Diabetes Care. 2014;37:3262–3269. doi: 10.2337/dc14-1529. [DOI] [PubMed] [Google Scholar]
- 3.Shapiro AL, Schmiege SJ, Brinton JT, Glueck D, Crume TL, Friedman JE, et al. Testing the fuel-mediated hypothesis: maternal insulin resistance and glucose mediate the association between maternal and neonatal adiposity, the Healthy Start study. Diabetologia. 2015;58:937–941. doi: 10.1007/s00125-015-3505-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang C, Tobias DK, Chavarro JE, Bao W, Wang D, Ley SH, et al. Adherence to healthy lifestyle and risk of gestational diabetes mellitus: prospective cohort study. BMJ. 2014;349:g5450. doi: 10.1136/bmj.g5450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crozier SR, Robinson SM, Godfrey KM, Cooper C, Inskip HM. Women’s dietary patterns change little from before to during pregnancy. J Nutr. 2009;139:1956–1963. doi: 10.3945/jn.109.109579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pereira MA, Rifas-Shiman SL, Kleinman KP, Rich-Edwards JW, Peterson KE, Gillman MW. Predictors of change in physical activity during and after pregnancy: Project Viva. Am J Prev Med. 2007;32:312–319. doi: 10.1016/j.amepre.2006.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hurley KM, Caulfield LE, Sacco LM, Costigan KA, Dipietro JA. Psychosocial influences in dietary patterns during pregnancy. J Am Diet Assoc. 2005;105:963–966. doi: 10.1016/j.jada.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 8.Poudevigne MS, O’Connor PJ. A review of physical activity patterns in pregnant women and their relationship to psychological health. Sports Med. 2006;36:19–38. doi: 10.2165/00007256-200636010-00003. [DOI] [PubMed] [Google Scholar]
- 9.Tooze JA, Kipnis V, Buckman DW, Carroll RJ, Freedman LS, Guenther PM, et al. A mixed-effects model approach for estimating the distribution of usual intake of nutrients: the NCI method. Stat Med. 2010;29:2857–2868. doi: 10.1002/sim.4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guenther PM, Reedy J, Krebs-Smith SM. Development of the Healthy Eating Index-2005. J Am Diet Assoc. 2008;108:1896–1901. doi: 10.1016/j.jada.2008.08.016. [DOI] [PubMed] [Google Scholar]
- 11.Chasan-Taber L, Schmidt MD, Roberts DE, Hosmer D, Markenson G, Freedson PS. Development and validation of a Pregnancy Physical Activity Questionnaire. Med Sci Sports Exerc. 2004;36:1750–1760. doi: 10.1249/01.mss.0000142303.49306.0d. [DOI] [PubMed] [Google Scholar]
- 12.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- 13.Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987;150:782–786. doi: 10.1192/bjp.150.6.782. [DOI] [PubMed] [Google Scholar]
- 14.Starling AP, Brinton JT, Glueck DH, Shapiro AL, Harrod CS, Lynch AM, et al. Associations of maternal BMI and gestational weight gain with neonatal adiposity in the Healthy Start study. Am J Clin Nutr. 2015;101:302–309. doi: 10.3945/ajcn.114.094946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3:32–35. doi: 10.1002/1097-0142(1950)3:1<32::aid-cncr2820030106>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 16.Zhang C, Ning Y. Effect of dietary and lifestyle factors on the risk of gestational diabetes: review of epidemiologic evidence. Am J Clin Nutr. 2011;94:1975S–1979S. doi: 10.3945/ajcn.110.001032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thangaratinam S, Rogozinska E, Jolly K, Glinkowski S, Roseboom T, Tomlinson JW, et al. Effects of interventions in pregnancy on maternal weight and obstetric outcomes: meta-analysis of randomised evidence. BMJ. 2012;344:e2088. doi: 10.1136/bmj.e2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dodd JM, Cramp C, Sui Z, Yelland LN, Deussen AR, Grivell RM, et al. The effects of antenatal dietary and lifestyle advice for women who are overweight or obese on maternal diet and physical activity: the LIMIT randomised trial. BMC Med. 2014;12:161. doi: 10.1186/s12916-014-0161-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Poston L, Bell R, Croker H, Flynn AC, Godfrey KM, Goff L, et al. Effect of a behavioural intervention in obese pregnant women (the UPBEAT study): a multicentre, randomised controlled trial. Lancet Diabetes Endocrinol. 2015;3:767–777. doi: 10.1016/S2213-8587(15)00227-2. [DOI] [PubMed] [Google Scholar]
- 20.Simmons D, Jelsma JG, Galjaard S, Devlieger R, van Assche A, Jans G, et al. Results From a European Multicenter Randomized Trial of Physical Activity and/or Healthy Eating to Reduce the Risk of Gestational Diabetes Mellitus: The DALI Lifestyle Pilot. Diabetes Care. 2015;38:1650–1656. doi: 10.2337/dc15-0360. [DOI] [PubMed] [Google Scholar]


