Abstract
Background
No data on the long-term ‘real-world’ use of fluvoxamine for the treatment of social anxiety disorder (SAD) in Japanese patients are currently available.
Objective
To evaluate the long-term safety and efficacy of fluvoxamine for SAD in the clinical setting.
Methods
Japanese patients with SAD who initiated treatment with fluvoxamine were enrolled in this 53-week post-marketing survey from 407 institutions nationwide. Data including rates of adverse drug reactions (ADRs) and efficacy were collected. Overall improvement was assessed using the Clinical Global Impression for Improvement. SAD symptoms and treatment responses were assessed with the Japanese version of the Liebowitz Social Anxiety Scale.
Results
From the 1,974 patients surveyed, 1,790 and 1,504 patients were eligible for analysis of safety and efficacy, respectively. ADRs were reported in 18.2 % of patients, with nausea, somnolence, and constipation the most common. Over 50 % of these ADRs developed in the first 4 weeks of treatment. Serious ADRs were reported in 0.8 % of patients and included six cases of suicide attempt and three cases of suicidal ideation. Response to fluvoxamine was reported in 78.4 % of patients. In patients comorbid with depression, improvement in SAD symptoms with fluvoxamine treatment was significantly affected by clinical improvement in the depression.
Conclusions
These findings support the long-term safety and efficacy of fluvoxamine in patients with SAD. Most ADRs developed during the early treatment phase, and higher doses during the later phase were not associated with an increase in ADRs.
Electronic supplementary material
The online version of this article (doi:10.1007/s40801-014-0005-2) contains supplementary material, which is available to authorized users.
Key Points
| Since the approval of fluvoxamine in Japan, no data on the long-term ‘real-world’ use of fluvoxamine for the treatment of social anxiety disorder in Japanese patients have been available. |
| To address this, a post-marketing survey of the long-term administration of fluvoxamine in patients with social anxiety disorder in the clinical setting was initiated. |
| This survey demonstrated that fluvoxamine is safe and efficacious for the long-term treatment of Japanese patients with social anxiety disorder in a clinical setting. |
Introduction
Fluvoxamine maleate (hereinafter referred to as fluvoxamine) was approved in April 1999 as Japan’s first selective serotonin reuptake inhibitor (SSRI) on the basis of efficacy and safety data in patients with depression/depressive state [1–3] and obsessive-compulsive disorder (OCD) [4, 5]. It became commercially available in May 1999 as a drug indicated for the treatment of these indications.
The efficacy and tolerability of fluvoxamine have also been investigated in a 10-week placebo-controlled, double-blind study in 265 Japanese patients with social anxiety disorder (SAD) followed by a 52-week open-label extension study [6]. However, such clinical trials with antidepressants were designed to include highly homogeneous samples in order to decrease heterogeneity in treatment response [7–13]. As a result, it has been pointed out that more than 70 % of typical patients with SAD would be excluded from participation in clinical trials on pharmacological efficacy for SAD by exclusion criteria [14]. Therefore, examination of treatment outcomes in a broader range of target population suffering from the disorder is required to establish better pharmacotherapy.
In this study, fluvoxamine significantly reduced the total score of the Japanese version of the Liebowitz Social Anxiety Scale (LSAS-J) compared with placebo [6]. On the basis of the positive results of this study and its open-label extension, the Japanese Ministry of Health, Labor and Welfare (MHLW) approved fluvoxamine as the first drug in Japan indicated for the treatment of SAD in October 2005. However, since the approval of fluvoxamine in Japan no data on the long-term ‘real-world’ use of fluvoxamine for the treatment of SAD in Japanese patients have been collected. To address this, a special post-marketing survey of the long-term administration of fluvoxamine in patients with SAD in the clinical setting was initiated.
Methods
Patients
Patients who visited the medical institutions participating in the survey, were diagnosed with SAD based on the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR) diagnostic criteria, and were initiating fluvoxamine for an expected treatment duration of at least 53 weeks were eligible for enrollment.
Study Design
This survey was conducted according to the MHLW Ordinance on Good Post-Marketing Study Practice (MHLW Ordinance Related to Standards for Conducting Post-Marketing Surveys and Studies on Drugs; Ministerial Ordinance No. 171 issued by the MHLW on 20 December 2004). Eligible patients were registered within 14 days after starting fluvoxamine using a central registration system, and patient data were recorded from baseline to the end of the observation period using case report forms (CRFs). The study was conducted between January 2006 and December 2008 with patient registration open between April 2006 and December 2007. Patients were planned to be followed for 53 weeks, but patients who discontinued or completed fluvoxamine treatment before week 53 were followed up from baseline to the end of their treatment.
Study Outcomes
The following data were recorded for each patient: (1) patient characteristics; (2) history of SAD treatment; (3) dosage regimen of fluvoxamine treatment, concomitant drug use, and non-drug treatments; (4) physical examinations including height, weight, pulse rate and blood pressure, electrocardiogram (ECG), and routine laboratory examinations; (5) safety; and (6) efficacy measures.
Safety analysis included recording the presence/absence, date of onset, and seriousness of adverse drugs reactions (ADRs; disorders, symptoms, and abnormal laboratory findings for which a causal relationship to fluvoxamine cannot be ruled out), the treatments received for ADRs, final outcome of ADRs, causal relationship between ADRs and fluvoxamine treatment and alternative etiology. Seriousness of ADRs was defined in accordance with the ICH E2A guideline (http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E2A/Step4/E2A_Guideline.pdf). If an ADR did not meet any of the criteria in the guideline, the ADR was defined as a non-serious ADR. In addition, the occurrence of ADRs related to suicide attempt, gastrointestinal hemorrhage, and cardiovascular ADRs (including abnormal ECG findings) was monitored.
Overall improvement in SAD during fluvoxamine treatment compared with baseline was assessed at weeks 12, 24, 36, 48, and 53 or at the discontinuation/conclusion of treatment using the 7-rank Clinical Global Impression for Improvement (CGI-I) scale: 1, very much improved; 2, much improved; 3, minimally improved; 4, no change; 5, minimally worse; 6, much worse; and 7, very much worse. Response was defined as the proportion of patients rated “very much improved” or “much improved” by investigators according to the overall improvement rating using the 7-rank CGI-I scale. Patients comorbid with depression/depressive state or OCD were also assessed for overall improvement in depression/depressive state or OCD at week 53 or at the discontinuation/conclusion of fluvoxamine treatment.
Severity of SAD symptoms and responses to treatment were also assessed using the LSAS-J. LSAS-J scores were recorded at baseline and weeks 12, 24, 36, 48, and 53 of treatment, or at the time of discontinuation/conclusion of fluvoxamine treatment. The Japanese version of the Sheehan Disability Scale (SDISS) was also used to assess the severity of psychosocial disorder (work/school, social life, home life, or family responsibilities), which was used as a measure of quality of life (QOL).
Statistical Analysis
Logistic regression analysis was used to assess the effects of factors that may affect occurrence of ADRs and improvement in SAD. The one-sample Wilcoxon signed rank test and t test were used to compare total LSAS-J and SDISS scores before and after treatment, and the chi-square test was used for other analyses. A two-sided p value less than 0.05 was considered significant.
Ethics Approval
The survey at each medical institution was conducted according to the clinical research agreement between the institution and Abbott Japan or Meiji Seika Pharma, and the protocol, including all ethical items, was approved by the institutional review board at each institution.
Results
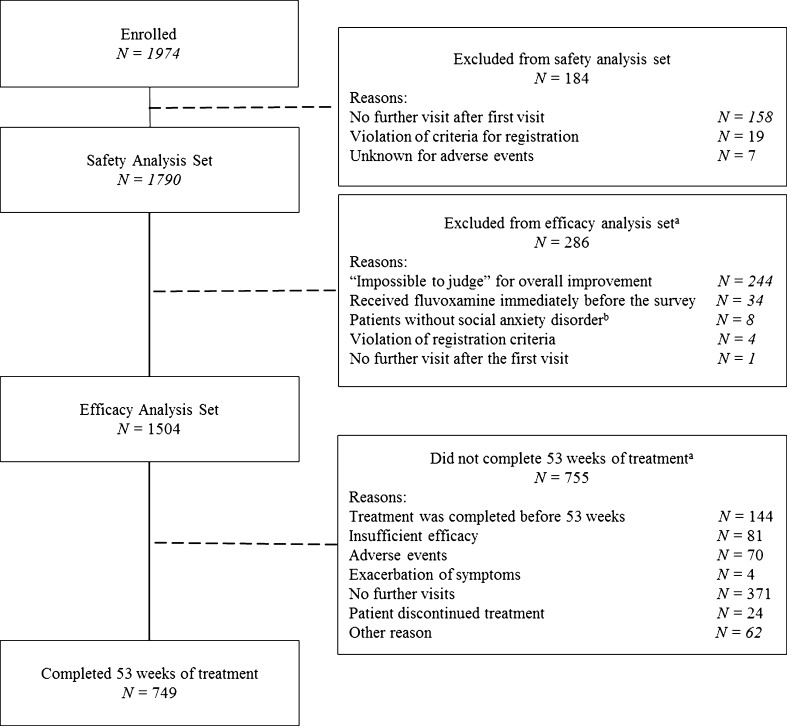
CRFs for 1,974 patients were retrieved from 407 medical institutions nationwide. Of these, 184 patients were excluded from the analysis (the majority because of lack of follow-up visits), leaving 1,790 patients in the safety analysis set (Fig. 1). Among the 1,790 patients eligible for safety analysis, 286 patients were excluded from the efficacy analysis set, with the remaining 1,504 patients analyzed for efficacy. A total of 749 patients received fluvoxamine for the full 53-week observation period.
Fig. 1.
Flow chart of patients through the study. aSome patients had more than one reason for exclusion; bpsychiatric illnesses included schizophrenia (n = 6), borderline personality disorder (n = 1), and obsessive-compulsive disorder (n = 1)
The baseline characteristics of the 1,790 patients included in the safety analysis set are shown in Table 1. Patients had a mean age of 32.6 ± 11.3 years and 49.4 % of patients were male. Comorbid disorders were present in 41.1 % of patients, with approximately half of these patients comorbid with depression or depressive state. In the majority of patients (75.4 %), fluvoxamine was given in combination with an antianxiety drug such as alprazolam, etizolam, and ethyl loflazepate. The mean daily dose of fluvoxamine reported for each patient was 25–50 mg in 24.0 %, 75–100 mg in 23.0 %, and more than 100 mg in 33.7 % (Table 1).
Table 1.
Baseline characteristics of the safety analysis set
| Characteristic | Safety analysis set [N = 1,790; n (%)] |
|---|---|
| Age, years | |
| Mean ± SD | 32.6 ± 11.3 |
| Range | 11–83 |
| Sex | |
| Male | 885 (49.4) |
| Comorbid disordersa | 736 (41.1) |
| Depression or depressive state | 377 (21.1) |
| Obsessive compulsive disorder | 58 (3.2) |
| Hepatic diseases | 47 (2.6) |
| Renal diseases | 7 (0.4) |
| Other | 489 (27.3) |
| Concomitant therapies | 1,349 (75.4) |
| Alprazolam | 361 (20.2) |
| Etizolam | 237 (13.2) |
| Ethyl loflazepate | 221 (12.3) |
| Mosapride citrate hydrate | 220 (12.3) |
| Sulpiride | 211 (11.8) |
| Mean daily dose of fluvoxamine, mg | |
| ≤25 | 65 (3.6) |
| 25–50 | 429 (24.0) |
| 50–75 | 277 (15.5) |
| 75–100 | 412 (23.0) |
| 100–125 | 150 (8.4) |
| 125–150 | 343 (19.2) |
| >150 | 109 (6.1) |
| Not known | 5 (0.3) |
| Total LSAS-J score | |
| ≤29 | 97 (5.4) |
| 30–59 | 384 (21.5) |
| 60–89 | 626 (35.0) |
| ≥90 | 536 (29.9) |
| Not assessed | 147 (8.2) |
All values are n (%) unless otherwise stated
LSAS-J Liebowitz Social Anxiety Scale-Japanese version, SD standard deviation
aData missing for 11 (0.6 %) patients
In addition, over the observation period, the mean daily dose of fluvoxamine was higher among patients with higher baseline total LSAS-J scores (Supplementary Tables 1, 2). Furthermore, the prevalence of comorbidity with depression/depressive state was higher among patients with higher total LSAS-J scores at baseline (Supplementary Table 3).
Safety
Over the observation period, 526 ADRs were reported in 326 patients. The most common ADRs included nervous system or gastrointestinal disorder-related events such as nausea (112 events), somnolence (85 events), constipation (22 events), and diarrhea (20 events) (Table 2). Non-serious ADRs accounted for 95.1 % of all the ADRs reported; 26 serious ADRs were reported in 15 patients, including six events of suicide attempt, three events of suicide ideation, and two events each of malaise, sedation, and somnolence.
Table 2.
Nervous system or gastrointestinal event-related adverse drug reactions reported in patients receiving fluvoxamine
| Any ADR | 326 (18.2) | Any gastrointestinal disorder | 173 (9.66) |
| Any nervous system disorder | 112 (6.26) | Abdominal discomfort | 11 (0.61) |
| Akathisia | 3 (0.17) | Abdominal distension | 1 (0.06) |
| Altered state of consciousness | 1 (0.06) | Abdominal pain | 5 (0.28) |
| Convulsion | 1 (0.06) | Abdominal pain upper | 9 (0.50) |
| Disturbance in attention | 2 (0.11) | Constipation | 22 (1.23) |
| Dizziness | 14 (0.78) | Diarrhoea | 20 (1.12) |
| Dizziness postural | 2 (0.11) | Dyspepsia | 1 (0.06) |
| Drug withdrawal headache | 1 (0.06) | Enteritis | 1 (0.06) |
| Head discomfort | 1 (0.06) | Flatulence | 1 (0.06) |
| Headache | 13 (0.73) | Gastritis | 6 (0.34) |
| Hypoesthesia | 4 (0.22) | Gastroduodenal ulcer | 1 (0.06) |
| Sedation | 6 (0.34) | Nausea | 112 (6.26) |
| Sensory loss | 1 (0.06) | Oral discomfort | 2 (0.11) |
| Somnolence | 85 (4.75) | Reflux esophagitis | 1 (0.06) |
| Tremor | 1 (0.06) | Stomach discomfort | 3 (0.17) |
| Poor quality sleep | 1 (0.06) | Vomiting | 2 (0.11) |
| Intestinal functional disorder | 1 (0.06) |
Safety analysis set [N = 1,790; n (%)]. Some patients had more than one ADR
ADR adverse drug reaction
Of the 526 ADRs reported during the observation period, 33.5 % (176/526 events) developed in the first week of treatment and 55.7 % (293/526 events) developed in the first 4 weeks of treatment (Supplementary Table 4). The incidence of ADRs reported in the second half of the observation period (week 25–53) was lower than that during the first 24 weeks of treatment, and there was no tendency toward an increase in the incidence of ADRs in patients receiving long-term treatment with fluvoxamine. ADRs were totally improved in 86.5 % of cases.
Factors possibly affecting the occurrence of ADRs with fluvoxamine treatment include gender, comorbid disorders or complications, hepatic diseases, and allergies. Over the observation period, the incidence of ADRs was 16.2 % in men and 20.2 % in women, and was higher in patients with comorbid disorders or complications, hepatic diseases, or history of allergy than those without the corresponding factor (Supplementary Table 5). The analysis of the relationship between dose and incidence of ADRs based on the total dose at the onset of these ADRs revealed a higher incidence among patients with a smaller total dose (Table 3), which indicates that patients experienced ADRs mainly in the first weeks of fluvoxamine treatment at lower doses.
Table 3.
Effect of fluvoxamine dose and baseline LSAS-J scores on the occurrence of adverse drug reactions (ADRs)
| N a | ADRs, n (%) | Logistic regression analysis | |||
|---|---|---|---|---|---|
| OR | 95 % CI | p value | |||
| Total dose of fluvoxamine, gb | |||||
| ≤2 | 328 | 207 (63.1) | 0.631 | 0.594, 0.670 | <0.0001 |
| 2–4 | 126 | 26 (20.6) | |||
| 4–8 | 160 | 22 (13.8) | |||
| 8–12 | 118 | 19 (16.1) | |||
| 12–16 | 95 | 11 (11.6) | |||
| 16–20 | 157 | 10 (6.4) | |||
| 20–24 | 75 | 5 (6.7) | |||
| 24–28 | 81 | 11 (13.6) | |||
| 28–32 | 79 | 3 (3.8) | |||
| 32–36 | 68 | 2 (2.9) | |||
| 36–40 | 91 | 3 (3.3) | |||
| >40 | 408 | 4 (1.0) | |||
| Unknown | 4 | 3 (75.0) | |||
| Maximum daily dose of fluvoxamine, mgb | |||||
| ≤25 | 65 | 18 (27.7) | 0.714 | 0.663, 0.768 | <0.0001 |
| 25–50 | 423 | 142 (33.6) | |||
| 50–75 | 177 | 22 (12.4) | |||
| 75–100 | 447 | 79 (17.7) | |||
| 100–125 | 14 | 2 (14.3) | |||
| 125–150 | 505 | 46 (9.1) | |||
| >150 mg | 155 | 14 (9.0) | |||
| Final daily dose of fluvoxamine, mgb | |||||
| ≤25 | 118 | 22 (18.6) | 0.775 | 0.721, 0.833 | <0.0001 |
| 25–50 | 505 | 146 (28.9) | |||
| 50–75 | 172 | 23 (13.4) | |||
| 75–100 | 457 | 82 (17.9) | |||
| 100–125 | 11 | 3 (27.3) | |||
| 125–150 | 407 | 3 (8.6) | |||
| >150 | 116 | 12 (10.3) | |||
| Baseline LSAS-J total score | |||||
| ≤29 | 97 | 16 (16.5) | 1.007 | 0.956, 1.060 | 0.7961 |
| 30–39 | 81 | 12 (14.8) | |||
| 40–49 | 130 | 25 (19.2) | |||
| 50–59 | 173 | 35 (20.2) | |||
| 60–69 | 191 | 35 (18.3) | |||
| 70–79 | 224 | 40 (17.9) | |||
| 80–89 | 211 | 43 (20.4) | |||
| 90–99 | 213 | 37 (17.4) | |||
| ≥100 | 323 | 59 (18.3) | |||
| ≤59 | 481 | 88 (18.3) | 1.008 | 0.766, 1.327 | 0.9540 |
| ≥60 | 1,162 | 214 (18.4) | |||
ADR adverse drug reaction, CI confidence interval, LSAS-J Liebowitz Social Anxiety Scale-Japanese version, N number of patients, OR odds ratio
aPatients with no relevant information were excluded from analysis
bTotal dose of fluvoxamine for patients with ADRs was calculated as total dose by the onset of the first ADR
Four ADRs were considered important items of investigation in this survey (ADRs related to suicide attempt, gastrointestinal hemorrhage, cardiovascular ADRs, and sexual function disorders). No patients experienced gastrointestinal hemorrhage during the observation period.
Over the observation period, 12 ADRs related to suicide attempt were reported in nine patients and consisted of six suicide attempts, three events of suicidal ideation, two events of intentional overdose, and one event of self-injurious behavior. The 12 ADRs related to suicide attempt consisted of two non-serious events of intentional overdose and ten serious ADRs. Patients recovered from five events, the remaining events were considered “improved” (one event), “not recovered” (three events), and “unknown” (one event). Patient characteristics analysis showed that age and comorbidity with depression/depressive state were significantly associated with the occurrence of attempted suicide. Among the nine patients in this study who attempted suicide, five patients were 24 years of age or younger, and three of them had depression/depressive state (Supplementary Table 6).
An ECG evaluation was requested as an important but non-mandatory item in CRFs and conducted in 281 patients. However, only 62 of these patients underwent ECG both before and after fluvoxamine treatment and the other 219 patients lacked any of data to be evaluated. Completed data of the 62 patients showed no prolonged QT interval which had been reported as a rare finding of clinically problematic abnormality of fluvoxamine before initiation of this survey [15, 16]. One patient was reported to show “ventricular extrasystoles” at 1 month after the start of administration but no adverse event affecting the cardiovascular system was reported for this patient. Independently from this ECG evaluation, cardiovascular ADRs were reported in eight patients and included six events of palpitation and one event each of sinus tachycardia and tachycardia. The eight cardiovascular ADRs were not serious, and the outcome was “recovered” or “improved”. An analysis of factors influencing the rate of cardiovascular ADRs revealed that a lower total dose of fluvoxamine at the onset of cardiovascular ADRs was significantly associated with a higher incidence of cardiovascular ADRs (Supplementary Table 7). No ECG data were available for these eight patients.
Eight events of sexual function disorders were reported in six patients, including delayed ejaculation, ejaculation disorder, sexual dysfunction, and erectile dysfunction with two of each event reported. All eight ADRs were not considered serious. Two events were not considered recovered (delayed ejaculation and sexual dysfunction) while the remaining events were all considered “recovered” or “improved”. Patient characteristic analysis showed that the presence of past illness and lower baseline total LSAS-J scores were significantly associated with a greater incidence of sexual function disorders (Supplementary Table 8).
Efficacy
Overall Improvement Rating
A clinical response rate was reported in 62.6 % of the 1,504 patients analyzed for efficacy and 78.4 % of the 749 patients who received fluvoxamine treatment for the entire 53-week observation period. In the patients who received 53 weeks of fluvoxamine treatment, the response rate to fluvoxamine treatment was higher in patients who did not have any complications or comorbid disorders and also in patients without comorbidity with depression/depressive state. In contrast, concomitant drug treatment had no significant effect on the response rate (Supplementary Table 9). The response rate was also higher in patients with mild SAD (indicated by lower total LSAS-J and SDISS scores at baseline; Table 4). Similar results were obtained in the 1,504 patients eligible for efficacy analysis.
Table 4.
Relationship of responder rate to fluvoxamine dose, baseline LSAS-J, and SDISS scores
| N a | Response n (%) | Logistic regression analysis | |||
|---|---|---|---|---|---|
| OR | 95 % CI | p value | |||
| Total dose of fluvoxamine, gb | |||||
| ≤2 | 0 | 0 (0) | 0.944 | 0.873, 1.020 | 0.1440 |
| 2–4 | 0 | 0 (0) | |||
| 4–8 | 0 | 0 (0) | |||
| 8–12 | 12 | 10 (83.3) | |||
| 12–16 | 6 | 5 (83.3) | |||
| 16–20 | 81 | 66 (81.5) | |||
| 20–24 | 43 | 37 (86.0) | |||
| 24–28 | 42 | 35 (83.3) | |||
| 28–32 | 42 | 30 (71.4) | |||
| 32–36 | 44 | 33 (75.0) | |||
| 36–40 | 81 | 67 (82.7) | |||
| >40 | 398 | 304 (76.4) | |||
| Maximum daily dose of fluvoxamine, mgb | |||||
| ≤25 | 10 | 8 (80.0) | 0.894 | 0.805, 0.992 | 0.0355 |
| 25–50 | 110 | 93 (84.5) | |||
| 50–75 | 73 | 57 (78.1) | |||
| 75–100 | 174 | 141 (81.0) | |||
| 100–125 | 6 | 5 (73.3) | |||
| 125–150 | 283 | 214 (75.6) | |||
| >150 mg | 93 | 69 (74.2) | |||
| Final daily dose of fluvoxamine, mgb | |||||
| ≤25 | 33 | 29 (87.9) | 0.876 | 0.795, 0.966 | 0.0080 |
| 25–50 | 151 | 125 (82.8) | |||
| 50–75 | 74 | 57 (77.0) | |||
| 75–100 | 190 | 154 (81.1) | |||
| 100–125 | 6 | 5 (83.3) | |||
| 125–150 | 224 | 166 (74.1) | |||
| >150 | 71 | 51 (71.8) | |||
| Baseline LSAS-J total score | |||||
| ≤29 | 39 | 36 (92.3) | 0.893 | 0.826, 0.966 | 0.0048 |
| 30–39 | 29 | 20 (69.0) | |||
| 40–49 | 52 | 46 (88.5) | |||
| 50–59 | 71 | 60 (84.5) | |||
| 60–69 | 83 | 68 (81.9) | |||
| 70–79 | 100 | 75 (75.0) | |||
| 80–89 | 92 | 68 (73.9) | |||
| 90–99 | 94 | 74 (78.7) | |||
| ≥100 | 147 | 106 (72.1) | |||
| ≤59 | 191 | 162 (84.8) | 0.560 | 0.359, 0.873 | 0.0104 |
| ≥60 | 516 | 391 (75.8) | |||
| Baseline SDISS total score | |||||
| ≤9 | 168 | 139 (82.7) | 0.665 | 0.502, 0.882 | 0.0046 |
| 10–19 | 362 | 294 (81.2) | |||
| ≥20 | 134 | 92 (68.7) | |||
CI confidence interval, LSAS-J Liebowitz Social Anxiety Scale-Japanese version, N number of patients, OR odds ratio, SDISS Sheehan Disability Scale
aPatients with no relevant information were excluded from analysis
bTotal dose of fluvoxamine for patients with ADRs was calculated as total dose by the onset of the first ADR
Change Over Time in Overall Improvement Rating
The response rates at weeks 12, 24, 36, 48, and 53 in the 749 patients who received fluvoxamine for the full 53 weeks of observation were 39.6, 57.3, 68.5, 74.3, and 78.4 %, respectively, showing an increase in response over time (Supplementary Fig. 1). Similarly, the response rates at weeks 12, 24, 36, 48, and 53 in the 1,504 patients analyzed for efficacy were 39.7, 56.0, 67.6, 74.1, and 78.4 %, respectively, indicating an increase in response rate in association with a longer duration of treatment.
Change Over Time in the LSAS-J Score
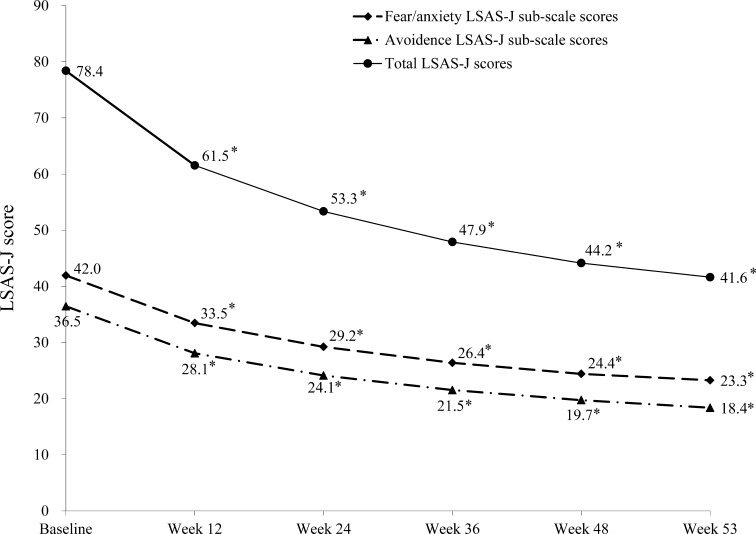
Over the 53-week fluvoxamine treatment period, total LSAS-J scores decreased rapidly during the first 12 weeks, and continued to significantly decrease over the remainder of the treatment period (Fig. 2; all p < 0.0001, n = 184). Similarly, the fear/anxiety and avoidance LSAS-J sub-scale scores substantially decreased over the first 12 weeks of fluvoxamine treatment and continued to decrease over the remainder of the trial (p < 0.0001 at all time points).
Fig. 2.
Change from baseline over the 53-week study period in total, fear/anxiety sub-scale, and avoidance sub-scale scores on the Liebowitz Social Anxiety Scale-Japanese version (LSAS-J) with fluvoxamine treatment. *p < 0.0001 vs. baseline
Change Over Time in SDISS
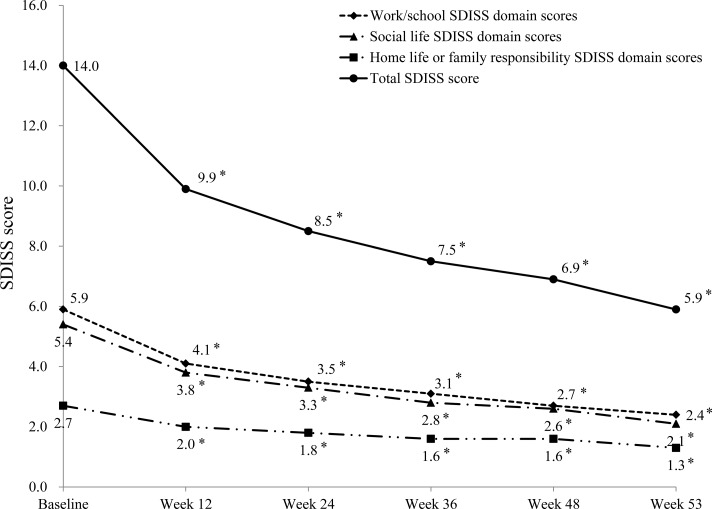
Among 749 patients who continued fluvoxamine for 53 weeks, 167 patients were evaluated using the SDISS. At baseline, the proportion of patients rated as “unimpaired” or “slightly impaired” was 15.0, 21.6, and 67.7 % in the “work/school”, “social life”, and “home life or family responsibilities” domains, respectively. With fluvoxamine treatment the proportion of patients rated as unimpaired or slightly impaired increased to 38.3, 46.1, and 77.8 % of patients at week 12, and to 80.2, 83.2, and 91.0 % at week 53, respectively, indicating a continuous improvement in QOL during treatment with fluvoxamine (Fig. 3; all p < 0.0001).
Fig. 3.
Change from baseline over the 53-week study period in total, work/study domain, social life, and home life or family responsibilities domain scores on the Sheehan Disability Scale (SDISS) with fluvoxamine treatment. *p < 0.0001 vs. baseline
Fluvoxamine Efficacy in Patients Comorbid with Depression or Depressive State
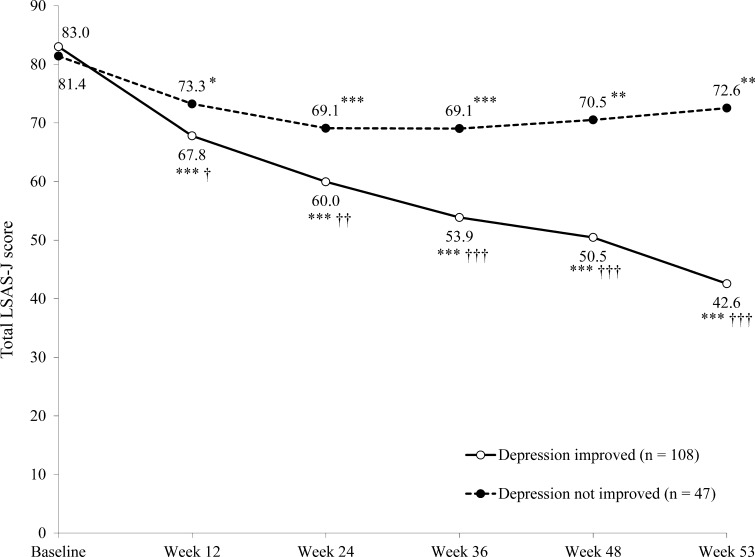
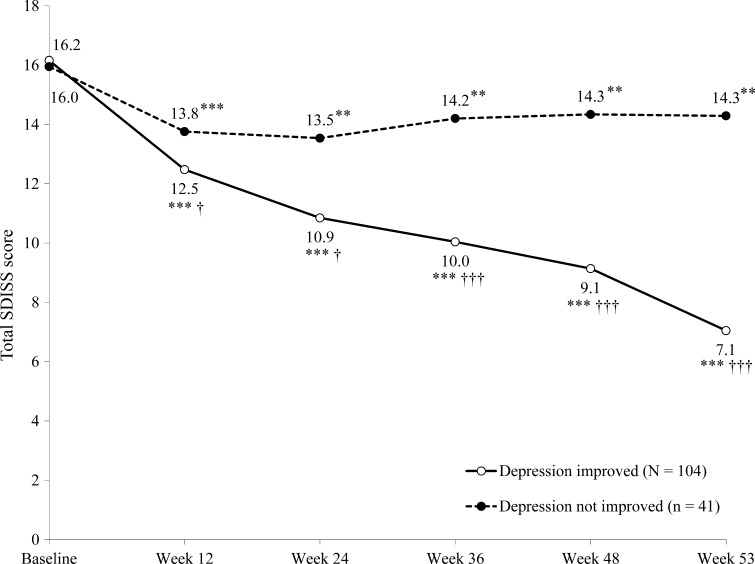
In the 163 patients comorbid with depression at baseline who completed 53 weeks of fluvoxamine treatment, significantly more patients whose depression improved over the observation period had a response in SAD with fluvoxamine treatment (86.4 vs. 35.8 % of patients in the depression improvement and non-improvement groups, respectively; p < 0.0001). Furthermore, patients whose depression improved over the observation period had a significantly greater reduction in total LSAS-J score from baseline to week 53 than those whose depression did not improve (Fig. 4). Similar results were observed with the change in total SDISS scores over the observation period (Fig. 5).
Fig. 4.
Change from baseline over the 53-week study period in total Liebowitz Social Anxiety Scale-Japanese version (LSAS-J) score in patients who did or did not report an improvement in depression (n = 155). *p < 0.05 vs. baseline; **p < 0.01 vs. baseline; ***p < 0.001 vs. baseline; † p < 0.05 vs. patients whose depression did not improve; †† p < 0.01 vs. non-improved patients; ††† p < 0.0001 vs. non-improved patients
Fig. 5.
Change from baseline over the 53-week study period in total Sheehan Disability Scale (SDISS) score in patients who did or did not report an improvement in depression (n = 145). *p < 0.05 vs. baseline; **p < 0.01 vs. baseline; ***p < 0.001 vs. baseline; † p < 0.05 vs. patients whose depression did not improve; †† p < 0.01 vs. non-improved patients; ††† p < 0.0001 vs. non-improved patients
Discussion
The efficacy of fluvoxamine for the treatment of SAD has previously been demonstrated in both non-Japanese [17, 18] and Japanese [6] patients in studies of 10–12 weeks duration. Furthermore, the long-term treatment (24 weeks) of patients with SAD with a controlled-release formulation of fluvoxamine (fluvoxamine CR) has also been shown and confirmed the benefit of the pharmacotherapy for SAD [19]. The current report describes for the first time the results of a survey on the longer-term (53 weeks) safety and efficacy of fluvoxamine in a sufficiently large number of Japanese patients with SAD in the clinical setting. Indeed, a total of 1,974 patients from 407 nationwide medical institutions participated in the survey to cover a wide range of age (11–83 years old) and comorbidity with depression/depressive state. SAD often develops in patients in their mid-teens and early 20s, and many patients have SAD for several years before visiting the clinic [20]. The patient population in the present survey, in which patients in their 20s were the largest age group, and those in their 20s and 30s accounted for about 65 % of participants, well represents patients with SAD in Japan. Therefore, the current report well represents outcomes of SAD treatment with fluvoxamine in the ‘real world’.
Of the 1,790 patients analyzed for safety, 18.2 % experience an ADR. Nausea, somnolence, and constipation were most commonly observed, which was consistent with previous studies [1–5, 21]. Although 26 serious ADRs occurred in 15 patients, 95.1 % of the ADRs reported in the survey were not serious. Of the 526 ADRs reported during the observation period, the majority of the events developed in the first 4 weeks of fluvoxamine treatment. There was no tendency for the incidence of ADRs to increase over time during treatment. An analysis of the incidence of ADRs by maximum daily dose of fluvoxamine did not reveal a dose-related increase in the incidence of ADRs. In fact, the incidence of ADRs was higher during the first few weeks of treatment, during which patients received fluvoxamine at lower doses. This suggests that physicians should carefully observe their patients for occurrence of ADRs during the first weeks of treatment.
In this observational study, four ADRs were considered important items of investigation in this survey (ADRs related to suicide attempt, gastrointestinal hemorrhage, cardiovascular ADRs, and sexual function disorders). ADRs related to suicide attempt were considered an important item, as it has been shown previously that some antidepressants increase the risk of suicide ideation and behavior in children, adolescents, and young adults (aged 18–24) during initial treatment [22]. It has also been reported that the combination of SSRIs and non-steroidal anti-inflammatory drugs increases the risk of upper gastrointestinal hemorrhage [23–25]. Although the effects of fluvoxamine on the cardiovascular system are reported to be less severe than those of tricyclic antidepressants [26], and the incidence of cardiovascular ADRs associated with fluvoxamine was significantly lower as compared with imipramine [21], there have been spontaneous reports of cardiovascular ADRs such as prolonged QT interval in association with fluvoxamine treatment in Japan [27]. As such we carefully monitored these ADRs during this study. In addition to the above important items of investigation, occurrence of sexual dysfunction was investigated, as a high rate of this ADR has been reported in a Western clinical study [28] but was low in a study in Japan [21].
Age and the presence of depression/depressive state were identified as significant factors potentially affecting the rate of suicide attempt in this study. As such close observation of the condition and change of pathophysiology during the early phase of treatment with fluvoxamine, or after changing its dose, is important in patients under 24 years of age with SAD comorbid with depression/depressive state.
All cardiovascular ADRs observed in the survey were not serious and no patient showed QT prolongation or other ECG findings. Since the total dose of fluvoxamine was analyzed by the onset of the ADRs and many of them developed in the first 4 weeks of treatment, the incidence of ADRs was significantly higher in patients with a smaller total dose. Therefore, physicians should carefully observe their patients for cardiovascular ADRs to fluvoxamine which tend to develop more frequently in the early phase of treatment, as in the case of other types of ADRs.
The response to fluvoxamine observed in this survey was similar to that shown in another 52-week open-label long-term clinical study of SAD in Japan [6]. For overall improvement rating, the response rate in 749 patients who continued fluvoxamine treatment for 53 weeks was 39.6 % at week 12, and 78.4 % at week 53, showing an increase in response rate over time. The total LSAS-J score also significantly decreased from 78.4 ± 26.4 points at baseline to 61.5 ± 26.6 points at week 12, and 41.6 ± 27.8 points at week 53 (p < 0.0001). Both overall improvement rating and total LSAS-J score significantly improved in the first 12 weeks of fluvoxamine treatment and continued to improve by week 53. Therefore, long-term efficacy of fluvoxamine in the treatment of SAD was confirmed. It is notable that there was no difference between patient groups with or without concomitant drugs, suggesting efficacy of long-term monotherapy with fluvoxamine.
SSRIs are commonly used as first-line pharmacotherapy for SAD on the basis of their clinical merits compared to placebo, and response rates in controlled studies were reported to range from 43 to 70 % in active arms (7–32 % in placebo) [17, 18, 29–33]. In agreement with such previous reports, overall response rate to fluvoxamine in this observation study was 62.6 % and the other 37.4 % of patients needed second-line treatment. No standardized guideline indicating treatment options for non-responder SAD patients to SSRIs is available, so far. Although some reports suggest positive effects of switching to a different SSRI or other class of pharmacotherapy, including serotonin–noradrenaline reuptake inhibitors, antiepileptic drugs, benzodiazepines, and antipsychotics, scientific evidence is limited to support their clinical merits [34, 35]. The value of cognitive behavioral therapy (CBT) for SSRI non-responder patients of other anxiety disorders such as obsessive-compulsive disorder or panic disorder was systematically reviewed [36]. However, clinical data to support the merits of CBT for SSRI non-responder SAD patients are not abundant enough to be discussed and we need to wait for upcoming results of controlled studies [37, 38]. In conclusion, fluvoxamine should be used for SAD treatment but with an understanding of its response rate described here, accompanied by careful monitoring of serious ADRs, such as suicide attempt in cases of young patients.
The response rate of SAD in the current survey was significantly affected by the presence of comorbidity of depression or depressive state. Indeed, SAD is often associated with depression: an epidemiological study revealed that 34 % of patients with SAD have comorbidity of depression as well [39]. The presence of depression/depressive state is believed to be an important predictor of poor response to treatment in patients with SAD [40, 41]. The response rate was high in both groups of patients but the response rate was about 10 % lower in patients with comorbidity of depression/depressive state: among patients who continued fluvoxamine for 53 weeks, the response rate was 80.7 % in patients without comorbidity of depression or depressive state and 69.9 % in patients with it. In order to clarify the treatment effect of fluvoxamine on SAD patients with depression, 163 out of 749 patients who had depression/depressive state and continued fluvoxamine treatment for 53 weeks were stratified into those whose depression/depressive state was improved over the study period versus those whose depression did not improve, and analyzed for the improvement rate of SAD. The response rate was 35.8 % in patients whose depression/depressed state did not improve and 86.4 % in patients with its improvement. The analysis of change over time of total LSAS-J score revealed that the score did not decrease at week 36 and thereafter in patients without improvement of depression/depressive state, whereas it continued to decrease significantly at all points from week 12 through to 53 in patients with the improvement, resulting in significantly higher treatment response to fluvoxamine. The total SDISS score, a measure of QOL, also continued to decrease significantly from week 12 through to week 53 in patients with improvement in depression/depressive state, demonstrating their good response to fluvoxamine.
It is generally accepted that depression–anxiety comorbidity results in poor treatment outcomes and requires a more careful treatment strategy [42]. The results of the current survey indicate that the response rate of SAD patients with depression/depressive state to fluvoxamine was significantly lower and improvement in their depression/depressive state by fluvoxamine in SAD patients could predict better improvement in symptoms of SAD in a real-world setting. It has also been reported that improvement in depression/depressive state is followed by improvement in SAD [40]. Therefore, the survey indicates again the importance of the treatment of depression/depressive state in addition to that of SAD in patients where these conditions coexist. On the other hand, a previous report showed no significant difference of treatment response to another SSRI, escitalopram, between SAD patients with and without depressive state during up to 24 weeks of treatment [43]. The longer observation period (53 weeks) of our survey might be better to find the difference and/or exclusion criteria of such controlled studies that might cause a kind of mask compared to the real-world survey. Alternatively, pharmacological differences between fluvoxamine and escitalopram could bring different outcomes on this point. Indeed, fluvoxamine has a unique property as a sigma-1 agonist that seems to contribute its clinical effects in part [44]. Continued effects of fluvoxamine on responders with depression/depressive state might come from network remodeling through sigma-1 agonism [45], although it requires more studies to be proved.
Finally, it should be noticed that there are limitations in this study caused by its design. All of the observations in this study do not have independent controls because the study was performed under clinical settings in the “real world”. Concerning comorbidity with depression/depressive state, no detailed data were obtained in this survey, because the protocol did not contain full assessment of depression by structured interview. Therefore, we cannot exclude possible cases of major depression, dysthymic disorder, or bipolar depression in this study.
Conclusions
This study confirms the long-term efficacy and safety of fluvoxamine for the treatment of SAD in Japanese patients in a clinical setting. Most ADRs were observed in the early treatment phase, and improvement of coexisting depression/depressive state was confirmed as an important factor influencing the treatment response of SAD itself. The findings in this study are an important addition to the literature on SAD, showing how to treat the disorder appropriately with fluvoxamine.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
This study was required by the Pharmaceuticals and Medical Devices Agency and funded by Abbott Japan Co., Ltd. and Meiji Seika Pharma Co., Ltd., the marketing authorization holders (MAHs) of fluvoxamine in Japan. These two companies cooperatively designed and operated the study. AbbVie GK (Japan) has been the MAH instead of Abbott Japan Co., Ltd. since 2013 and funded development of this report together with Meiji Seika Pharma Co., Ltd. Authors S.A. and T.K. have no conflict of interest on this study. Authors H.K., T.H., and Y.K. are employees of Meiji Seika Pharma Co., Ltd., Abbott Japan Co., Ltd., and AbbVie GK (Japan), respectively. The authors gratefully acknowledge the cooperation of the participating medical institutions and investigators that provided important data on patients participating in this special surveillance of fluvoxamine. The authors would also like to thank Simone Boniface of inScience Communications, Springer Healthcare, who provided medical writing assistance funded by AbbVie GK (Japan) and Meiji Seika Pharma Co., Ltd.
References
- 1.Murakami M, Mori A, Miura S, Kurihara M, Yamashita I, Asai M, et al. Clinical evaluation of SME3110 (fluvoxamine maleate), a selective serotonin reuptake inhibitor, in depression or depressive state: a double blind study versus amitriptyline hydrochloride [in Japanese] Rinsho Iyaku. 1998;14(5):951–980. [Google Scholar]
- 2.Namiki M, Muto E, Minemoto H, Ishikawa Y, Okuse T, Hisamura M, et al. A phase III clinical study of SME3110 (fluvoxamine maleate) in depression in the field of internal medicine: a double blind study versus trazodone hydrochloride [in Japanese] Rinsho Iyaku. 1996;12(4):651–677. [Google Scholar]
- 3.Oka I, Ito K, Narita H, Matsubara S, Matsubara R. Clinical evaluation of SME3110 (fluvoxamine maleate) in depression and depressive state: a long-term treatment study [in Japanese] Rinsho Iyaku. 1996;12(3):471–487. [Google Scholar]
- 4.Nakajima T, Kudo Y, Yamashita I, Asai M, Kamijima K, Murasaki M, et al. A long-term treatment study of fluvoxamine maleate (SME3110), a selective serotonin reuptake inhibitor, in obsessive-compulsive disorder [in Japanese] Rinsho Iyaku. 1996;12(4):679–700. [Google Scholar]
- 5.Nakajima T, Kudo Y, Yamashita I, Asai M, Kamijima K, Murasaki M, et al. A late phase II clinical study of fluvoxamine maleate (SME3110), a selective serotonin reuptake inhibitor, in obsessive-compulsive disorder [in Japanese] Rinsho Iyaku. 1998;14(3):589–616. [Google Scholar]
- 6.Asakura S, Tajima O, Koyama T. Fluvoxamine treatment of generalized social anxiety disorder in Japan: a randomized double-blind, placebo-controlled study. Int J Neuropsychopharmacol. 2007;10(2):263–274. doi: 10.1017/S1461145706006602. [DOI] [PubMed] [Google Scholar]
- 7.Gaynes BN, Warden D, Trivedi MH, Wisniewski SR, Fava M, Rush AJ. What did STAR*D teach us? Results from a large-scale, practical, clinical trial for patients with depression. Psychiatr Serv. 2009;60(11):1439–1445. doi: 10.1176/appi.ps.60.11.1439. [DOI] [PubMed] [Google Scholar]
- 8.Hoertel N, Le Strat Y, Limosin F, Dubertret C, Gorwood P. Prevalence of subthreshold hypomania and impact on internal validity of RCTs for major depressive disorder: results from a national epidemiological sample. PLoS One. 2013;8(2):e55448. doi: 10.1371/journal.pone.0055448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blanco C, Olfson M, Goodwin RD, Ogburn E, Liebowitz MR, Nunes EV, et al. Generalizability of clinical trial results for major depression to community samples: results from the National Epidemiologic Survey on Alcohol and Related Conditions. J Clin Psychiatry. 2008;69(8):1276–1280. doi: 10.4088/JCP.v69n0810. [DOI] [PubMed] [Google Scholar]
- 10.Hoertel N, Le Strat Y, De Maricourt P, Limosin F, Dubertret C. Are subjects in treatment trials of panic disorder representative of patients in routine clinical practice? Results from a national sample. J Affect Disord. 2013;146(3):383–389. doi: 10.1016/j.jad.2012.09.023. [DOI] [PubMed] [Google Scholar]
- 11.Hoertel N, Le Strat Y, Blanco C, Lavaud P, Dubertret C. Generalizability of clinical trial results for generalized anxiety disorder to community samples. Depress Anxiety. 2012;29(7):614–620. doi: 10.1002/da.21937. [DOI] [PubMed] [Google Scholar]
- 12.Zimmerman M, Mattia JI, Posternak MA. Are subjects in pharmacological treatment trials of depression representative of patients in routine clinical practice? Am J Psychiatry. 2002;159(3):469–473. doi: 10.1176/appi.ajp.159.3.469. [DOI] [PubMed] [Google Scholar]
- 13.Zimmerman M, Chelminski I, Posternak MA. Generalizability of antidepressant efficacy trials: differences between depressed psychiatric outpatients who would or would not qualify for an efficacy trial. Am J Psychiatry. 2005;162(7):1370–1372. doi: 10.1176/appi.ajp.162.7.1370. [DOI] [PubMed] [Google Scholar]
- 14.Hoertel N, de Maricourt P, Katz J, Doukhan R, Lavaud P, Peyre H, et al. Are participants in pharmacological and psychotherapy treatment trials for social anxiety disorder representative of patients in real-life settings? J Clin Psychopharmacol. 2014 [Epub ahead of print]. [DOI] [PubMed]
- 15.Laird LK, Lydiard RB, Morton WA, Steele TE, Kellner C, Thompson NM, Ballenger JC. Cardiovascular effects of imipramine, fluvoxamine, and placebo in depressed outpatients. J Clin Psychiatry. 1993;54(6):224–228. [PubMed] [Google Scholar]
- 16.Claghorn JL, Earl CQ, Walczak DD, Stoner KA, Wong LF, Kanter D, Houser VP. Fluvoxamine maleate in the treatment of depression: a single-center, double-blind, placebo-controlled comparison with imipramine in outpatients. J Clin Psychopharmacol. 1996;16(2):113–120. doi: 10.1097/00004714-199604000-00003. [DOI] [PubMed] [Google Scholar]
- 17.van Vliet IM, den Boer JA, Westenberg HG. Psychopharmacological treatment of social phobia; a double blind placebo controlled study with fluvoxamine. Psychopharmacology (Berl). 1994;115(1–2):128–134. doi: 10.1007/BF02244762. [DOI] [PubMed] [Google Scholar]
- 18.Stein MB, Fyer AJ, Davidson JR, Pollack MH, Wiita B. Fluvoxamine treatment of social phobia (social anxiety disorder): a double-blind, placebo-controlled study. Am J Psychiatry. 1999;156(5):756–760. doi: 10.1176/ajp.156.5.756. [DOI] [PubMed] [Google Scholar]
- 19.Stein DJ, Westenberg HG, Yang H, Li D, Barbato LM. Fluvoxamine CR in the long-term treatment of social anxiety disorder: the 12- to 24-week extension phase of a multicentre, randomized, placebo-controlled trial. Int J Neuropsychopharmacol. 2003;6(4):317–323. doi: 10.1017/S146114570300364X. [DOI] [PubMed] [Google Scholar]
- 20.Keller MB. Social anxiety disorder clinical course and outcome: review of Harvard/Brown Anxiety Research Project (HARP) findings. J Clin Psychiatry. 2006;67(Suppl 12):14–19. [PubMed] [Google Scholar]
- 21.Asakura M, Tajima O. A randomized, double-blind study of fluvoxamine maleate in patents with depression or depressive state: a comparison in anticholinergic effects and cardiovascular adverse reactions between fluvoxamine maleate and imipramine hydrochloride [in Japanese] Jpn Pharmacol Ther. 2005;33(8):773–787. [Google Scholar]
- 22.United States Food and Drug Administration (FDA). Antidepressant use in children, adolescents, and adults. http://www.fda.gov/Drugs/DrugSafety/InformationbyDrugClass/ucm096273. Accessed 30 May 2013.
- 23.de Abajo FJ, Rodríguez LA, Motero D. Association between selective serotonin reuptake inhibitors and upper gastrointestinal bleeding: population based case-control study. BMJ. 1999;319(7217):1106–1109. doi: 10.1136/bmj.319.7217.1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dalton SO, Johansen C, Mellemkjer L, et al. Use of selective serotonin reuptake inhibitors and risk of upper gastrointestinal tract bleeding. Arch Intern Med. 2003;163(1):59–64. doi: 10.1001/archinte.163.1.59. [DOI] [PubMed] [Google Scholar]
- 25.Tada K, Uehara J, Matsuda E, Suzuki T, Watanabe M, Kojima T. Three cases of serious gastrointestinal hemorrhage during treatment with SSRIs and NSAIDs [in Japanese] Seishin Igaku. 2003;45(2):187–189. [Google Scholar]
- 26.Hochberg HM, Kanter D, Houser VP. Electrocardiographic findings during extended clinical trials of fluvoxamine in depression: one years experience. Pharmacopsychiatry. 1995;28(6):253–256. doi: 10.1055/s-2007-979612. [DOI] [PubMed] [Google Scholar]
- 27.Pharmaceuticals and Medical Devices Agency. Information on case reports of possible adverse drug reactions [in Japanese]. http://www.info.pmda.go.jp/fsearchnew/jsp/menu_fukusayou_base.jsp. Accessed 30 May 2013.
- 28.Montejo AL, Llorca G, Izquierdo JA, Rico-Villademoros F. Incidence of sexual dysfunction associated with antidepressant agents: a prospective multicenter study of 1022 outpatients. Spanish Working Group for the Study of Psychotropic-Related Sexual Dysfunction. J Clin Psychiatry. 2001;62(Suppl 3):10–21. [PubMed] [Google Scholar]
- 29.Stein MB, Liebowitz MR, Lydiard RB, Pitts CD, Bushnell W, Gergel I. Paroxetine treatment of generalized social phobia (social anxiety disorder): a randomized controlled trial. JAMA. 1998;280(8):708–713. doi: 10.1001/jama.280.8.708. [DOI] [PubMed] [Google Scholar]
- 30.Allgulander C. Paroxetine in social anxiety disorder: a randomized placebo-controlled study. Acta Psychiatr Scand. 1999;100(3):193–198. doi: 10.1111/j.1600-0447.1999.tb10845.x. [DOI] [PubMed] [Google Scholar]
- 31.Baldwin D, Bobes J, Stein DJ, Scharwächter I, Faure M. Paroxetine in social phobia/social anxiety disorder. Randomised, double-blind, placebo-controlled study. Paroxetine Study Group. Br J Psychiatry. 1999;175:120–126. doi: 10.1192/bjp.175.2.120. [DOI] [PubMed] [Google Scholar]
- 32.Katzelnick DJ, Kobak KA, Greist JH, Jefferson JW, Mantle JM, Serlin RC. Sertraline for social phobia: a double-blind, placebo-controlled crossover study. Am J Psychiatry. 1995;152(9):1368–1371. doi: 10.1176/ajp.152.9.1368. [DOI] [PubMed] [Google Scholar]
- 33.Van Ameringen MA, Lane RM, Walker JR, Bowen RC, Chokka PR, Goldner EM, et al. Sertraline treatment of generalized social phobia: a 20-week, double-blind, placebo-controlled study. Am J Psychiatry. 2001;158(2):275–281. doi: 10.1176/appi.ajp.158.2.275. [DOI] [PubMed] [Google Scholar]
- 34.Davidson JR. Pharmacotherapy of social anxiety disorder: what does the evidence tell us? J Clin Psychiatry. 2006;67(Suppl 12):20–26. [PubMed] [Google Scholar]
- 35.Canton J, Scott KM, Glue P. Optimal treatment of social phobia: systematic review and meta-analysis. Neuropsychiatr Dis Treat. 2012;8:203–215. doi: 10.2147/NDT.S23317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rodrigues H, Figueira I, Gonçalves R, Mendlowicz M, Macedo T, Ventura P. CBT for pharmacotherapy non-remitters—a systematic review of a next-step strategy. J Affect Disord. 2011;129(1–3):219–228. doi: 10.1016/j.jad.2010.08.025. [DOI] [PubMed] [Google Scholar]
- 37.Yoshinaga N, Ohshima F, Matsuki S, Tanaka M, Kobayashi T, Ibuki H, et al. A preliminary study of individual cognitive behavior therapy for social anxiety disorder in Japanese clinical settings: a single-arm, uncontrolled trial. BMC Res Notes. 2013;6:74. doi: 10.1186/1756-0500-6-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yoshinaga N, Niitsu T, Hanaoka H, Sato Y, Ohshima F, Matsuki S, et al. Strategy for treating selective serotonin reuptake inhibitor-resistant social anxiety disorder in the clinical setting: a randomised controlled trial protocol of cognitive behavioural therapy in combination with conventional treatment. BMJ Open. 2013;3(2). doi:10.1136/bmjopen-2012-002242. [DOI] [PMC free article] [PubMed]
- 39.Grant BF, Hasin DS, Blanco C, Stinson FS, Chou SP, Goldstein RB, et al. The epidemiology of social anxiety disorder in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. J Clin Psychiatry. 2005;66(11):1351–1361. doi: 10.4088/JCP.v66n1102. [DOI] [PubMed] [Google Scholar]
- 40.Schneier FR, Blanco C, Campeas R, Lewis-Fernandez R, Lin SH, Marshall R, et al. Citalopram treatment of social anxiety disorder with comorbid major depression. Depress Anxiety. 2003;17(4):191–196. doi: 10.1002/da.10112. [DOI] [PubMed] [Google Scholar]
- 41.Ballenger JC. Clinical guidelines for establishing remission in patients with depression and anxiety. J Clin Psychiatry. 1999;60(Suppl 22):29–34. [PubMed] [Google Scholar]
- 42.Hofmeijer-Sevink MK, Batelaan NM, van Megen HJ, Penninx BW, Cath DC, van den Hout MA, et al. Clinical relevance of comorbidity in anxiety disorders: a report from the Netherlands Study of Depression and Anxiety (NESDA) J Affect Disord. 2012;137(1–3):106–112. doi: 10.1016/j.jad.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 43.Stein DJ, Kasper S, Andersen EW, Nil R, Lader M. Escitalopram in the treatment of social anxiety disorder: analysis of efficacy for different clinical subgroups and symptom dimensions. Depress Anxiety. 2004;20(4):175–181. doi: 10.1002/da.20043. [DOI] [PubMed] [Google Scholar]
- 44.Hindmarch I, Hashimoto K. Cognition and depression: the effects of fluvoxamine, a sigma-1 receptor agonist, reconsidered. Hum Psychopharmacol. 2010;5(3):193–200. doi: 10.1002/hup.1106. [DOI] [PubMed] [Google Scholar]
- 45.van Waarde A, Ramakrishnan NK, Rybczynska AA, Elsinga PH, Ishiwata K, et al. The cholinergic system, sigma-1 receptors and cognition. Behav Brain Res. 2011;221(2):543–554. doi: 10.1016/j.bbr.2009.12.043. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.