Abstract
Nine oxygenated cannabinoids were isolated from a high potency Cannabis sativa L. variety. Structure elucidation was achieved using spectroscopic techniques, including 1D and 2D NMR, HRMS and GC–MS. These minor compounds include four hexahydrocannabinols, four tetrahydrocannabinols, and one hydroxylated cannabinol, namely 9α-hydroxyhexahydrocannabinol, 7-oxo-9α-hydroxyhexa-hydrocannabinol, 10α-hydroxyhexahydrocannabinol, 10aR-hydroxyhexahydrocannabinol, Δ9-THC aldehyde A, 8-oxo-Δ9-THC, 10aα-hydroxy-10-oxo-Δ8-THC, 9α-hydroxy-10-oxo-Δ6a,10a-THC, and 1′S-hydroxycannabinol, respectively. The latter compound showed moderate anti-MRSa (IC50 10.0 μg/mL), moderate antileishmanial (IC50 14.0 μg/mL) and mild antimalarial activity against Plasmodium falciparum (D6 clone) and P. falciparum (W2 clone) with IC50 values of 3.4 and 2.3 μg/mL, respectively.
Keywords: Cananbis, Cannabis sativa, Cannabaceae, High potency, Oxygenated cannabinoids, Anti-bacterial, Anti-leishmanial, Anti-malarial
1. Introduction
Cannabinoids are the most distinctive and specific class of compounds known to exist only in the cannabis plant, which are responsible for the majority of the biological activities of the cannabis plant. The best-known and the most specific class of cannabis constituents is the C21 terpenophenolic cannabinoids, with (−)-Δ9-trans-(6aR, 10aR)-tetrahydrocannabinol (Δ9-THC) being the most psychologically active constituent (Mechoulam and Gaoni, 1967a,b). Although several subclasses of cannabinoids have been identified, the skeletons of these subclasses do not differ greatly from one another. Modification of the structures are limited to changes in the side-chain and the terpenoid portion of the molecule (ElSohly and Slade, 2005). The total number of natural cannabinoids identified in C. sativa L. was 66 in 1995, 70 in 2005 and 105 in 2014 (Ahmed et al., 2008a,b; Appendino et al., 2008; Radwan et al., 2008a,b, 2009; ElSohly and Slade, 2005; ElSohly and Gul, 2014; Ross and ElSohly, 1995).
In efforts to study the chemistry of high potency cannabis, a variety of new constituents were isolated (Radwan et al., 2008a,b, 2009; Ahmed et al., 2008a,b). Herein reported are the isolation and structure elucidation of nine new oxygenated cannabinoids (1–9) namely, 9α-hydroxyhexahydrocannabinol (1), 7-oxo-9α-hydroxy-hexahydrocannabinol (2), 10α-hydroxyhexa-hydrocannabinol (3), 10aR-hydroxyhexa-hydrocannabinol (4), Δ9-THC aldehyde A (5), 8-oxo-Δ9-THC (6), 10aR-hydroxy-10-oxo-Δ8-THC (7), 9α-hydroxy-10-oxo-Δ6a,10a-THC (8), and 1′S-hydroxycannabinol (9) along with other previously identified constituents.
2. Results and discussions
Compound 1 was obtained as a yellow oil and its molecular formula was determined to be C21H32O3 from GC–MS (m/z 332 at Rt 12.23 min) and HRESIMS (m/z 333.2495 [M+H]+), representing six degrees of unsaturation The 13C NMR spectrum showed signals indicating four methyl, seven methylene, four methine and six quaternary carbons [two oxyaryl (C-1, C-4a), two oxygenated sp3 (C-6, C-9) and two aryl sp2 (C-3, C-10b) carbons]. Comparing the 1H and 13C NMR spectroscopic data of 1 (Tables 1 and 2) with Δ9-THC indicated that 1 is a hexahydrocannabinol derivative. Significant differences between 1 and Δ9-THC were observed in the NMR spectra. This included the absence of the olefinic carbon resonances at δC 134.6 (C-9), and δC 123.6 (C-10) in the carbon spectrum, the lack of a broad olefinic resonance at δH 6.41 (1H, s, H-10) in the proton spectrum, and the appearance of an oxygenated sp3 carbon at δC 71.2 (C-9) and a methylene carbon at δC 42.3 (C-10) in the carbon spectrum of 1. Oxygenation of C-9 led to changes in the chemical shifts of the nearest methyl protons of carbon C-11 from δH 1.67 (3H, s) to δH 1.28 s (3H, s). This assumption was supported by the 1H–1H COSY correlations of H-9 with H-10 and H-10 with H-10a and HMBC correlations of H-10a with C-9 and H3-11 with C-9 (Fig. 1). The molecular formula, degrees of unsaturation and 2D NMR spectroscopic analysis (Fig. 1), pointed towards a presence of free hydroxyl group at C-9, which was supported by the presence of hydroxyl absorption band in IR spectrum at vmax 3460 cm−1. The 9α-hydroxyhexahydrocannabinol configuration assignment was supported by ROESY correlations of H3-13, H-6a and H3-11 (Fig. 1).
Table 1.
1H NMR spectroscopic data (400 MHz, CDCl3) for compounds (1–9).
| No. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
|---|---|---|---|---|---|---|---|---|---|
| 2 | 6.20 s | 6.20 s | 6.20 | 6.24 s | 6.23 s | 6.34 s | 6.32 s | 6.55s | |
| 4 | 6.22 s | 6.4s | 6.22 | 6.25 s | 6.18 s | 6.27 s | 6.47 s | 6.47 | 6.46 |
| 6a | 1.43 | 1.92 (d, J = 8.0) | 1.66 m | 1.81 | 1.88 | 2.2 | 2.38 | ||
| 7 | 1.40 | 1.51 m | 1.40 | 1.40 | 2.69 | 2.70 | 2.62 | 7.10 (d, J = 7.6) | |
| 1.70 | 1.79 m | 1.92 | 1.92 | 2.34 | |||||
| 8 | 1.90 | 2.10 s | 2.75 m | 1.77 | 2.15 | 6.89 br. S | 2.13 | 7.13 (d, J = 7.6) | |
| 9 | 1.83 m | 1.38 | |||||||
| 10 | 1.90 | 1.85 | 3.42 | 2.01 | 6.41 s | 7.83 s | 8.27 s | ||
| 10a | 1.54 | 3.52 | 1.66 m | 3.22 | 3.52 | ||||
| 11 | 1.28 s | 1.41s | 0.88 (d, J = 6.8) | 0.87 | 1.67 s | 1.81 s | 1.86 s | 2.36 s | |
| 12 | 1.35 s | 1.40 s | 1.21 s | 1.31 s | 1.10 s | 1.14 s | 1.33 s | 1.56 s | |
| 13 | 1.00 s | 1.50 s | 1.21 s | 1.35 s | 1.43 s | 1.36 s | 1.40 s | 1.59 s | |
| 1′ | 2.40 (t, J = 7.4) | 2.42 (t, J = 7.4) | 2.40 (t, J = 7.4) | 2.45 (t, J = 7.4) | 2.30 (t, J = 7.4) | 2.43 (t, J = 7.4) | 2.47 (t, J = 7.4) | 2.47 (t, J = 7.4) | 4.59 (t, J = 6.8) |
| 2′ | 1.56 | 1.55 | 1.56 | 1.58 | 1.57 | 1.54 | 1.57 | 1.57 | 1.65 |
| 3′ | 1.30 | 1.30 | 1.29 | 1.31 | 1.33 | 1.29 | 1.29 | 1.27 | 1.29 |
| 4′ | 1.30 | 1.30 | 1.29 | 1.31 | 1.33 | 1.29 | 1.29 | 1.27 | 1.29 |
| 5′ | 0.86 (t, J = 6.8) | 0.87 (t, J = 6.8) | 0.85 (t, J = 6.8) | 0.87 (t, J = 6.4) | 0.87 (t, J = 6.4) | 0.86 (t, J = 6.4) | 0.87 (t, J = 6.6) | 0.87 (t, J = 6.4) | 0.87 (t, J = 6.4) |
| CHO | 10.01 s |
Table 2.
13C NMR spectroscopic data (400 MHz, CDCl3) for compounds (1–9).
| No. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 154.8 | 153.7 | 153.9 | 154.3 | 161.3 | 154.9 | 154.6 | 153.6 | 154.8 |
| 2 | 108.6 | 108.3 | 106.9 | 107.8 | 102.6 | 108.1 | 112.7 | 110.0 | 107.3 |
| 3 | 142.9 | 146.1 | 142.7 | 143.6 | 147.0 | 144.0 | 146.6 | 146.7 | 146.0 |
| 4 | 110.1 | 110.8 | 109.2 | 107.9 | 111.5 | 110.3 | 112.2 | 113.5 | 108.2 |
| 4a | 155.1 | 154.7 | 154.2 | 157.1 | 158.2 | 154.6 | 155.4 | 153.5 | 154.5 |
| 6 | 77.0 | 77.4 | 76.2 | 74.5 | 79.6 | 76.2 | 77.3 | 77.5 | 77.3 |
| 6a | 49.4 | 56.4 | 46.9 | 51.8 | 45.5 | 47.5 | 50.0 | 163.3 | 137.0 |
| 7 | 24.2 | 213.7 | 17.9 | 22.5 | 25.1 | 41.3 | 24.8 | 25.7 | 122.7 |
| 8 | 39.4 | 39.6 | 28.3 | 38.4 | 31.4 | 199.9 | 146.2 | 33.4 | 127.7 |
| 9 | 71.2 | 75.2 | 27.5 | 27.8 | 134.3 | 134.8 | 133.1 | 73.4 | 137.0 |
| 10 | 42.3 | 19.0 | 78.5 | 40.0 | 123.2 | 150.5 | 199.3 | 206.0 | 128.0 |
| 10a | 30.6 | 35.3 | 46.9 | 74.3 | 33.3 | 35.1 | 72.2 | 124.6 | 127.2 |
| 10b | 110.0 | 110.8 | 110.0 | 110.0 | 110.0 | 106.3 | 109.7 | 106.1 | 110.4 |
| 11 | 31.9 | 25.0 | 19.9 | 24.3 | 23.5 | 16.1 | 16.7 | 25.0 | 21.8 |
| 12 | 19.2 | 27.4 | 19.9 | 27.3 | 19.9 | 19.5 | 20.5 | 22.3 | 27.4 |
| 13 | 27.8 | 27.1 | 14.5 | 28.9 | 27.6 | 27.1 | 28.3 | 25.2 | 27.4 |
| 1′ | 35.5 | 36.0 | 36.0 | 36.0 | 36.3 | 35.8 | 35.8 | 35.7 | 74.7 |
| 2′ | 31.8 | 30.6 | 31.1 | 30.9 | 31.2 | 30.9 | 30.5 | 30.5 | 38.4 |
| 3′ | 30.9 | 31.7 | 31.7 | 31.9 | 32.1 | 31.7 | 31.7 | 31.7 | 28.1 |
| 4′ | 22.7 | 22.7 | 22.7 | 22.8 | 22.7 | 22.8 | 22.7 | 22.8 | 22.8 |
| 5′ | 14.2 | 14.2 | 14.3 | 14.3 | 14.2 | 14.3 | 14.2 | 14.3 | 14.3 |
| CHO | 193.0 |
Fig. 1.
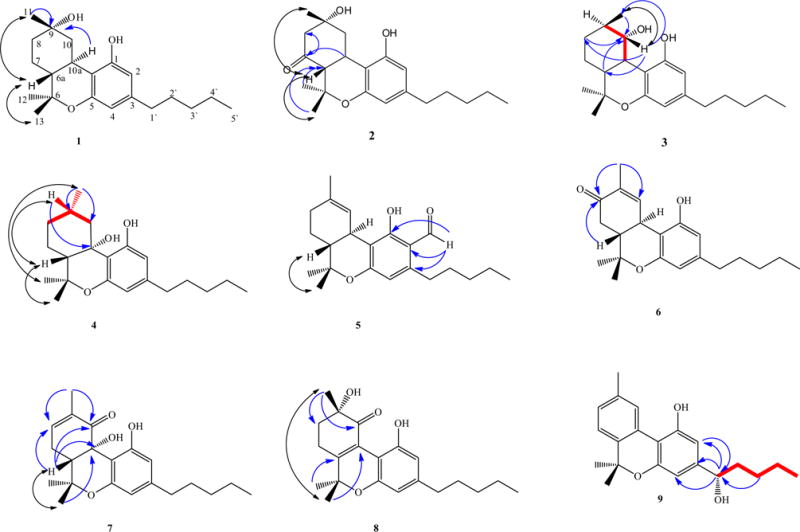
Important HMBC (blue), COSY (red) and ROESY (violet) correlations for 1–9. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Compound 2 was obtained as a yellow oil and its molecular formula was determined to be C21H30O4 by HRESIMS (m/z 347.2235 [M+H]+), representing seven degrees of unsaturation. The 13C NMR spectrum showed signals for four methyl, six methylene, four methine and seven quaternary carbons [two oxyaryl (C-1, C-4a), two oxygenated sp3 (C-6, C-9) and two aryl sp2 (C-3, C-10b) and one carbonyl (C-7)]. Comparison of the 1H and 13C NMR spectroscopic data (Tables 1 and 2) with 1 and Δ9-THC indicated that compound 2 belongs to the hexahydrocannabinol series. Significant differences between 2 and 1 were observed in the NMR spectra in which a carbonyl carbon appears at δC 213.7 in the spectrum of 2 instead of a methylene carbon in 1. HMBC correlations of H2-8/C-7 (2JCH), H-10a/C-7 (3JCH), H-12/C-6a (3JCH) and H-13/C-6a (3JCH) support that the oxo substitution existed on C-7 (Fig. 1). The 9α-hydroxyhexahydrocannabinol configuration assignment was supported by the ROESY correlations of H3-13, H-6a and H3-11 (Fig. 1).
Compound 3 was obtained as a yellow oil and its molecular formula was determined to be C21H30O3 by GC–MS (m/z 332 at Rt 13.46 min) and HRESIMS (m/z 333.2413 [M+H]+), representing six degrees of unsaturation. The NMR spectra was similar to those of Δ9-THC except for the disappearance of olefin carbon resonances at δC 134.6 (C-9) and δC 123.6 (C-10), as well as a broad olefinic resonance at δC 6.41 (1H, s, H-10) and the appearance of a sp3 methine and oxygenated methane at δC 28.3 (C-9) and δC 78.5 (C-10) respectively. This indicated the structure of 3 belongs to the hexahydrocannabinol series. The 1H- and 13C NMR, DEPT and HMQC data of 3 indicate the presence of a hydroxy group (CHOH) [δH 3.42 (dd, J = 3.6, 10.8); δC 78.5 (C-10)] The hydroxy group position on C-10 was determined by the HMBC correlations of H3-11, H2-8 and H-6a with C-10 and H-10 with C-11, C-8 and C-6a (Fig. 1). The absolute configuration of 3 at the chiral centers (C-9 and C-10) was assigned by comparing its specific rotation and 1H NMR (CDCl3) with the analogs as reported in the literature; the optical rotation [−55.6 (c 0.05, CH3Cl)] and the chemical shifts of H-10 δH 3.42 were in good agreement with C(10β) proton at δH 3.57 which appears relatively upfield compared to the C(10α) proton which appears at δH 4.98 in known synthetic canabinoids.11 Further support for the α orientation of 10-OH was established via the Mosher ester analysis protocol (Dale and Mosher, 1973; Sullivan et al., 1973; Hoye et al., 2007; Seco et al., 2004). ROESY correlations of H-10 and H3-11 indicated that both the C-10 proton and C-11 methyl were in the same direction (Fig. 1). This is the first report of 3 from a natural source with full NMR spectroscopic data; however, it was previously prepared synthetically (Theodor et al., 1976).
Compound 4 was obtained as a yellow oil and its molecular formula was determined to be C21H32O3 by GC–MS (m/z 332 at Rt 12.04) and HRESIMS (m/z 333.2495 [M+H]+), representing six degrees of unsaturation. The NMR spectra of 5 were indicative of an oxygenated hexahydrocannabinol structure. The 1H- and 13C NMR, DEPT and HMQC data of 4 supported the presence of a hydroxy group on a quaternary carbon (δC 74.3). The placement of the hydroxyl group on C-10a was determined by HMBC correlations of H-9 with C-10a and H3-11 with C-10 (Fig. 1). The configuration assignment at C-9 was supported by the ROESY correlations, where H3-13, H-6a and H-9 showed a good correlation with each other, while H3-12 showed a good correlation with H3-11 (Fig. 1). The 6aR, 10aR configuration was provisionally established for Δ9-THC (Mechoulam and Gaoni, 1967a,b; ElSohly and Slade, 2005). Based on the fact that all Δ9-THC compounds have a 10a R configuration or its equivalent, the configuration of hydroxyl group at C-10a is suggested to be biosynthetically in the R configuration.
Compound 5 was obtained as a yellow oil and its molecular formula was determined to be C22H30O3 by GC–MS (m/z 342 at Rt 39.66) and HRESIMS (m/z 343.2240 [M+H]+), representing eight degrees of unsaturation. The spectroscopic data of 5 were similar to those of Δ9-tetrahydrocannabinolic acid A (Δ9-THCAA). It has four characteristic methyls resonating at δ 1.67 (3H, s, H-11), 1.43 (3H, s, H-13), 1.10 (3H, s, H-12) and 0.87 (3H, t, J = 6.4 Hz, H-5′), and an aromatic proton at δ 6.18 (1H, s, H-4) shifted upfield from δ 108.6 to δC 102.6 (Table 2). The differences between compound 5 and Δ9-THCAA were observed in the NMR spectra where the carbonyl resonance was shifted downfield from δ 176.4 to δ 193.0 These data point to the presence of an aldehyde group in C-2 instead of a carboxylic acid group. This was further confirmed by the disappearance of the carboxylic acid proton at δH 12.18 and the appearance of an aldehydic proton at δH 10.01 ppm. Full assignment of the 1H and 13C NMR resonances were completed via analysis of the COSY, HMQC, HMBC and ROESY spectra (Tables 1 and 2, 5Fig. 1) confirming as Δ9-THC aldehyde A.
Compound 6 was obtained as a yellow oil and its molecular formula was determined to be C21H28O3 by GC–MS (m/z 328 at Rt 40.44 min) and HRESIMS (m/z 329.2145 [M+H]+), representing eight degrees of unsaturation. The 13C NMR spectrum showed signals indicating four methyl, five methylene, five methine and seven quaternary carbons [two oxyaryl (C-1, C-4a), one oxygenated sp3 (C-6), two aryl sp2 (C-3, C-10b), one olefinic sp2 (C-9) and one carbonyl (C-8)]. Comparison of the 1H and 13C NMR spectroscopic data (Tables 1 and 2) with Δ9-THC indicated that 7 belongs to the tetrahydrocannabinol series. Significant difference between 6 and Δ9-THC was observed in the NMR spectra where a carbonyl carbon appears at δC 199.9 instead of a methylene carbon. HMBC correlations of H2-7/C-8 (2JCH), H-6a/C-8 (3JCH H-10/C-8 (3JCH)) and H-11/C-8 (3JCH) support the interpretation that the roxo substitution existed on C-8 (δC 199.9) (Fig. 1). Full assignment of the 1H and 13C NMR resonances were completed via analysis of the COSY, HMQC, HMBC and ROESY spectra (Tables 1 and 2, 6Fig. 1) confirming as 8-oxo-Δ9-THC. This is the first report of 6 from a natural source; however, it was previously prepared synthetically (Gurny et al., 1972).
Compound 7 was obtained as a yellow oil and its molecular formula was determined to be C21H28O4 by HRESIMS (m/z 345.2096 [M+H]+), representing eight degrees of unsaturation. GC–MS analysis of the trimethylsilyl-derivative of 8 yielded a molecular ion at m/z 488 at Rt 38.68 min, indicating the presence of two hydroxyl groups. The 13C NMR spectrum showed signals indicating four methyl, five methylene, four methine and eight quaternary carbons [two oxyaryl (C-1, C-4a), two oxygenated sp3 (C-6, C-10a), two aryl sp2 (C-3, C-10b), one olefinic sp2 (C-9) and one carbonyl (C-10)] indicating that the structure of 7 is a substituted tetrahydrocannabinol with one oxo and one hydroxyl groups. Analysis of the 1H–1H COSY, HMQC and HMBC spectra led to the assignment of proton and carbon resonances for 8. The position of the δC 146.2 (C-8) and broad olefinic resonance at δ 6.89 (1H, bs, H-8), carbonyl carbon δC 199.3 (C-10) and oxygenated sp3 carbon δC 72.2 (C-10a) were confirmed by 1H–1H COSY spectrum between H2-7/H-6a and H2-7/H-8 and also from HMBC of H-8/C-10 (3JCH), H-8/C-6a (3JCH), H-8/C-11 (3JCH), H2-7/C-10a (3JCH), H-6a/C-10 (3JCH), H3-11/C-8 (3JCH) and H3-11/C-10 (3JCH). Full assignment of the 1H and 13C NMR resonances were completed via analysis of the COSY, HMQC, HMBC and ROESY spectra (Tables 1 and 2, 7Fig. 1) confirming as 10a-hydroxy-10-oxo-Δ8-THC. Based on the fact that all Δ8-THC have the 10a R configuration or its equivalent (ElSohly and Slade, 2005), the configuration of hydroxyl group at C-10a is suggested to be biosynthetically in the R configuration.
Compound 8 was obtained as a yellow oil and its molecular formula was determined to be C21H28O4 by GC–MS (m/z 344 at Rt 39.50) and HRESIMS (m/z 345.2033 [M+H]+), representing eight degrees of unsaturation. The 13C NMR spectrum showed signals indicating four methyl, six methylene, two methine and nine quaternary carbons [two oxyaryl (C-1, C-4a), two oxygenated sp3 (C-6, C-10), two aryl sp2 (C-3, C-10b), two olefinic sp2 (C-6a, C-10a) and one carbonyl (C-10)]. This indicates that the structure of 8 belongs to the tetrahydrocannabinol series with oxo and hydroxyl groups. The 13C NMR spectrum showed resonances at δC 163.3 and 124.6 corresponding to the C6a–C10a double bond, a carbonyl carbon at δC 206.0 (C-10) and an oxygenated sp3 carbon at δC 73.4 (C-9). The position of the double bond, carbonyl carbon and the oxygenated sp3 carbon were confirmed by 1H–1H COSY spectrum between H2-7/H2-8 and also from HMBC of H2-8/C-10 (3JCH), H2-8/C-6a (3JCH), H2-8/C-11 (3JCH), H2-7/C-10a (3JCH), H2-7/C-9 (3JCH), H3-11/C-8 (3JCH) and H3-11/C-10 (3JCH). The additional hydroxyl group in compound 8 generates a stereogenic center at C-9. Through the use of a ROESY experiment, the stereochemistry was assigned as 9S-hydroxy-10-oxo-Δ6a,10a-THC through space correlations between the C-9 methyl protons and the C-13 methyl protons. Comparison of the 13C NMR spectrum of the oxidation product 9α-hydroxy-10-oxo-hexahydrocannabinol resulting from selective oxidation reaction of 9S,10S-dihydroxy-hexahydrocannabinol (Cannabiripsol) with pyridinium chlorochromate (PCC), further supported the S configuration of C-9 where both have δC at 73 (Fig. 2) (Fan et al., 2006). Thus compound 8 was established as 9α-hydroxy-10-oxo-Δ6a,10a-THC.
Fig. 2.
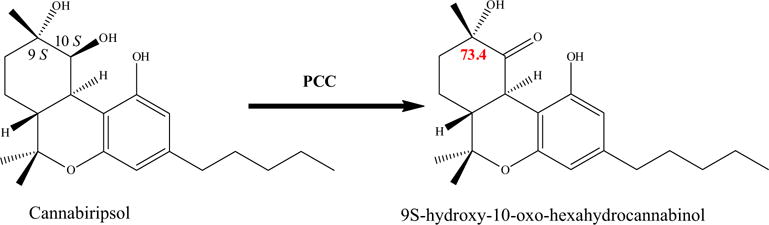
Selective oxidation of cannabiripsol (PCC).
Compound 9 was obtained as a yellow oil and its molecular formula was determined to be C21H26O3 by GC–MS (m/z 326 at Rt 13.39) and HRESIMS (327.1931 [M+H]+), representing eight degrees of unsaturation. The trimethylsilyl derivative of 9 had a molecular ion at m/z 470 in the GCMS, confirming the HRESIMS result and the presence of two hydroxyl groups. The 1H NMR spectroscopic data showed four methyl singlets, five aromatic protons and three methylene protons (Table 1). The 13C and DEPT NMR data indicated that 9 contains 21 carbons [four methyls, three methylenes, five methines (four aryl sp2 and one oxygenated methine) and nine quaternary carbons]. The 1H and 13C NMR spectra, as well as the GC–MS data of 9, suggested a close similarity to cannabinol (Ahmed et al., 2008a) with an additional hydroxyl group δC 74.7 and δH 4.59 (1H, t, J = 6.8). The location of this hydroxyl group at C-1′ was determined by 1H–1H COSY spectrum between H-1′/H2-2′, H2-2′/H2-3′, H2-3′/H2-4′ and H2-4′/H3-5′ and also from HMBC of H-1′/C-2 (3JCH), H-1′/C-4 (3JCH), H-1′/C-3 (2JCH), H-1′/C-3′ (3JCH), H-2/C-1′ (3JCH), H-4/C-1′ (3JCH) and H2-3′/C-1′ (3JCH). The absolute configuration of C-1′ was determined as S via the Mosher ester analysis protocol (Fig. 3) (Dale and Mosher, 1973; Sullivan et al., 1973; Hoye et al., 2007; Seco et al., 2004). Thus, compound 9 was established as 1′S-hydroxycannabinol.
Fig. 3.
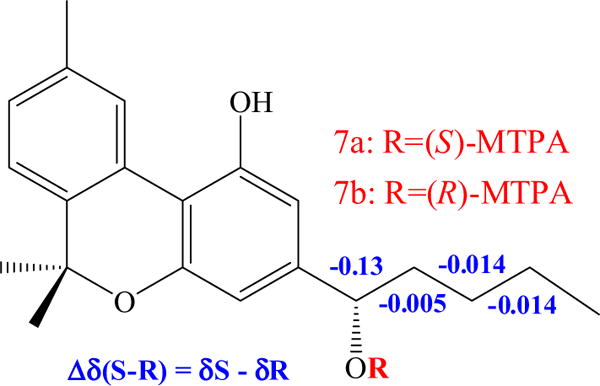
Mosher ester analysis of 9.
The antimicrobial, antileishmanial, and antimalarial of the isolated compounds were tested. Compound 9 showed moderate anti-MRSa (IC50 10.0 μg/mL), moderate antileishmanial (IC50 14.0 μg/mL) and mild antimalarial activity against Plasmodium falciparum (D6 clone) and P. falciparum (W2 clone) with IC50 values of 3.4 and 2.3 μg/mL, respectively.
3. Conclusion
Nine new oxygenated cannabinoids (1–9) were isolated from a high potency Cannabis sativa L. variety. Compound 9 showed moderate activity against methicillin resistant Staphylococcus aureus, Leishmania donovani, P. falciparum (D6 clone) and P. falciparum (W2 clone).
4. Experimental
4.1. General experimental procedures
1D and 2D NMR spectra were recorded in CDCl3 on a Varian AS 400 spectrometer, whereas IR spectra were obtained using a Bruker Tensor 27 spectrophotometer. UV spectra were acquired on a Varian Cary 50 Bio UV–Visible spectrophotometer. Optical rotations were measured at ambient temperature using a Rudolph Research Analytical Autopol IV automatic polarimeter. HRESIMS was obtained using a Bruker Bioapex FTMS in ESI mode. TLC was carried out on aluminum-backed plates precoated with silica gel F254 (20 × 20 cm, 200 μm, 60 Å, Merck) and on glass-backed plates precoated with C18 silica gel F254 (10 × 10 cm, 200 μm, 60 Å, 11% carbon loading, Silicycle). Visualization was accomplished by spraying with Fast blue B salt (0.5% w/w in H2O) or p-anisaldehyde [0.5 mL in glacial AcOH (50 mL) and H2SO4 (97%, 1 mL)] spray reagent followed by heating. Flash silica gel (40–63 μm, 60 Å, Silicycle) and SiliaBond C18 silica gel (40– 63 μm, 60 Å, 17% carbon loading, Silicycle) were used for column chromatography (CC). Analytical HPLC was performed on a Waters 2695 Separations Module [Empower Pro 2 Software (Build 2154)] connected to a Waters 2996 photodiode array (PDA) detector (190–500 nm) and a Sedere Sedex 75 evaporative light scattering detector (ELSD) (3.5 psi N2, 50 °C) using a Phenomenex Luna C18(2) column (150 × 4.6 mm, 5 μm, 100 Å) [MeCN (100%), 1.0 mL/min] and a Phenomenex Luna Silica (2) column (150 × 4.6 mm, 5 μm, 100 Å) [n-hexane/EtOH (99:1), 1.0 mL/min]. Semi-preparative HPLC was performed on a Waters Delta Prep 4000 Preparative Chromatography System [Empower Pro Software (Build 1154)] connected to a Waters 486 Tunable Absorbance detector (206 nm) using a Phenomenex Luna C18(2) column (250 × 21.2 mm, 5 μm, 100 Å) [MeCN (100%), 35.4 mL/min] and a Phenomenex Luna Silica (2) column (250 × 21.2 mm, 5 μm, 100 Å) [n-hexane/EtOH (99:1), 35.4 mL/min]. GCMS analyses were carried out on a ThermoQuest Trace 2000 GC, equipped with a single split/splitless capillary injector, a ThermoQuest AS2000 autosampler and a Phenomenex ZB-5 column (30 m × 0.25 mm × 0.25 μm), interfaced to a ThermoQuest-Finnigan Trace MS quadrupole ion trap detector. The injector temperature was 250 °C and 1 μL injections were performed in splitless mode, with the splitless time set at 60 s, the split flow set at 50 mL/min and the septum purge valve set to close 60 s after the injection occurred. The oven temperature was raised from 70 to 270 °C (hold 20 min) at a rate of 5 °C/min, for a total run time of 60 min; the transfer line temperature was 250 °C. Helium was used as the carrier gas at a constant pressure of 20 psi. The mass spectrometer was operated in the electron impact mode (EI+) and scanned from 40 to 800 amu at 1 scan/s, with an ionizing voltage of 70 eV and an emission current of 350 μA. Data was recorded using an IBM Netfinity 3000 Workstation with Microsoft Windows NT 4.0 operating system (Build 1381, Service pack 6) and Xcalibur data acquisition and analysis software (Version 1.2). The NIST Mass Spectral Search Program (Version 1.7, Build 11/05/1999) for the NIST/EPA/NIH.
4.2. Plant material
Plants were grown from high potency Mexican seeds (variety code CHPF-01). The seeds and plants were authenticated by Dr. Suman Chandra, The University of Mississippi, and a specimen (S1310V1) is deposited at the Coy Waller Complex, The University of Mississippi. Whole buds of mature female plants were harvested, air-dried, packed in barrels and stored at low temperature (−24 °C).
4.3. Extraction and isolation
Dried buds and small leaves of C. sativa (9.0 kg) was sequentially extracted with hexanes (2 × 60 L), CH2Cl2 (48 L), EtOAc (40 L), EtOH (37.5 L), EtOH/H2O (36 L, 1:1) and H2O (40 L) at room temperature. The extracts were evaporated under reduced pressure at 40 °C to afford hexanes (1.48 kg), CH2Cl2 (0.15 kg), EtOAc (0.13 kg), EtOH (0.09 kg), EtOH/H2O (0.77 kg) and H2O (0.54 kg) extracts, respectively. The hexanes extract (0.96 kg) was subjected to VLC on flash silica gel eluting with hexanes, EtOAc and MeOH gradient to afford 32 fractions. Fractions (f1–f3) eluted with hexanes were combined according to TLC profiles to afford a reddish green residue (35 g). This fraction was subsequently subjected to flash silica gel CC eluting with hexanes to afford large quantities of delta-9-tetrahydrocannabinol (Δ9-THC), delta-9-tetrahydrocannabinolic acid A (Δ9-THCAA), delta-8-tetrahydrocannabinol (Δ8-THC) and cannabinol (CBN). Fractions with an Rf higher than THC according to TLC (hexanes/EtOAc, 9:1) were combined and purified by semi-preparative reversed-phase HPLC (CH3CN as eluent) to afford compound 5 (4 mg).
Fractions (f24–f25) were combined according to TLC profiles to afford a reddish green residue (26 g). This fraction was subsequently applied to a flash silica gel column, with the eluent further subjected to a C18 SPE CC followed by final purification by semi-preparative reversed-phase HPLC (H2O:CH3CN 25:75, v/v as eluent) to afford compounds 1 (5 mg), 3 (10 mg), 4 (8 mg), 6 (9 mg) and 9 (10 mg) while, using semi-preparative reversed-phase HPLC (H2O:CH3CN 40:60, v/v as eluent) afforded compounds 2 (3 mg), 7 (15 mg), 8 (4 mg) and cannabiripsol (10) (150 mg).
4.4. Selective oxidation of cannabiripsol (10) using (PCC) (Fan et al., 2006)
Cannabiripsol (10) (10.2 mg, 15 mmol) was dissolved in dry CH2Cl2 (10 mL), and PCC (6.4 mg, 15 mmol) was added at 0 °C. The reaction mixture was stirred at room temperature and after completion it was filtered through Celite. The filtrate was concentrated and the residue was purified by silica gel CC to give 9S-hydroxy-10-oxo-hexahydrocannabinol (8). The reaction was carried out under anhydrous conditions, monitored by TLC.
4.5. Antimicrobial, antileishmanial and antimalarial assay
Isolated compounds were evaluated for antimicrobial (Candida albicans ATCC 90028, Escherichia coli ATCC 35218, Pseudomonas aeruginosa ATCC 27853, Mycobacterium intracellulare ATCC 23068, Aspergillus fumigatus ATCC 90906, Methicillin Resistant S. aureus ATCC 43300), antileishmanial and antimalarial activity [P. falciparum (D6 clone) and P. falciparum (W2 clone)] (Radwan et al., 2008a,b), respectively.
8α-hydroxyhexahydrocannabinol (1): yellow oil; UV (MeOH) λmax 280, 227 nm; ; IR (neat) vmax 3460, 2820, 1624, 1457, 1057 cm−1; For 1H NMR and 13C NMR spectroscopic data, see Tables 1 and 2; GCMS m/z 332 [M]+, 299 (100%); HRESIMS m/z 333.2495 [M+H]+ (calcd for C21H33O3, 333.2430).
7-oxo-8α-hydroxyhexahydrocannabinol (2): yellow oil; UV (MeOH) λmax 220, 267, 330 nm; ; IR (neat) vmax 3460, 2877, 1732, 1624, 1457 cm−1; For 1H NMR and 13C NMR spectroscopic data, see Tables 1 and 2; HRESIMS m/z 347.2235 [M+H]+ (calcd for C21H31O4, 345.2222).
10-α-hydroxyhexahydrocannabinol (3): yellow oil; UV (MeOH) λmax 280, 227 nm; ; IR (neat) vmax 3460, 2820, 1624, 1457, 1057 cm−1; For 1H NMR and 13C NMR spectroscopic data, see Tables 1 and 2; GCMS m/z 332 [M]+, 193 (100%); HRESIMS m/z 333.2413 [M+H]+ (calcd for C21H33O3, 333.2430).
10a-hydroxyhexahydrocannabinol (4): yellow oil; ; UV (MeOH) λmax 275, 225 nm; IR (neat) vmax 3460, 2930, 1624, 1457 cm−1; For 1H NMR and 13C NMR spectroscopic data, see Tables 1 and 2; GCMS m/z 332 [M]+, 231 (100%); HRESIMS m/z 333.2495 [M+H]+ (calcd for C21H33O3, 333.2430).
Δ9-THC aldehyde A (5): yellow oil; ; UV (MeOH) λmax 310, 255, 215 nm; IR (neat) vmax 3455, 2929, 1722, 1624, 1457 cm−1; For 1H NMR and 13C NMR spectroscopic data, see Tables 1 and 2; GCMS m/z 342 [M]+, 327 (100%); HRESIMS m/z 343.2240 [M+H]+ (calcd for C22H31O3, 343.2273).
8-oxo-Δ9-THC (6): yellow oil; ; UV (MeOH) λmax 280, 270, 225 nm; IR (neat) vmax 3361, 2929, 1740, 1655, 1428, 1048 cm−1; For 1H NMR and 13C NMR spectroscopic data, see Tables 1 and 2; GCMS m/z 328 [M]+, 271 (100%); HRESIMS m/z 329.2145 [M+H]+ (calcd for C21H29O3, 329.2117).
10aα-hydroxy-10-oxo-Δ8-THC (7): yellow oil; ; UV (MeOH) λmax 310, 255, 215 nm; IR (neat) vmax 3464, 2929, 1732, 1624, 1457 cm−1; For 1H NMR and 13C NMR spectroscopic data, see Tables 1 and 2; GCMS-TMS m/z 488 [M]+, 391 (100%);
HRESIMS m/z 345.2119 [M+H]+ (calcd for C21H29O4, 345.2066). 9α-hydroxy-10-oxo-Δ6a,10a-THC (8): yellow oil; UV (MeOH) λmax 315, 255, 215 nm; ; IR (neat) vmax 3468, 2929, 2857, 1732, 1624, 1457 cm−1; For 1H NMR and 13C NMR spectroscopic data, see Tables 1 and 2; GCMS m/z 444 [M]+, 301 (100%); HRESIMS m/z 345.2033 [M+H]+ (calcd for C21H29O4, 345.2066).
(S)-1′-hydroxycannabinol (9): yellow oil; UV (MeOH) λmax 260, 225, 205 nm; ..; IR (neat) vmax 3446, 2920, 2825, 1718, 1636, 1541, 1457, 1418, 1467, 1057 cm−1; For 1H NMR and 13C NMR spectroscopic data, see Tables 1 and 2; GCMS m/z 326 [M]+, 311 (100%); GCMS-TMS m/z 470 [M]+, 455 (100%); HRESIMS m/z 327.1931 [M+H]+ (calcd for C21H25O3, 327.1960).
Supplementary Material
Acknowledgments
The project described was supported by Grant Number 5P20RR021929 from the National Center for Research Resources and in part by the National Institute on Drug Abuse, contract # N01DA-10-7773. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health. The Authors are grateful to Drs. Melissa Jacob, Shabana Khan and Babu Tekwani for conducting the biological testing and to Dr. Baharthi Avula for assistance with HRESIMS.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.phytochem.2015.04.007.
References
- Ahmed SA, Ross SA, Slade D, Radwan MM, Zulfiqar F, ElSohly MA. Cannabinoid ester constituents from high-potency Cannabis sativa. J Nat Prod. 2008a;71:536–542. doi: 10.1021/np070454a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed SA, Ross SA, Slade D, Radwan MM, Ikhlas AK, ElSohly MA. Structure determination and absolute configuration of cannabichromanone derivatives from high potency Cannabis sativa. Tetrahedron Lett. 2008b;49:6050–6053. doi: 10.1016/j.tetlet.2008.07.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appendino G, Giana A, Gibbons S, Maffei M, Gnavi G, Grassi G, Sterner O. A polar cannabinoid from Cannabis sativa var Carma. Nat Prod Commun. 2008;3:1977–1980. [Google Scholar]
- Dale JA, Mosher HSJ. Nuclear magnetic resonance enantiomer regents. Configurational correlations via nuclear magnetic resonance chemical shifts of diastereomeric mandelate, O-methylmandelate, and .alpha.-methoxy-.alpha.-trifluoromethylphenylacetate (MTPA) esters. J Am Chem Soc. 1973;95:512. [Google Scholar]
- ElSohly MA, Gul W. Constituents of Cannabis Sativa. In: Pertwee RG, editor. Handbook of Cannabis. Oxford University Press; 2014. pp. 3–22. [Google Scholar]
- ElSohly MA, Slade D. Chemical constituents of marijuana: the complex mixture of natural cannabinoids. Life Sci. 2005;78:539–548. doi: 10.1016/j.lfs.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Fan Q, Ni N, Li Q, Zhang L, Ye Xin-Shan. New one-carbon degradative transformation of β-alkyl-β-azido alcohols. Org Lett. 2006;8:1007–1009. doi: 10.1021/ol0601996. [DOI] [PubMed] [Google Scholar]
- Gurny O, Maynard DE, Pitcher RG, Kieerstead RW. Metabolism of (−)-DELTA.9- and (−)-.DELTA.8-tetrahydrocannabinol by monkey liver. J Am Chem Soc. 1972;94(22):7928–7935. doi: 10.1021/ja00777a048. [DOI] [PubMed] [Google Scholar]
- Hoye TR, Jeffrey CS, Shao F. Mosher ester analysis for the determination of absolute configuration of stereogenic (a.k.a Chiral) carbinol carbons. Nat Protoc. 2007;2:2451–2458. doi: 10.1038/nprot.2007.354. [DOI] [PubMed] [Google Scholar]
- Mechoulam R, Gaoni Y. Recent advances in the chemistry of hashish. Fortschr Chem Org Naturst. 1967a;25:175–213. doi: 10.1007/978-3-7091-8164-5_6. [DOI] [PubMed] [Google Scholar]
- Mechoulam R, Gaoni Y. The absolute configuration of Δ1-tetrahydrocannabinol, the major active constituent of hashish. Tetrahedron Lett. 1967b;8(12):1109–1111. doi: 10.1016/s0040-4039(00)90646-4. [DOI] [PubMed] [Google Scholar]
- Radwan MM, ElSohly MA, Slade D, Ahmed SA, Wilson L, El-Alfy AT, Khan IA, Ross SA. Non-cannabinoid constituents from a high potency Cannabis sativa variety. Phytochemistry. 2008a;69:2627. doi: 10.1016/j.phytochem.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radwan MM, Ross SA, Ahmed SA, Slade D, Zulfiqar F, ElSohly MA. Isolation and characterization of new cannabis constituents from a high potency variety. Planta Med. 2008b;74:267–272. doi: 10.1055/s-2008-1034311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radwan MM, ElSohly MA, Slade D, Ahmed SA, Khan IA, Ross SA. Biologically active cannabinoids from high-potency Cannabis sativa. J Nat Prod. 2009;72(5):906–911. doi: 10.1021/np900067k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross SA, ElSohly MA. Constituents of Cannabis sativa L. XXVII. A review of the natural constituents: 1980–1994. Zagazig J Pharm Sci. 1995;4:1–10. [Google Scholar]
- Seco JM, Quinoa E, Riguera R. The assignment of absolute configuration by NMR. Chem Rev. 2004;104:17–117. doi: 10.1021/cr2003344. [DOI] [PubMed] [Google Scholar]
- Sullivan GR, Dale JA, Mosher HSJ. Selectivity, strategy, and efficiency in modem organic chemistry. J Org Chem. 1973;38:2143. [Google Scholar]
- Theodor P, Kapa K, Gerard S. Transformations of 9α,10α-epoxyhexahydrocannabinol acetate helv. Chim Acta. 1976;59(6):1963. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


